Abstract
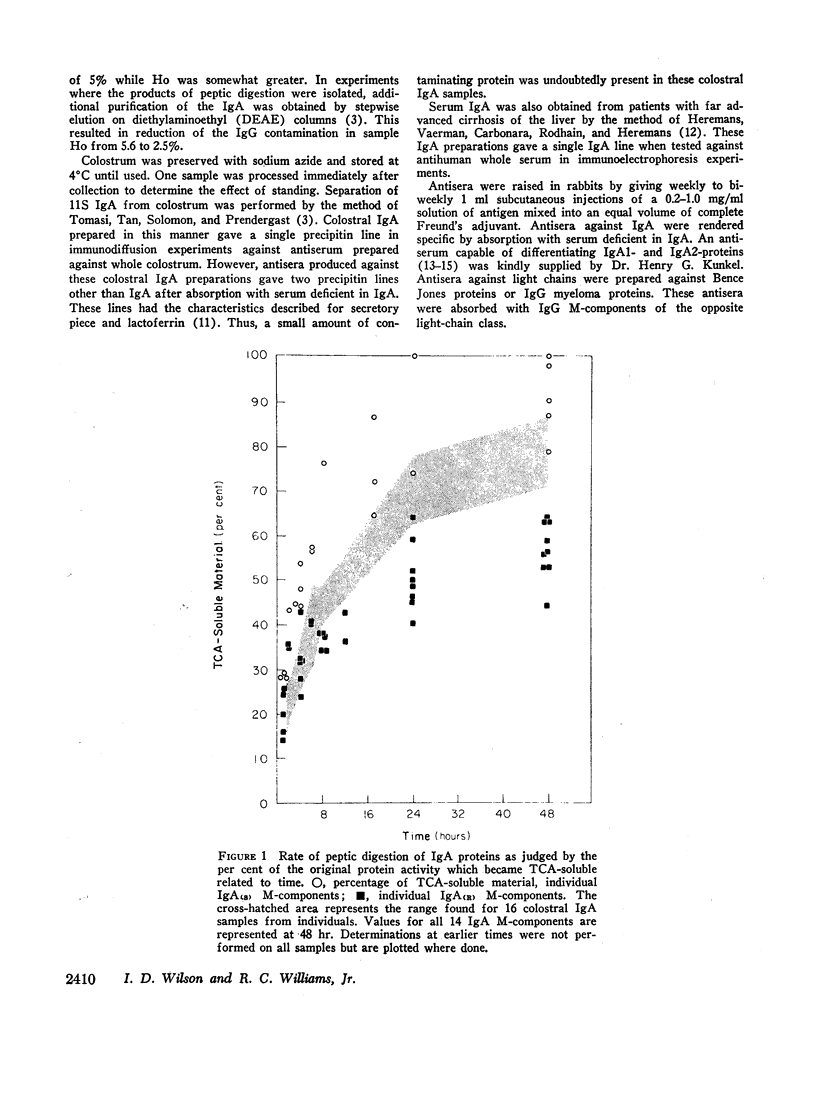
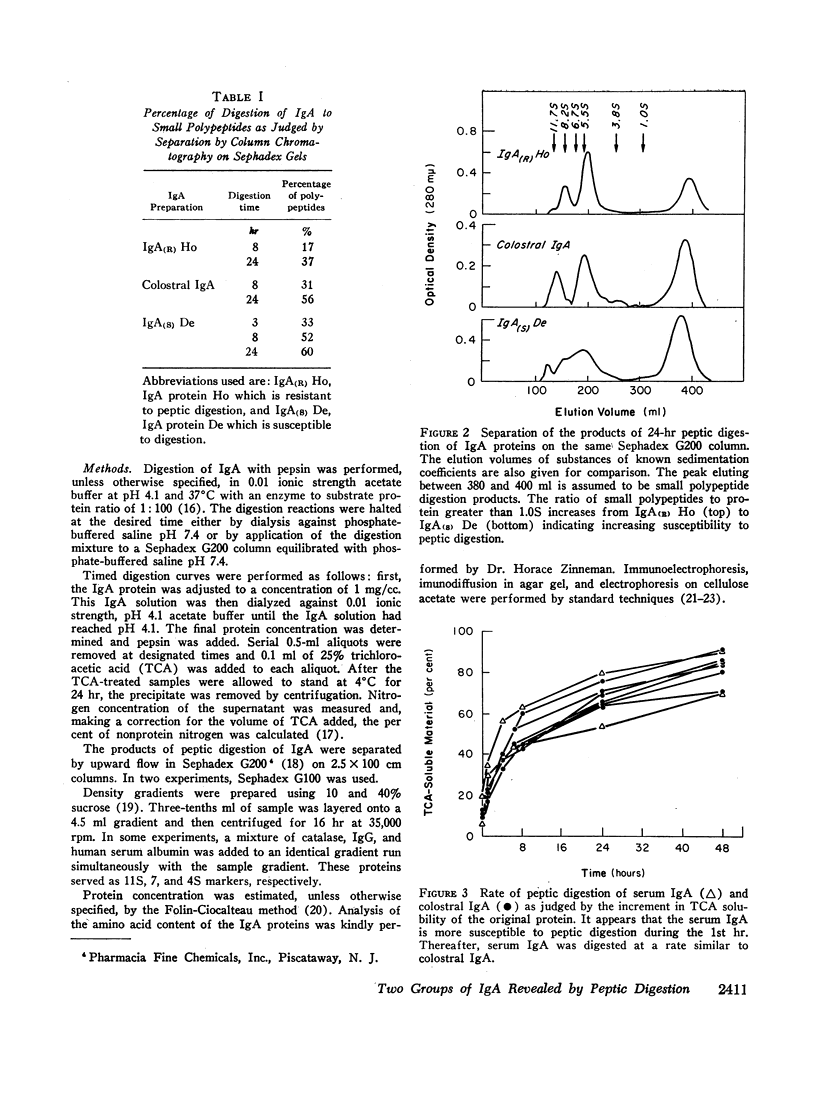
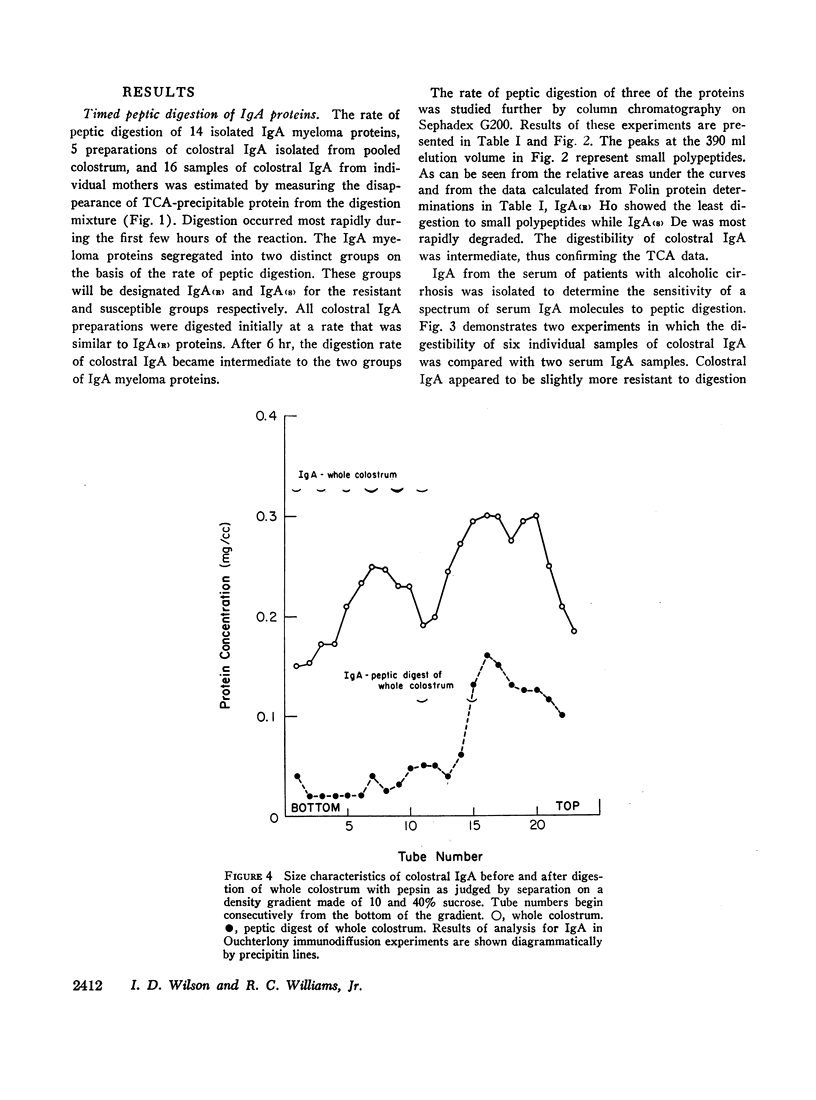
Serum IgA M-components, secretory IgA separated from colostrum, and IgA from serum of patients with cirrhosis of the liver were digested with pepsin at pH 4.1. The IgA M-components segregated into two groups on the basis of their relative rates of peptic digestion. Serum and colostral IgA were digested at a total rate intermediate to that of the two groups of IgA myeloma proteins. It appeared, however, that colostral IgA may have been initially more resistant to peptic digestion than serum IgA. The variability in the rate of peptic digestion was not related to electrophoretic mobility, light-chain type, or IgA subclass. Experimental conditions related to enzyme to substrate ratio or to the pH of the reaction mixture did not appear to explain the differences found.
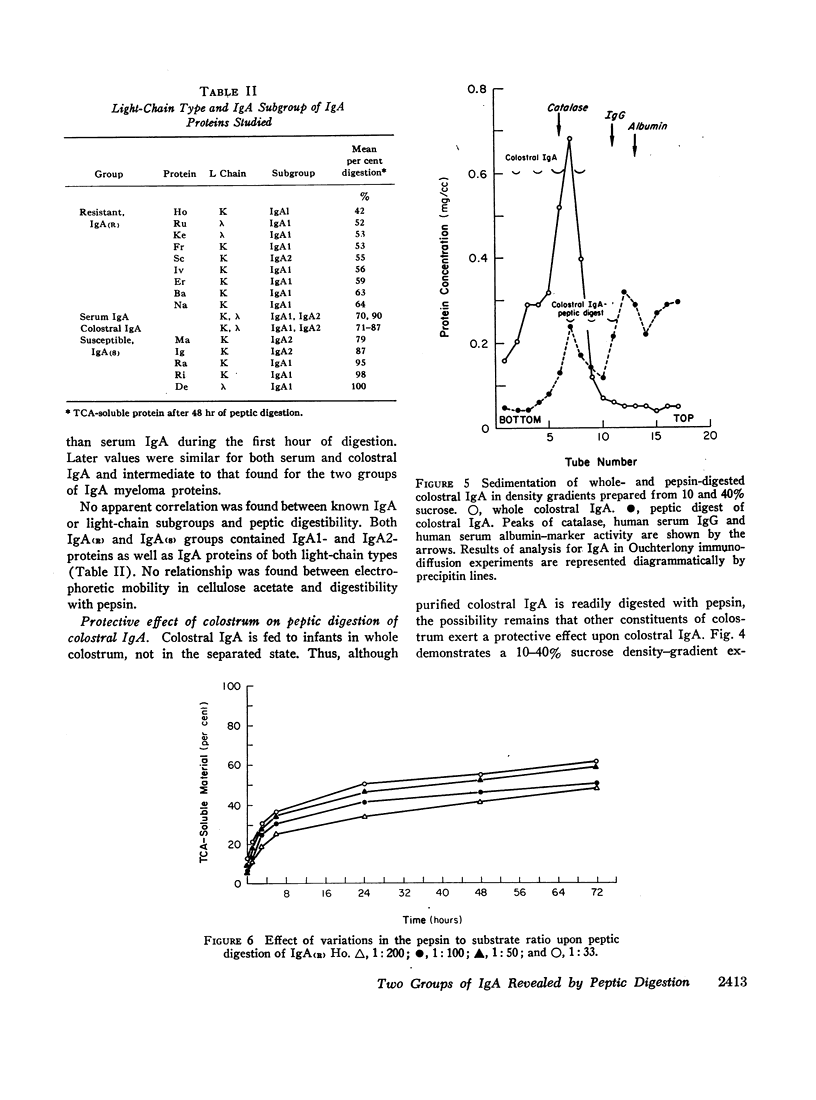
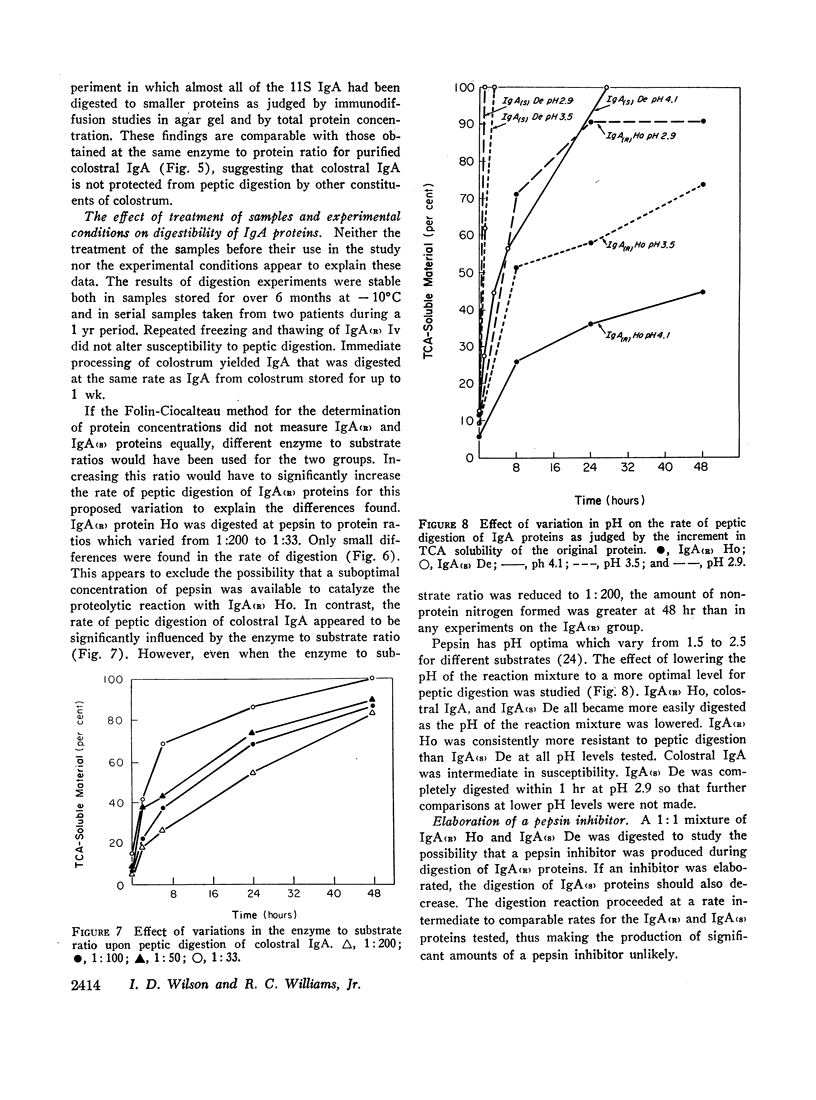
These findings indicate that (a) two groups of IgA proteins can be distinguished on the basis of susceptibility to proteolysis with pepsin, and (b) secretory piece confers, at most, only a minor increase in stability to the IgA molecule against the digestive action of pepsin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brambell F. W. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966 Nov 19;2(7473):1087–1093. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- Cederblad G., Johansson B. G., Rymo L. Reduction and proteolytic degradation of immunoglobulin A from human colostrum. Acta Chem Scand. 1966;20(9):2349–2357. doi: 10.3891/acta.chem.scand.20-2349. [DOI] [PubMed] [Google Scholar]
- EDELMAN G. M., HEREMANS J. F., HEREMANS M. T., KUNKEL H. G. Immunological studies of human gamma-globulin. Relation of the precipitin lines of whole gamma-globulin to those of the fragments produced by papain. J Exp Med. 1960 Jul 1;112:203–223. doi: 10.1084/jem.112.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTLEY C. C. Simple gel diffusion micromethod for gamma-globulin determination. Pediatrics. 1963 Jan;31:123–129. [PubMed] [Google Scholar]
- Jefferis R., Weston P. D., Stanworth D. R., Clamp J. R. Relationship between the papain sensitivity of human gammaG immunoglobulins and their heavy chain subclass. Nature. 1968 Aug 10;219(5154):646–649. doi: 10.1038/219646b0. [DOI] [PubMed] [Google Scholar]
- KOHN J. A cellulose acetate supporting medium for zone electrophoresis. Clin Chim Acta. 1957 Aug;2(4):297–303. doi: 10.1016/0009-8981(57)90005-0. [DOI] [PubMed] [Google Scholar]
- KUNKEL H. G. Zone electrophoresis. Methods Biochem Anal. 1954;1:141–170. doi: 10.1002/9780470110171.ch6. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Prendergast R. A. Subgroups of gamma-A immune globulins. Proc Soc Exp Biol Med. 1966 Jul;122(3):910–913. doi: 10.3181/00379727-122-31287. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Yount W. J., Litwin S. D. Genetically determined antigen of the Ne subgroup of gamma-globulin: detection by precipitin analysis. Science. 1966 Nov 25;154(3752):1041–1043. doi: 10.1126/science.154.3752.1041. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence T. G., Jr, WIilliams R. C., Jr Reactions of human anti-gamma-globulin factors with digested and urea-reduced gamma-chains. J Immunol. 1966 Sep;97(3):319–330. [PubMed] [Google Scholar]
- Litwin S. D., Kunkel H. G. The genetic control of gamma-globulin heavy chains. Studies of the major heavy chain subgroup utilizing multiple genetic markers. J Exp Med. 1967 May 1;125(5):847–862. doi: 10.1084/jem.125.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960 Dec 9;132(3441):1770–1771. doi: 10.1126/science.132.3441.1770. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. Separation and isolation of fractions of rabbit gamma-globulin containing the antibody and antigenic combining sites. Nature. 1958 Sep 6;182(4636):670–671. doi: 10.1038/182670a0. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- South M. A., Cooper M. D., Wollheim F. A., Hong R., Good R. A. The IgA system. I. Studies of the transport and immunochemistry of IgA in the saliva. J Exp Med. 1966 Apr 1;123(4):615–627. doi: 10.1084/jem.123.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, ZIGELBAUM S. THE SELECTIVE OCCURENCE OF GAMMA-1A GLOBULINS IN CERTAIN BODY FLUIDS. J Clin Invest. 1963 Oct;42:1552–1560. doi: 10.1172/JCI104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry W. D., Roberts M. S. Antigenic heterogeneity of human immunoglobulin A proteins. Science. 1966 Aug 26;153(3739):1007–1008. doi: 10.1126/science.153.3739.1007. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F. Subclasses of human immunoglobulin a based on differences in the alpha polypeptide chains. Science. 1966 Aug 5;153(3736):647–649. doi: 10.1126/science.153.3736.647. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lawrence T. G., Jr Variations among gamma-globulins at the antigenic site revealed by pepsin digestion. J Clin Invest. 1966 May;45(5):714–723. doi: 10.1172/JCI105386. [DOI] [PMC free article] [PubMed] [Google Scholar]



