Abstract
Ranolazine modulates the cardiac voltage-gated sodium channel (Nav 1.5) and is FDA-approved for the treatment of ischemic heart disease. Ranolazine also targets neuronal (Nav 1.7, 1.8) isoforms that are implicated in neuropathic pain. Therefore, we determined the analgesic efficacy of ranolazine in a pre-clinical animal model of neuropathic pain. Both i.p. and oral administration of ranolazine dose-dependently inhibited the mechanical and cold allodynia associated with spared nerve injury, without producing ataxia or other behavioral side effects. These data warrant clinical investigation of the potential use of ranolazine in the treatment of neuropathic pain.
Keywords: spared nerve injury, hyperalgesia, allodynia, rat, Nav 1.7, Nav 1.8
Ranolazine (Ranexa®, Gilead Sciences, Palo Alto, CA) was recently FDA-approved as a cardiac, anti-ischemic, agent (Nash, 2005; Marx and Sweeney, 2006; Pham and Mehta, 2007; Sossalla et al., 2008). Ranolazine blocks skeletal muscle (Nav 1.4), cardiac muscle (Nav 1.5), and neuronal (Nav 1.7, 1.8) sodium channel subtypes and produces a profound use-dependent blockade of the tetrodotoxin-sensitive, late sodium channel current associated with Nav 1.7 (Ju et al., 1996a, 1996b; Haufe et al., 2005; Belardinelli et al., 2006; Hale et al., 2008; Rajamani et al., 2008; Wang et al., 2008). Many patients spontaneously report a significant reduction in characteristic signs of neuropathic pain including burning dysesthesia in the lower extremities when treated with ranolazine for ischemic heart disease (C. Walker, personal communication). Since recent studies link sodium channels to inflammatory pain (Gould et al., 2000, 2004) and several inherited neuropathic pain states (Nassar et al., 2004; Dib-Hajj et al., 2005; Cox et al., 2006; Fertleman et al., 2006; Waxman, 2006; Dib-Hajj et al., 2007; Drenth and Waxman, 2007; Goldberg et al., 2007), we asked whether ranolazine could reduce the mechanical and cold hypersensitivity produced in a preclinical model of acquired peripheral neuropathic pain (Decosterd and Woolf, 2000).
Methods
Subjects
All experiments were conducted in accordance with protocols that were approved and monitored by the Tulane Health Sciences Center and the University of Kentucky Institutional Animal Care and Use Committee. Male Sprague Dawley rats, (Harlan Sprague Dawley, Inc., Indianapolis, IN) received at 220-240g, were housed 1 animal to a cage and maintained at 25°C and 60% humidity, on a 12 hour light/dark cycle beginning at 6 a.m. and allowed free access to food and water.
Procedure
Rats were allowed to acclimate to the testing apparatus by placing them into Plexiglas boxes on a stainless steel grid for 30-60 min. Baseline determinations of responses to mechanical or cold stimuli applied to the lateral aspect of the plantar surface of the hindpaw were measured using von Frey monofilaments (Stoelting, Inc., Wood Dale, IL) or acetone, respectively (Decosterd and Woolf, 2000; Taylor et al., 2007; Intondi et al., 2008). Mechanical thresholds were assessed first. Hind paws were stimulated on the lateral aspect of the plantar surface within the distribution of the sural nerve with a series of 8 von Frey monofilaments of logarithmic stiffness. A modified version of the 50% withdrawal threshold was determined using the up–down method of Dixon, modified by Chaplan et al. (1994). First, an intermediate monofilament (number 4.31, exerts 2.0 g of force) was gently applied with enough force to cause it to bend. This was repeated up to three times in distinct areas along the lateral paw. In case of a positive response (rapid withdrawal of the paw within 2 s), a smaller filament was tested. If no response was recorded in any of the three different areas, a larger filament was tested.
Paw withdrawal responses to cold allodynia were then measured using an acetone-loaded syringe connected to PE-90 tubing, flared at the tip to a diameter of 3½ mm. Surface tension maintained the delivery volume of the acetone drop to 10–12 μl. The length of time the animal lifted or shook its paw was recorded for the first 30 s. Three observations were averaged.
Following the determination of pre-injury responses, anesthesia was induced in rats weighing approximately 250 g with 5% isoflurane and maintained for surgical manipulation with 2-3% isoflurane. An incision was made in the skin at the level of the trifurcation of the left sciatic nerve. The overlying muscles were retracted, exposing the common peroneal, tibial, and sural nerves. With care taken to avoid mechanical stimulation of the sural branch, the common peroneal and tibial nerves were ligated with 6.0 silk (Ethicon, Somerville, NJ), followed by transection of the intervening 1 mm segment of nerve adjacent to both the proximal and distal ligatures. The muscle was sutured with 5-0 sutures, and the skin was closed with 9 mm metal clips. Sensitivity to stimulation was reassessed 7-14 days post-operatively. Rats that showed signs of allodynia received either vehicle (i.p. injection- propylene glycol and dichloromethane; oral gavage- distilled water titrated to pH 4.0 with dilute acetic acid and sodium hydroxide) or ranolazine (10, 30, and 100mg/kg), administered in an experimenter-blinded, randomized design. Animals were again tested 15, 30, 60, and 90 minutes (i.p.) or 30, 60, 90, 120, and 180 minutes (p.o.) after drug administration. Occasionally, animals did not develop mechanical allodynia (VF threshold > 5.0 g on the nerve-injured side) or exhibit cold allodynia on the day of pharmacological testing after nerve injury. In such cases (5%), testing was either terminated or the data were not included in the final analysis.
To evaluate possible ataxic effects of ranolazine administration, the ability of un-operated animals to remain on an accelerating rotarod was assessed before and after i.p. administration. Rats were placed on the rotarod, set at an initial rotating speed of 4 rpm. Immediately thereafter, speed increased at a rate of 0.5 rpm every 5 sec to a maximum of 40 rpm. Each rat was trained in this procedure 3-9 times, until latency to fall was approximately 180 sec. Animals that failed to remain on the rotarod for at least 100 s (two) or that remained on the rotarod for more than 300 s (one) were removed from the study. Testing of a vehicle group (n=4), 30 mg/kg group (n=6) or 100 mg/kg ranolazine group (n=5) began within 1-2 days of training. Triplicate measurements were taken before and then 30, 60, 90, 120, 150, and 180 min after injection. Values are reported as mean±SEM.
Statistical analysis
Significance was determined with a mixed design, two-way analysis of variance (ANOVA) with Drug as the between-subjects factor and Time as the repeated measure. If the overall F-value was significant, further Bonferroni post-hoc tests were conducted to determine the effect of Drug relative to vehicle at each time point.
Results
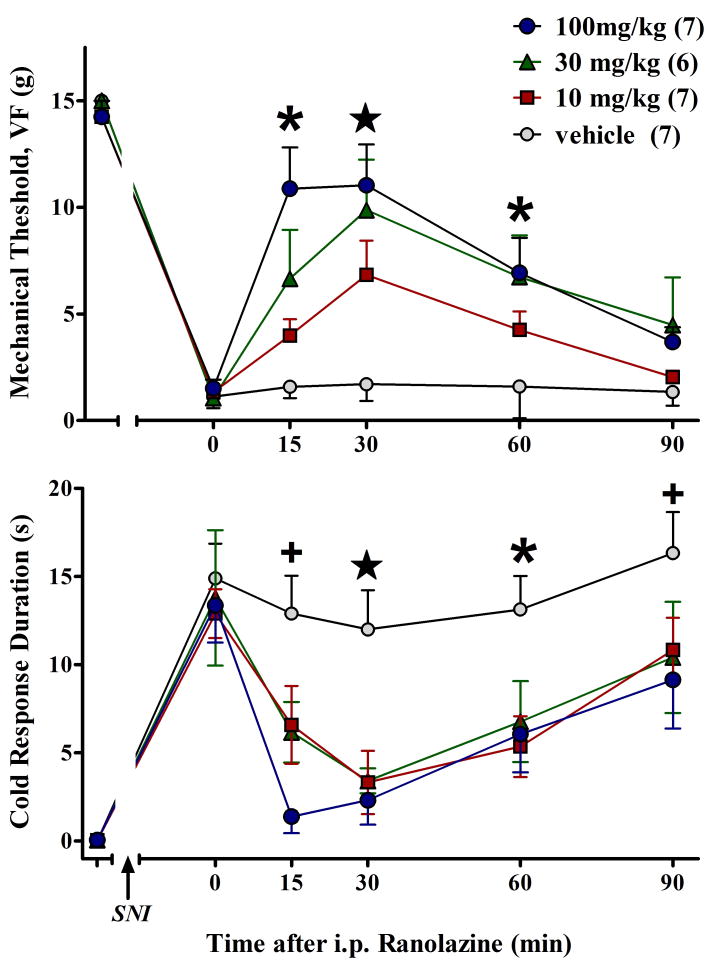
Spared nerve injury (SNI) increased sensitivity to mechanical and cold stimulation within the ipsilateral sural nerve distribution. As illustrated in Figure 1, i.p. administration of ranolazine dose-dependently reduced both mechanical allodynia [F(3,115)=5.7, p<0.005] and cold allodynia [F(3,115)=4.3, p<0.05]. These anti-allodynic effects peaked at 15-30 min and lasted 60- 90 min. Vehicle had no effect.
Figure 1.
I.p. administration of ranolazine dose-dependently increases paw withdrawal threshold to a mechanical stimulus (A) and reduces response duration to a cool stimulus (B). Values represent mean ± SEM. Number of animals per group are in parentheses. +p<0.05 saline vs 100; *p<0.05 saline vs 100 and 30; (star) p<0.05 saline vs 100 and 30 and 10.
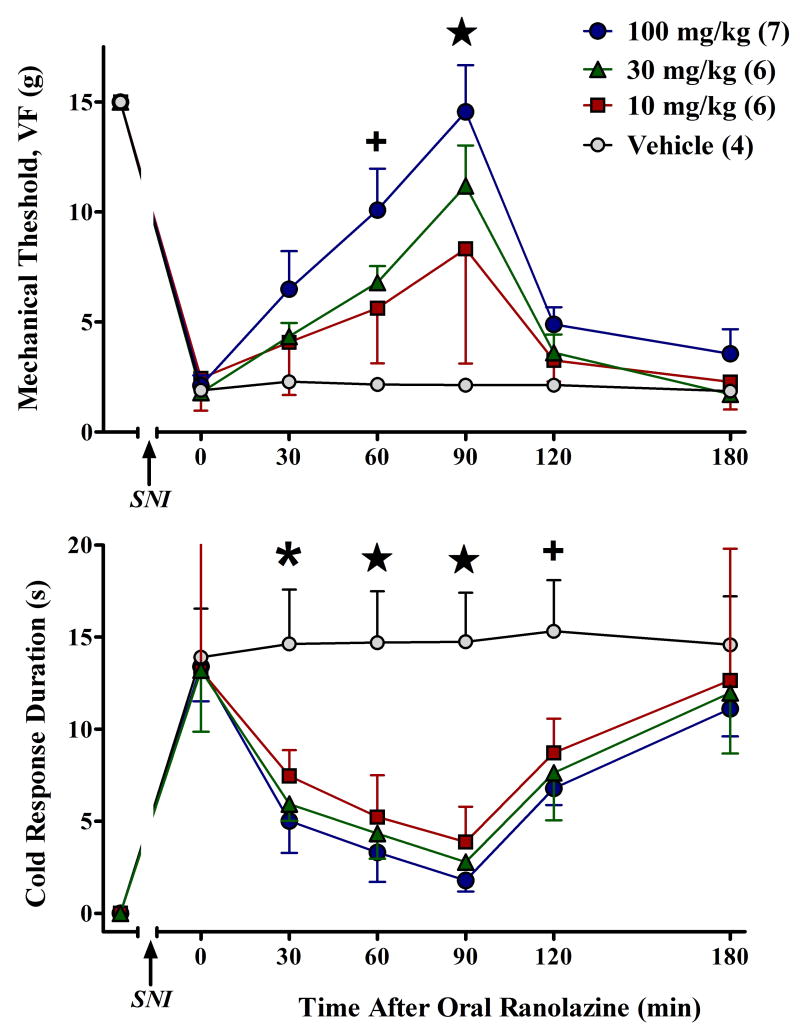
Only the oral route of administration of ranolazine has been approved by the FDA. Therefore, we determined whether oral ranolazine would also reduce SNI-induced allodynia. As illustrated in Figure 2, oral ranolazine dose-dependently reduced both mechanical allodynia [F(3,114)=5.1, p<0.01] and cold allodynia [F(3,108)=4.5, p<0.05]. These effects peaked at 90 min and lasted 90-180 min. Vehicle had no effect.
Figure 2.
Oral administration of ranolazine dose-dependently increases paw withdrawal threshold to a mechanical stimulus (A) and reduces response duration to a cool stimulus (B). Values represent mean ± SEM. Number of animals per group are in parentheses. +p<0.05 saline vs 100; *p<0.05 saline vs 100 and 30; (star) p<0.05 saline vs 100 and 30 and 10.
Non-selective sodium channel blockers are an integral part of the pharmacotherapy for peripheral neuropathic pain; however, severe side effects limit their use. Throughout these studies with 10-30 mg/kg ranolazine, we observed no overt signs of adverse effects. To evaluate more subtle effects on behavior, we assessed performance on an accelerating rotarod. Prior to drug testing, baseline latency was balanced between the three groups (vehicle, 193±10 s; 30 mg/kg, 188±6 s; 100 mg/kg, 189±14 s). ANOVA revealed no effect of oral ranolazine treatment [F(2,60)=0.6, NS] at any time point throughout the 3-hr test session (p>0.05). For example, when tested at the peak of anti-hyperalgesic effect (90 min, Fig 2), rotarod latency in the vehicle, 30 mg/kg, and 100 mg/kg groups was 205±28 s, 225±17 s, and 207±14 s, respectively. These data indicate that oral ranolazine did not produce ataxia.
Discussion
Ranolazine is FDA-approved for the treatment of ischemic cardiac disease and angina at a dose of 500-1000 mg bid (∼2000 mg/day for a 70kg patient). The present experiments used this dosing range in a pre-clinical model of neuropathic pain. We report that ranolazine reduces nerve injury-induced mechanical allodynia and cold hypersensitivity.
Systemic administration of non-selective sodium channel blockers, such as lidocaine, clearly reduce behavioral signs of neuropathic pain in humans (Backonja, 2001, Devor, 2006). Due to ubiquitous distribution of the 9 known sodium channels in many tissues of the body and the essential role of these channels in the function of excitable membranes, however, recipients risk dangerous cardiac and central nervous system effects. For example, lidocaine produces cardiac arrhythmia and ataxia at the same dosages required to yield analgesia in neuropathic pain models (Challapalli et al., 2005; Gil-Gouveia and Goadsby, 2009). Potentially, these risks could be minimized by using agents that influence just a subset of sodium channel subtypes. One such agent is ranolazine, the actions of which appear restricted to NaV 1.4, 1.5, 1.7, and 1.8. In the current study, ranolazine exhibited anti-allodynic efficacy at doses that do not produce significant adverse effects. We suggest that ranolazine may provide a better option for treating neuropathic pain with a greater therapeutic window of efficacy than is currently available with broad-spectrum sodium channel blockers. This remains to be studied in controlled clinical trials.
Neuropathic pain is a multifaceted condition that is driven by a constellation of complex central and peripheral mechanisms. The pharmacotherapy of clinical neuropathic pain is highly variable, perhaps due to large differences in selectivity of a particular medication for a particular pain modality (Zimmerman, 2001; Erichsen et al., 2003; Paul et al., 2008). We speculate that the anti-allodynic actions of ranolazine are mediated at least in part by Nav 1.7 and Nav 1.8 sodium channels located on primary sensory neurons of the peripheral nervous system. Interestingly, in the current study, dose-response curves reveal that ranolazine more potently reduced cold allodynia as compared to mechanical allodynia. For example, 10 mg/kg ranolazine essentially reversed cold allodynia but only partially reduced mechanical allodynia. We speculate that ranolazine is more effective at reducing peripheral mechanisms that drive cold allodynia (such as loss of A-δ suppression of C-fiber input to lamina 1) than those that drive mechanical allodynia (such as enhancement of spontaneous oscillatory activity in A-β input) (Bennett, 1994; Koltzenburg, 1996; Amir et al., 1999, 2002a, 2002b). Further investigation is warranted to determine whether ranolazine could also be an important new option in the management of the cold or mechanical allodynia that is associated with peripheral nerve injury.
Acknowledgments
HJG and DP are supported by a grant from Gilead Sciences and by the LSU Health Sciences Center Departments of Neurology and Pharmacology. BKT was supported by 5R29DA010356-04, 5K02DA019656-03, and 5R01NS045954-04.
Footnotes
Conflicts: HJG is a research consultant for Gilead Sciences. ID is Vice President for Neuroscience for Gilead Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: Role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8559–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Burst discharge in primary sensory neurons: Triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci. 2002a;22:1187–1198. doi: 10.1523/JNEUROSCI.22-03-01187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Liu CN, Kocsis JD, Devor M. Oscillatory mechanism in primary sensory neurons. Brain. 2002b;125:421–435. doi: 10.1093/brain/awf037. [DOI] [PubMed] [Google Scholar]
- Backonja M. Anticonvulsants and antiarrhythmics in the treatment of neuropathic pain syndromes. In: Hansson PT, Fields HL, Hill RG, Marchettini P, editors. Neuropathic Pain: Pathophysiology and Treatment, Progress in Pain Research and Management. Vol. 21. Seattle: IASP Press; 2001. pp. 285–202. [Google Scholar]
- Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92:6–14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GJ. Neuropathic pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Vol. 3. Edinburgh UK: Churchill Livingstone; 1994. pp. 201–224. [Google Scholar]
- Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 2005;19:CD003345. doi: 10.1002/14651858.CD003345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, et al. An SNC9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf C. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Rush AM, Cummins TR, Hisama FM, Novella S, Tyrrell L, et al. Gain-of-function mutation in Nav 1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain. 2005;128:1847–1854. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Nav 1.7 and human pain disorders. Trends in Neurosci. 2007;30:555–563. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Drenth JPH, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007;117:3603–3609. doi: 10.1172/JCI33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen HK, Hao JX, Xu XJ, Blackburn-Munro G. A comparison of the antinociceptive effects of voltage-activated Na+ channel blockers in two rat models of neuropathic pain. Eur J Pharmacol. 2003;458:275–282. doi: 10.1016/s0014-2999(02)02792-9. [DOI] [PubMed] [Google Scholar]
- Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, et al. SCN9A mutations in paroxysmal extreme pain disorder: Allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–774. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Gil-Gouveia R, Goadsby PJ. Neuropsychiatric side-effects of lidocaine: examples from the treatment of headache and a review. Cephalalgia. 2009;29:496–508. doi: 10.1111/j.1468-2982.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice MP, et al. Loss-of-function mutations in the Nav 1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71:311–319. doi: 10.1111/j.1399-0004.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- Gould HJ, III, Gould TN, England JD, Paul D, Liu ZP, Levinson SR. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res. 2000;854:19–29. doi: 10.1016/s0006-8993(99)02216-7. [DOI] [PubMed] [Google Scholar]
- Gould HJ, III, England JD, Soignier RD, Nolan P, Minor LD, Liu ZP, et al. Ibuprofen blocks changes in Nav 1.7 and 1.8 sodium channels associated with complete Freund's adjuvant-induced inflammation in rat. J Pain. 2004;5:270–280. doi: 10.1016/j.jpain.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardiol. 2008;44:954–967. doi: 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Haufe V, Camacho JA, Dumaine R, Günther B, Bollensdorff C, Von Banchet GS, et al. Expression pattern of neuronal and skeletal muscle voltage-gated Na+ channels in the developing mouse heart. J Physiol. 2005;564:683–696. doi: 10.1113/jphysiol.2004.079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intondi AB, Dhalgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137:352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YK, Gage PW, Saint DA. Tetrodotoxin-sensitive inactivation-resistant sodium channels in pacemaker cells influence heart rate. Pflugers Arch. 1996a;431:868–875. doi: 10.1007/s004240050079. [DOI] [PubMed] [Google Scholar]
- Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol (Lond) 1996b;497:337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M. Afferent mechanisms mediating pain and hyperalgesias in neuralgia. In: Jänig W, Stanton-Hicks M, editors. Reflex Sympathetic Dystrophy: A Reappraisal, Progress in Pain Research and Management. Vol. 6. Seattle: IASP Press; 1996. pp. 123–150. [Google Scholar]
- Marx C, Sweeney M. Mechanism of action of ranolazine. Arch Intern Med. 2006;166:1325–1326. doi: 10.1001/archinte.166.12.1325-b. [DOI] [PubMed] [Google Scholar]
- Nash DT. The case for medical treatment in chronic stable coronary artery disease. Arch Intern Med. 2005;165:2587–2589. doi: 10.1001/archinte.165.22.2587. [DOI] [PubMed] [Google Scholar]
- Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, et al. Nociceptor-specific gene deletion reveals a major role for Nav 1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci (USA) 2004;101:12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D, Gould HJ, III, Dunne-Reilly A. Predictive validity of a model of peripheral nerve demyelination-induced neuropathic pain. 12th World Congress on Pain; 2008. Abstract:PW40. [Google Scholar]
- Pham DQ, Mehta M. Ranolazine: a novel agent that improves dysfunctional sodium channels. Int J Clin Pract. 2007;61:864–872. doi: 10.1111/j.1742-1241.2007.01348.x. [DOI] [PubMed] [Google Scholar]
- Rajamani S, Shryock JC, Belardinelli L. Block of tetrodotoxin-sensitive, Nav 1.7, and tetrodotoxin-resistant, Nav 1.8, Na+ channels by ranolazine. Channels. 2008;2:449–460. doi: 10.4161/chan.2.6.7362. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schöndube FA, et al. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts--role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Abhyankar SS, Vo NTT, Kriedt CL, Churi SB, Urban JH. Neuropeptide Y acts at Y1 receptors in the rostral ventral medulla to inhibit neuropathic pain. Pain. 2007;131:83–95. doi: 10.1016/j.pain.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Calderon J, Wang SY. State- and use-dependent block of muscle Nav 1.4, and neuronal Nav 1.7 voltage-gated Na+ channel isoforms by ranolazine. Mol Pharmacol. 2008;73:940–948. doi: 10.1124/mol.107.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. A channel sets the gain on pain. Nature. 2006;444:831–832. doi: 10.1038/444831a. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]