Abstract
A characteristic of acute pancreatitis is the premature activation and retention of enzymes within the pancreatic acinar cell. Because ligands linked to cAMP production may prevent some forms of pancreatitis, we evaluated the effects of increased intracellular cAMP in the rat pancreatic acinar cell. Specifically, this study examined the effects of the cholinergic agonist carbachol and agents that increase cAMP [secretin and 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP)] on zymogen activation (trypsin and chymotrypsin), enzyme secretion, and cellular injury in isolated pancreatic acini. Although cAMP agonists affected the responses to physiological concentrations of carbachol (1 μM), their most prominent effects were observed with supraphysiological concentrations (1 mM). When secretin was added to 1 mM carbachol, there was a slight increase in zymogen activation, but no change in the secretion of amylase or chymotrypsin. Furthermore, coaddition of secretin increased parameters of cell injury (trypan blue exclusion, lactic dehydrogenase release, and morphological markers) compared with carbachol (1 mM) alone. Although directly increasing cellular cAMP by 8-Br-cAMP caused much greater zymogen activation than carbachol (1 mM) alone or with secretin, 8-Br-cAMP cotreatment reduced all parameters of injury to the level of unstimulated acini. Furthermore, 8-Br-cAMP dramatically enhanced the secretion of amylase and chymotrypsin from the acinar cell. This study demonstrates that increasing acinar cell cAMP can overcome the inhibition of enzyme secretion caused by high concentrations of carbachol and eliminate acinar cell injury.
Keywords: 8-bromoadenosine 3′,5′-cyclic monophosphate; pancreas; protease
Acute pancreatitis is associated with aberrant intracellular zymogen activation and retention of active enzymes within the acinar cell. Agonists, such as the CCK analog caerulein, which bind to pancreatic acinar cell receptors, can cause zymogen activation and pancreatitis. Because human acinar cells appear to lack the CCK1 receptor, relevance of results obtained with CCK to clinical disease has been questioned (8, 18). Muscarinic receptors, which, like CCK receptors, signal through Ca2+-mediated pathways, are expressed both by human and rodent acinar cells. Our lab has shown that the muscarinic agonist carbachol can cause zymogen activation in isolated rat acinar cells (20). However, unlike high concentrations of CCK, carbachol does not increase acinar cell cAMP levels.
In addition to G protein-coupled receptors linked to Ca2+ signaling, the pancreatic acinar cell has receptors that stimulate adenylate cyclase and cAMP generation. These include secretin, VIP, and pituitary adenylate cyclase-activating peptide (PACAP) receptors. Previous studies have shown that cAMP agonists enhance the effects of secretion elicited by physiological concentrations of CCK (2). Similarly, we have shown that cAMP agonists enhance caerulein-induced zymogen activation (11). These effects were observed with secretin, VIP, and PACAP. Bypassing receptor-mediated events with the cell-permeable cAMP analog, 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) also enhances caerulein-induced zymogen activation. Preliminary clinical studies suggest that the cAMP agonist secretin can reduce the severity of pancreatitis. The present study examines the effects of cAMP agonists on carbachol-induced zymogen activation, enzyme secretion, and cellular injury.
Previous studies (5) have suggested that acute acinar cell injury requires two key pathological events: zymogen activation and the retention of active enzymes within the pancreatic acinar cell. We hypothesized that cAMP agonists might reduce acinar cell injury associated with zymogen activation by stimulating secretion of activated enzymes from the acinar cell. In testing this hypothesis, we first demonstrated that cAMP agonists sensitize the acinar cell to carbachol-induced zymogen activation. We also found that cAMP agonists enhance secretion after stimulation with physiological concentrations of carbachol. Surprisingly, the suppression of secretion observed with supraphysiological concentrations of carbachol was overcome when acinar cell cAMP was directly increased. Furthermore, acinar cell injury was reduced by increasing intracellular cAMP. The protective effect of cAMP was associated with enhanced secretion of activated enzymes from the acinar cell. These findings suggest that cAMP agonists may reduce acinar cell injury by enhancing the secretion of active enzymes.
MATERIALS AND METHODS
Preparation of isolated pancreatic acini
Acini were isolated as previously described (11). Briefly, fasted male Sprague-Dawley rats ~50–150 g (Charles River Laboratories, Wilmington, MA) were killed by CO2 using a protocol approved by the Yale University and the Veterans Administration Animal Care and Use Committees. The pancreas was removed and placed into 6 ml of acinar medium (in mM): 40 Tris (pH 7.4), 95 NaCl, 4.7 KCl, 0.6 MgCl2, 1.3 CaCl2, 1 NaH2PO4, 10 glucose, 2 glutamine, plus 0.1% BSA, 1× MEM-amino acids (GIBCO-BRL, San Jose, CA) containing 50 U/ml of type-4 collagenase (Worthington, Freehold, NJ). The collagenase medium was injected into the pancreas, and, after 5 min, the pancreas was minced and placed into a 50-ml flask with 12 ml of collagenase medium for 60 min at 37°C with shaking (120 rpm). The digest was filtered through a 300- to 400-μm mesh (Sefar American, Depew, NY). Isolated acini were distributed among the 24 wells (0.5 ml suspension/well) of a 24-well Falcon tissue culture plate (Becton Dickinson, Franklin Lakes, NJ). All reagents were purchased from Sigma, St. Louis, MO, unless otherwise noted.
Acinar experimental protocol
Tissue culture plates containing acini were incubated for 60 min at 37°C under constant O2 with shaking (80 rpm). After exchanging the medium, acini were incubated for an additional 60 min. At this time, acini were treated without changing medium. Treatments included carbachol (1 μM and 1 mM), atropine (1 μM), secretin (0.1–100 nM), 8-Br-cAMP (1 μM–1 mM), and forskolin 10 μM. Acini were incubated for 60 min (unless otherwise noted) at which time samples (medium and cells) were collected, placed in 1.5-ml centrifuge tubes (USA Scientific, Waltham, MA) and centrifuged for 1 min at 30 g. Of the resulting cell-free supernatant, 50 μl was removed to a 0.5-ml microcentrifuge tube. All samples were stored at −80°C.
Enzymatic activity assays
Samples containing 450 μl of cells and medium were thawed on ice, homogenized in a conical 1-ml Eppendorf tube with a pestle, and centrifuged at 1,000 g for 1 min. From the resulting postnuclear supernatant, 100 μl was added to wells of a 24-well tissue culture plate containing 350 μl of trypsin assay buffer [50 mM Tris (pH 8.1), 150 mM NaCl, 1 mM CaCl2, 0.01% BSA]. Finally, 50 μl of 400 μM enzyme substrate (trypsin 3135; Peptides International, Louisville, KY and chymotrypsin; Calbiochem, San Diego, CA) diluted in trypsin assay buffer (40 μM final) was added to each well. The plate was read by using a fluorometric microtiter plate reader (model HTS 7000; Perkin-Elmer Analytical Instruments, Shelton, CT) using an 380-nm excitation wavelength and 440-nm emission for 20 reads over 10 min. The slope of the line, which represents enzyme activity of the homogenate, was normalized to total amylase activity and expressed as relative fluorescence units per second per microgram total amylase.
Amylase assay
Samples were thawed on ice. The cell-free supernatant was assayed for secreted amylase. The remaining sample (450 μl of cells + medium) was homogenized in 1.5-ml centrifuge tubes with a pestle and centrifuged at 1,000 g for 1 min. The resulting postnuclear supernatant was assayed for total amylase. Amylase activity was determined by using a commercial kit (Phaebadas kit; Pharmacia Diagnostic, Rochester, NY). Amylase secretion was calculated as the percent total release (medium/medium + cells).
Lactate dehydrogenase assay
The assay was performed by using the commercially available Cytotox 96 nonradioactive cytotoxicity assay kit according to the manufacturer’s instructions (Promega, Madison, WI). In brief, acinar cells were treated with 1 μM or 1 mM carbachol alone or in the presence of either 8-Br-cAMP (100 μM), secretin (100 nM), or atropine (1 μM) for 2 h. Samples were collected and centrifuged at 30 g for 30 s. A 50-μl aliquot of medium was then removed to measure lactate dehydrogenase (LDH) release from the cells. A manufacturer-provided lysis reagent was then added to the remaining 450 μl of cells and medium to determine total LDH. Both cell and medium samples were assayed. The results are expressed as the percent LDH released into the medium.
Trypan blue retention
Acinar cells were treated with 1 μM or 1 mM carbachol alone or in the presence of either 8-Br-cAMP (100 μM) or secretin (100 nM) for 2 h. Samples were then allowed to sediment by gravity. The medium was removed, leaving a cell pellet. The cell pellet was treated with 100 μl of 0.4% trypan blue stain (GIBCO-BRL) for exactly 2 min. The samples were then washed with 1 ml of 1× PBS four times. Samples were frozen at −80°C. After thawing, 100 μl of 1% Triton X-100 was added to the cells, which were then sonicated. The samples were then read on a plate reader at a wavelength of 583. This result was normalized to total nucleic acid content, which was determined by reading the samples at a wavelength of 260. Results were then expressed as fold vs. control.
cAMP assay
cAMP content in acinar homogenates was determined by using a commercial ELISA assay (Amersham Biosciences, Piscataway, NJ). Acini were preincubated with 1 mM IBMX for 15 min to inhibit endogenous cAMP-phosphodiesterases. The cells were then treated with specified agents for 10 min. cAMP content was calculated by using a standard curve and normalized to total amylase content.
Chymotrypsin antibody production and purification
Trypsin cleaves chymotrypsinogen between arginine 15 and isoleucine 16. The sequence for the first 11 amino acids of human chymotrypsin-B at the amino end of the cleavage site (16–26) IVNGEDAVPGS was used to immunize rabbits. Serum was collected, and affinity was purified by using an affinity column with immobilized peptide. With the use of immunoblot analysis, the antibody recognizes a major band at ~13 kDa that corresponds to chymotrypsin. Intensity of the band was determined to directly correlate with chymotrypsin activity.
Chymotrypsin immunoblot analysis
Because preliminary data demonstrated that our fluorogenic assays for trypsin and chymotrypsin could not reliably measure secreted enzymes, an immunoblot assay for active enzyme secretion was developed. After treatment, cells and medium (300 μl) were collected. A 50-μl aliquot of cells and medium was collected and assayed for total amylase to ensure equal cell number in each sample. To the remaining 250 μl of cells and medium, 100 mM EGTA and 500 mM benzamidine were added to a final concentration of 1 mM and 5 mM, respectively. Samples were allowed to sediment by gravity and all of the cell-free medium was removed. A sample of 150 μl of 1× Laemmli loading buffer was added to 150 μl cell-free medium. The remaining cell-free medium was discarded. A sample of 300 μl of 1× Laemmli loading buffer was added to the medium-free cell pellet. All samples were heated to 95°C for 5 min and then stored at −80°C. Western blot analysis was performed to detect the presence of precursor or active forms of chymotrypsinogen.
Briefly, samples were separated on 15% SDS-PAGE gels (Bio-Rad, Hercules, CA) and transferred to Immobilon-P membranes (Millipore, Billerica, MA). Membranes were blocked for 1 h at room temperature with Blotto (PBS, 5% nonfat dry milk, 0.05% Tween-20). Membranes were then probed with primary antibody (1:500) in Blotto for 2 h at room temperature. Membranes were washed and then probed with horseradish peroxidase-labeled goat anti-rabbit IgG (Sigma, St. Louis, MO) for 1 h at room temperature. After treatment with the secondary antibody, membranes were washed in PBS, and autoradiography was performed by using a SuperSignal West Pico chemiluminescence kit (Pierce, Rockford, IL).
Statistical analysis
Data represent means ± SE of at least 3 individual experiments unless otherwise noted, with each experiment being performed in at least duplicate. A Student’s t-test analysis was used to determine statistical significance and P values of <0.05 were assigned significance.
RESULTS
Zymogen activation
Carbachol-induced zymogen activation was investigated first. Adding physiological (1 μM) doses of carbachol to isolated acinar cells caused a small enhancement in zymogen activation with a 1.3- and 2-fold increase in trypsin and chymotrypsin activities, respectively, compared with un-stimulated controls (Figs. 1A and 2A). Supraphysiological concentrations of carbachol (1 mM) stimulated trypsin and chymotrypsin activities by 2.5- and 5.5-fold, respectively (Figs. 1B and 2B). Because the effects of carbachol were reduced to background levels by a 15-min pretreatment with 1 μM atropine, these actions of carbachol were specific (data not shown).
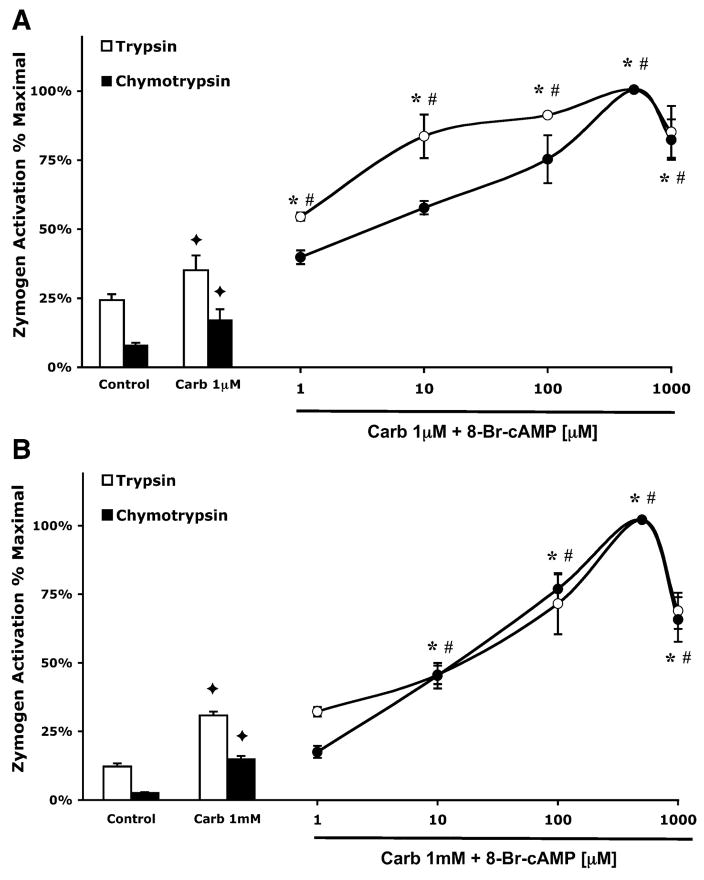
Fig. 1.
8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) enhances carbachol-stimulated zymogen activation. Acini were incubated with or without 8-Br-cAMP (1–1,000 μM) in the presence of 1 μM carbachol (Carb; A) or 1 mM carbachol (B) for 1 h. Samples were assayed for trypsin and chymotrypsin activity. Results are expressed as a percentage of the treatment that produced maximum zymogen activation (1 μM or 1 mM carbachol + 500 μM 8-Br-cAMP). All samples represent the means ± SE from 3 separate experiments. ✦ P < 0.05 vs. control; *P < 0.05 vs. corresponding dose of carbachol alone for trypsin; #P < 0.05 vs. corresponding dose of carbachol alone for chymotrypsin.
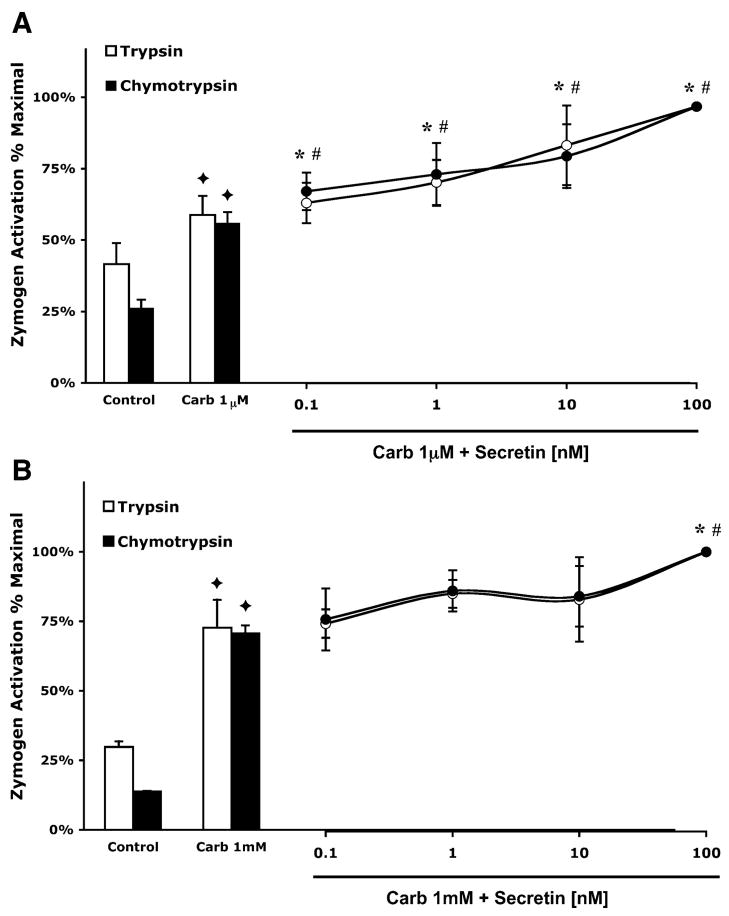
Fig. 2.
Secretin enhances zymogen activation stimulated by physiological doses of carbachol. Secretin has a minimal effect on zymogen activation induced by supraphysiological carbachol. Acini were incubated with or without secretin (0.1–100 nM) in the presence of 1 μM carbachol (A) or 1 mM carbachol (B) for 1 h. Samples were assayed for trypsin and chymotrypsin activity. Results are expressed as a percentage of the treatment that produced maximum zymogen activation (1 μM or 1 mM carbachol + 100 nM secretin). All samples represent the means ± SE from 3 separate experiments. ✦ P < 0.05 vs. control; *P < 0.05 vs. corresponding dose of carbachol alone for trypsin; #P < 0.05 vs. corresponding dose of carbachol alone for chymotrypsin.
To test the effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretin and carbachol were added to cells simultaneously. At 1 μM carbachol, increasing doses of secretin caused a dose-dependent increase in zymogen activation (Fig. 1A). The largest increases were observed with 100 nM secretin; it enhanced trypsin and chymotrypsin activation by 1.7- and 1.8-fold compared with cells treated with carbachol alone. A different pattern was noted when secretin treatment was combined with 1 mM carbachol (Fig. 1B). Under these conditions, only the highest concentration of secretin (100 nM) produced a significant increase in carbachol-induced zymogen activation. Compared with cells treated with 1 mM carbachol alone addition of 100 nM secretin caused a 1.4-fold increase in zymogen activation.
When intracellular cAMP was directly increased by the membrane permeable analog of cAMP, 8-Br-cAMP, a different pattern of sensitization was observed. At both 1 μM and 1 mM doses of carbachol, 8-Br-cAMP caused a concentration-dependent, biphasic enhancement of both trypsin and chymotrypsin activation, peaking at 500 μM (Fig. 2, A and B). The addition of 500 μM 8-Br-cAMP increased trypsin and chymotrypsin activation by 2.9- and 5.8-fold, respectively, compared with cells treated with 1 μM carbachol alone. At 1 mM carbachol, 500 μM 8-Br-cAMP caused a 3.2-fold increase in trypsin activation and a 6.8-fold increase in chymotrypsin activation compared with cells treated with carbachol alone.
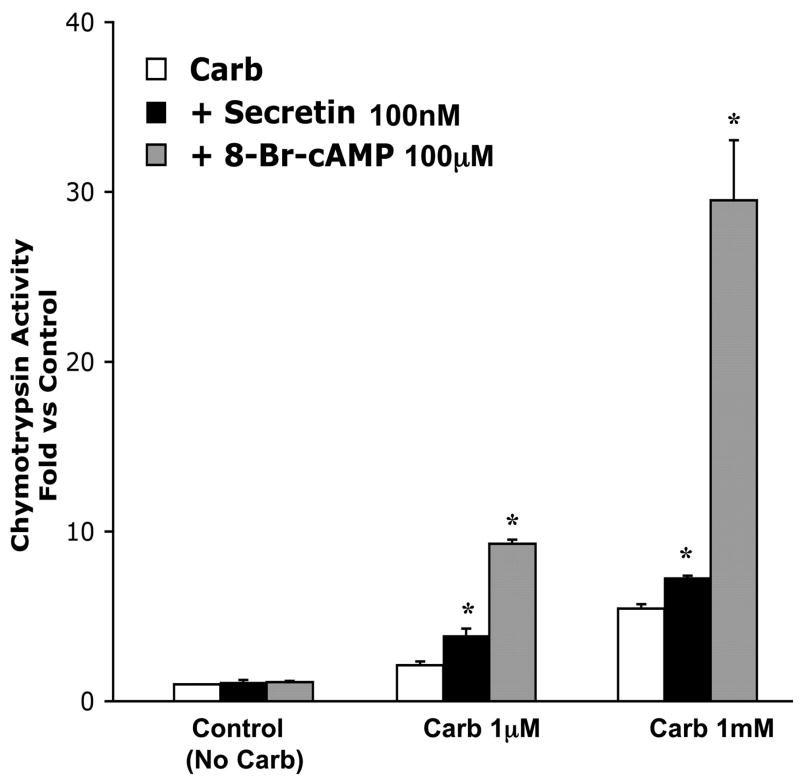
Note that the magnitude of zymogen activation caused by 8-Br-cAMP is greater than that caused by secretin. Figure 3 compares a near maximal dose of 8-Br-cAMP (100 μM) to the dose of secretin (100 nM) that had the largest effect on zymogen activation. At 1 μM carbachol, secretin caused a 1.8-fold increase in chymotrypsin activation, and 8-Br-cAMP caused a larger 4.4-fold increase in chymotrypsin activation compared with carbachol alone. Whereas 100 nM secretin caused a 1.4-fold increase in chymotrypsin activation compared with 1 mM carbachol, 8-Br-cAMP caused a 5.1-fold enhancement. Neither secretin (100 nM) nor 8-Br-cAMP (100 μM) increased chymotrypsin activation when added to cells not treated with carbachol. These studies demonstrate that cAMP agonists and analogs sensitize the acinar cell to the effects of carbachol on zymogen activation.
Fig. 3.
Magnitude of chymotrypsin activation caused by 8-Br-cAMP (100 μM) is greater than that caused by secretin (100 nM). Acini were incubated with or without 8-Br-cAMP (100 μM) or secretin (100 nM) in the presence of carbachol (1 μM and 1 mM) for 1 h and samples assayed for chymotrypsin activity. Results are expressed as fold vs. control. Note that control indicates no carbachol. Neither 100 μM 8-Br-cAMP nor 100 nM secretin had a significant effect on control cells. All samples represent the means ± SE from 3 separate experiments. *P < 0.05 vs. corresponding dose of carbachol alone.
Secretion
Effects of carbachol and cAMP on acinar cell amylase secretion were then evaluated. Physiological carbachol alone enhanced amylase secretion from a basal level of 3 to 17% (Fig. 4). Supraphysiological carbachol reduced this amylase secretion to 12% (Fig. 4). The increases in amylase secretion caused by carbachol were inhibited to control levels by the addition of 1 μM atropine (data not shown).
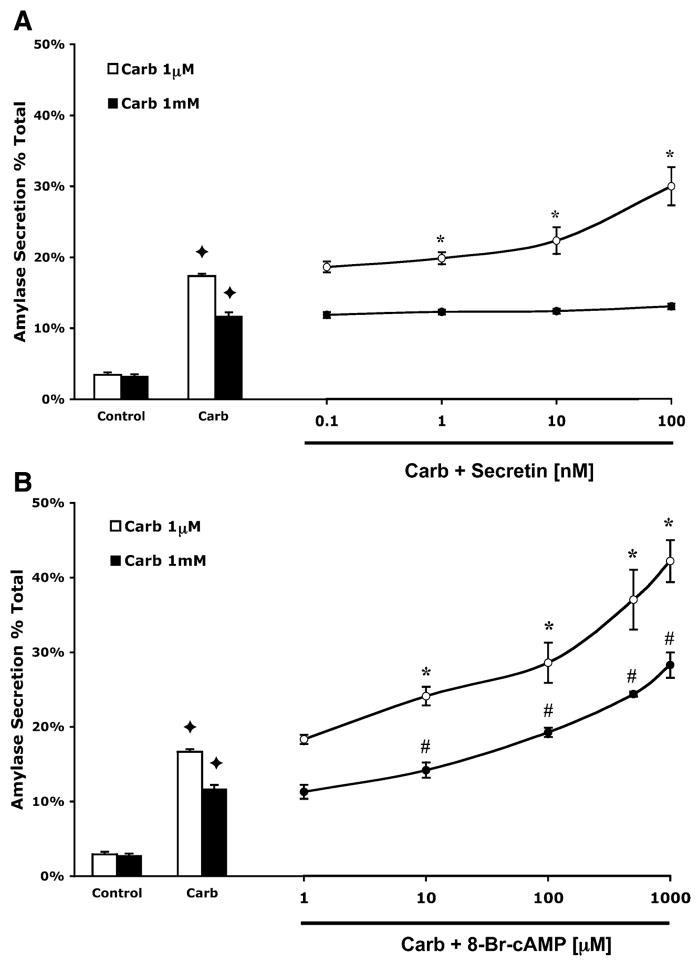
Fig. 4.
8-Br-cAMP enhances amylase secretion stimulated by physiological and supraphysiological carbachol. Secretin enhances amylase secretion induced by physiological, but not supraphysiological carbachol. Acini were incubated with or without either 1–1,000 μM 8-Br-cAMP (A) or 0.1–100 nM secretin (B) in the presence of carbachol (1 μM or 1 mM) and assayed for amylase secretion. All samples represent the means ± SE from 3 separate experiments. ✦ P < 0.05 vs. control; *P < 0.05 vs. 1 μM carbachol alone; #P < 0.05 vs. 1 mM carbachol alone.
Addition of secretin to cells treated with 1 μM carbachol produced a concentration-dependent enhancement in amylase secretion (Fig. 4A). The maximum response was observed at 100 nm secretin, which doubled amylase secretion. However, at 1 mM carbachol, secretin did not produce a significant increase in amylase secretion at any dose (Fig. 4A). When secretin was added to cells not treated with carbachol, amylase secretion increased to 14% (data not shown).
When 8-Br-cAMP was added to cells treated with carbachol, a dose-dependent enhancement in amylase secretion, peaking at 1 mM 8-Br-cAMP was seen at both physiological and supraphysiological concentrations of carbachol (Fig. 4B). When added to 1 μM and 1 mM carbachol, 1 mM 8-Br-cAMP caused an enhancement in amylase secretion to 45 and 30%, respectively. Note that inhibition of secretion produced by supraphysiological carbachol was reversed by 8-Br-cAMP, which increased secretion above levels seen with physiological carbachol alone. The addition of 8-Br-cAMP to control cells had no effect on secretion (data not shown).
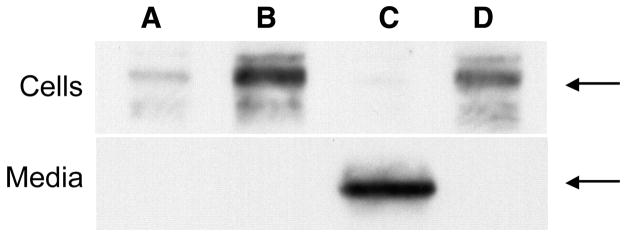
Because it is unclear whether activated enzymes will exhibit the same secretory responses as amylase, the release of chymotrypsin in acini and the medium was evaluated by using immunoblot assay (Fig. 5). Whereas supraphysiological carbachol alone caused an increase in the active enzyme form in the cell pellet compared with control, no chymotrypsin was detected in the medium of these samples. The addition of secretin to cells treated with supraphysiological carbachol did not affect these results. However, the addition of 8-Br-cAMP to cells treated with supraphysiological carbachol caused chymotrypsin to appear in the medium. Furthermore, the chymotrypsin in the cell pellet decreased to control levels. These data provide the first evidence that cAMP can overcome the inhibitory effects of carbachol on the secretion of activated enzymes from the acinar cell and suggest that it might protect against acinar cell injury.
Fig. 5.
Addition of 8-Br-cAMP to isolated acini treated with supraphysiological carbachol causes the release of chymotrypsin into the medium. Autoradiograph depicting presence of chymotrypsin in cells (top) and medium (bottom). Bands indicated by arrows correspond to chymotrypsin in unstimulated control (A) and samples treated with 1 mM carbachol (B), 1 mM carbachol with 100 μM 8-Br-cAMP (C), and 1 mM carbachol with 100 nM secretin (D). Note that although all treatments (B–D) increased the levels of chymotrypsin over control (A), the enzyme appeared in the medium only after treatment with carbachol and 8-Br-cAMP (C). This autoradiograph is representative of 3 studies.
Injury
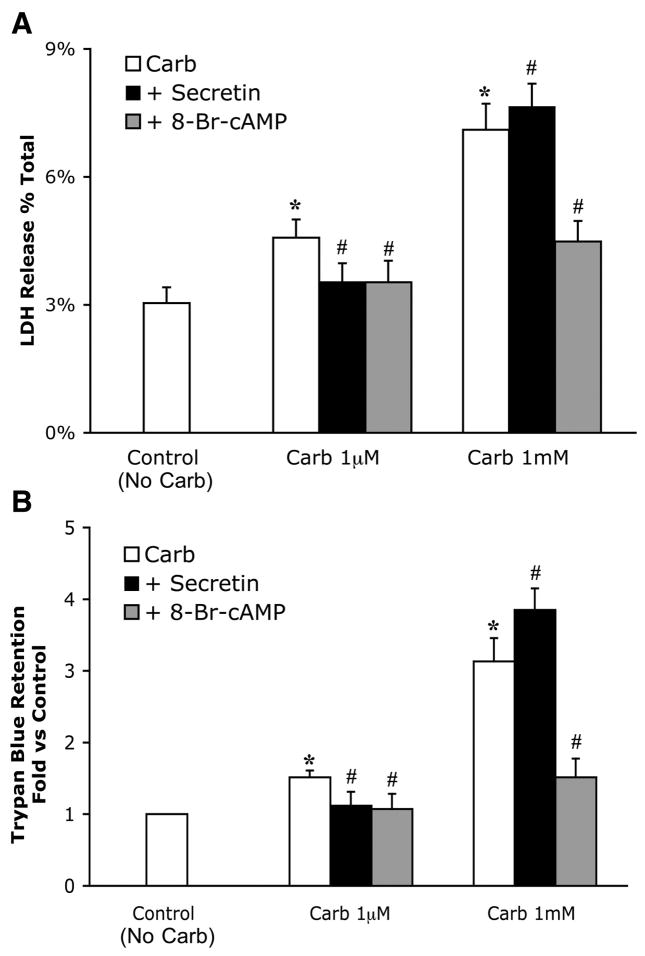
The effects of secretin and 8-Br-cAMP on cellular injury were evaluated in several ways. To perform these experiments, the only dose of secretin (100 nM) that had significant effects on zymogen activation and a submaximal dose of 8-Br-cAMP (100 μM) were chosen. LDH release was the first index of cell damage evaluated (Fig. 6A). Acini were treated with carbachol in the presence or absence of either secretin or 8-Br-cAMP for 2 h, and the percent LDH release into the cell culture medium was determined. After treatment with 1 μM carbachol, LDH release rose from a control level of 3 to 4.6%; the value increased to 7.1% with 1 mM carbachol. Pretreatment with 1 μM atropine prevented this injury (data not shown). When acini were treated with both 1 μM carbachol and secretin (100 nM), LDH release by physiological carbachol was reduced from 4.6 to 3.5%. Interestingly, secretin slightly increased LDH release caused by supraphysiological carbachol from 7.1 to 7.6%. In contrast, 8-Br-cAMP (100 μM) significantly reduced LDH release caused by both physiological (4.5–3.5%) and supraphysiological carbachol (7.1–4.5%). These studies were confirmed when trypan blue was used as an indicator of cell injury (Fig. 6B). Notably, some enhancement of trypan blue retention was observed after 1 μM carbachol. When these acini were examined by light microscopy, trypan retention was observed only in a few discrete acini. In contrast, trypan blue was retained in most cells after supraphysiological carbachol. These findings indicate that the enhanced injury observed after physiological stimulation occurs in only a small subset of cells and is not a generalized phenomena.
Fig. 6.
8-Br-cAMP and secretin reduce the small amount of injury caused by physiological carbachol. 8-Br-cAMP, but not secretin, reduces injury caused by supraphysiological carbachol. Acini were incubated with or without 8-Br-cAMP (100 μM) or secretin (100 nM) in the presence of carbachol (1 μM and 1 mM) for 2 h. Samples were assayed for LDH release as a percentage of total LDH (A). Trypan blue retention was determined as the ratio of λ583:λ260 and expressed as fold vs. control (B). Note that control indicates no carbachol. All samples represent the means ± SE from 3 separate experiments. *P < 0.05 vs. control; #P < 0.05 vs. carbachol alone.
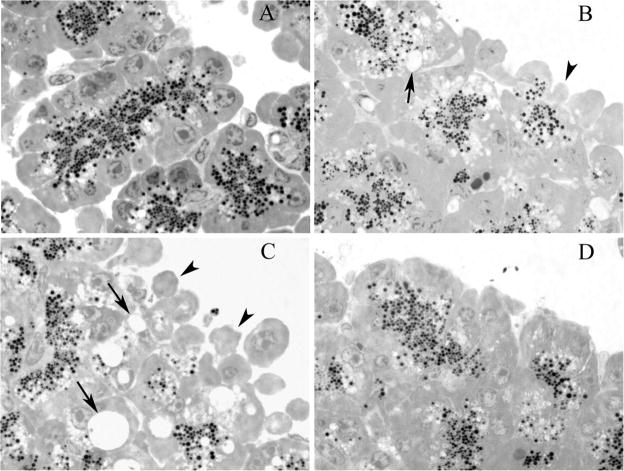
To further evaluate the cellular injury caused by supraphysiological doses of carbachol, morphological indicators of injury were evaluated (Fig. 7). First, the appearance of large cytoplasmic vacuoles, a hallmark of acute pancreatitis, was evaluated. Whereas untreated control cells or those exposed to secretin or 8-Br-cAMP alone (data not shown) did not exhibit this change, cells treated with 1 mM carbachol displayed prominent vacuoles. The effect of carbachol was reduced by the addition of 8-Br-cAMP but appeared to be enhanced by secretin. Blebbing of the basolateral membranes, a characteristic of injury in isolated acini, exhibited the same pattern as that described for vacuolization. These morphological results confirm that increasing cellular cAMP reduces cellular injury.
Fig. 7.
Morphological indicators demonstrate that 8-Br-cAMP, but not secretin, ameliorates injury caused by supraphysiological carbachol. Representative photograph showing basolateral blebbing (arrowheads) and vacuolization (arrows) in unstimulated controls (A) and samples treated with 1 mM carbachol (B), 1 mM carbachol with 100 nM secretin (C), and 1 mM carbachol with 100 μM 8-Br-cAMP (D). Note that the injury produced by 1 mM carbachol is ameliorated by the addition of 100 μM 8-Br-cAMP.
cAMP generation and specificity
To evaluate the distinct effects of secretin on activation, secretion, and injury when combined with 1 μM and 1 mM carbachol, cAMP levels were assayed (Table 1). Untreated control cells and those treated with low or high concentrations of carbachol alone exhibited similar levels of cAMP (~200–300 fmol/μg amylase). When secretin was combined with 1 μM carbachol, intracellular cAMP increased approximately twofold. In contrast, when secretin was combined with 1 mM carbachol, cAMP levels remained near control values. These studies suggest that the generation of cAMP by acinar cell is inhibited when supraphysiological but not physiological carbachol is combined with secretin.
Table 1.
Secretin enhances cAMP production stimulated by physiologic but not supraphysiologic carbachol
| Treatment | cAMP, fmol/μg amylase |
|---|---|
| Control | 199±32 |
| Carb (1 μM) | 303±22 |
| Carb (1 mM) | 285±67 |
| Carb (1 μM) + secretin | 558±46* |
| Carb (1 mM) + secretin | 305±20 |
All samples represent the means ± SE from 3 separate experiments. Acini were incubated with 1 μM or 1 mM carbachol (Carb) in the presence or absence of 100 nM secretin for 10 min. cAMP levels were determined by ELISA and normalized to total amylase content.
P < 0.05 vs. corresponding dose of carbachol.
To further ensure that the effects on activation and secretion were specific to cAMP, activation and secretion assays were performed on cells treated with 1 mM carbachol and 8-Br-cGMP (10, 100, and 500 μM, and 1 mM). However, 8-Br-cGMP had only slight effects on carbachol-induced activation and secretion. For example 100 μM 8-Br-cGMP produced only 30% of the enhancement in activation and secretion observed with 100 μM 8-Br-cAMP (data not shown).
To verify that the effects of 8-Br-cAMP on injury were specific, cells were pretreated for 30 min with 10 μM forskolin, a potent stimulator of the catalytic subunit of adenylate cyclase, before the addition of supraphysiological carbachol (1 mM). Effects of forskolin on carbachol-induced activation, secretion, and LDH release were similar to those seen with 8-Br-cAMP. Compared with 1 mM carbachol alone, the addition of forskolin enhanced trypsin and chymotrypsin activation by approximately twofold, increased amylase by ~33%, and decreased LDH release by 30% (P value for each comparison <0.05; data not shown). These studies confirm that the effects of 8-Br-cAMP on activation, secretion, and injury are specific to cAMP.
DISCUSSION
The early activation of zymogens and their retention within the acinar cell are key factors in the evolution of acute pancreatitis. The relationship between secretion and acinar cell injury is highlighted by the ability of various secretagogues to stimulate zymogen activation and inhibit secretion, causing pancreatitis. In the rat pancreatic acinar cell, supraphysiological concentrations of both caerulein, a CCK analog, and the muscarinic receptor agonist carbachol stimulate zymogen activation and cause enzyme retention and cell injury. In vivo, both caerulein and carbachol have been used to induce pancreatitis. The present study makes two central observations relevant to the mechanisms of acute pancreatitis. First, it demonstrates that cAMP agonists can modulate effects of carbachol on acinar cell secretion and zymogen activation. Of particular note is the ability of 8-Br-cAMP to overcome the inhibition of secretion caused by supraphysiological carbachol. Second, it suggests that cAMP agonists that increase acinar cell secretion can reduce the cell injury associated with zymogen activation.
Acinar cell secretion was evaluated in two ways in this study: 1) by measuring amylase secretion and 2) by using immunoblot analysis to detect the active form of chymotrypsinogen in the medium. The release of chymotrypsin into the medium was taken as surrogate for the release of other activated enzymes. Because the compartment(s) in which zymogens are converted to active enzymes remains unclear, the relationship between the secretion of amylase and active enzymes is also uncertain. Whereas two studies (6, 14) have suggested that zymogens become activated in a compartment that codistributes with lysosomal markers, another suggested activation occurs in zymogen granules (15). Whether this disparity results from differences in detection methods, the timing of analysis, or other factors awaits further study. In the present study, conditions that enhanced carbachol-induced amylase secretion also caused an increase in activated enzyme secretion. However, increases in activated enzyme secretion caused by 8-Br-cAMP appeared to be more prominent than the increases seen in amylase secretion. Thus it remains unclear whether secretion of amylase and activated enzymes is regulated by a common mechanism and/or is localized to a common compartment.
A novel and surprising result of this study is that 8-Br-cAMP was able to overcome the inhibition of amylase secretion and retention of active enzymes observed with supraphysiological carbachol. A dramatic decrease in enzyme secretion is observed early in the course of experimental pancreatitis and is thought to be required for acinar cell injury (5). The mechanism(s) responsible for the overall reduction in acinar cell secretion and retention of active enzymes observed with supraphysiological concentrations of CCK or carbachol is uncertain. Studies (3, 13a) have noted disruption of the apical acinar cell actin cytoskeleton early in the course of caerulein-induced pancreatitis or after supraphysiological caerulein-treatment in vitro. A study in permeabilized acinar cells concluded that the acinar cell has two actin networks that regulate secretion (12). Whereas one network may act as a regulated barrier to exocytosis, the other may maintain zymogen granules in a distribution required for exocytosis. It has been suggested that a redistribution of zymogen granules away from the apical membrane might account for the suppression of secretion observed with supraphysiological caerulein or carbachol. High doses of cAMP have been shown to partially reverse the effects of caerulein hyperstimulation on the actin cytoskeleton (21). Additional studies are needed to examine whether the effects of cAMP agonists on the apical actin cytoskeleton are responsible for the enhancement of secretion seen with cAMP in this study.
Another interesting finding of this study is the distinct effects of the two cAMP agonists, secretin and 8-Br-cAMP. Secretin and 8-Br-cAMP enhanced amylase secretion and reduced injury when combined with physiological concentrations of carbachol. However, with supraphysiological carbachol stimulation, directly increasing cellular cAMP with 8-Br-cAMP or forskolin was required to enhance secretion and reduce injury. Our data suggest that these differences are likely due to insufficient cAMP generation with secretin when it is combined with supraphysiological carbachol. The blunted production of cAMP under these conditions is likely the result of inhibition of adenylate cyclase by high dose carbachol (1). Because 8-Br-cAMP directly increases intracellular cAMP, it can have similar actions at both physiological and supraphysiological concentrations of carbachol.
The present study also further elucidates the interaction among zymogen activation, secretion, and injury. Our data indicate that conditions, like supraphysiological carbachol, which enhance zymogen activation and cause retention of active enzymes, lead to increased injury. Whereas it might be predicted that treatments that sensitize to zymogen activation may lead to increased cellular injury, this study confirms that these outcomes are not always generated in parallel. For example, we report that activation induced by carbachol is enhanced by both secretin and 8-Br-cAMP. However, under the same conditions, the addition of 8-Br-cAMP produces a decrease in injury. The dissociation between the effects of cAMP agonists on zymogen activation and injury may be related to enhanced secretion of activated enzymes.
The relationship among activation, secretion, and injury suggested by the present study supports previous results with another secretagogue, bombesin (5). When bombesin binds to the G protein-linked, gastrin-releasing peptide receptor, intracellular calcium and cAMP are increased (19). Like caerulein and carbachol, bombesin causes zymogen activation. However, unlike supraphysiological caerulein and carbachol, active enzymes are secreted from acinar cell after bombesin treatment. Bombesin does not cause cellular injury in vitro or pancreatitis in vivo. That bombesin causes zymogen activation without leading to injury or pancreatitis reinforces the hypothesis that retention of active enzymes is also necessary for damage to the acinar cell. The present study builds on these findings by demonstrating that the injurious effects of a treatment like carbachol, which enhances zymogen activation and causes the retention of active enzymes, may be prevented. This effect presumably occurs through the secretion of active enzymes.
The significance of our finding that physiological doses of carbachol also cause some injury is not clear. Our data confirm previous findings that physiological doses of secretagogue can produce a small level of zymogen activation (10). It is possible that this modest increase in zymogen activation or another event, like secretagogue-induced consumption of ATP, causes stress in our in vitro system that may or may not reflect the in vivo condition. That discrete populations of cells were observed to retain trypan blue after physiological stimulation suggests that a subset of isolated acini is more susceptible to secretagogue-induced injury. Notably, secretin and 8-Br-cAMP, which both enhance carbachol-induced amylase secretion, significantly reduce this injury.
Although we believe this study is the first to demonstrate a direct effect of secretin on acinar cell injury, secretin has been used to treat or prevent acute pancreatitis without an established rationale. In animal studies (7, 13, 16, 17, 22) involving rodents and dogs, there is no consensus about the ability of secretin to prevent or ameliorate pancreatitis. In humans, there has been interest in the ability of secretin to reduce the incidence and severity of post-ERCP-induced pancreatitis. In the few clinical studies (13, 22) evaluating secretin prophylaxis for ERCP-induced pancreatitis, results have been mixed. However, a preliminary randomized, double-blind, placebo-controlled trial suggested that secretin reduces the incidence of post-ERCP pancreatitis when given at the time of the procedure (9). That secretin has a protective effect in our studies when combined with physiological, but not supraphysiological carbachol, might have therapeutic implications. Furthermore, our results showing that secretin can have differing effects may help to explain the divergent results of these animal and human studies. Although the present study provides a rationale for the therapeutic use of secretin to prevent ERCP-induced pancreatitis, it also suggests that an agent with actions more similar to 8-Br-cAMP might be more effective.
In summary, we have shown that increasing intracellular cAMP augments both zymogen activation and secretion in carbachol-treated acinar cells. Notably, cAMP agonists enhance secretion of amylase and markers of activated enzymes. When secretion is increased, the cell injury associated with carbachol treatment is significantly reduced. These results suggest that cAMP-stimulated pathways may reduce cellular injury by causing the secretion of active enzymes from the pancreatic acinar cell. The findings support the hypothesis that zymogen activation and the retention of active enzymes are both required for acinar cell injury.
Acknowledgments
These studies were supported by a Ruth I. Kirschstein National Research Service Award (to A. Chaudhuri) and a Veterans Administration Career Development sabbatical award and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK5–4021 (to F. S. Gorelick).
References
- 1.Akiyama T, Hirohata Y, Okabayashi Y, Imoto I, Otsuki M. Supramaximal CCK and CCh concentrations abolish VIP potentiation by inhibiting adenylyl cyclase activity. Am J Physiol Gastrointest Liver Physiol. 1998;275:G1202–G1208. doi: 10.1152/ajpgi.1998.275.5.G1202. [DOI] [PubMed] [Google Scholar]
- 2.Burnham DB, McChesney DJ, Thurston KC, Williams JA. Interaction of cholecystokinin and vasoactive intestinal polypeptide on function of mouse pancreatic acini in vitro. J Physiol. 1984;349:475–482. doi: 10.1113/jphysiol.1984.sp015168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallon M, Gorelick F, Anderson J, Mennone A, Saluja A, Steer M. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology. 1995;108:1863–1872. doi: 10.1016/0016-5085(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 5.Grady T, Mah’moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol Gastrointest Liver Physiol. 1998;275:G1010–G1017. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- 6.Hofbauer B, Saluja AK, Lerch MM, Bhagat L, Bhatia M, Lee HS, Frossard JL, Adler G, Steer ML. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. 1998;275:G352–G362. doi: 10.1152/ajpgi.1998.275.2.G352. [DOI] [PubMed] [Google Scholar]
- 7.Infantino A, Dodi G, Basso D, Munaretto S, Fabris C, Pregnolato P, Del Favero G, Fassina A, Lise M, Naccarato R. Failure of secretin to exert an ameliorative effect on acute reflux pancreatitis in the rat. Res Exp Med (Berl) 1990;190:89–93. doi: 10.1007/pl00020010. [DOI] [PubMed] [Google Scholar]
- 8.Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology. 2001;121:1380–1390. doi: 10.1053/gast.2001.29557. [DOI] [PubMed] [Google Scholar]
- 9.Jowell PS, Branch M, Robuck-Magnum G, Fein S, Purich E, Stiffler H, Baillie J. Synthetic secretin administered at the start of the procedure significantly reduces the risk of post-ERCP pancreatitis: a randomized, double-blind, placebo controlled trial (Abstract) Gastoenterology. 2003 [Google Scholar]
- 10.Leach SD, Modlin IM, Scheele GA, Gorelick FS. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991;87:362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Z, Kolodecik TR, Karne S, Nyce M, Gorelick F. Effect of ligands that increase cAMP on caerulein-induced zymogen activation in pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2003;285:G822–G828. doi: 10.1152/ajpgi.00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muallem S, Kwiatlowska K, Xu X, Yin H. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundorf J, Jowell P, Branch MS, Affronti J, Cotton P, Baillie J. Reduced incidence of post-ERCP pancreatitis (PEP) in non-pancreas divisum (non-PD) patients who receive intravenous secretin (sec) during ERCP (Abstract) Am J Gastroenterol. 1995;90:1611. [Google Scholar]
- 13a.O’Konski MS, Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest. 1990;86:1649–1657. doi: 10.1172/JCI114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otani T, Chepilko SM, Grendell JH, Gorelick FS. Co-distribution of trypsinogen activation peptide and the granule membrane protein, GRAMP-92, in rat cerulein-induced pancreatitis. Am J Physiol Gastroin-test Liver Physiol. 1998;275:G999–G1009. doi: 10.1152/ajpgi.1998.275.5.G999. [DOI] [PubMed] [Google Scholar]
- 15.Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renner IG, Wisner JR., Jr Ceruletide-induced acute pancreatitis in the dog and its amelioration by exogenous secretin. Int J Pancreatol. 1986;1:39–49. doi: 10.1007/BF02795238. [DOI] [PubMed] [Google Scholar]
- 17.Renner IG, Wisner JR, Jr, Rinderknecht H. Protective effects of exogenous secretin on ceruletide-induced acute pancreatitis in the rat. J Clin Invest. 1983;72:1081–1092. doi: 10.1172/JCI111033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reubi JC, Waser B, Gugger M, Friess H, Kleeff J, Kayed H, Buchler MW, Laissue JA. Distribution of CCK1 and CCK2 receptors in normal and diseased human pancreatic tissue. Gastroenterology. 2003;125:98–106. doi: 10.1016/s0016-5085(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 19.Rozengurt E, Sinnett-Smith J. Bombesin stimulation of fibroblast mitogenesis: specific receptors, signal transduction and early events. Philos Trans R Soc Lond B Biol Sci. 1990;327:209–221. doi: 10.1098/rstb.1990.0055. [DOI] [PubMed] [Google Scholar]
- 20.Schmid S, Modlin IM, Stoch A, Tang L, Rhee S, Nathanson M, Scheele GA, Gorelick FS. Telenzepine-sensitive muscarinic receptors on the rat pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol. 1998;274:G734–G741. doi: 10.1152/ajpgi.1998.274.4.G734. [DOI] [PubMed] [Google Scholar]
- 21.Singh VP, Saluja AK, Bhagat L, Hietaranta AJ, Song A, Mykoniatis A, Van Acker GJ, Steer ML. Serine protease inhibitor causes F-actin redistribution and inhibition of calcium-mediated secretion in pancreatic acini. Gastroenterology. 2001;120:1818–1827. doi: 10.1053/gast.2001.24883. [DOI] [PubMed] [Google Scholar]
- 22.Tympner F, Rosch W. Effect of secretin and gabexate-mesilate (synthetic protease inhibitor) on serum amylase level after ERCP. Z Gastroenterol. 1982;20:688–693. [PubMed] [Google Scholar]