Abstract
Intramedullary pressure (ImP) and low-level bone strain induced by oscillatory muscle stimulation (MS) has the potential to mitigate bone loss induced by disuse osteopenia, i.e., hindlimb suspension (HLS). To test this hypothesis, we evaluated a) MS induced ImP and bone strain as function of stimulation frequency, and b) the adaptive responses to functional disuse, and disuse plus 1Hz and 20Hz stimulation in vivo. Femoral ImP and bone strain generated by MS were measured in the frequencies of 1Hz-100Hz in four rats. Forty retired breeder rats were used for the in vivo HLS study. The quadriceps muscle was stimulated at frequencies of 1 Hz and 20 Hz, 10min/d for 4 weeks. The metaphyseal trabecular bone quantity and microstructure at the distal femur were evaluated using μCT, while bone formation indices were analyzed using histomorphometric techniques. Oscillatory MS generated a maximum ImP of 45±9 mmHg at 20 Hz and produced a maximum matrix strain of 128±19 με at 10 Hz. Our analyses from the in vivo study showed that MS at 20 Hz was able to attenuate trabecular bone loss and partially maintain the microstructure induced by HLS. Conversely, there was no evidence of an adaptive effect of stimulation at 1 Hz on disused skeleton. The results suggested that oscillatory MS regulates fluid dynamics and mechanical strain in bone, which serves as a critical mediator of adaptation. These results clearly demonstrated the ability of MS in attenuating bone loss from the disuse osteopenia and could hold potential in mitigating skeletal degradation imposed by conditions of disuse, which may serve as a biomechanical intervention in clinic application.
Keywords: muscle stimulation, bone fluid flow, intramedullary pressure, osteopenia, loading frequency
Introduction
The morphology and function of bone and muscle are strongly related (Allen et al., 2006; LeBlanc et al., 2000). As a direct consequence of aging and exposure to microgravity, elderly and astronauts experience a number of physiological changes in their musculoskeleton, including osteopenia (Akima et al., 2005; Lang et al., 2004; McCarthy, 2005). Studies of mechanical influences on tissue morphology have demonstrated that the removal of functional loads leads to a loss of bone mass (Rubin and Lanyon, 1987), whereas an increase of activity, such as exercise, results in the augmentation of muscle strength and bone mineral density (BMD) (Krolner et al., 1983). However, the mechanotransductive mechanism and interface interaction between bone and muscle remain unclear.
Skeletal muscle contraction can increase blood flow within musculoskeletal tissues and generate bone strain within the physiological range (Midura et al., 2005; Valic et al., 2005). Dynamic stimulation in altering the intramedullary fluid pressure (ImP) and bone strain simultaneously maybe the two key determinants responsible for the mechanotransductive signals in bone. It is widely accepted that dynamic loading through various frequencies plays a critical role in the skeletal adaptive response and further enhance cellular level perfusion (Wang et al., 2004). Previous in vivo studies have demonstrated that the rate of the osteogenic response in bone tissue increases at higher frequency (Qin et al., 2003; Zhang et al., 2007). Vibration studies have shown that low-level mechanical signals at 45 Hz lower osteoclastic activity and enhance the rate of trabecular bone formation in the tibia of either growing animals or functional disused animals (Garman et al., 2007). Stimulation of the knee region at 15 Hz increased the cortical mineralizing surface and apposition rate in the femur (Zhang et al., 2007). Alternatively, by inducing a 20 Hz of fluid pressure into the marrow cavity of turkey ulna, the formation of periosteal and endosteal new bone was augmented at the cortex (Qin et al., 2003).
Identifying regulatory components of the mechanical milieu between muscle and bone may prove instrumental in devising a biomechanically based intervention for many serious clinical conditions including the prevention of osteopenia. Identifying stimulation parameter, i.e., frequency, within the regimen may generate beneficial adaptive responses and alleviate the consequence of bone loss. To test the hypothesis - oscillatory muscle stimulation (MS) has the potential to attenuate bone loss induced by disuse osteopenia, the objectives for this study were: (1) to evaluate the immediate effects on ImP and bone strain induced by dynamic muscle stimulation in response to a broad range of loading frequencies and (2) to characterize the regulatory role of dynamic MS at low and high frequencies on bone adaptation under conditions of functional disuse, e.g., hindlimb suspension (HLS).
Materials and Methods
All surgical and experimental procedures were approved by University’s Laboratory Animal Use Committee.
ImP and Strain Measurements
Four 6 to 9-months-old Sprague Dawley retired breeder rats with a mean body weight of 387g ± 41g (Taconic, NY) were used to measure the relationships between ImP, bone strain, and induced-muscle contraction. Rats were anesthetized using standard isoflurane inhalation. For the ImP measurement, an incision of 3 mm was made at the knee region to expose the distal femur. An 1 mm hole was carefully drilled into the marrow cavity from the distal end of the femur between medial and lateral condyles. A micro-cardiovascular pressure transducer (Millar Instruments, SPR-524, Houston, TX) was inserted into the femoral marrow cavity, guided via a 16-guage catheter. The drill hole was completely sealed with the pressure transducer and catheter apparatus. For bone strain measurement, a single element strain gauge (120Ω, factor 2.06, Kenkyojo Co., Tokyo) was firmly attached onto the lateral surface of the same femur at the mid-diaphyseal region, with minimal disruption to the quadriceps. Both ImP and strain were measured simultaneously.
Two disposable needle-sized electrodes (L-type guage #3, Seirin, Weymouth, MA) were inserted into the quadriceps, about 5mm anterior from the femur. One electrode was placed at the height of the greater trochanter. The second electrode was inserted about 10mm superior to the femoral condyles. The electrodes were then connected to a 100MHz arbitrary waveform generator (Model 395, Wavetek) to conduct MS at frequencies of 1, 2.5, 5, 10, 15 20, 30, 40, 50, 60 and 100 Hz. Stimulation was induced at 2V with 1ms square pulse with duty of one second active followed by a rest of 4 seconds. For each animal, the entire frequency spectrum was repeated three times. Three signals (ImP, bone strain, load feedback) were collected simultaneously using a strain gauge amplifier (National Instruments) with a 160 Hz low-pass filter and A/D conversion at 1000 Hz with 16-bit resolution.
In Vivo Experiment
A hindlimb suspension rat model was used to mimic the condition of weightlessness of the hindlimbs. Forty 6-months-old Sprague Dawley female retired breeder rats with an average body weight of 250-350g were divided into five groups (n=8 each): baseline control (sacrificed at the beginning of the experiment), age-matched control, HLS control, HLS+1Hz MS, and HLS+20Hz MS. Each animal was housed individually in a 18”×18”×24” (L×W×H) stainless steel cage in a temperature-controlled room with a 12:12 hours light:dark cycle. Animals were provided standard rodent chow and water ad libitum.
The preparation of HLS was performed under isoflurane anesthesia at the beginning of the experiment. Briefly, the rat’s tail was cleaned with 70% ethanol and applied with a thin coat of benzoin. Once dried, a piece of surgical tape, attached to a plastic tab, was applied to the sides of the tail, covering ¾ of its length. Two pieces of elastic adhesive were used to secure the tape. The plastic tab was then attached to the fishline swivel, which was suspended from the top of the cage to allow height adjustment of the animal’s tail. The extended hindlimbs were approximately 2 cm off the ground and the forelimbs were allowed full access to the entire cage bottom.
The right quadriceps was stimulated at a frequency of 1 Hz or 20 Hz for 10 minutes once daily, 5 days per week, for 4 weeks. Each animal, including the controls, was anesthetized using standard isoflurane inhalation during daily stimulation. Once each day, two disposable needle-sized electrodes were inserted into the quadriceps as described above. The electrodes were then connected to the waveform generator to induce contraction. Stimulation was applied at 2V with 1ms square pulse for two seconds followed by a rest of eight seconds to avoid fatigue. The number of pulses applied per day to the skeletal muscle was 120 for the 1 Hz MS and 2,400 for the 20 Hz. Calcein (10mg/kg) was administered intraperitoneally to each animal two days and 16 days prior to euthanasia for histomorphometric analysis.
Microcomputed Tomography (μCT) Analysis
Using a high resolution μCT scanner (μCT-40, SCANCO Medical AG, Switzerland), the distal portions of each femur, including both experimental and contralateral limbs, were scanned at 15 μm resolution. A 750 μm metaphyseal region, 750 μm above the growth plate, was selected for evaluation. A single operator drew a set of contour lines that included only the trabecular bone within the metaphyseal region. The region was evaluated with specific sigma, support, and threshold values of 0.5, 1, and 347, respectively. Bone volume fraction (BV/TV, given as %), connectivity density (Conn.D, 1/mm3), structural model index (SMI), trabecular number (Tb.N, 1/mm), thickness (Tb.Th, mm), and separation (Tb.Sp, mm) were measured. After the initial evaluation, we noticed that the contralateral femurs from the experimental group were shown similar response to the MS stimulated side by the daily MS. For example with the 20 Hz stimulation, the average trabecular BV/TV was 0.164 ± 0.08% and 0.167 ± 0.08% for the stimulated femur and its contralateral, respectively. Likewise, the trabecular number was 3.77 ± 0.67 mm−1 for the stimulated femur and 3.89 ± 0.67 mm−1 for the contralateral. This was not surprising to us since during the daily loading, the MS induced by 20 Hz contraction caused contralateral limb movement as well. Other MS studies have showed similar phenomenon and detected that muscle activity was induced in the non-stimulated hindlimb (Wei et al., 1998). Thus, we have focused all in vivo data analyses on the stimulated limb only, in which MS generated responses at 1Hz and 20Hz were compared to HLS alone group.
Histomorphometric analysis
The 10-mm distal portion of each femur was cut and embedded in a solution of polymethyl methacrylate. Longitudinal 8 μm sections were cut with a Leica 2165 microtome (Leica, Wetzlar, Germany). Using the Osteomeasure software (OsteoMetrics Inc, Decatur, GA), trabecular bone surface and calcein labels were traced in the metaphyseal region (3mm2) that was 750 μm above the growth plate. Histomorphometric bone volume fraction (BV/TV-Histo, %), trabecular number (Tb.N-Histo, 1/mm), trabecular separation (Tb.Sp-Histo, μm), mineral apposition rate (MAR, μm/day), and bone formation rate (BFR/BV, %/yr) were determined.
Statistical analysis
Values were reported as mean ± SD for the ImP and bone strain experiments, μCT, and histomorphometric analyses. Statistical analyses were performed using SigmaStat v. 2.03 (Systat Software Inc, San Jose, CA). The effects of treatments on ImP and bone strain were evaluated using a Kruskal-Wallis one-way analysis of variance (ANOVA) on ranks and Dunn’s pairwise comparison test. Effects of treatments on μCT and histomorphometric measurements were evaluated using a one-way ANOVA with Tukey’s pairwise comparison test. In each test, the null hypothesis was rejected if p < 0.05.
Results
ImP induced by MS
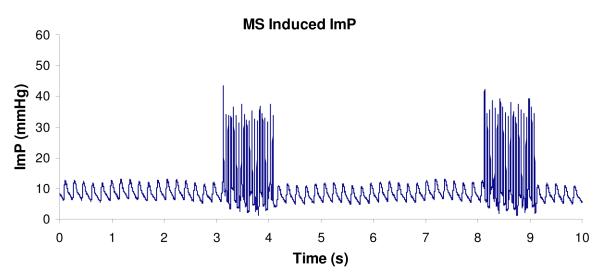
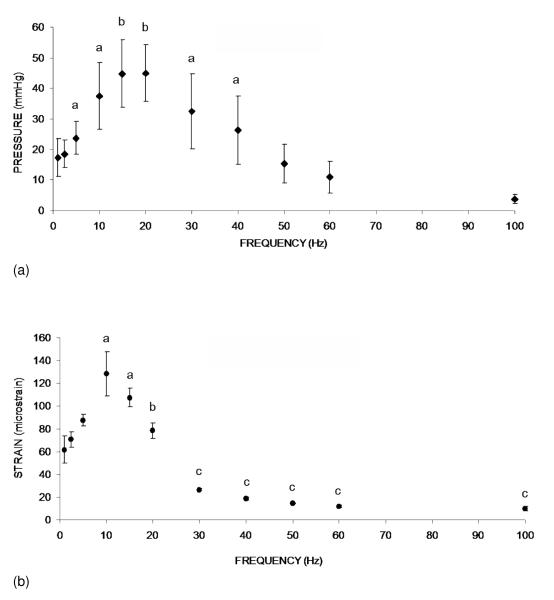
Oscillatory MS has shown significant effect in increasing ImP. A representative ImP profile induced by MS at 20 Hz is shown in Figure 1. Normal heart beat generated approximately 5 mmHg of ImP in the femur at a frequency of 5.37±0.35 Hz. The ImP value (peak-peak) was increased by dynamic MS at 5, 10, 15, 20, 30, and 40 Hz (p < 0.05 for 5, 10, 30, and 40 Hz, p < 0.01 for 15 and 20 Hz). The response trend of the ImP against frequency was nonlinear; the ImP reached a maximum value of 45±9.3 mmHg (peak-peak) at 20 Hz (Fig. 2a), although there was no significant difference between 10, 20, and 30 Hz. The MS generated ImP values of 17.4±6.2, 24±5.4, 37.5±11.0, 26.3±11.1, and 3.7±1.5 mmHg at frequencies of 1, 5, 10, 40, and 100 Hz, respectively.
Figure 1.
Representative ImP profile induced by electrical muscle stimulation at 20Hz. Stimulation was applied for 1 seconds followed by 4 seconds rest. Normal heart beat generated approximately 4 mmHg of ImP (mean) in the femur. Electrically induced muscle contraction increased ImP to 45 mmHg.
Figure 2.
(a). Graphs show mean ± SD values from the ImP measurement. ImP in femur increased significantly with electrical frequency at 5, 10, 15, 20, 30, and 40 Hz. In the loading spectrum from 1 to 100 Hz, stimulation at 1 Hz generated an ImP of 18 mmHg. A maximum ImP of 45 mmHg was measured at 20 Hz, which was 2.5 folds higher than 1 Hz. a p <0.05 vs. baseline ImP; b p <0.01 vs. baseline ImP.
(b). Graphs show mean ± SD values from the bone surface strain measurement. Dynamic muscle stimulation applied at various frequencies significantly increased bone strain. In the loading spectrum from 1 to 100 Hz, stimulation at 1 Hz produced a strain of 62 με. Peak strain of 128 με was recorded at 10 Hz stimulation. The strain magnitude was reduced by >75% of the peak strain for stimulation frequencies greater than 30 Hz. a p <0.01 vs. 1, 2.5, and 5 Hz; b p <0.01 vs. 10 Hz; c p <0.001 vs. stimulation 20 Hz and below.
Bone strain generated by MS
The response of matrix strain to the MS frequency also was nonlinear (Fig. 2b). The MS generated femoral matrix strains of 61.8±6.2, 87.5±5.1, 128.4±19.2, 78.3±6.8, 18.7±1.3, and 10.1±1.8 με at frequencies of 1, 5, 10, 20, 40, and 100 Hz, respectively. While the peak ImP in the pressure trend was observed at 20 Hz, the maximum matrix strain was measured at 10 Hz. MS induced bone strain at 10 Hz was significant higher (p < 0.01) than the strain values at other frequencies with the exception of 15 Hz. In addition, the strains generated by MS above 30 Hz were significantly lower than those values stimulated at and below 20 Hz (p < 0.005). Matrix strains, when loaded above 30 Hz, decreased by more than 75% of the peak strain measured at 10 Hz, in which induced strains at frequencies of 40~100 Hz were less than 20 με.
μCT Analysis
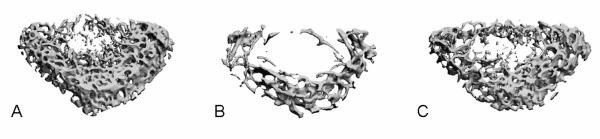
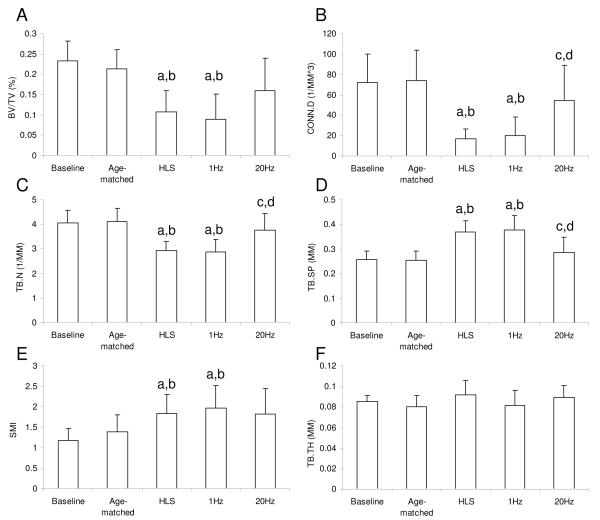
When muscle was stimulated at a frequency of 1 Hz, the level of in vivo bone loss and structural deterioration using μCT measurement was similar to those under HLS alone sham control (Fig. 3 and Fig. 4). Dynamic stimulation of the quadriceps at 20Hz demonstrated partial preventive effects at the metaphyseal region of the distal femur (Fig. 3). Compared to the age-matched control, 4 weeks of HLS alone significantly reduced trabecular bone quantity and quality, in which BV/TV, Conn.D, and Tb.N, decreased by 49%, 77%, and 29%, respectively, and SMI and Tb.Sp increased by 33% and 45%, respectively (p < 0.001) (Fig. 4). Compared to the baseline control, similar results were observed, in which BV/TV, Conn.D, and Tb.N were reduced by 53%, 77%, and 28%, respectively, and SMI and Tb.Sp were increased by 44% and 56%, respectively (p < 0.001). Trabecular bone loss induced by disuse was attenuated with daily MS at 20Hz. Compared to the HLS control, values of BV/TV, Conn.D, Tb.N, Tb.Sp, SMI, and Tb.Th were changed by 48%, 224%, 29%, −23%, −1%, and 2%, respectively. Effects of MS on Conn.D, Tb.N, and Tb.Sp were statistically significant.
Figure 3.
Representative 3D μCT images of trabecular bone in the distal metaphyseal region of the femur. The region was 750 μm above the growth plate. A. Age-matched control, B. HLS control, C. HLS+20 Hz MS
Figure 4.
Graphs show mean + SD values from the μCT analysis in the distal metaphyseal region of the femur. A. Trabecular bone volume fraction (BV/TV, %), B. Connectivity density (Conn.D, 1/mm3), C. Trabecular number (Tb.N, 1/mm), D. Trabecular separation (Tb.Sp, mm), E. Structural Model Index (SMI), and F. Trabecular thickness (Tb.Th, mm). MS at 20Hz showed significant effects on Conn.D, Tb.N, and Tb.Sp against 4 weeks of HLS. b p <0.001 vs. baseline; a p <0.001 vs. age-matched; c p <0.005 vs. HLS; d p <0.005 vs. 1 Hz MS.
Histomorphometric Analysis
Effects of bone loss by functional disuse were also observed via 2-D histomorphometric analysis; BV/TV-Histo, and TB.N-Histo were reduced by 41% and 50%, respectively, while Tb.Sp-Histo was 167% greater in HLS than the values in the age-matched control (p < 0.01). Similarly, the bone formation indices, MAR and BFR/BV declined by 80% and 91% (p < 0.01), respectively in HLS animals. We found no evidence that Tb.Th was affected by the HLS or MS. At 1 Hz, MS did not ameliorate effects of disuse. Effects on bone formation activity were partially ameliorated at 20 Hz (Fig. 5). Compared to the HLS control, stimulation at 20Hz increased levels of BV/TV-Histo and Tb.N-Histo (p < 0.01) but reduced Tb.Sp-Histo within the metaphyseal region (29.5 ± 3.1%, 4.18 ± 0.30 /mm, 178 ± 21μm, respectively for 20Hz MS, compared to 23.0 ± 3.1%, 2.38 ± 0.20 /mm, 359 ± 57μm for HLS). Values of MAR and BFR/BV were 155% and 118% greater in the 20 Hz MS group than in the HLS animals but changes were not statistically significant. Similarly, values of MAR and BFR/BV were 48% and 81% greater in 20 Hz MS group than in the age-matched control, although these changes also were not statistically significant.
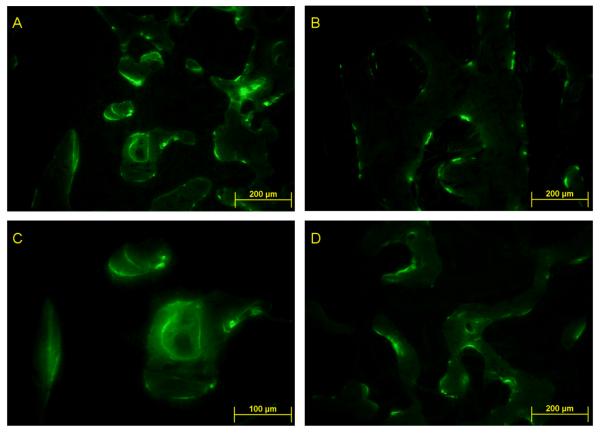
Figure 5.
Representative 2D histomorphometric images of calcein labeled trabecular bone in the distal metaphyseal region of the femur. A. Age-matched control at 10X magnification, B. Age-matched control at 20X, C. HLS control at 10X, D. HLS + 1 Hz MS at 10X. Mineralizing bone surfaces were labeled by the calcein (green line). Trabecular bone formation was clearly indicated by the double calcein labels in A & B.
Discussion
The results indicated that dynamic muscle stimulation generates fluid pressure in bone with simultaneously low-level bone strain. MS adjacent to the rat femur induces a peak ImP at 20-30 Hz. Similarly, bone strain was peaked at approximately 10 Hz. In the optimized loading rates (e.g., 20-50 Hz), relatively high ImP value and low bone strain were observed as a function of loading frequency. Both ImP and strain at 10 and 20 Hz are higher than the values at the lower frequency, i.e., 1 Hz. There is significant strain difference between 10 Hz and 20 Hz, while no significant ImP difference was observed between corresponding frequencies. MS can potentially produce high fluid pressure gradients within the femoral marrow cavity and high strain value in bone. Loading generated matrix strain and fluid pressure in bone may have combined effects in attenuating bone loss if loaded at proper frequencies. The result is consistent with previous in vivo results, in which mechanical loading at frequencies of 20 to 45 Hz were shown to be anti-catabolic to cortical bone (Garman et al., 2007; Qin et al., 1998; Qin et al., 2003). This sensitivity was even more apparent in the trabecular bone, perhaps due to the increased surface area in the trabecular network, which exposes it to the rapid changes in fluid pressure (Qin et al., 2002). For example, trabecular osteoblast surface in the tibia was increased by 26% when a MS protocol at 10 Hz was applied for 3 weeks (Zerath et al., 1995).
Both ImP and matrix strain have indicated nonlinear response in the MS spectrum between 1 Hz and 100 Hz, though peaked differently at 20 Hz (ImP) and 10 Hz (strain). From the characteristics of tissue material point of view, e.g., viscoelastic property in the tissues, both muscle and bone could quickly damp the loads at high frequencies. However, due to the difference in material densities and viscosities within hard and soft tissues, MS induced ImP and matrix strain could result in different frequency responses. Secondly, mechanotransductive pathway through different connective tissues during MS may attenuate the high frequency response in bone, e.g., via the connective pathway from muscle, tendon to bone, resulting in peak strain and peak ImP at varied frequencies. Future research on such complex interrelation between muscle kinematics, bone fluid flow, and matrix strain is necessary to further elucidate the mechanism.
Previous data from our group has shown that ImP alone can induce bone adaptation (Qin et al., 2003). Using an avian ulna model, disuse resulted in a 5.7% loss of cortical bone. Direct fluid loading at 20 Hz for 4 weeks increased cortical bone mass by 18% (Qin et al., 2003). Transcortical fluid pressure gradient and total bone formation were strongly correlated. In light of mechanotransductive role in triggering bone remodeling (Wang et al., 2003; Wang et al., 2004), strong evidence suggests that interstitial fluid flow in bone interacts strongly with external muscular activities via various mechanisms (Stevens et al., 2006; Valic et al., 2005). According to a muscle pump hypothesis, an arteriovenous pressure gradient enhances muscle perfusion (Laughlin, 2005). This process may in turn increase the hydraulic pressure in skeletal nutrient vessels and amplify the capillary filtration in bone tissue (Laughlin, 2005; Otter et al., 1999; Winet, 2003).
MS on spinal cord-injured patients can cause partial reversal of disuse osteopenia and recovery of muscular strength (Belanger et al., 2000). Other in vivo studies have also reported positive effects of using muscle stimulation to inhibit muscle atrophy. Immobilization studies using MS at 50 to 100 Hz have shown to minimize the reduction of the cross-sectional area of muscle fiber and to restore mechanical properties (Kim et al., 2007). Stimulation of distal nerve stumps had similar action potential response between normal and muscle innervated (O’Gara et al., 2006). Although the response of ImP and bone mass by MS under such periphery nerve block conditions is still remained unknown, MS could serve as a mitigating agent to retain bone mass under chronic nerve damage conditions, e.g., spinal cord injury.
In conclusion, dynamic muscle stimulation can generate low-level bone strain and ImP as a function of stimulation frequency. Induced dynamic ImP may ultimately enhance interstitial fluid flow in bone. MS, if applied at an optimal frequency, has preventive potential in attenuating bone loss in disuse osteopenia. The results of this study provide an impetus for further development of a biomechanically based intervention for preventing and/or treating osteoporosis and muscle atrophy.
Acknowledgments
This work is kindly supported by The National Institute of Health (R01 AR52379 and R01 AR49286), the US Army Medical Research and Materiel Command, and the National Space Biomedical Research Institute through NASA NCC 9-58. The authors are grateful to Mr. Meng Zhang for technical assistance, and Drs. C. Rubin and S. Judex for critical discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akima H, Katayama K, Sato K, Ishida K, Masuda K, Takada H, Watanabe Y, Iwase S. Intensive cycle training with artificial gravity maintains muscle size during bed rest. Aviat.Space Environ.Med. 2005;76:923–929. [PubMed] [Google Scholar]
- Allen MR, Hogan HA, Bloomfield SA. Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J.Musculoskelet.Neuronal.Interact. 2006;6:217–225. [PubMed] [Google Scholar]
- Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch.Phys.Med.Rehabil. 2000;81:1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J.Orthop.Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Roy RR, Zhong H, Suzuki H, Ambartsumyan L, Haddad F, Baldwin KM, Edgerton VR. Electromechanical stimulation ameliorates inactivity-induced adaptations in the medial gastrocnemius of adult rats. J.Appl.Physiol. 2007;103:195–205. doi: 10.1152/japplphysiol.01427.2006. [DOI] [PubMed] [Google Scholar]
- Krolner B, Toft B, Pors NS, Tondevold E. Physical exercise as prophylaxis against involutional vertebral bone loss: a controlled trial. Clin.Sci.(Lond) 1983;64:541–546. doi: 10.1042/cs0640541. [DOI] [PubMed] [Google Scholar]
- Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner.Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. The muscle pump: what question do we want to answer? J Appl.Physiol. 2005;99:774. doi: 10.1152/japplphysiol.00578.2005. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl.Physiol. 2000;89:2158–2164. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- McCarthy ID. Fluid shifts due to microgravity and their effects on bone: a review of current knowledge. Ann.Biomed.Eng. 2005;33:95–103. doi: 10.1007/s10439-005-8967-6. [DOI] [PubMed] [Google Scholar]
- Midura RJ, Dillman CJ, Grabiner MD. Low amplitude, high frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med.Eng Phys. 2005;27:285–293. doi: 10.1016/j.medengphy.2004.12.014. [DOI] [PubMed] [Google Scholar]
- O’Gara T, Urban W, Polishchuk D, Pierre-Louis A, Stewart M. Continuous stimulation of transected distal nerves fails to prolong action potential propagation. Clin.Orthop.Relat Res. 2006;447:209–213. doi: 10.1097/01.blo.0000203481.11797.0f. [DOI] [PubMed] [Google Scholar]
- Otter MW, Qin YX, Rubin CT, McLeod KJ. Does bone perfusion/reperfusion initiate bone remodeling and the stress fracture syndrome? Med. Hypotheses. 1999;53:363–368. doi: 10.1054/mehy.1998.0782. [DOI] [PubMed] [Google Scholar]
- Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, are correlated with the formation of bone and inhibition of intracortical porosity. J.Biomech. 2003;36:1427–1437. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Qin YX, Lin W, Rubin CT. Load-Induced Bone Fluid Flow Pathway as Definded by In-vivo Intramedullary Pressure and Streaming Potentials Measurements. Ann.Biomed.Eng. 2002;30:693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J.Orthop.Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J.Orthop.Res. 1987;5:300–310. doi: 10.1002/jor.1100050217. [DOI] [PubMed] [Google Scholar]
- Stevens HY, Meays DR, Frangos JA. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone. 2006;39:565–572. doi: 10.1016/j.bone.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J.Appl.Physiol. 2005;98:72–76. doi: 10.1152/japplphysiol.00151.2004. [DOI] [PubMed] [Google Scholar]
- Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone’s interstitial fluid pathway in vivo. Bone. 2004;34:499–509. doi: 10.1016/j.bone.2003.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fritton SP, Weinbaum S, Cowin SC. On bone adaptation due to venous stasis. J.Biomech. 2003;36:1439–1451. doi: 10.1016/s0021-9290(03)00241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CN, Ohira, Tanaka T, Yonemitsu H, Ueda A. Does electrical stimulation of the sciatic nerve prevent suspension-induced changes in rat hindlimb bones? Jpn.J.Physiol. 1998;48:33–37. doi: 10.2170/jjphysiol.48.33. [DOI] [PubMed] [Google Scholar]
- Winet H. A bone fluid flow hypothesis for muscle pump-driven capillary filtration: II. Proposed role for exercise in erodible scaffold implant incorporation. Eur.Cell Mater. 2003;6:1–10. doi: 10.22203/ecm.v006a01. [DOI] [PubMed] [Google Scholar]
- Zerath E, Canon F, Guezennec CY, Holy X, Renault S, Andre C. Electrical stimulation of leg muscles increases tibial trabecular bone formation in unloaded rats. J.Appl.Physiol. 1995;79:1889–1894. doi: 10.1152/jappl.1995.79.6.1889. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tanaka SM, Sun Q, Turner CH, Yokota H. Frequency-dependent enhancement of bone formation in murine tibiae and femora with knee loading. J.Bone Miner.Metab. 2007;25:383–391. doi: 10.1007/s00774-007-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]