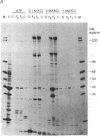
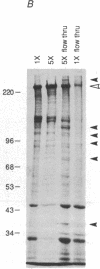
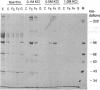
Abstract
We have developed stable and easy to use filamentous actin (F-actin) affinity-chromatography columns that selectively purify proteins that bind to actin filaments from cell extracts. Most traditional assays for actin-associated proteins screen for their effects on actin polymerization or actin filament crosslinking. Because our technique requires only actin-filament binding, it can identify additional types of proteins involved in the function of the actin cytoskeleton. By chromatographing extracts of several types of cells on these columns, we show that known actin-binding proteins are selectively retained as a subset of a larger group of actin-binding proteins that have not been identified previously.
Full text
PDF

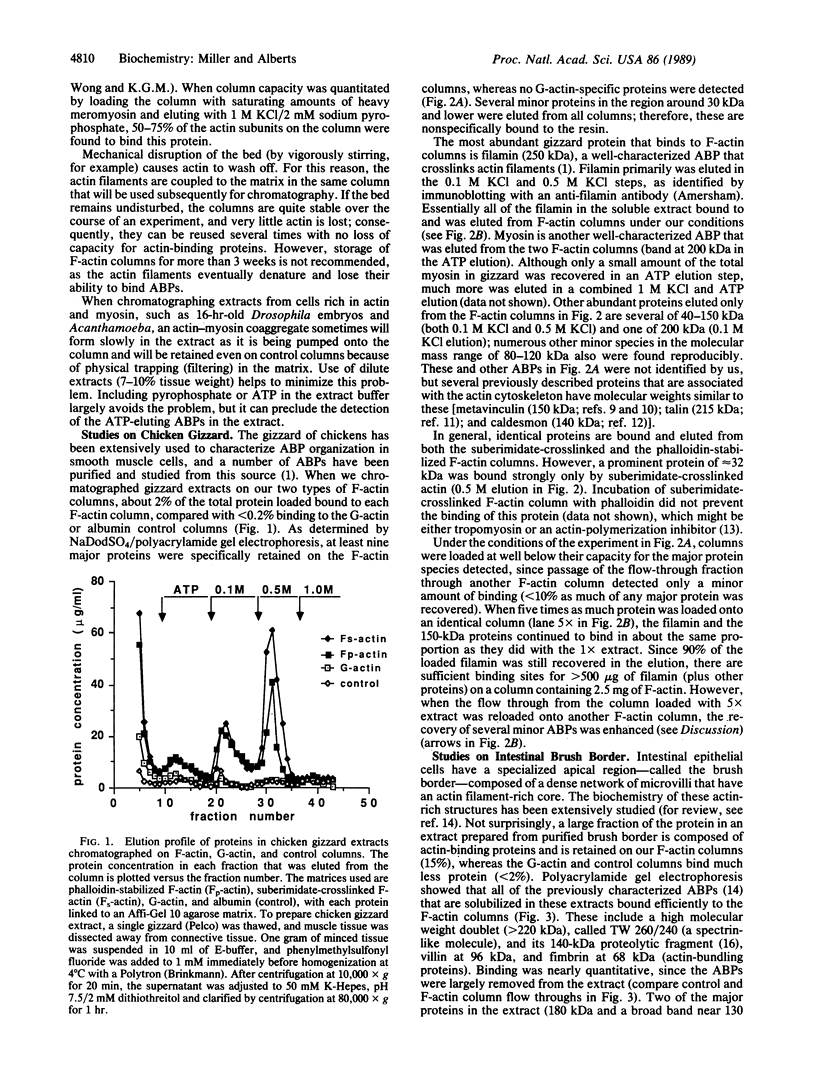

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Burridge K., Connell L. A new protein of adhesion plaques and ruffling membranes. J Cell Biol. 1983 Aug;97(2):359–367. doi: 10.1083/jcb.97.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio L. M., Tilney L. G. Phalloidin enhances actin assembly by preventing monomer dissociation. J Cell Biol. 1984 Aug;99(2):529–535. doi: 10.1083/jcb.99.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Miller K. G., Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988 Dec;107(6 Pt 2):2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Grandmont-Leblanc A., Gruda J. Affinity chromatography of myosin, heavy meromyosin, and heavy meromyosin subfragment one on F-actin columns stabilized by phalloidin. Can J Biochem. 1977 Sep;55(9):949–957. doi: 10.1139/o77-142. [DOI] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Mooseker M. S. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J Cell Biol. 1982 Dec;95(3):943–959. doi: 10.1083/jcb.95.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Acanthamoeba castellanii: methods and perspectives for study of cytoskeleton proteins. Methods Cell Biol. 1982;25(Pt B):313–332. doi: 10.1016/s0091-679x(08)61431-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Goodloe-Holland C. M., Ingalls H. M. A membrane cytoskeleton from Dictyostelium discoideum. II. Integral proteins mediate the binding of plasma membranes to F-actin affinity beads. J Cell Biol. 1984 Jul;99(1 Pt 1):58–70. doi: 10.1083/jcb.99.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E. J., Wang Y. L., Voss E. W., Jr, Branton D., Taylor D. L. A stable, high capacity, F-actin affinity column. J Biol Chem. 1982 Nov 10;257(21):13095–13100. [PubMed] [Google Scholar]
- Lynch W. P., Riseman V. M., Bretscher A. Smooth muscle caldesmon is an extended flexible monomeric protein in solution that can readily undergo reversible intra- and intermolecular sulfhydryl cross-linking. A mechanism for caldesmon's F-actin bundling activity. J Biol Chem. 1987 May 25;262(15):7429–7437. [PubMed] [Google Scholar]
- Miller K. G., Karr T. L., Kellogg D. R., Mohr I. J., Walter M., Alberts B. M. Studies on the cytoplasmic organization of early Drosophila embryos. Cold Spring Harb Symp Quant Biol. 1985;50:79–90. doi: 10.1101/sqb.1985.050.01.012. [DOI] [PubMed] [Google Scholar]
- Ohara O., Takahashi S., Ooi T., Fujiyoshi Y. Cross-linking study on skeletal muscle actin: properties of suberimidate-treated actin. J Biochem. 1982 Jun;91(6):1999–2012. doi: 10.1093/oxfordjournals.jbchem.a133893. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Microtubule-associated proteins. Annu Rev Cell Biol. 1986;2:421–457. doi: 10.1146/annurev.cb.02.110186.002225. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Assembly and dynamics of the actin filament system in nonmuscle cells. J Cell Biochem. 1986;31(2):87–95. doi: 10.1002/jcb.240310202. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Schröer E., Wegner A. Purification and characterization of a protein from chicken gizzard, which inhibits actin polymerization. Eur J Biochem. 1985 Dec 16;153(3):515–520. doi: 10.1111/j.1432-1033.1985.tb09332.x. [DOI] [PubMed] [Google Scholar]
- Siliciano J. D., Craig S. W. Properties of smooth muscle meta-vinculin. J Cell Biol. 1987 Mar;104(3):473–482. doi: 10.1083/jcb.104.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley M. A., Trayer H. R., Trayer I. P. Role of the myosin light chains in binding to actin. FEBS Lett. 1977 May 15;77(2):239–242. doi: 10.1016/0014-5793(77)80242-1. [DOI] [PubMed] [Google Scholar]