Abstract
Background
Numerous signaling molecules have been shown to participate in the dynamic process of orofacial development. Amongst these signal mediators, members of the transforming growth factor ß (TGFß) superfamily have been shown to play critical roles. Developing orofacial tissue expresses TGFß and BMP mRNAs, their protein isoforms and TGFß- and BMP-specific receptors. All these molecules display unique temporo-spatial patterns of expression in embryonic orofacial tissue, suggesting functional roles in orofacial development. For example, the TGFßs and BMPs regulate maxillary mesenchymal cell proliferation and extracellular matrix synthesis. This is particularly noteworthy in that perturbation of either process results in orofacial clefting. Although the cellular and phenotypic effects of the TGFß superfamily of growth factors on embryonic orofacial tissue have been extensively studied, the specific genes that function as effectors of these cytokines in orofacial development has not been well defined.
Methods
In the present study, oligonucleotide-based microarray technology was utilized to provide a comprehensive analysis of the expression of the panoply of genes related to the TGFß superfamily, as well as those encoding diverse groups of proteins functionally associated with this superfamily, during orofacial ontogenesis.
Results
Of the ~7000 genes whose expression was detected in the developing orofacial region, 249 have been identified that encode proteins related to the TGFß superfamily. Expression of several (27) of these genes was temporally regulated. In addition, several candidate genes, whose precise role in orofacial development is still unknown, were also identified. Examples of genes constituting this cluster include: TGFß1-induced anti-apoptotic factor-1 and -2, TGFß-induced factor 2, TGFß1 induced transcript -1 and -4, TGFß inducible early growth response 1, follistatin-like 1, follistatin-like 3, Tmeff (transmembrane protein with EGF-like and two follistatin-like domains) -1 and -2, nodal modulator 1, various isoforms of Stat (signal transducers and activators of transcription), notch and growth and differentiation factors (GDFs).
Conclusions
Elucidation of the precise physiological roles of these proteins in orofacial ontogenesis should provide unique insights into the intricacies of the TGFß superfamily signal transduction pathways utilized during orofacial development.
Keywords: TGFß, orofacial, microarray, fetal, development
INTRODUCTION
The orofacial region in mammalian embryos develops from the first pharyngeal arch and normal development of this region is contingent on proper spatio-temporal patterns of mesenchymal cell proliferation, apoptosis, tissue differentiation and the synthesis and degradation of extracellular matrix components (Ferguson 1998; Greene and Pisano, 2005; Greene and Pisano, 2004). Epithelial-mesenchymal interactions, as well as a number of molecular autocrine and paracrine interactions are also known to be critical (Ferguson 1998; Greene and Pisano, 2005; Greene and Pisano, 2004; Greene et al., 1998; Richman and Tickle, 1989). In mammals, the bilateral maxillary processes of the first arch enlarge and fuse with the medial nasal processes thereby forming the primary palate and upper lip. This fusion is dependent upon proper growth of the facial processes and localized tissue differentiative events. Such directed growth of the orofacial processes is dependent, at least in avian embryos, on epithelial-derived cues (Wedden 1987; Shuler 1995). Later in embryonic development, the secondary palate originates as bilateral extensions from the oral aspect of the maxillary processes. These extensions grow in size due to cell proliferation and the elaboration of extracellular matrix, elevate to a horizontal plane, and fuse with one another in the midline thereby separating the oral and nasal cavities. Significantly, abnormal development of the embryonic midfacial region is often associated with a variety of orofacial clefts of both the primary and/or secondary palates. These malformations can manifest themselves as isolated defects, or as part of a syndrome (such as DiGeorge, Treacher Collins and Apert). Thus, the embryonic orofacial region has proven to be an excellent model system, of human clinical significance, with which to investigate regulation of the sequential expression and interaction of molecular signals that effect morphogenesis and cellular differentiation.
The transforming growth factor ß (TGFß) superfamily represents a class of signaling molecules that plays a central role in development of the orofacial region. Members of this family, especially the TGFßs and the bone morphogenetic proteins (BMPs), are vital to development of orofacial region wherein they regulate mesenchymal and epithelial cell proliferation, differentiation, apoptosis and extracellular matrix synthesis (Greene and Pisano, 2004; Francis-West et al., 1998; Bennett et al., 1995; Zhang et al., 2002; Potchinski et al., 1997; Lu et al., 2000). The importance of this growth factor family is further emphasized by the observations that perturbation of TGFß or BMP expression results in a variety of orofacial malformations (Lu et al., 2000; Sanford et al., 1997; Kaartinen et al., 1995; Proetzel et al., 1995). Other members of the TGFß superfamily, such as activins, and their related protein, follistatin, are also important contributors to orofacial morphogenesis, as mice with targeted deletion of these genes exhibit various orofacial defects (Matzuk et al., 1995a; Matzuk et al., 1995b). Inactivation or deletion of genes encoding various TGFß or BMP type I (Alk-2, Alk-5) and type II (ActRII, TgfbrII) receptors (Dudas et al., 2004a; Matzuk et al., 1995c; Dudas et al., 2004b; Ito et al., 2003), or inactivation of genes encoding the TGFß and BMP signaling mediators (Smad2, Smad5) (Chang et al., 1999; Nomura and Li, 1998; Cacheux et al., 2001), also result in a spectrum of orofacial developmental abnormalities.
The BMP subfamily of TGFß signaling molecules was originally identified in, and isolated from, demineralized bone matrix and characterized by its ability to induce ectopic bone formation in vivo (Wozney, 1993; Rittenberg et al., 2005; Urist, 1965; Wozney et al., 1990; Wozney et al., 1988). Subsequently, it was demonstrated that BMPs, similar to TGFßs, are widely expressed in the vertebrate embryo and fetus (Rosen and Thies, 1992; Lyons et al., 1990; Lyons et al., 1991) where they regulate various aspects of development including patterning of mesoderm, neurogenesis, ossification, organogenesis and tissue growth (Kishigami et al., 2004; Hogan, 1996a). TGFßs and BMPs function through a Smad-mediated signaling mechanism which regulates the level of target gene expression. The induction of TGFß and BMP target genes represents a balance between recruitment of transcriptional activators such as CBP, p300 and OAZ (Takizawa et al., 2003; Nishimura et al., 2003; Lin et al., 2002; Postigo et al., 2003; Hata et al., 2000) and transcriptional repressors such as c-Ski, SnoN, BF2 and HIPK2 (Wang et al., 2000; Baker et al., 1999; Mariani and Harland, 1998; Harada et al., 2003; Wotton and Massague, 2001). Our laboratory has recently demonstrated the presence of a Smad-containing-transcriptional complex, in embryonic orofacial tissue, which contains, among other proteins, phosphorylated-CREB, phosphorylated-Smad, the co-activator CBP, and the co-repressors c-Ski and SnoN (Warner et al., 2003).
Over the past several years significant advances have been made in our understanding of the role of the TGFß superfamily signaling pathways in embryonic orofacial morphogenesis. It remains unclear however, precisely how members of this family orchestrate the unique repertoire of expressed genes that execute normal orofacial development. To identify and comprehensively catalog genes that are members of, and/or functionally related to, the TGFß superfamily of growth factors, as a function of stages of orofacial development, a comprehensive “molecular fingerprint” of developing murine orofacial tissue has been established through utilization of an oligonucleotide-based microarray chip technology. Exploiting such an embryogenomic approach in the current study, the expression of a wide range of genes encoding signaling mediators belonging to, as well as numerous transcription factors and target genes functionally linked with, the TGFß superfamily of growth factors has been detected during murine orofacial development. The experimental findings from this study offer valuable information concerning the expression of genes encoding various members of this superfamily of growth factors, along with those encoding functionally associated proteins, in embryonic orofacial tissue.
METHODS
Animals
Mature male and female ICR mice (Harlan, Indianapolis, IN) were housed in a climate-controlled room with a 12-h alternating dark-light cycle and were mated overnight. The presence of a vaginal plug the following morning (day 0 of gestation) was considered as evidence of mating. On gestational days (GD) 12, 13 and 14, which represent the period of secondary palate development in the mouse, female mice were euthanized by asphyxiation and embryos were dissected from uteri in sterile calcium/magnesium-free phosphate buffered saline. Extraembryonic membranes were removed from the embryos and first branchial arch derived tissue, including primary palatal tissue and secondary palatal processes were dissected as demarcated in Fig. 1 (Gehris et al., 1991; Mukhopadhyay et al., 2004), was excised, minced and stored at −20°C in RNALater solution (Qiagen, Chatsworth, CA) for subsequent extraction of total RNA. For each day of gestation, three independent pools of 20 to 25 staged embryos were used to procure embryonic orofacial tissues for preparation of three distinct RNA samples (n=3 biological replicates). Those three distinct RNA samples were processed to prepare three separate sets of target RNAs (n=3) from fetal orofacial tissue for each day of gestation. The three biological replicate RNA samples, for each of the days of gestation, were then applied to individual GeneChips (i.e. nine samples and nine GeneChips total).
Figure 1. Photomicrographs of ventral views of the developing orofacial region of a GD-13 mouse embryo.
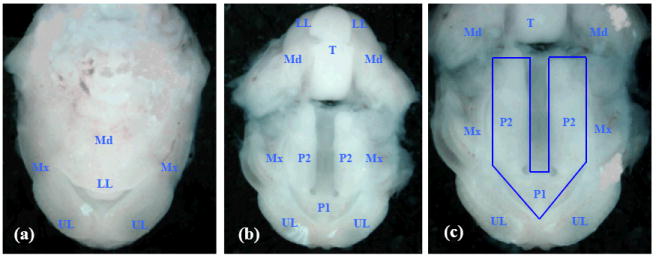
(a) upper and lower lips and jaws (maxilla and mandible); (b) the embryonic oral cavity; the lower half of the photo contains the roof of the oral cavity with the maxillary processes, primary palate and secondary palatal processes; the upper half contains the base/floor of the oral cavity showing the tongue and the mandible; (c) a magnified view of the roof of the oral cavity: note that the upper lip and the primary palate are completely formed, and the developing secondary palatal shelves are derived from the medial aspect of each maxillary process. The region demarcated by the blue line was excised from GD-13 embryos for extraction of total RNA. Corresponding regions were dissected from the developing orofacial region of GD-12 and GD-14 embryos. (UL) upper lip; (LL) lower lip; (Mx) maxilla; (Md) mandible; (P1) primary palate; (P2) secondary palate; (T) tongue.
RNA Extraction
Total RNA from excised tissue samples was isolated using the RNeasy Protect Mini Kit (Qiagen) following the manufacturer’s recommendations. The quality and quantity of the extracted total RNAs were assessed by formaldehyde agarose gel electrophoresis and spectrophotometric UV absorbance at 260/280 nm, respectively. Synthesis of poly (A)+ mRNA, double stranded cDNA, biotin-labeled cRNA and GeneChip hybridization were performed as described previously (Mukhopadhyay et al., 2004).
Microarray Data Analysis and Presentation
Analysis of microarray data was accomplished as previously described (Mukhopadhyay et al., 2004). Briefly, images from the scanned murine U74Av2 GeneChip arrays were processed using Affymetrix Microarray Analysis Suite (MAS) 5.0. Independent fetal orofacial tissue samples were prepared and analyzed in triplicate for each day of gestation (gd-12, -13 and -14). Sample loading and variations in staining were standardized by scaling the average of the fluorescence intensities of all genes on an array to constant target intensity (250) for all GeneChip arrays utilized. All array data report files demonstrated acceptable 3//5/ signal ratios for the internal housekeeping controls: GAPDH and ß-actin, and comparable values for various quality control parameters such as “Noise”, “Scaling factor” and “Number of genes present”. Mean fold change values were determined for each gene represented on the GeneChip array. On the murine U74Av2 GeneChip, each gene is represented by the use of 16 perfectly matched (PM) and mismatched (MM) control probes. The MM probes acted as specificity controls allowing direct subtraction of both background and cross-hybridization signals. The number of instances in which the PM hybridization signal was larger than the MM signal was computed along with the average of the logarithm of the PM:MM ratio (after background subtraction) for each probe set. These values were used to make a matrix-based decision concerning the presence or absence of a RNA molecule. To quantitate RNA abundance in the experimental sample, the average of the differences representing PM minus MM for each gene-specific probe family was calculated, after discarding the maximum, the minimum and any outliers beyond three standard deviations. For analysis of the three different embryonic orofacial tissue target RNA samples (GD-12 vs GD-13 vs GD-14), the GeneChip image of the GD-12 sample was normalized to the corresponding image of the GD-13 sample and that of the GD-13 sample was normalized to the corresponding image of GD-14 sample across all probe pair sets. Difference call, fold change, average difference value, and absolute call data from each of the three embryonic orofacial samples were exported. The full dataset was obtained using Affymetrix MAS 5.0 and contained expression levels at GD 12, 13, and 14 for all 12,488 genes and ESTs as well as logarithms (base 2) of the estimated fold changes. Of the ~7000 genes found to be expressed in developing orofacial tissue on the basis of the aforementioned microarray analysis, we have delineated several hundred genes encoding various members of the TGFß superfamily of growth factors, their signaling mediators, and other proteins that are functionally associated with this superfamily. Within this latter group, genes whose expression was increased or decreased two-fold or more on successive days of gestation have also been identified. Hierarchical clustering analysis was performed using the GeneSpring v6.1 software (Silicon Genetics, Redwood City, CA) to generate dendrograms (Fig. 2) representing developmentally regulated TGFß superfamily associated genes, based on their expression profiles. A heat map (Fig. 2) was generated by dividing each measurement by the 50th percentile of all measurements in that sample, then setting the average value of expression level for each gene across the samples to 1.0, and plotting the resulting normalized signal value for each sample (values <0.01 were set to 0.01). The list of genes comprising the heat map is listed in Table 2. Finally, gene expression profiling results obtained by the microarray analyses were independently validated using TaqMan quantitative real-time PCR (Bustin, 2000). The experimental data obtained from this microarray analysis of developing orofacial tissue is available from the GEO gene expression database (http://www.ncbi.nlm.nih.gov/geo/info/linking.html; accession no. GSE1624).
Figure 2. Heat map illustrating the statistically significant alterations in expression of the TGFß superfamily of genes during murine orofacial development.
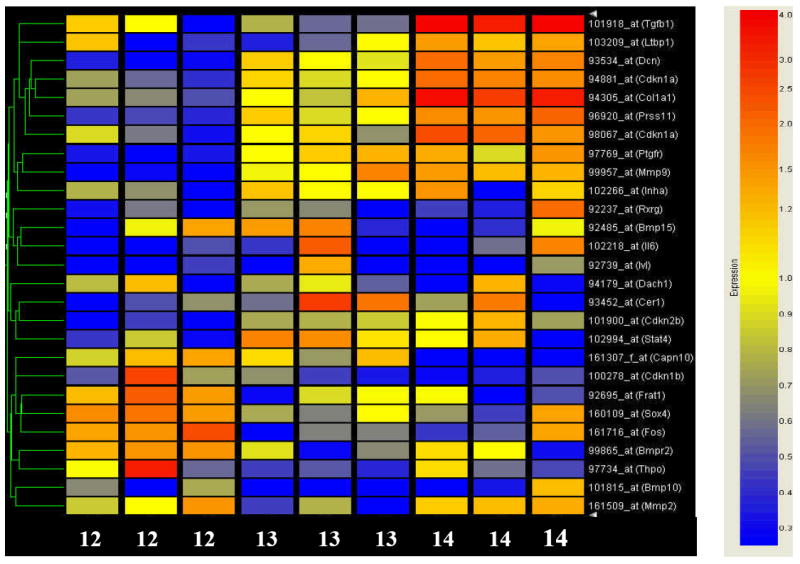
Each row of the heat map represents a gene, and each column represents a time point in development (gestational day labeled along the bottom). The color saturation represents the level of gene expression. Red indicates an increase in gene expression, whereas blue indicates a decrease. Genes whose expression demonstrated a two-fold or greater increase or decrease are depicted. The list of genes comprising the heat map is listed in Table 2.
Table 2.
Genes Encoding Members of the TGFß Superfamily and their Associated Signaling Proteins which are Differentially Expressed During Murine Orofacial Ontogenesis.
| Probe Set ID | Gene | 1Fold Change GD-13 vs. GD-12 | 1Fold Change GD-14 vs. GD-13 |
|---|---|---|---|
| 101918_at | TGF beta1 | -1.12 | 6.50 |
| 103209_at | Latent transforming growth factor beta binding protein 1 | 1.10 | 2.14 |
| 161307_f_at | Calpain 10 | 1.05 | -5.04 |
| 92237_at | Retinoid X receptor gamma | -1.05 | 2.10 |
| 92695_at | Frequently rearranged in advanced T-cell lymphomas (Frat-1) (Wnt signaling; cell growth & maintenance) | -2.20 | -1.20 |
| 97769_at | Prostaglandin F receptor | 2.83 | 1.07 |
| 93534_at | Decorin | 3.33 | 1.62 |
| 94179_at | Dachshund-1 | 1.35 | -2.30 |
| 101815_at | BMP10 | -2.52 | 1.63 |
| 92485_at | BMP15 or GDF-9B | 1.41 | -2.70 |
| 96920_at | Protease, serine, 11 (Prss11) (Igf binding) [Negative regulation of BMP signaling] | 2.20 | 2.25 |
| 99865_at | BMP receptor, type II | -2.14 | 1.18 |
| 93452_at | Cerberus 1 | 3.18 | -2.14 |
| 102266_at | Inhibin alpha | 2.25 | -1.55 |
| 97734_at | Thrombopoietin | -2.41 | 1.18 |
| 160109_at | Sox-4 | -2.05 | -1.023 |
| 102994_at | Signal transducer and activator of transcription 4 (Stat4) | 2.56 | -2.00 |
| 100278_at | Cyclin-dependent kinase inhibitor p27kip1 (Cdkn1b) | -1.91 | -1.15 |
| 98067_at | Cyclin-dependent kinase inhibitor 1A (p21) | 1.78 | 2.20 |
| 101900_at | Cyclin dependent kinase inhibitor 2B (Cdkn2b or p15) | 3.18 | 1.18 |
| 94881_at | Cyclin-dependent kinase inhibitor 1A (p21) | 1.90 | 2.00 |
| 94305_at | Alpha 1 type I procollagen | 1.80 | 3.03 |
| 161716_at | Fos (FBJ osteosarcoma oncogene) | -3.33 | 1.50 |
| 92739_at | Involucrin (keratinocyte differentiation) | 1.55 | -2.10 |
| 102218_at | Interleukin 6 | 2.50 | -1.45 |
| 161509_at | MMP2 (gelatinase A) | -2.41 | 2.35 |
| 99957_at | MMP9 (gelatinase B) | 6.96 | 1.12 |
Triplicate gene expression data sets from orofacial tissue from each of GD-12, GD-13 and GD-14 embryos were filtered and the average fold change for each gene was calculated from three different comparisons of the gene expression patterns of GD-13 vs. GD-12 and GD-14 vs. GD-13 orofacial tissue. Only those genes which demonstrated a statistically significant (p<0.005) increase or decrease in expression of at least two-fold in all three biological replicates for either the GD-13 vs. GD-12 expression comparison or the GD-14 vs. GD-13 expression comparison were included in this table. Note that GD-13 vs. GD-12 means that expression on gestation day 12 was utilized as the baseline, and that GD-14 vs. GD-13 means that expression on gestation day 13 was utilized as the baseline. Therefore, negative numbers indicate a decrease in expression, whereas positive numbers indicate an increase in expression.
Quantitative Real-Time PCR
Total RNA prepared from GD-12, GD-13 or GD-14 orofacial tissue was treated with DNase I in the presence of RNaseOUT (Invitrogen Life Technologies, Inc., Carlsbad, CA) to remove contaminating DNA before cDNA synthesis. cDNA was synthesized with random hexamer primers and Superscript II reverse transcriptase (Invitrogen Life Technologies, Inc.). Real-time PCR analysis was performed on a TaqMan ABI Prism 7000 Sequence Detector (Applied Biosystems; Foster City, CA). Primers and their corresponding fluorescence probes (Assays-on-Demand) were purchased from Applied Biosystems. For each gene analyzed, both forward and reverse primers were used at a concentration of 900 nM and the final fluorescent probe concentration was 200 nM. The PCR reaction was performed in a total volume of 25 μl with 0.2 mM dATP, dCTP, and dGTP, 0.4 mM dUTP, 0.625 unit of Amplitaq Gold and 2 μl of cDNA template. Cycling parameters were: 50°C for 2 min for probe and primer activation, 95°C for 10 min for denaturation of DNA strands, followed by 40 cycles of denaturation at 95°C for 15 s, and primer extension at 60°C for 1 min. For each reaction, a parallel reaction lacking template was performed as a negative control. Raw data were acquired and processed with ABI Sequence Detector System software, version 1.0 (Applied Biosystems, UK). Each determination of mRNA amount for the genes analyzed was normalized to GAPDH mRNA present in each sample by using TaqMan GAPDH PCR primers and probe.
RESULTS
A high-density oligonucleotide-based microarray was utilized to define the expression profiles of genes functionally related to the TGFß superfamily of growth factors during mammalian orofacial development. Of the greater than 12,000 genes represented on each of the Affymetrix Murine GeneChip arrays, approximately 7000 genes and ESTs demonstrated a detectable level of expression in murine embryonic orofacial tissue from the three gestational ages examined (Mukhopadhyay et al., 2004). Among these ~7000 genes whose expression was detected, 249 genes belonged to, or were targets of, the TGFß superfamily. These genes, encoding members of the TGFß superfamily of growth factors, as well as other proteins functionally associated with this superfamily, are listed in Table 1. Expression of all of these genes was reproducible in triplicate biological samples from each stage of gestation. This group includes expression of a panoply of genes encoding a number of growth factors/ligands belonging to the TGFß superfamily such as the TGFßs, BMPs, growth/differentiation factors (GDFs), follistatins, inhibins and activins; a range of type I and type II receptors for TGFß superfamily ligands; signaling mediators such as the Smads; transcriptional co-activators such as CBP, p300, SKIP; corepressors such as c-ski, sno, sin-3A and -3B, NcoRs; modulators of TGFß superfamily signaling such as the latent TGFß binding proteins (Ltbps), endoglin, SARA (Smad anchor for receptor activation), gremlin-1 and -2, chordin, cerberus-1, noggin; and various target genes of TGFß superfamily signaling pathways such as genes encoding cell division cycle 20 homolog (cdc 20), CD79A antigen, procollagens type I, alpha 1 and alpha 2, type III, alpha 1, cyclin-dependent kinase inhibitor 1A (p21), 1B (p27) and 2B (p15), Id1-4, Runx-1 and -2, several Msx and Dlx family of homeobox transcription factors, and oncoproteins such as c-fos, c-jun, c-myc. These results clearly indicate that, the developing orofacial region expresses numerous genes functionally associated with the TGFß superfamily signaling pathway, encoding a variety of molecular markers, such as growth and differentiation factors, signal transduction modulators and effectors, cytoskeletal and extracellular matrix proteins and transcription factors. Moreover, expression of a number of genes linked to the TGFß superfamily was differentially regulated during orofacial growth and morphogenesis (Table 2). Heat maps (Fig. 2) generated by hierarchical clustering analysis of the data with the GeneSpring v6.1 software (Silicon Genetics) demonstrated diverse patterns of expression of those TGFß superfamily and functionally associated genes (listed in Table 2) during murine orofacial morphogenesis. The developmentally regulated genes shown in Table 2, exhibited an average increase or reduction in expression of 2.0-fold or greater in at least one set of comparisons (e.g. either in GD-13 vs. GD-12 or in GD-14 vs. GD-13) in all three biological replicates, and hence were considered as having undergone a significant and reproducible alteration in their expression levels during orofacial ontogenesis. Examples of such differentially expressed genes include those encoding TGFß1, BMP10, BMP15, BMP receptor type II, Ltbp-1, cerberus-1, inhibin alpha, dachshund-1, Stat-1, Sox-4, cyclin-dependent kinase inhibitors 1A (p21), 1B (p27), 2B (p15) and the oncoprotein, c-fos. These findings reinforce the notion that components of the TGFß signal transduction machinery are integral to the regulation of cellular processes such as cell proliferation, tissue differentiation, and elaboration of extracellular matrix critical for orofacial morphogenesis.
Table 1.
Genes Expressed in Developing Orofacial Tissue Encoding Members of the TGFß Superfamily and their Associated Signaling Proteins.
| 1Gene | Affymetrix Probe ID# | Gen Bank Accession Number |
|---|---|---|
| Transcription factor 1 | 100082_at | M57966 |
| Jun oncogene | 100130_at | X12761 |
| T-complex protein 10c | 100359_at | M22602 |
| IQ motif containing GTPase activating protein 1 | 100561_at | AW209098 |
| Calpain, small subunit 1 | 100610_at | AF058298 |
| Luteinizing hormone/choriogonadotropin receptor | 100705_at | M81310 |
| Formyl peptide receptor, related sequence 2 (lipoxin A4 receptor-like protein) | 101800_at | AF071180 |
| Follicle stimulating hormone receptor | 101810_at | AF095642 |
| Mitogen activated protein kinase 3 | 101834_at | Z14249 |
| Transforming growth factor, beta 1 | 101918_at | AJ009862 |
| TGFß1-induced anti-apoptotic factor 1 | 102080_at | AF104984 |
| TGFß1-induced anti-apoptotic factor 2 (co-activator of nuclear receptors) | 102613_at | AF075717 |
| Tumor necrosis factor alpha | 102629_at | D84196 |
| Transforming growth factor, beta receptor III / betaglycan | 102637_at | AF039601 |
| Transforming growth factor, beta 3 | 102751_at | M32745 |
| E2F transcription factor 1 | 102963_at | L21973 |
| MAD homolog 1 (Drosophila) | 102983_at | U58992 |
| MAD homolog 1 (Drosophila) | 102984_g_at | U58992 |
| Latent transforming growth factor beta binding protein 1 | 103209_at | AF022889 |
| Budding uninhibited by benzimidazoles 1 homolog (S. cerevisiae) | 104097_at | AF002823 |
| IQ motif containing GTPase activating protein 1 | 104300_at | AI117936 |
| src homology 2 domain-containing transforming protein C1 | 104350_at | AI050321 |
| MAD homolog 2 (Drosophila) | 104536_at | U60530 |
| Eukaryotic translation initiation factor 5 | 160265_at | AW123979 |
| Thrombospondin 1 (activator of TGFß) | 160469_at | M62470 |
| Harvey rat sarcoma virus oncogene 1 | 160536_at | Z50013 |
| Low density lipoprotein receptor | 160832_at | Z19521 |
| Mitogen activated protein kinase kinase kinase 7 | 160854_at | D76446 |
| Transforming growth factor, beta induced | 161157_r_at | AV231282 |
| Calpain 10 | 161307_f_at | AV350809 |
| Tryptophanyl-tRNA synthetase | 161337_f_at | AV121930 |
| Transforming growth factor, beta 3 | 161382_at | AV246832 |
| E2F transcription factor 1 | 161427_f_at | AV301683 |
| Prostaglandin F receptor | 161713_f_at | AV248803 |
| Budding uninhibited by benzimidazoles 1 homolog (S. cerevisiae) | 162264_s_at | AV359014 |
| Retinoid X receptor gamma | 92237_at | X66225 |
| Latent transforming growth factor beta binding protein 2 | 92335_at | AF004874 |
| Transforming growth factor alpha | 92369_at | M92420 |
| Transforming growth factor, beta receptor I/ALK-5 | 92427_at | D25540 |
| Origin recognition complex, subunit 1-like (S.cereviaiae) | 92458_at | AJ00313 |
| Frequently rearranged in advanced T-cell lymphomas | 92695_at | U58974 |
| Transforming growth factor, beta induced | 92877_at | L19932 |
| Cyclin A1 | 92911_at | X84311 |
| Connective tissue growth factor | 93294_at | M70642 |
| Transforming growth factor, beta 2 | 93300_at | X57413 |
| Chemokine (C-C motif) receptor 2 | 93397_at | U56819 |
| MAD homolog 3 (Drosophila) | 93613_at | AB008192 |
| TGFß-induced factor 2 (TALE family homeobox) | 93621_at | AA914350 |
| src homology 2 domain-containing transforming protein C1 | 93713_at | U15784 |
| Transforming growth factor beta 1 induced transcript 4 | 93728_at | X62940 |
| IQ motif containing GTPase activating protein 1 | 93850_at | AI642553 |
| Glial cell line derived neurotrophic factor family receptor alpha 1 | 93872_at | AF014117 |
| Serine (or cysteine) proteinase inhibitor, clade E, member 1/ PAI-1 (SERPIN-1) | 94147_at | M33960 |
| TGFßRII | 94702_at | D32072 |
| WNT1 inducible signaling pathway protein 2 / connective tissue growth factor related protein WISP-2 | 94704_at | AF100778 |
| Cell division cycle 20 homolog (S. cerevisiae) | 96319_at | AW061324 |
| Oxoglutarate dehydrogenase (lipoamide) | 96879_at | AI852338 |
| RIKEN cDNA 5730427N09 gene | 97229_at | AW061042 |
| Calpain 10 | 97331_at | AW049679 |
| Latent transforming growth factor beta binding protein 4 | 97347_at | AA838868 |
| Eukaryotic translation elongation factor 2 | 97559_at | M76131 |
| Prostaglandin F receptor | 97769_at | D17433 |
| Forkhead box H1 | 97789_at | AF069303 |
| Transforming growth factor beta 1 induced transcript 1 | 98019_at | L22482 |
| Nuclear factor of kappa light chain gene enhancer in B-cells 1, p105 | 98427_s_at | M57999 |
| Tryptophanyl-tRNA synthetase | 98606_s_at | X69656 |
| Glycerol phosphate dehydrogenase 2, mitochondrial | 98984_f_at | D50430 |
| Fumarate hydratase 1 | 99148_at | AI852862 |
| Interferon gamma | 99334_at | K00083 |
| Guanine nucleotide binding protein (G protein), gamma 12 | 99477_at | AI842738 |
| TGFß inducible early growth response 1 (Kruppel-like factor 10) | 99602_at | AF064088 |
| TGFß inducible early growth response 1 (Kruppel-like factor 10) | 99603_g_at | AF064088 |
| Prostaglandin F2 receptor negative regulator | 99894_at | AF006201 |
| MAD homolog 5 (Drosophila) | 102865_at | U58993 |
| MAD homolog 1 (Drosophila) | 102983_at | U58992 |
| MAD homolog 1 (Drosophila) | 102984_g_at | U58992 |
| MAD homolog 6 (Drosophila) | 104220_at | AF010133 |
| Smad nuclear interacting protein 1 | 104349_at | AA870301 |
| MAD homolog 2 (Drosophila) | 104536_at | U60530 |
| MAD homolog 4 (Drosophila) | 160440_at | U79748 |
| MAD homolog 7 (Drosophila) | 92216_at | AF015260 |
| Spectrin beta 2 | 93571_at | M74773 |
| MAD homolog 3 (Drosophila) | 93613_at | AB008192 |
| Zinc finger, FYVE domain containing 16 | 96491_at | AI035632 |
| Decorin (TGFb inhibitor) | 93534_at | X53929 |
| Biglycan (inhibitor) | 96049_at | X53928 |
| alpha 2-Macroglobulin (Clearance factor) | 104486_at | AI850558 |
| Endoglin (signal modulator of TGFßs, Activins, BMPs etc.) | 100134_at | X77952 |
| Nodal | 99801_at | X70514 |
| Nodal Modulator 1 (Nomo 1) | 104140_s_at | AI846989 |
| SARA (Smad anchor for receptor activation) | 96491_at | AI035632 |
| Apolipoprotein J / Clusterin (role in TGFß signaling &/or processing) | 95286_at | D14077 |
| Furin (Proprotein convertase subtilisin/kexin type 3) (TGFß precursor cleavage and activation) | 100515_at | X54056 |
| Dachshund-1 | 94179_at | AJ005669 |
| SKIP | 104158_at | AW046671 |
| SnoN | 94752_s_at | U10531 |
| Lefty-1 (Leftb) | 102345_at | D83921 |
| Fkbpl; FK506 binding protein-like | 102206_at | AF030001 |
| Notch-1 | 97497_at | Z11886 |
| Notch-2 | 104188_at | AI853703 |
| Notch-3 | 92956_at | X74760 |
| Notch-4 | 92652_at | AF030001 |
| Evi-1 | 100754_at | X54989 |
| Evi-1 | 99391_at | M21829 |
| Jagged2 | 92510_at | Y14331 |
| Sin3A | 103011_at | L36831 |
| Sin3B | 93790_at | L38622 |
| Cited-2 | 101973_at | Y15163 |
| Cited-1 | 160705_at | U65091 |
| CREM (cAMP responsive element modulator) | 100533_s_at | M60285 |
| CREM (cAMP responsive element modulator) | 160526_s_at | M60285 |
| CREBBP/EP300 inhibitory protein 1 or Cri1 | 99191_at | AI844939 |
| CD79A antigen (immunoglobulin-associated alpha; TGFß family receptor) | 102778_at | X13450 |
| Integrin beta-5 | 100601_at | AF022110 |
| Integrin beta 7 | 100906_at | M68903 |
| Nuclear receptor subfamily 0, group B, member 1 (NrOb1) | 93141_at | U41568 |
| Cyclin-dependent kinase inhibitor 1A (P21 or Cdkn1a) | 94881_at | AW048937 |
| Cyclin-dependent kinase inhibitor 1A (P21 or Cdkn1a) | 98067_at | U09507 |
| Cyclin dependent kinase inhibitor 2B (P15 or Cdkn2b) | 101900_at | AF059567 |
| Cyclin dependent kinase inhibitor p16INK4a (P16 or Cdkn2a) | 98789_at | AF044336 |
| Procollagen type I, alpha 1 | 94305_at | U03419 |
| Procollagen, type I, alpha 2 | 101130_at | X58251 |
| Procollagen, type III, alpha 1 | 102990_at | AA655199 |
| Procollagen, type III, alpha 1 | 98331_at | X52046 |
| Fos (FBJ osteosarcoma oncogene) | 160901_at | V00727 |
| Fos (FBJ osteosarcoma oncogene) | 161716_at | AV252296 |
| FosB (FBJ osteosarcoma oncogene B) | 103990_at | X14897 |
| Goosecoid | 94187_at | M85271 |
| Involucrin (keratinocyte differentiation) | 92739_at | L28819 |
| Jun oncogene | 100130_at | X12761 |
| Plasminogen activator, tissue | 93981_at | J03520 |
| Urokinase-type plasminogen activator protein gene | 97772_at | M17922 |
| TGIF (TG interacting factor) | 101502_at | X89749 |
| Tissue inhibitor of metalloproteinase 1 (Timp1) | 101464_at | V00755 |
| c-myc | 104712_at | L00039 |
| FK506-binding protein 1b (protein folding) | 103248_at | AF060872 |
| Leucine aminopeptidase 3; Lap3; (proteolysis and peptidolysis) | 98112_r_at | AI839225 |
| HDAC-1 | 97706_at | AA474655 |
| HDAC-1 | 96046_at | X98207 |
| HDAC-2 | 160643_at | U31758 |
| HDAC-3 | 103655_at | AF074882 |
| HDAC-5 | 104376_at | AF006602 |
| HDAC-6 | 104471_at | AF006603 |
| HDAC-7A | 97550_at | AW047228 |
| CBP | 95886_g_at | AA177826 |
| p300/CBP-associated factor (Pcaf) | 104070_at | AW047728 |
| NCoR-1 | 101536_at | U35312 |
| NCoR-2 | 95129_at | AW121057 |
| MMP2 (gelatinase A) | 161509_at | AV145762 |
| MMP9 (gelatinase B) | 99957_at | X72795 |
| Bone morphogenetic protein 8b | 101657_at | U39545 |
| Gremlin 1 | 101758_at | AF045801 |
| Bone morphogenetic protein 10 | 101815_at | AF101440 |
| Twisted gastrulation homolog 1 (Drosophila) | 102032_at | AW060819 |
| Bone morphogenetic protein 2 | 102559_at | L25602 |
| Bone morphogenetic protein 6 | 161028_at | AI850533 |
| Solute carrier family 29 (nucleoside transporters), member 1 | 161687_r_at | AV217246 |
| Bone morphogenetic protein 6 | 92372_at | X80992 |
| Bone morphogenetic protein 15 or GDF-9B | 92485_at | AJ010259 |
| Bone morphogenetic protein 1 | 92701_at | AA518586 |
| Bone morphogenetic protein receptor, type 1A or ALK-3 | 92767_at | D16250 |
| Solute carrier family 29 (nucleoside transporters), member 2 | 92950_at | X86682 |
| Bone morphogenetic protein 8a | 92982_at | M97017 |
| Bone morphogenetic protein 7 | 93243_at | X56906 |
| BMP2 inducible kinase | 93376_at | AA673486 |
| Bone morphogenetic protein 4 | 93455_s_at | X56848 |
| Bone morphogenetic protein 4 | 93456_r_at | L47480 |
| Bone morphogenetic protein 1 | 95557_at | L24755 |
| Solute carrier family 29 (nucleoside transporters), member 1 | 95733_at | AI838274 |
| Protease, serine, 11 (Igf binding) | 96920_at | AW125478 |
| Bone morphogenetic protein receptor, type 1B or ALK-6 | 97725_at | Z23143 |
| Noggin | 97727_at | U79163 |
| Bone morphogenetic protein 5 | 99393_at | L41145 |
| Transducer of ErbB-2.1 | 99532_at | D78382 |
| Bone morphogenetic protein receptor, type II (serine/threonine kinase) | 99865_at | AF003942 |
| Runx1 (Core binding factor alpha 2) | 92399_at | D26532 |
| Runx2 (Core binding factor alpha 1) | 92676_at | D14636 |
| Runx2 | 92677_s_at | AF010284 |
| Runx2 | 161378_r_at | AV245229 |
| Chordin (inhibitor of BMP signaling/ ligand-receptor interaction) | 103249_at | AF069501 |
| Cerberus 1(inhibitor of BMP & activin signaling/ ligand-receptor interaction) | 93452_at | AF031896 |
| DAN or NBL1 (inhibitor of BMP signaling; like Gremlin); | 101969_at | D50263 |
| Gremlin 2 or PRDC | 103975_at | AB011030 |
| Bone gamma-carboxyglutamate protein 2 | 102801_at | L24430 |
| Idb-1 | 100050_at | M31885 |
| Idb-2 | 93013_at | AF077861 |
| Idb-3 | 92614_at | M60523 |
| Idb-4 | 96144_at | AJ001972 |
| Sox-4 | 160109_at | X70298 |
| Signal transducer and activator of transcription 1 (Stat1) | 101465_at | U06924 |
| Signal transducer and activator of transcription 4 (Stat4) | 102994_at | U06923 |
| Signal transducer and activator of transcription 3 (Stat3) | 99099_at | U08378 |
| PIAS1 (protein inhibitor of activated STAT protein 1) | 99029_at | AF077950 |
| PIAS3 (protein inhibitor of activated STAT protein 3) | 93708_at | AF034080 |
| Signal transducer and activator of transcription 5B (Stat 5b) | 92199_at | U21110 |
| Signal transducer and activator of transcription 5A (Stat 5a) | 100422_i_at | AJ237939 |
| Signal transducer and activator of transcription 5A (Stat 5a) | 100423_f_at | AJ237939 |
| Zinc finger homeobox 1a (Zfhx1a or Zfhep) or Transcription factor 8 | 99052_at | D76432 |
| Cyclin-dependent kinase inhibitor p27kip1 (Cdkn1b) | 100278_at | U09968 |
| Msx1 | 101526_at | X14759 |
| Msx2 | 102956_at | X59252 |
| Msx3 | 92912_at | X96518 |
| Msx-interacting-zinc finger protein 1 (Miz1) (Transcription Factor) | 100941_at | AF039567 |
| Dlx2 | 92332_at | M80540 |
| Dlx3 | 99328_at | U79738 |
| Dlx5 | 92930_at | U67840 |
| Dlx6 | 98866_at | U67841 |
| Homeodomain-interacting protein kinase 2 (HIPK-2) [BMP Co-repressor] | 103833_at | AF077659 |
| Homeodomain-interacting protein kinase 3 (HIPK-3) | 103233_at | AF077660 |
| Inhibin beta-A | 100277_at | X69619 |
| Activin A receptor, type II-like 1 (ALK-1) | 100448_at | Z31664 |
| Activin A receptor, type II-like 1 | 100449_g_at | Z31664 |
| Activin A receptor, type II-like 1 | 100450_r_at | L48015 |
| Activin A receptor, type 1B or ALK-4 (binds Activins & Nodal) | 101177_at | Z31663 |
| Synaptojanin 2 binding protein | 101548_at | AI846345 |
| Inhibin beta-C | 103986_at | X90819 |
| RIKEN cDNA 1810020G14 gene | 160556_at | AW048976 |
| Inhibin beta-B | 160828_at | X69620 |
| Activin A receptor, type 1 / TGFb type I receptor (ALK-2, ALK-8) | 93460_at | L15436 |
| Activin receptor IIB | 93903_at | M84120 |
| Synaptojanin 2 binding protein | 96734_at | AA674143 |
| Inhibin beta E | 97153_at | U96386 |
| Forkhead box H1 (Fast2; forkhead-activin signal transducer 2) | 97789_at | AF069303 |
| Activin receptor IIA | 98841_at | M65287 |
| Beta-microseminoprotein (beta-inhibin; prostatic inhibin protein) | 100715_at | U89840 |
| Inhibin alpha | 102266_at | X55957 |
| Transmembrane protein with EGF-like and two follistatin-like domains 2 | 103536_at | AB017270 |
| Follistatin-like 3 | 104452_at | AI845896 |
| Transmembrane protein with EGF-like and two follistatin-like domains 1 | 160472_r_at | AI837838 |
| Follistatin-like 1 | 94833_at | M91380 |
| Follistatin | 98817_at | Z29532 |
| Growth differentiation factor 11 | 101814_at | AF100906 |
| Growth differentiation factor 15 | 104646_at | AJ011967 |
| Growth differentiation factor 9 | 160567_at | L06444 |
| Growth differentiation factor 10 | 160860_at | AI853332 |
| Growth differentiation factor 3 | 161144_r_at | AV217223 |
| Growth differentiation factor 3 | 92476_at | L06443 |
| Growth differentiation factor 1 /// longevity assurance homolog 1 (S. cerevisiae) | 93642_at | M62301 |
| Growth differentiation factor 8 / Myostatin | 97155_at | U84005 |
| Thrombopoietin | 97734_at | L34169 |
| Hepatoma-derived growth factor, related protein 2 | 98599_at | D63850 |
| Growth differentiation factor 5 | 98793_at | U08337 |
| Anti-Mullerian hormone | 101765_at | X63240 |
| IGF-1 | 95545_at | X04480 |
| IGFBP-3 | 95083_at | X81581 |
| Interleukin 6 | 102218_at | X54542 |
| PDGFs | 99890_at | M64849 |
All constitutively- and differentially-expressed genes encoding members of the TGFß superfamily and their functionally associated signaling proteins from GD-12, GD-13 and GD-14 developing orofacial tissue (n=3 for each gestational day). Only those genes which demonstrated statistically significant expression in the tissue on at least one of the three days of gestation in all three biological replicates (n=3) are included in this table.
Using TaqMan quantitative real-time PCR (Bustin, 2000), a specific and sensitive method permitting detection and quantification of mRNA species, the gene expression profile of TGFß superfamily members in developing orofacial tissue obtained by the microarray analyses was independently validated. Expression levels of 14 candidate mRNAs of the TGFß superfamily and functionally associated proteins, that showed diverse patterns of differential regulation during the three days of orofacial development, were quantified by TaqMan quantitative real-time PCR and compared to those levels determined by the microarray technique. The overall expression profiles of 12 of the 14 mRNAs examined were found to be in consistent agreement between the two methodologies (Table 3).
Table 3.
Verification of GeneChip Microarray Data by TaqMan Quantitative Real-Time PCR1
| Gene2 | 3Fold Change GD-13 vs. GD-12 | 3Fold Change GD-14 vs. GD-13 | 4Concordance |
|---|---|---|---|
| TGFß1 | -1.32 | 2.05 | +/+ |
| Latent transforming growth factor beta binding protein 1 | 1.21 | 2.03 | +/+ |
| Dachshund-1 | 1.23 | -2.10 | +/+ |
| BMP10 | -2.70 | 2.30 | +/+ |
| BMP15 or GDF-9B | 2.30 | -2.00 | +/+ |
| BMP receptor, type II | -2.15 | 1.05 | +/+ |
| Cerberus 1 | 2.06 | 1.73 | +/- |
| Inhibin alpha | 2.03 | -1.36 | +/+ |
| Sox-4 | -2.01 | -1.41 | +/+ |
| Signal transducer and activator of transcription 4 (Stat4) | 2.15 | 2.05 | +/- |
| Cyclin-dependent kinase inhibitor 1A (p21) | 1.70 | 3.00 | +/+ |
| Cyclin-dependent kinase inhibitor 1B(p27) | -2.15 | -1.30 | +/+ |
| Cyclin dependent kinase inhibitor 2B (p15) | 2.01 | 1.36 | +/+ |
| MMP9 | 3.03 | 1.19 | +/+ |
Developmental expression patterns of 14 genes in embryonic orofacial tissue were compared using Affymetrix GeneChip Arrays and TaqMan Quantitative Real-Time PCR as detailed in Materials and Methods. All analyses were performed in triplicate.
Target genes were selected based on results from Affymetrix GeneChip Arrays.
Note that GD-13 vs. GD-12 means that expression on gestation day 12 was utilized as the baseline, and that GD-14 vs. GD-13 means that expression on gestation day 13 was utilized as the baseline. Therefore, negative numbers indicate a decrease in expression, whereas positive numbers indicate an increase in expression.
+/+ indicates full concordance in the pattern/level of gene expression obtained during both the GD-12 to GD-13 developmental interval and the GD-13 to GD-14 developmental interval using Affymetrix GeneChip Arrays and TaqMan Quantitative Real-Time PCR. “+/-” indicates a comparable pattern of gene expression was detected in the gd-12 to gd-13 developmental interval and a differing pattern of gene expression was detected in the gd-13 to gd-14 developmental interval using Affymetrix GeneChip Arrays and TaqMan Quantitative Real-Time PCR.
DISCUSSION
The TGFß superfamily includes nearly 30 proteins in mammals, e.g. TGFßs (three isoforms; TGFß-1,-2 and -3), activins and inhibins, nodal, bone morphogenetic proteins (BMPs), growth/differentiation factors (GDFs; includes myostatin), and anti-Mullerian hormone (AMH, also called Mullerian inhibiting substance or MIS). Earlier studies reported the expression of a number of members of this family and their receptors in the developing orofacial region (Ferguson, 1998; Greene and Pisano, 2005; Greene and Pisano, 2004; Francis-West et al., 1998; Dewulf et al., 1995; Roelen et al, 1997). Members of the TGFß superfamily of signaling molecules, as well as their transporter/effector Smad molecules, regulate growth, differentiation and tissue morphogenesis in species as diverse as worms and mammals, eliciting a variety of biological responses including morphogenesis, cell proliferation, cell differentiation, apoptosis and extracellular matrix synthesis (Kingsley et al., 1994; Hogan, 1996b; Moses and Serra, 1996). They also play a critical role in the developing mammalian orofacial region as discussed below.
While the cellular and phenotypic effects of TGFß during mammalian orofacial development have been investigated, the specific genes that function as downstream mediators of TGFß in morphogenesis of the tissue remain poorly defined. Genomic array technology has been utilized increasingly in a wide spectrum of biological systems in order to identify individual genes as well as pathways critical for embryonic development (Jochheim et al., 2003; Richards et al., 2002). In the present study, oligonucleotide-based microarray chips were utilized to profile expressed genes, directly and indirectly associated with the TGFß superfamily in the developing murine orofacial complex in vivo, by comparing mRNA derived from the tissue on each of GD-12, GD-13, and GD-14 of development. Three mRNA samples were prepared from each of the three days of gestation. This approach enabled delineation of genes encoding members of the TGFß superfamily and other proteins (as defined in the methods section) functionally associated with the signal transduction system of this growth factor superfamily. Murine embryonic orofacial tissues and cells have been utilized in the past to address questions of maxillary/palatal mesenchymal and epithelial cell function. Abundant experimental evidence, especially that from gene ‘loss of function’ studies, indicates that the TGFß superfamily of growth factors play a critical role in morphogenesis of the embryonic orofacial region (Kaartinen et al., 1995; Proetzel et al., 1995).
Molecular genetic analyses have documented over 30 genes that, when disrupted, result in orofacial dysmorphogenesis (Francis-West et al., 1998; Schutte and Murray, 1999). Many of these genes encode members of the TGFß superfamily of growth factors and their receptors (TGF-ß2 & -ß3, BMP4, Alk2, TGFßRII, Activin ßA, ActRIIa), TGFß signaling mediators (such as, Smad2, Smad5, SMADIP1) or TGFß target genes (such as Dlx-1 and -2, Msx-1 and -2) (for a review see Francis-West et al., 1998). In the current study, gene expression profiling of the developing orofacial tissue enabled detection of expression of all of the aforementioned genes. Additionally, a number of other genes, encoding members or associated proteins of the TGFß superfamily, were detected as being expressed in the developing orofacial complex. However, the precise biological functions of many of these genes in orofacial ontogenesis are still unclear. Examples of some of these genes include TGFß1-induced anti-apoptotic factor-1 and -2, TGFß-induced factor 2, TGFß1 induced transcript -1 and -4, TGFß inducible early growth response 1, follistatin-like 1, follistatin-like 3, Tmeff (transmembrane protein with EGF-like and two follistatin-like domains) -1 and -2, Stat (signal transducers and activators of transcription) -1,-3,-4,-5A, 5B, a number of GDFs, and several isoforms of notch (Table 1). Understanding the specific functional roles of these proteins in orofacial maturation would greatly enhance our knowledge of embryonic orofacial ontogenesis and the molecular/genetic mechanisms whereby development is perturbed with a resultant orofacial cleft.
TGFß superfamily signals are initiated upon binding of the ligand to cell surface serine/threonine kinase receptors, inducing the stable assembly of heteromeric complexes of transmembrane types I and II receptors (Derynck and Feng, 1997). Embryonic orofacial tissue contains functional types I, II and III TGFß receptors (Linask et al., 1991; Cui and Shuler 2002) that, when activated, elicit changes in palatal cell proliferation (Linask et al., 1991), glycosaminoglycan synthesis (D’Angelo and Greene, 1991), collagen metabolism (D’Angelo et al., 1994) and the remodeling of the extracellular matrix via matrix metalloproteinases such as MMP2 and MMP9 (also known as gelatinases A & B, respectively) (D’Angelo et al., 1994, Brown et al., 2002). Moreover, the TGFßs exhibit unique spatio-temporal patterns of expression in the developing orofacial region (Fitzpatrick et al., 1990; Gehris et al., 1991; Jaskoll et al., 1996; Pelton et al., 1991).
Functionality of the TGFßs has been documented through genetic deletion studies. Murine embryos, in which TGFß2 or TGFß3 are deleted by homologous recombination, exhibit a cleft palate, though the penetrance and etiology of the cleft differs between the two lines of knockout mice (Sanford et al., 1997; Kaartinen et al., 1995; Proetzel et al., 1995; Doetschman, 1999). In addition, an association between TGFß3 and the incidence of cleft palate in humans has been proposed, though not confirmed, for all allelic forms of the gene (Lidral et al., 1997; Romitti et al., 1999; Sato et al., 2001). Moreover, evidence from animal studies suggests that the TGFßs play a central role in various aspects of secondary palate development including palate epithelial differentiation (Kaartinen et al., 1995; Brunet et al., 1993a; Gehris and Greene, 1992; Bhattacherjee et al., 2003; Melnick et al., 2000; Nugent and Greene, 1994) and mesenchymal (D’Angelo and Greene, 1991; D’Angelo et al., 1994) cell differentiation. These processes are critical to normal palatal ontogenesis in that perturbation of either palate medial edge epithelial (MEE) cell differentiation (Brunet et al., 1993b) or mesenchymal cell growth (Greene and Pisano, 1989) results in a cleft of the palate. An interesting finding of the current study is that, during the time period examined, genes encoding TGF-ß2 and -ß3 were constitutively expressed (Table 1) whereas, there was a 6.5-fold increase in TGFß1 mRNA expression from GD-13 to GD-14 (Table 2). These findings confirm that the three TGFß isoforms are expressed throughout the critical period of orofacial ontogenesis, and that during this interval transcription of their genes may be differentially regulated in the developing tissue.
Supporting the presence of a functional (cellular membrane to nucleus) TGFß signaling system in the developing orofacial region, expression of the following repertoire of genes has been detected in the present microarray analysis of developing orofacial tissue: genes encoding Type I TGFß receptors such as ALK-1 (activin A receptor, type II-like 1), ALK-5, Type II TGFß receptor such as TGFßRII, Type III TGFß receptor such as TGFßRIII or betaglycan, TGFß-specific Smads such as Smad-2, -3, the co-Smad Smad-4, inhibitory Smads such as Smad-6 and -7, Smad nuclear interacting protein 1 (Snip1, a corepressor), SARA (Smad anchor for receptor activation), several modulators of TGFß synthesis and signaling such as decorin, biglycan, endoglin, involucrin, latent transforming growth factor beta binding protein (Ltbp) -1,-2,-4, FK506-binding protein 1b, FK506 binding protein-like (Fkbpl), leucine aminopeptidase 3 (Lap3), thrombospondin 1 (activator of TGFß), furin (TGFß precursor cleavage and activation), α2-Macroglobulin (clearance factor) (Table 1). Furthermore, expression of the genes encoding extracellular proteins such as Ltbp-1 and decorin, was found to be differentially regulated (Table 2) in the developing tissue. A number of TGFß signaling pathway-regulated genes were also found to be expressed in GD-12, -13 and -14 orofacial tissue: MMP2, MMP9, tissue inhibitor of metalloproteinase 1 (Timp1), procollagens type I, α1 and α2, type III, α1, cyclin-dependent kinase inhibitor -1A (p21 or Cdkn1a), -2A (p16INK4a or Cdkn2a), -2B (p15 or Cdkn2b), involucrin, tissue plasminogen activator, urokinase-type plasminogen activator protein, TGIF (TG interacting factor), Goosecoid, TGFß1-induced anti-apoptotic factor-1 and -2, TGFß-induced factor 2, TGFß1 induced transcript -1 and -4, TGFß inducible early growth response 1 and oncogenes such as c-myc, fos, fosB, jun, among others (Table 1). Among these TGFß-inducible genes, those encoding MMP-2 and -9, procollagen type I, alpha 1, cyclin-dependent kinase inhibitor -1A (p21 or Cdkn1a) and -2B (p15 or Cdkn2b), fos and invoulcrin were found to be differentially regulated during the course of orofacial ontogenesis (Table 2).
It is now well documented that Smads - the TGFß and BMP signaling mediators - bind DNA with poor affinity and that high affinity binding requires additional cofactors for a robust transcriptional response (Derynck et al., 1998). Thus, induction of TGFß and BMP target genes represents a balance between recruitment of transcriptional co-activators (such as CBP, p300 and OAZ) and co-repressors (such as TGIF, c-Ski, SnoN, BF2 HIPK2, and Evi1) (Pouponnot et al., 1998; Shim et al., 2002; Stroschein et al., 1999; Xu et al., 2000; Mariani and Harland, 1998; Alliston et al., 2005; Harada et al., 2003; Attisano and Wrana, 2000). Moreover, by preferentially associating with a wide spectrum of nuclear proteins, including a number of other transcription factors, Smad proteins are capable of eliciting both positive and negative regulation of a variety of genes (Attisano and Wrana, 2000; ten Dijke et al., 2000). In the current study, expression of a number of genes encoding transcriptional co-activators and co-repressors of TGFß superfamily signaling have been detected in the developing orofacial tissue. Examples of such transcriptional regulators include CBP, p300, Cited-1 and -2, CREM (cAMP responsive element modulator), p300/CBP-associated factor (Pcaf), c-Ski, SnoN, Skip, TGIF, Dachshund-1, Evi-1, Sin-3A and -3b, HDAC-1, -2, -3, -5, -6, -7a, NCoR-1, -2, HIPK-2 (co-repressor of BMP signaling) (Table 1). Moreover, expression of the genes encoding Sin3B, Dachshund-1 and HIPK-2 was differentially regulated during the critical period of orofacial development (Table 2).
Bone morphogenetic proteins (BMPs) belong to a distinct subfamily of the TGFß superfamily. Similar to TGFßs, BMPs exert their effects on cell growth, differentiation and tissue morphogenesis by first binding to two types of serine/threonine kinase cell surface receptors, activation of which leads to phosphorylation, and translocation into the nucleus, of intracellular signaling molecules, including Smad1, Smad5, and Smad8 (“canonical” BMP signaling pathway) (Balemans and Van Hul, 2002). BMP effects are also mediated by activation of the mitogen-activated protein (MAP) kinase pathway (“noncanonical” BMP signaling pathway) (Gallea et al., 2001; Lou et al., 2000; Monzen et al., 1999). As mentioned earlier, vertebrate orofacial development is dependent, in part, on growth of the facial primordia, which has been shown to be regulated by members of the BMP family (Francis-West et al., 1994). Following migration of craniofacial progenitor cells – the neural crest – into the branchial arches, BMPs exhibit distinct spatiotemporal patterns of expression in developing orofacial processes contributing to the development of the midface (Bennett et al., 1995; Gong and Guo 2003; Lu et al., 2000;). Further, BMP-mediated epithelial-mesenchymal interactions in this region induce a differentiation cascade leading to bone and cartilage formation (Bennett et al., 1995) and odontogenesis (Wang et al., 1999). Treatment with the BMP antagonist noggin, results in proliferation and outgrowth of the avian frontonasal mass and maxillary prominences and ultimately to the deletion of the maxillary and palatine bones (Ashique et al., 2002), thereby demonstrating a requirement for endogenous BMP in the proliferation of facial mesenchyme. Further evidence for functionality of the BMPs in orofacial development comes from the observation that expression of Bmp4 and Bmp2 in developing palate mesenchyme requires expression of the MSX1 homeobox gene (Zhang et al., 2002). The functional importance of this resides in the fact that mutations in the MSX1 homeobox gene are associated with non-syndromic cleft palate and tooth agenesis in humans (van den Boogaard et al., 2000), and that transgenic expression of human BMP4 in Msx1-/- murine embryonic palatal mesenchyme rescues the cleft palate phenotype as well as restores Shh and BMP2 expression and normal levels of mesenchymal cell proliferation (Zhang et al., 2002). There is a growing body of evidence implicating integration of the sonic hedgehog (Shh) and BMP signal transduction pathways in morphogenesis of the craniofacial complex. Shh is essential for growth and morphogenesis of the facial processes. Underexpression of Shh results in facial clefting, holoprosencephaly and cyclopia, while overexpression of Shh leads to hyperteleorism (Hu and Helms, 1999). Moreover, mutations in the human and murine Shh genes result in holoprosencephaly and cyclopia (Muenke and Cohen, 2000; Roessler et al., 1996). Shh – expressed in embryonic palate epithelial tissue – activates BMP2 expression in the palatal mesenchyme which functions as a mitogen to stimulate mesenchymal cell proliferation (Zhang et al., 2002). In the present study, expression of Shh was detected in embryonic orofacial tissue during the course of its development (Table 1). Moreover, expression of genes encoding several members of the BMP subdivision of the TGFß superfamily was detected in developing orofacial tissue. This includes BMP-1, -2, -4, -5, -6, -7, -8a, -8b, -10 and -15 (or GDF-9b) (Table 1). Detection of genes encoding Type I BMP receptors [BMPRIa (ALK3), BMPRIb (ALK6), activin receptor Ia (ALK2; binds to BMP-6 and -7)], the Type II BMP receptors [BMPRII] and BMP-specific signaling mediators [Smad-1,-5], in embryonic orofacial tissue from GD12-14 reinforces the existence and functionality of the BMP signaling pathway during orofacial morphogenesis (Table 1). Expression of the genes encoding BMP10, BMP15 and BMPRII was differentially regulated during orofacial development (Table 2). Expression of genes encoding a range of BMP signaling modulators such as noggin, chordin, tob-1, gremlin-1 and -2, DAN, cerberus1 (inhibitor of BMP and activin signaling) and expression of several other BMP target genes including Sox-4, Stat-1,-3,-4,-5A, 5B, PIAS-1,-3, Zfhep, cyclin-dependent kinase inhibitor p27kip1, twisted gastrulation homolog 1 (Drosophila), was detected in the orofacial region of GD-12, -13 and -14 embryo. Amongst these, genes encoding Sox-4, Stat-1 and -5B were found to be developmentally regulated (Table 2). Genes encoding a series of BMP-regulated transcription factors such as Runx-1 and -2, Id-1,-2,-3.-4, Msx-1,-2,-3, Dlx-2,-3,-5,-6,-7, most of which are well-known for their vital roles in orofacial ontogenesis, were also detected in developing orofacial tissue (Table 1).
The growth/differentiation factors (GDFs) are a subfamily of the highly conserved group of bone morphogenetic protein (BMP) signaling molecules and are known to play diverse developmental roles, especially in the musculoskeletal system (Francis-West et al., 1994; King et al., 1996; Miyazawa et al., 2002). Myostatin, also known as GDF-8, is produced by cells of skeletal muscle lineage, and inhibits their growth whereas GDF-11 is expressed in neuronal lineages and inhibits olfactory epithelium neurogenesis (Sakuma et al., 2000; Wu et al., 2003). GDF-5, also known as cartilage-derived morphogenetic protein 1 (CDMP-1), is structurally related to the BMPs, and participates in skeletal morphogenesis (Everman et al., 2002). Heterozygous mutations in GDF5, which maps to human chromosome 20, occur in individuals with autosomal dominant brachydactyly type C (Everman et al., 2002). The disorder, characterized by skeletal abnormalities restricted to the limbs, is phenotypically similar to murine brachypodism which is due to mutations in GDF5 (Everman et al., 2002). Although little is known at present regarding their role in orofacial development, expression of genes encoding a range of GDFs (e.g. GDF-1,-3,-5,-8,-9,-10,-11, and -15) were detected in GD-12, -13 and -14 embryonic orofacial tissue (Table 1).
The TGFß superfamily member nodal, plays critical roles in the induction of dorsal mesoderm, anterior patterning, and formation of left–right asymmetry (Schier, 2003). The notch signaling pathway functions upstream of the nodal gene (Raya et al., 2004). The importance of nodal signaling in craniofacial development is evident by the observation that mice trans-heterozygous for both Smad2 and nodal deletion display a range of phenotypes, including complex craniofacial abnormalities such as cyclopia (Nomura and Li, 1998). This finding indicates that Smad2 may mediate nodal signaling during craniofacial development. The Jagged2 (Jag2) gene, encodes a ligand for the Notch family of transmembrane receptors. Mice homozygous for Jag2 deletion exhibit cleft palate, fusion of the tongue with the palatal shelves and die perinatally demonstrating that Notch signaling mediated by Jag2 plays an essential role during craniofacial development in mice (Jiang et al., 1998). In the present study expression of genes encoding nodal, nodal modulator 1 (Nomo-1), and those encoding a number of notch proteins such as notch-1,-2,-3 and -4 and the notch receptor ligand, jagged2, has been detected in embryonic orofacial tissue during its development (Table 1). Demonstration, in the current study, of the expression of notch and nodal proteins suggests potential functional interactions between these two signaling pathways mediating orofacial morphogenesis.
Members of the TGFß superfamily, such as activins, inhibins and their related protein follistatin, are known mediators of orofacial development, as mice with targeted deletions of these genes demonstrate various orofacial defects (Vale et al., 2004; Lambert-Messerlian et al., 2004; Ferguson et al., 1998). Inhibins, dimers of α and ß inhibin chains, function as cytokines that inhibit the secretion of follicle-stimulating hormone (FSH) from the pituitary gland whereas activins, dimers of ß chains, stimulate the production of FSH by the same gland (Gregory and Kaiser, 2004). Activins also play crucial roles in the induction of dorsal mesoderm during early embryogenesis and act on epithelial as well as haematopoietic cells regulating their growth, differentiation and apoptosis (Fukui and Asashima, 1994; Dawid et al., 1992). Developing mammalian facial mesenchymal tissues express activin βA, (Feijen et al., 1994; Roberts and Barth, 1994) and functional deletion of this gene in mice results in cleft palate formation and loss of lower incisors (Matzuk et al., 1995a). Interestingly, such cleft palate occurs devoid of any skeletal abnormalities suggesting that the palatal cleft in activin βA knockout mice is a primary defect that arises due to aberrant formation of the orofacial primordia (Matzuk et al., 1995a). Deletion of the murine activin type 2 receptor gene, ActRIIa, also results in craniofacial defects, including hypoplasia of the mandible and cleft palate (Matzuk et al., 1995c). In the present microarray study, constitutive expression of various members of the activin/inhibin signaling pathway was apparent in the developing orofacial region including inhibin-βA,-βB,-βC,-βE and inhibin-α, Type I activin receptor (ActRIB or ALK4), Type II activin receptors [ActRIIA and -IIB], forkhead box H1 (Fast2 or forkhead-activin signal transducer 2; mediator of TGFß/Nodal/Activin signaling), synaptogamin 2 binding protein (activin receptor interacting protein 2) (Table 1). Moreover, expression of the genes encoding inhibin-α and activin receptor IIB was found to be increased 2.25-fold and decreased 2.0-fold respectively, between GD-12 and GD-13 of orofacial development (Table 2). Developmentally regulated expression of these genes in embryonic orofacial tissue might reflect the roles of inhibin-α and activin receptor IIB, in controlling epithelial and mesenchymal cell proliferation, apoptosis and tissue differentiation during orofacial ontogenesis.
Follistatin acts as an activin antagonist in vitro but as an agonist in vivo. This observation is supported by the report that functional deletion of the follistatin gene in mice results in hard palate abnormalities comparable to those reported in activin βA knockout mice (Matzuk et al., 1995b). In addition, follistatin can modulate the activity of several other members of the TGFß family (e.g. it can act as a BMP-2 and BMP-4 antagonist). In the present study genes encoding follistatin and other related proteins such as follistatin-like 1, follistatin-like 3, Tmeff (transmembrane protein with EGF-like and two follistatin-like domains) -1 and -2, were constitutively expressed during orofacial development (Table 1).
Despite significant advances in our understanding of the contributions of the TGFß superfamily members and their functionally associated proteins during orofacial ontogenesis, the means by which perturbation of the TGFß superfamily signaling pathways, or the means by which alterations in expression of their downstream genes, results in orofacial dysmorphology remains enigmatic. The present study, offers a comprehensive “molecular fingerprint” of developing murine orofacial tissue with regard to the expression of the TGFß superfamily of growth, differentiation and morphogenesis factors. The results of the current gene expression profiling study demonstrate that genes encoding various TGFß superfamily members and functionally associated proteins are expressed in developing orofacial tissue where they are either known to play crucial roles or have as yet unidentified roles in morphogenesis, tissue growth and cellular differentiation during development. The embryogenomic approach utilized in the present study provides an interface between genomics and developmental biology and offers the opportunity to initiate studies investigating the complexity of cellular signal transduction mediated by the TGFß superfamily of growth factors as well as examine novel interactions between the members of this superfamily and their functionally associated proteins, during mammalian orofacial development.
Acknowledgments
We wish to thank Ms. Sabine Waigel for expert technical assistance in the preliminary analysis of our microarray data.
This research was supported in part by NIH grants DE05550, DE12858 and ES11775, CDC grant RO6CCR420170, The Commonwealth of Kentucky Research Challenge Trust Fund, The Kentucky Science and Engineering Foundation and P20 RR017702 from the COBRE program of the National Center for Research Resources.
References
- Alliston T, Ko TC, Cao Y, Liang YY, Feng XH, Chang C, Derynck R. Repression of bone morphogenetic protein and activin-inducible transcription by Evi-1. J Biol Chem. 2005;280:24227–24237. doi: 10.1074/jbc.M414305200. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana J. Smads as transcriptional co-modulators. Curr Opin Cell Biol. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Bennett JH, Hunt P, Thorogood P. Bone morphogenetic protein-2 and -4 expression during murine orofacial development. Arch Oral Biol. 1995;40:847–854. doi: 10.1016/0003-9969(95)00047-s. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee V, Greene RM, Michele Pisano M. Divergence of epidermal growth factor - transforming growth factor beta signaling in embryonic orofacial tissue. In Vitro Cell Dev Biol Anim. 2003;39:257–261. doi: 10.1290/1543-706X(2003)039<0257:DOEGFG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Brunet CL, Sharpe PM, Ferguson MW. The distribution of epidermal growth factor binding sites in the developing mouse palate. Int J Dev Biol. 1993a;37:451–458. [PubMed] [Google Scholar]
- Brunet CL, Sharpe PM, Ferguson MW. Inhibition of TGFß3 (but not TGFß1 or TGFß2) actively prevents normal mouse embryonic palate fusion. Int J Dev Biol. 1993b;39:345–355. [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Cacheux V, Dastot-Le Moal F, Kaariainen H, Bondurand N, Rintala R, Boissier B, Wilson M, Mowat D, Goossens M. Loss-of-function mutations in SIP1 Smad interacting protein 1 result in a syndromic Hirschsprung disease. Hum Mol Genet. 2001;10:1503–1510. doi: 10.1093/hmg/10.14.1503. [DOI] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- Cui X, Shuler C. The TGF-beta type III receptor is localized to the medial edge epithelium during palatal fusion. Int J Dev Biol. 2002;44:397–402. [PubMed] [Google Scholar]
- D’Angelo M, Chen J-M, Ugen K, Greene RM. TGFß1 regulation of collagen metabolism by embryonic palate mesenchymal cells. J Exp Zool. 1994;270:189–201. doi: 10.1002/jez.1402700208. [DOI] [PubMed] [Google Scholar]
- D’Angelo M, Greene RM. Transforming growth factor-ß modulation of glycosaminoglycan production by mesenchymal cells of the developing murine secondary palate. Dev Biol. 1991;145:374–378. doi: 10.1016/0012-1606(91)90136-q. [DOI] [PubMed] [Google Scholar]
- Dawid IB, Taira M, Good PJ, Rebagliati MR. The role of growth factors in embryonic induction in Xenopus laevis. Mol Reprod Dev. 1992;32:136–144. doi: 10.1002/mrd.1080320209. [DOI] [PubMed] [Google Scholar]
- Derynck R, Feng X. TGFß receptor signaling. Biochim Biophys Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng X-H. Smads: transcriptional activators of TGF-ß responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, More′n A, Grimsby S, Vande-Spiegle K, Miyazono K, Huylebroeck D, ten Dijke P. Distinct spatial and temporal expression patterns of two type 1 receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Doetschman T. Interpretation of phenotype in genetically engineered mice. Lab Anim Sci. 1999;49:137–43. [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004a;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Dudas M, Nagy A, Laping NJ, Moustakas A, Kaartinen V. TGF-beta3-induced palatal fusion is mediated by Alk-5/Smad pathway. Dev Biol. 2004b;266:96–108. doi: 10.1016/j.ydbio.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Everman DB, Bartels CF, Yang Y, Yanamandra N, Goodman FR, Mendoza-Londono JR, Savarirayan R, White SM, Graham JM, Jr, Gale RP, Svarch E, Newman WG, Kleckers AR, Francomano CA, Govindaiah V, Singh L, Morrison S, Thomas JT, Warman ML. The mutational spectrum of brachydactyly type C. Am J Med Genet. 2002;112:291–296. doi: 10.1002/ajmg.10777. [DOI] [PubMed] [Google Scholar]
- Feijen A, Goumans MJ, van den Eijinden-van Raaij AJM. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development. 1994;120:3621–3637. doi: 10.1242/dev.120.12.3621. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 1998;12:2636–2649. doi: 10.1101/gad.12.16.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. Palate development. Development. 1998;103:S41–S60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Denhez F, Kondaiah P, Akhurst R. Differential expression of TGFß isoforms in murine palatogenesis. Development. 1990;109:585–595. doi: 10.1242/dev.109.3.585. [DOI] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A, Graveson A. Signaling interactions during facial development. Mech Dev. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Francis-West P, Tatla T, Brickell P. Expression patterns of the bone morphogenetic protein genes Bmp-4 and Bmp-2 in the developing chick face suggest a role in outgrowth of the primordia. Dev Dyn. 1994;201:168–178. doi: 10.1002/aja.1002010207. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Parish J, Lee K, Archer CW. BMP/GDF-signaling interactions during synovial joint development. Cell Tissue Res. 1999;296:111–119. doi: 10.1007/s004410051272. [DOI] [PubMed] [Google Scholar]
- Fukui A, Asashima M. Control of cell differentiation and morphogenesis in amphibian development. Int J Dev Biol. 1994;38:257–266. [PubMed] [Google Scholar]
- Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–498. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- Gehris AL, D’Angelo M, Greene RM. Immunodetection of the transforming growth factors ß1 and ß2 in the developing murine palate. Int J Dev Biol. 1991;35:1–8. [PubMed] [Google Scholar]
- Gehris AL, Greene RM. Regulation of murine embryonic epithelial cell differentiation by transforming growth factors ß. Differentiation. 1992;49:167–173. doi: 10.1111/j.1432-0436.1992.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Gong SG, Guo C. Bmp4 gene is expressed at the putative site of fusion in the midfacial region. Differentiation. 2003;71:228–236. doi: 10.1046/j.1432-0436.2003.710304.x. [DOI] [PubMed] [Google Scholar]
- Greene RM, Pisano MM. Analysis of cell proliferation in developing orofacial tissue. In: Kimmel GL, Kochhar DM, editors. Vitro Techniques in Developmental Toxicology: Use in Defining Mechanisms and Risk Parameters. NewYork: CRC Press; 1989. pp. 91–101. [Google Scholar]
- Greene RM, Weston W, Nugent P, Potchinsky M, Pisano M. Signal transduction pathways as targets for induced embryotoxicity. In: Slikker W, Chang L, editors. Handbook of developmental neurotoxicology. San Diego: Academic Press; 1998. pp. 119–139. [Google Scholar]
- Greene RM, Pisano MM. Perspectives on growth factors and orofacial development. Curr Pharm Des. 2004;10:2701–2717. doi: 10.2174/1381612043383647. [DOI] [PubMed] [Google Scholar]
- Greene RM, Pisano MM. Recent advances in understanding transforming growth factor ß regulation of orofacial development. Hum Exp Toxicol. 2005;24:1–12. doi: 10.1191/0960327105ht492oa. [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22:253–267. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- Harada J, Kokura K, Kanei-Ishii C, Nomura T, Khan MM, Kim Y, Ishii S. Requirement of the co-repressor homeodomain-interacting protein kinase 2 for ski-mediated inhibition of bone morphogenetic protein-induced transcriptional activation. J Biol Chem. 2003;278:38998–39005. doi: 10.1074/jbc.M307112200. [DOI] [PubMed] [Google Scholar]
- Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, Massague J. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell. 2000;100:229–240. doi: 10.1016/s0092-8674(00)81561-5. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996a;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996b;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–4884. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Choy HA, Chen H, Melnick M. Developmental expression and CORT-regulation of TGF-beta and EGF receptor mRNA during mouse palatal morphogenesis: correlation between CORT-induced cleft palate and TGF-beta 2 mRNA expression. Teratology. 1996;54:34–44. doi: 10.1002/(SICI)1096-9926(199607)54:1<34::AID-TERA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochheim A, Cieslak A, Hillemann T, Cantz T, Scharf J, Manns MP, et al. Multistage analysis of differential gene expression in BALB/C mouse liver development by high-density microarrays. Differentiation. 2003;71:62–72. doi: 10.1046/j.1432-0436.2003.700606.x. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- King JA, Storm EE, Marker PC, Dileone RJ, Kingsley DM. The role of BMPs and GDFs in development of region-specific skeletal structures. Ann N Y Acad Sci. 1996;785:70–79. doi: 10.1111/j.1749-6632.1996.tb56245.x. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGFß superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276:185–193. doi: 10.1016/j.ydbio.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Lambert-Messerlian GM, Pinar H, Laprade E, Tantravahi U, Schneyer A, Canick JA. Inhibins and activins in human fetal abnormalities. Mol Cell Endocrinol. 2004;225:101–108. doi: 10.1016/j.mce.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Lidral AC, Murray JC, Buetow KH, Basart AM, Schearer H, Shiang R, et al. Studies of the candidate genes TGFß-2, MSX1, TGF-a, and TGFß-3 in the etiology of cleft lip and palate in the Philippines. Cleft Palate Craniofac J. 1997;34:1–6. doi: 10.1597/1545-1569_1997_034_0001_sotcgt_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Lin Y, Martin J, Gruendler C, Farley J, Meng X, Li BY, Lechleider R, Huff C, Kim RH, Grasser WA, Paralkar V, Wang T. A novel link between the proteasome pathway and the signal transduction pathway of the bone morphogenetic proteins (BMPs) BMC Cell Biol. 2002;3:15–25. doi: 10.1186/1471-2121-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask KK, D’Angelo M, Gehris AL, Greene RM. Transforming growth factor-ß receptor profiles of human and murine embryonic palate mesenchymal cells. Exp Cell Res. 1991;192:1–9. doi: 10.1016/0014-4827(91)90149-o. [DOI] [PubMed] [Google Scholar]
- Lou J, Tu Y, Li S, Manske PR. Involvement of ERK in BMP-2 induced osteoblastic differentiation of mesenchymal progenitor cell line C3H10T1/2. Biochem Biophys Res Commun. 2000;268:757–762. doi: 10.1006/bbrc.2000.2210. [DOI] [PubMed] [Google Scholar]
- Lu H, Jin Y, Tipoe GL. Alteration in the expression of bone morphogenetic protein-2,3,4,5 mRNA during pathogenesis of cleft palate in BALB/c mice. Arch Oral Biol. 2000;45:133–140. doi: 10.1016/s0003-9969(99)00118-1. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Jones CM, Hogan BL. The DVR gene family in embryonic development. Trends Genet. 1991;7:408–412. doi: 10.1016/0168-9525(91)90265-r. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Harland RM. XBF-2 is a transcriptional repressor that converts ectoderm into neural tissue. Development. 1998;125:5019–5031. doi: 10.1242/dev.125.24.5019. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995a;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995b;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995c;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- Melnick M, Chen H, Zhou Y, Jaskoll T. Thrombospondin-2 gene expression and protein localization during embryonic mouse palate development. Arch Oral Biol. 2000;45:19–25. doi: 10.1016/s0003-9969(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signaling. Genes Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, et al. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses HL, Serra R. Regulation of differentiation by TGFß. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- Muenke M, Cohen MM. Genetic approaches to understanding brain development: holoprosencephaly as a model. Ment Retard Dev Disabil Res Rev. 2000;6:15–21. doi: 10.1002/(SICI)1098-2779(2000)6:1<15::AID-MRDD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Greene RM, Zacharias W, Weinrich M, Singh S, Young W, Pisano MM. Developmental gene expression profiling of mammalian fetal oro/facial tissue. Birth Defects Res (Part A) 2004;70:912–926. doi: 10.1002/bdra.20095. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Hata K, Ikeda F, Matsubara T, Yamashita K, Ichida F, Yoneda T. The role of Smads in BMP signaling. Front Biosci. 2003;8:s275–284. doi: 10.2741/1049. [DOI] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Nugent P, Greene RM. Interactions between the transforming growth factor beta (TGF beta) and retinoic acid signal transduction pathways in murine embryonic palatal cells. Differentiation. 1994;58:149–155. doi: 10.1046/j.1432-0436.1995.5820149.x. [DOI] [PubMed] [Google Scholar]
- Pelton R, Saxena B, Jones M, Moses H, Gold L. Immunohistochemical localization of TGF-B1, TGF-B2, and TGFB3 in the mouse embryo: Expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potchinsky MB, Weston WM, Lloyd MR, Greene RM. TGF-beta signaling in murine embryonic palate cells involves phosphorylation of the CREB transcription factor. Exp Cell Res. 1997;231:96–103. doi: 10.1006/excr.1996.3422. [DOI] [PubMed] [Google Scholar]
- Pouponnot C, Jayaraman L, Massague J. Physical and functional interaction of SMADs and p300/CBP. J Biol Chem. 1998;273:22865–22868. doi: 10.1074/jbc.273.36.22865. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- Richards J, Le Naour F, Hanash S, Beretta L. Integrated genomic and proteomic analysis of signaling pathways in dendritic cell differentiation and maturation. Ann NY Acad Sci. 2002;975:91–100. doi: 10.1111/j.1749-6632.2002.tb05944.x. [DOI] [PubMed] [Google Scholar]
- Richman JM, Tickle C. Epithelia are interchangeable between facial primordial of chick embryos and morphogenesis is controlled by mesenchyme. Dev Biol. 1989;136:201–210. doi: 10.1016/0012-1606(89)90142-5. [DOI] [PubMed] [Google Scholar]
- Rittenberg B, Partridge E, Baker G, Clokie C, Zohar R, Dennis JW, Tenenbaum HC. Regulation of BMP-induced ectopic bone formation by Ahsg. J Orthop Res. 2005;23:653–662. doi: 10.1016/j.orthres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Roberts VJ, Barth SL. Expression of messenger ribonucleic acids encoding the inhibin/activin system during mid- and late-gestation rat embryogenesis. Endocrinology. 1994;134:914–923. doi: 10.1210/endo.134.2.8299586. [DOI] [PubMed] [Google Scholar]
- Roelen BAJ, van Rooijen MA, Mummery CL. Expression of ALK-1, a type 1 serine/threonine kinase receptor, coincides with sites of vasculogenesis and angiogenesis in early mouse development. Dev Dyn. 1997;209:418–430. doi: 10.1002/(SICI)1097-0177(199708)209:4<418::AID-AJA9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, et al. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–360. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Romitti PA, Lidral AC, Munger RG, Daack-Hirsch S, Burns TL, Murray JC. Candidate genes for nonsyndromic cleft lip and palate and maternal cigarette smoking and alcohol consumption: evaluation of genotype-environment interactions from a population-based case-control study of orofacial clefts. Teratology. 1999;59:39–50. doi: 10.1002/(SICI)1096-9926(199901)59:1<39::AID-TERA9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Rosen V, Thies RS. The BMP proteins in bone formation and repair. Trends Genet. 1992;8:97–102. doi: 10.1016/0168-9525(92)90197-c. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Watanabe K, Sano M, Uramoto I, Totsuka T. Differential adaptation of growth and differentiation factor 8/myostatin, fibroblast growth factor 6 and leukemia inhibitory factor in overloaded, regenerating and denervated rat muscles. Biochim Biophys Acta. 2000;1497:77–88. doi: 10.1016/s0167-4889(00)00044-6. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFß2 knockout mice have multiple developmental defects that are non-overlapping with other TGFß knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Natsume N, Machida J, Suzuki S, Kawai T. Association between transforming growth factor beta 3 and cleft lip and/or cleft palate in the Japanese population. Plast Reconstruct Surg. 2001;107:1909–1910. [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schutte BC, Murray JC. The many faces and factors of orofacial clefts. Hum Mol Genet. 1999;8:1853–1859. doi: 10.1093/hmg/8.10.1853. [DOI] [PubMed] [Google Scholar]
- Shim S, Bae N, Han JK. Bone morphogenetic protein-4-induced activation of Xretpos is mediated by Smads and Olf-1/EBF associated zinc finger (OAZ) Nucleic Acids Res. 2002;30:3107–3117. doi: 10.1093/nar/gkf437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuler C. Programmed cell death and transformation in craniofacial development. Crit Rev Oral Biol Med. 1995;6:202–217. doi: 10.1177/10454411950060030301. [DOI] [PubMed] [Google Scholar]
- Stroschein S, Wang W, Zhou S, Zhou Q, Lu K. Negative feedback regulation of TGF-ß signaling by the SnoN oncoprotein. Science. 1999;286:771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Ochiai W, Nakashima K, Taga T. Enhanced gene activation by Notch and BMP signaling cross-talk. Nucleic Acids Res. 2003;31:5723–5731. doi: 10.1093/nar/gkg778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin C. Signaling inputs converge on nuclear effectors in TGF-ß signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Vale W, Wiater E, Gray P, Harrison C, Bilezikjian L, Choe S. Activins and inhibins and their signaling. Ann N Y Acad Sci. 2004;1038:142–147. doi: 10.1196/annals.1315.023. [DOI] [PubMed] [Google Scholar]
- van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet. 2000;24:342–343. doi: 10.1038/74155. [DOI] [PubMed] [Google Scholar]
- Wang W, Mariani FV, Harland RM, Luo K. Ski represses bone morphogenic protein signaling in Xenopus and mammalian cells. Proc Natl Acad Sci USA. 2000;97:14394–14399. doi: 10.1073/pnas.97.26.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Rutherford B, Upholt WB, Mina M. Effects of BMP-7 on mouse tooth mesenchyme and chick mandibular mesenchyme. Dev Dyn. 1999;216:320–335. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<320::AID-DVDY2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Warner DR, Pisano MM, Greene RM. Nuclear convergence of the TGFbeta and cAMP signal transduction pathways in murine embryonic palate mesenchymal cells. Cell Signal. 2003;15:235–242. doi: 10.1016/s0898-6568(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Wedden SE. Epithelial-mesenchymal interactions in the development of chick facial primordial and the target of retinoid actions. Development. 1987;99:341–351. doi: 10.1242/dev.99.3.341. [DOI] [PubMed] [Google Scholar]
- Wotton D, Massague J. Smad transcriptional corepressors in TGF beta family signaling. Curr Top Microbiol Immunol. 2001;254:145–164. [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Byrne M, Celeste AJ, Moutsatsos I, Wang EA. Growth factors influencing bone development. J Cell Sci Suppl. 1990;13:149–56. doi: 10.1242/jcs.1990.supplement_13.14. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wozney JM. Bone morphogenetic proteins and their gene expression. In: Noda M, editor. Cellular and Molecular Biology of Bone. New York: Academic Press; 1993. pp. 132–167. [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Xu W, Angelis K, Danielpour D, Haddad M, Bischof O, Campisi J, Stavnezer E, Medrano E. Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type ß transforming growth factor. Proc Natl Acad Sci USA. 2000;97:5924–5929. doi: 10.1073/pnas.090097797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]


