Abstract
Multipotent skin-derived stem cells represent neural-crest derived precursors which have neural and mesodermal potency and can generate neurons, glias, smooth muscle cells and adipocytes. Transcriptional profiling studies show that both intrinsic programs and extrinsic signaling pathways mediate their neural and mesodermal potency. In addition, recent progress implies that skin-derived stem cells may have a broader developmental potency than previously expected, of which is their potential to generate germline cells in vitro. In this review, we discuss the transcriptional profiling of multipotency and neural crest-derived characteristics of skin-derived stem cells, and argue for their potential germ-line competency in the view of nuclear and cellular reprogramming.
Keywords: stem cell, skin, chimera, somatic cell nuclear transfer
Introduction
Stem cells are a type of primitive cell arising during embryonic and postnatal development, which have the ability to self-renew and differentiate into multiple functional cell types. A number of pluripotent and multipotent stem cell lines have been derived from preimplantation embryos (1,2), post-implantation epiblast (3,4) and various sources of postnatal animals (5). Mammalian skin is a complex tissue that regenerates dynamically by continuing to turnover during the life of the animal. Thus, on-one-hand, epidermal skin-derived stem cells contribute to balance the epidermal homeostasis in the skin. Such stem cell populations maintain the self-renewing compartments of mammalian skin epidermis: the hair follicle, the sebaceous gland and the interfollicullar epidermis (6). On-the-other-hand, multipotent dermis-derived stem cells provide substantial support for wound healing and regeneration (7,8). A novel type of dermis-derived stem cell termed skin-derived progenitors (SKPs) were first isolated from juvenile and adult mammalian skin by Toma et al in 2001 (9,10). They used a serum-free medium (DMEM/F12 (1:1) + B27 + EGF + bFGF) originally developed for neural stem cell culture in vitro. The SKPs cells can survive as spheres in suspension culture and the population can double every 2–3 days. Under certain conditions SKPs have multiple lineage potential and can generate neural and mesodermal lineages: neuron, glias, smooth muscle cells and adipocytes (9).
With properties similar to rodent SKPs, human SKPs were successfully isolated from neonatal foreskin and adult trunk skin (11,12). In contrast to mesenchymal stem cells, SKPs express nestin (neural stem cell marker), p75 (neural crest stem cell marker), fibronection and Sox10. Several lines of evidence show that SKPs are neural crest derived and that they arise during embryogenesis and can persist into adulthood (12–14), thus sharing properties similar to embryonic neural crest stem cells. In pigs, several independent groups reported the isolation of porcine skin-derived progenitors (pSKP) from both embryonic and adult skin (13,15,16). Porcine SKPs express pluripotency-related genes (Oct3/4, Sox2, Stat3 and Nanog) and neural crest marker genes (p75NTR, Sox10, Snail), showing a pattern of marker gene expression similar to human and rodent SKPs in vitro (13,15). In addition, individual cells of SKP spheres show heterogeneous expression of specific marker genes, implying a metastable state inside the SKP spheres (13). Porcine SKPs are multipotent and can generate both neural and mesodermal progeny in vitro. However, porcine SKPs show distinct transcriptional profiles when compared to neural stem cells in the central nervous system (CNS) and skin-derived fibroblasts, indicating a novel type of multipotent stem cells derived from skin (17). Specifically, porcine SKPs can generate primordial germ cell-like and oocyte-like progeny in vitro, showing a promising in vitro model to investigate germ-cell formation and gametogenesis (18,19). In this review, we will discuss the neural crest-derived properties, neural and mesodermal differentiation, germline cell potency, and prospective biomedical application of skin-derived stem cells.
Neural crest derived properties of skin-derived stem cells
The neural crest cells are a group of transient and multipotent cells which are induced to migrate and give rise to various cell lineages: melanocytes, craniofacial cartilage, bone, smooth muscle, peripheral and enteric neurons and glias. In the past two decades, neural crest stem cells (NCSCs) or neural crest-derived progenitors have been isolated from neural crest explants (20), sciatic nerves (21), dorsal root ganglion (DRG) (22), skin (10,12), enteric ganglia (23–25), cornea (26), carotid bodies (27), whisker pads, bone marrow (28), and heart (29). Neural crest stem cells can generate both neural (neurons and Schwann cells) and mesodermal lineages (adipocytes, chondrogenic cells, osteogenic cells and smooth muscle cells) when stimulated by various extracellular signals (Figure 1) (30). Several lines of evidence suggest that SKP cells are neural crest-derived and show properties similar to neural crest stem cells. It appears that SKPs cells reflect the residual neural crest stem cells in adult skin, whose developmental potential is restricted in vivo by the niche it occupies, but could be revealed when cultured in vitro. The first evidence comes from the comparison of marker gene expression between neural crest cells and SKP cells. Rodent SKPs express several transcription factors genes (slug, snail, twist, pax3 and sox9) (10) which are involved in the migration and specification of neural crest cells (31). However, p75NTR, which is widely used in identification and isolation of neural crest stem cells by flow cytometry, was either not expressed or undetectable in rodent dorsal and facial SKPs (10), or in human neonatal foreskin SKPs (11). In contrast, multipotent SKP cells from human and mouse trunk skin co-express p75NTR and Sox10 (12), showing that neural crest marker gene expression may be associated with their tissue of origin. In pigs, we found that SKP cells expressed both pluripotency-related genes and neural crest markers (13), further demonstrating the neural crest origin of skin-derived stem cells.
Figure 1.
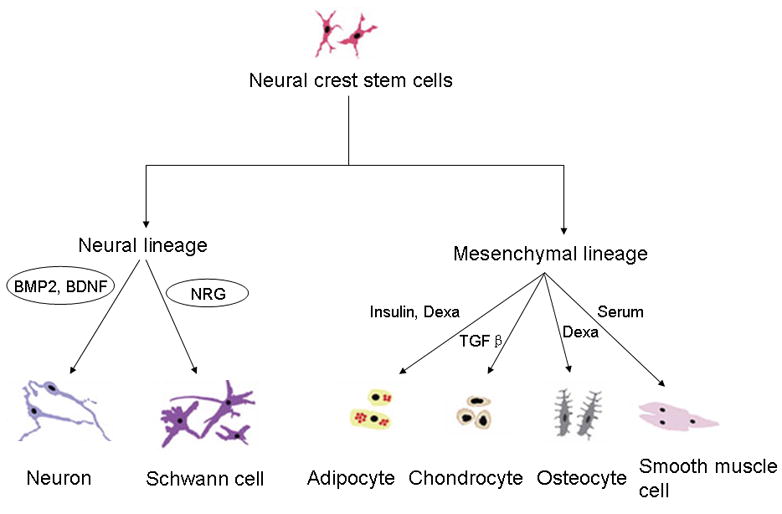
The developmental potential of neural crest-derived stem cells. Neural crest stem cells can generate neural (neurons and glias) and mesodermal progeny (adipocytes, chondrocytes, osteocyte and smooth muscle cells) when various extracellular signals are present. BMP2: bone morphogenetic protein 2; NRG: neuregulin; TGF-β: transforming growth factor-β; BDNF: brain derived neurotrophic factor; Dexa: dexamethasone.
The second evidence arises from the fate mapping approach by which transgenic reporter genes were exclusively expressed in cells of neural crest origin. Fernandes et al employed Wnt1-Cre/RosaR26R mice, where β-galactosidase expression was directed by a Wnt1 promoter and was only restricted to the progeny of neural crest stem cells. This demonstrated that follicle dermal papillae contain neural crest-derived cells and SKPs from facial skin are neural crest-derived (10). They also found that neural crest-derived SKP spheres from facial dermis (whisker papillae) could first be isolated as early as embryonic day 9 and persist into adulthood. Wong et al also used Wnt1-Cre/R26RLacZ transgenic mice to conclude that sphere-forming neural crest-derived cells reside in distinct structures of the adult skin and display different intrinsic properties with time and location (12). In whisker follicles of the face, neural crest-derived SKPs appear to be located in many mesenchymal structures; however, in the trunk skin they are restricted to the glial and melanocyte lineages. In addition, these neural crest-derived cells in adult skin have intrinsically different growth factor responsiveness from previously identified neural crest stem cells which exert exclusively neural or glial lineage differentiation in response to bone morphogenetic protein 2 (BMP2) or neuregulin 1 (NRG1) in vitro (12,32). Using a combined fate mapping and microdissection approach, Hunt et al identified a highly enriched niche of neural crest-derived SKP spheres in the dermal papilla (1,000 enriched compared with whole facial skin and termed papillaspheres) of the hair follicle in the adult skin (33). In addition, Schwann cell precursors are also derived from SKPs in adult skin, further illustrating that the gliogenic SKPs are neural crest derived (34).
The third evidence is from cell transplantation studies. When yellow fluorescent protein (YFP)-labeled SKP spheres were transplanted into the chick neural crest migratory stream in ovo at Hamburger-and-Hamilton stage 18, these SKP sphere derived cells migrated into the sympathetic ganglia, spinal nerve, dorsal root ganglion (DRG) and even the dermal layer of the skin, whereas very few cells went into neural tube (10). These results are consistent with the in vivo property of p75+P0− neural crest stem cells which can give rise to neurons and glias in peripheral nervous system (PNS) upon transplanting into chick embryos (21). Hence, three lines of evidence suggest that SKP spheres are neural crest-derived precursors that arise from embryogenesis and retain multipotency into adulthood (14).
Transcriptional characterization of porcine skin-derived stem cells: illustrating the neural and mesodermal potential by microarray analysis
In the past two decades, the genetic program of the “stemness” in multipotent/pluripotent stem cells has been extensively elucidated by high-throughput microarray or next-generation sequencing technologies (35). Recent studies show that the core transcriptional regulatory circuitry centered on the transcription factors Oct3/4, Sox2 and Nanog maintains the transcriptional program required for pluripotency in embryonic stem (ES) cells (36). The importance of transcriptional regulation on maintaining the “stemness” has been further demonstrated by the reprogramming of fibroblast (37) or even terminally differentiated B lymphocytes (38) into induced pluripotent stem (iPS) cells by defined factors (Oct3/4, Sox2, Klf4 and c-myc). However, the transcriptional regulation of somatic stem cells has still been elusive although the transcriptional profiling experiments on hematopoietic stem cells (39), neural stem cells (40), and neural crest stem cells (41,42) have been performed. This unsolved problem is confounded by the variation of transcriptional profiling caused by the noise of different genetic backgrounds and the heterogeneity of ES cells and adult stem cells (43). Different extracellular stimuli may also trigger stem cells to display various transcriptional profiling because transformation or reprogramming is likely to happen during long-term culture (44). Thus, a similar in vitro culture system and genetic background would be indispensible to describe the molecular basis of multipotency in porcine skin-derived stem cells.
Transcriptional characterization of neural and mesodermal potency in porcine skin-derived stem cells has recently been dissected (17). The general strategy is summarized in Figure 2. In order to eliminate any potential genetic background difference in describing the neural potency of porcine SKP spheres, neural stem cells (neuropsheres) (45) were isolated from the same fetuses as SKP spheres. Both SKP cells and neural stem cells were cultured in the same medium (DEME/F12 + B27 + N2 + EGF + bFGF) and they formed spheres after several days’ selection. After they formed spheres transcriptional analysis via a pig-specific microarray (46) was performed directly without any further culture. The common highly expressed genes in the SKP spheres and neurospheres are mainly ribosome, tight junction, gap junction, cell communication, calcium signaling, ErbB signaling, JAK-STAT signaling, MAPK signaling etc. The differentially expressed genes between SKP spheres and neurospheres are involved in ECM-receptor interaction and the TGF-β signaling pathway. Leukemia inhibitory factor (LIF) or MEK inhibitor treatment results in a distinctive impact on the “stemness” and differentiation-related genes in SKP spheres and neurospheres. Thus it is inferred that the cell-intrinsic genetic program may contribute to the innate “stemness” of tissue origin in SKP spheres and neurospheres in a similar local microenvironment (47).
Figure 2.
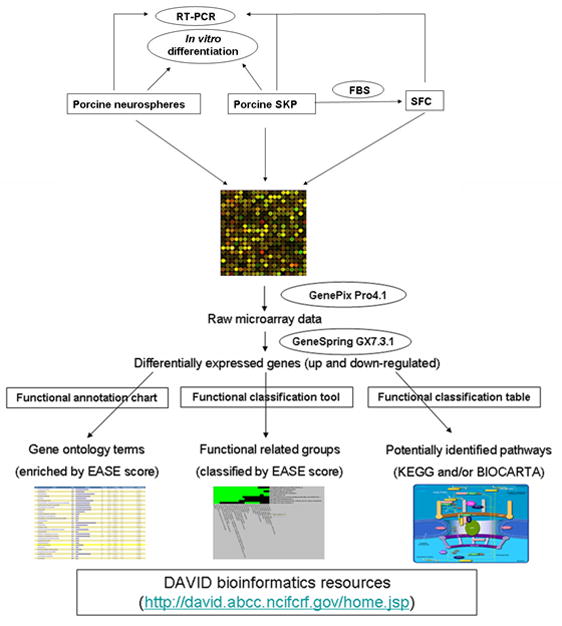
Strategy for deciphering the neural and mesodermal potency of porcine skin-derived stem cells by microarray analysis and functional annotation clustering. Porcine SKP spheres and neurospheres were isolated from the same fetuses and cultured in the same medium. SKP spheres differentiate into SKP-derived fibroblast-like cells (SFC) with serum. Reprinted with permission from (17). The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers.
To decipher the mesodermal potential of porcine SKP spheres, they were induced to differentiate into SKP-derived fibroblast-like cells (SFC) by culture in serum and adhesive dishes. The porcine SKP spheres gradually lost their neural potential but still retained mesodermal properties (17). By microarray and functional annotation clustering analysis of porcine SKP spheres and SFC, 305 genes were found to be up-regulated and 96 genes down-regulated. The down-regulated genes are mostly involved in intrinsic programs such as the Dicer pathway and asymmetric cell division; whereas up-regulated genes are more likely to participate in extrinsic signaling pathways such as ErbB signaling, MAPK signaling, ECM-receptor reaction, Wnt signaling, cell communication and TGF-beta signaling pathways. These intrinsic programs and extrinsic signaling pathways may collaborate to mediate the transcription-state transition between SKP spheres and SFC. Together with the neural potential assays, these transcriptional profiling data provide candidate signal pathways that may orchestrate the neural and mesodermal potency of porcine SKP spheres. Further studies would be to test the role of key molecules in signal transduction pathways in the self-renewal and multipotency of porcine SKP spheres by functional experiments.
Neural and mesodermal potency of skin-derived stem cells and their prospective clinical application
Since skin may provide an accessible source for autologous stem cell transplantation, SKP cells would be an idea experimental and therapeutic model for regenerative medicine (48). Strikingly, the neural progeny derived from SKP spheres may provide a new resource of neurons and glias for degenerative diseases in the nervous system. Potential clinical application is suggested by experiments that show that enriched skin-derived AC133+ (a cell surface marker) progenitors can migrate through the forebrain and give rise to astrocytes but not oligodendrocytes after injection into adult brain (49). Similarly, human skin-derived stem cells generate neurons when exposed to hippocampal astrocytes. They stably express neuronal makers (neurofilament and tubulin β-III) and show the presence of voltage-dependent calcium transients (50). This is consistent with Gingras’ report that human skin-derived neuronal precursors eventually became terminally differentiated mature neurons in vitro (51). In contrast, rodent SKP-derived neuron-like cells can maintain their peripheral phenotypes for more than 5 weeks when transplanted into the brain but still possess an immature electrophysiological profile, implying that further investigation is required to induce them into electrophysiological mature neurons (52). These studies illustrate the prospective clinical application of using SKP-derived neurons for brain therapy.
In addition, SKP-derived progeny also play a role in the regeneration of injury in the peripheral nervous system. Skin-derived stem cells can improve functional nerve regeneration after sciatic nerve resection when transplanted into resorbable guides (53). Similarly, rodent SKPs can generate myelinating Schwann cells for an injured peripheral nerve, promoting remyelination and functional recovery after contusion spinal cord injury (54). These SKPs produced Schwann cells which proliferated and induced myelin protein when in contact with sensory neuron axons. Furthermore, SKPs-derived Schwann cells can myelinate CNS axons or injured peripheral nerves in either wild type or shiverer mutant mice, thus providing an accessible source of myelinating cells for nervous system injury and dysmyelinating disorders (55). Nevertheless, SKPs or their derivatives are proposed to be the cell origin of dermal neurofibroma through loss of Nf1 tumor-suppressor gene (56). Furthermore, it is still not known whether SKPs are tumorigenic and can give rise to teratomas in vivo. Thus more comprehensive tests should be performed before any clinical application.
Another clinical application may be a result of the mesodermal potency of SKP cells. Mouse SKP cells can produce muscle progenitors and differentiated muscle cells both in vitro and in vivo (57). Both rodent and human SKPs generate skeletogenic cell types: chondrocytes and osteocytes in vitro, and can be induced into an osteogenic lineage when transplanted into injured bone marrow (58). This skeletal and osteogenic potency was also reported in porcine skin-derived stem cells (16,59). Interestingly, mouse skin-derived stem cells can be converted into insulin-producing cells in vitro (60), indicating another possible source of stem cells for cell-transplantation therapy of diabetes.
Germline potency and cellular reprogramming in porcine skin-derived stem cells
Although it is still controversial about the plasticity of adult/somatic stem cells (61), several studies reported a very broad developmental potential of somatic stem cells. For example, adult neural stem cells can contribute to chicken and mouse embryos and give rise to cells of all three germ layers (62). Bone marrow-derived multipotent adult progenitors cells (MAPCs) are also capable of generating cells of mesenchyme, neuroectoderm and endoderm in vitro and contributing to most somatic tissue when injected into blastocysts and engrafted (63). For skin-derived stem cells, epidermal stem cells have the ability to produce cells of ectodermal, mesenchymal, and neural crest-derived tissues when injected into day 3.5 C57BL/6 mouse blastocysts (64). In particular, hair follicle dermal stem cells are capable of repopulating the hematopoietic system after transplantation into lethally irradiated recipient mice (65). Porcine skin-derived stem cells are demonstrated to have the intrinsic ability to differentiate into oocyte-like and primordial germ-like cells in vitro (18,19). The oocyte-like cells can spontaneously develop into parthenogenetic embryo-like structures. However, they failed to be fertilized by sperm in vitro because of their unstable structure. Until now this germline potential of skin-derived stem cells has not been confirmed by other independent groups even though porcine SKP cells can potentially integrate into the genital ridge in vivo when injected into peri-morula embryos (66). A more stringent assay, such as production of a chimeric animal should be performed to test the germline potency of porcine skin-derived stem cells.
In theory, skin-derived stem cells have the possibility of producing germline cells based on the following strategies (Figure 3). First, somatic cells can be reprogrammed into a totipotent embryonic state and produce offspring by using somatic cell nuclear transfer technology (67). Skin-derived stem cells have been used as donor cells to generate live offspring (68). Somatic adult cells, even terminally differentiated B lymphocytes, can be reprogrammed into iPS cells by defined factors in vitro (37). The iPS cells have the ability to produce chimeric mice when injected into early blastocysts and even produce completely iPS-derived live offspring after tetraploid complementation (69). The original somatic cells, of course, develop into germline cells in either chimeric or completely iPS-derived mice. Second, both male (70,71) and female gametes (72) can be derived from embryonic stem cells in vitro, and the ES cell-derived sperm can produce live mice. It may be possible to reprogram skin-derived stem cells into an intermediate pluripotent state and then differentiate them into gametes in vitro. Third, direct cell reprogramming may be an alternative strategy to interconvert cell states between different cell types. Recent progress includes directly converting mouse embryonic fibroblast cells into functional neurons by defined factors in vitro (Ascl1, Brn2 and Myt1l) (73) and in vivo reprogramming of adult pancreatic exocrine cells to β-cells by a combination of three factors (Ngn3, Pdx1 and Mafa) (74). Thus it is conceivable to convert skin-derived stem cells into germline cells by unknown factors in metaphase II oocytes and early embryos.
Figure 3.
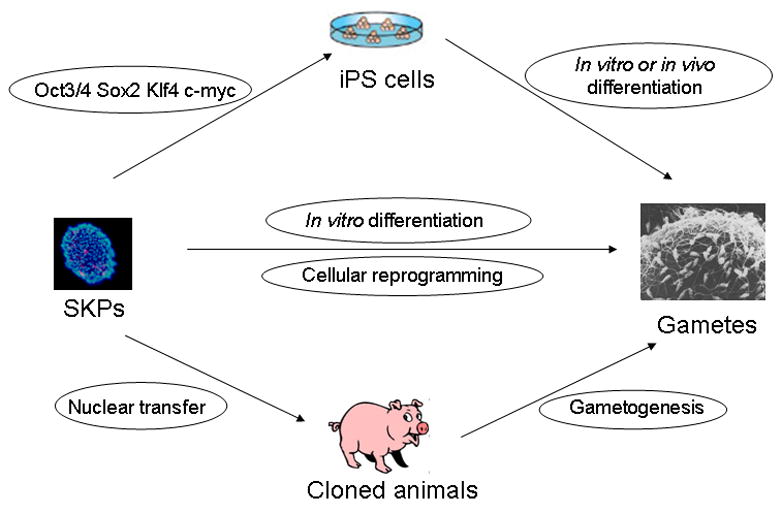
Prospective strategies to reprogram porcine skin-derived stem cells into germ-line cells. Porcine SKPs can generate oocyte-like cells in vitro (middle panel). Alternatively, they may be used as donor cells for nuclear transfer to produce cloned animals which can generate normal gametes in vivo (lower panel). Finally they might be reprogrammed into iPS cells by defined factors and then derive gametes by in vitro induction or in vivo chimera production and tetraploid complementation (upper panel).
Conclusion
Multipotent skin-derived stem cells have neural and mesodermal lineage potency and represent a prospective stem cell source for autologous cell transplantation. They are neural crest-derived but display intrinsic transcriptional profiling reflecting their tissue origin and developmental stage. Endogenous SKP can be isolated from embryonic skin and they persist into adulthood. It seems that these skin-derived stem cells have a broader developmental potency including generating primordial germ cells and gamete-like cells. Recent progress on nuclear and cellular reprogramming implies that it is possible to convert skin-derived stem cells into germline cells or any other cell type of interest. However, rigorous cellular and transplantation tests are needed before any clinical application because SKP cells are considered to be the cell origin of particular tumors in the nervous system. Therefore, further studies are needed to assess their developmental potency of skin-derived stem cells and illustrate the molecular basis which establishes and maintains the multipotency.
Acknowledgments
This study was supported by grants from NIH National Center for Research Resources (R01RR013438) and Food for the 21st Century at the University of Missouri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun BW, Lopes SMCD, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–U197. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 4.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–U110. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 5.Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- 6.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD. SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Fukunaga-Kalabis M, Yu H, Xu XW, Kong J, Lee JT, Herlyn M. Human dermal stem cells differentiate into functional epidermal melanocytes. Journal of Cell Science. 2010;123:853–860. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 11.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 12.Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U, Zimmermann DR, Dufour S, Thiery JP, Meijer D, Beermann F, Barrandon Y, Sommer L. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao M, Isom SC, Lin H, Hao Y, Zhang Y, Zhao J, Whyte JJ, Dobbs KB, Prather RS. Tracing the stemness of porcine skin-derived progenitors (pSKP) back to specific marker gene expression. Cloning Stem Cells. 2009;11:111–122. doi: 10.1089/clo.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008;363:185–198. doi: 10.1098/rstb.2006.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyce PW, Zhu H, Craig J, Li J. Stem cells with multilineage potential derived from porcine skin. Biochem Biophys Res Commun. 2004;316:651–658. doi: 10.1016/j.bbrc.2004.02.093. [DOI] [PubMed] [Google Scholar]
- 16.Lermen D, Gorjup E, Dyce PW, von Briesen H, Muller P. Neuro-Muscular Differentiation of Adult Porcine Skin Derived Stem Cell-Like Cells. Plos One. 2010;5(1):e8968. doi: 10.1371/journal.pone.0008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao MT, Whitworth KM, Zhang X, Zhao JG, Miao YL, Zhang Y, Prather RS. Deciphering the Mesodermal Potency of Porcine Skin-Derived Progenitors (SKP) by Microarray Analysis. Cellular Reprogramming. 2010;12:161–173. doi: 10.1089/cell.2009.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linher K, Dyce P, Li JL. Primordial Germ Cell-Like Cells Differentiated In Vitro from Skin-Derived Stem Cells. Plos One. 2009;4 doi: 10.1371/journal.pone.0008263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyce PW, Wen L, Li J. In vitro germline potential of stem cells derived from fetal porcine skin. Nat Cell Biol. 2006;8:384–390. doi: 10.1038/ncb1388. [DOI] [PubMed] [Google Scholar]
- 20.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 21.Morrison SJ, White PM, Zock C, Anderson DJ. Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell. 1999;96:737–749. doi: 10.1016/s0092-8674(00)80583-8. [DOI] [PubMed] [Google Scholar]
- 22.Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25:2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 23.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–656. doi: 10.1016/s0896-6273(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 25.Mosher JT, Yeager KJ, Kruger GM, Joseph NM, Hutchin ME, Dlugosz AA, Morrison SJ. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Dev Biol. 2007;303:1–15. doi: 10.1016/j.ydbio.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, Okano H, Tsubota K. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714–2722. doi: 10.1634/stemcells.2006-0156. [DOI] [PubMed] [Google Scholar]
- 27.Pardal R, Ortega-Saenz P, Duran R, Lopez-Barneo J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131:364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y, Mabuchi Y, Katoh H, Okada S, Fukuda K, Suda T, Matsuzaki Y, Toyama Y, Okano H. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, Ogawa S, Osumi N, Okano H, Fukuda K. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170:1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee G, Kim H, Elkabetz Y, Al Shamy G, Panagiotakos G, Barberi T, Tabar V, Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nature Biotechnology. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 31.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 32.Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 33.Hunt DP, Morris PN, Sterling J, Anderson JA, Joannides A, Jahoda C, Compston A, Chandran S. A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells. 2008;26:163–172. doi: 10.1634/stemcells.2007-0281. [DOI] [PubMed] [Google Scholar]
- 34.Hunt DP, Sajic M, Phillips H, Henderson D, Compston A, Smith K, Chandran S. Origins of gliogenic stem cell populations within adult skin and bone marrow. Stem Cells Dev. doi: 10.1089/scd.2009.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgantas RW, 3rd, Tanadve V, Malehorn M, Heimfeld S, Chen C, Carr L, Martinez-Murillo F, Riggins G, Kowalski J, Civin CI. Microarray and serial analysis of gene expression analyses identify known and novel transcripts overexpressed in hematopoietic stem cells. Cancer Res. 2004;64:4434–4441. doi: 10.1158/0008-5472.CAN-03-3247. [DOI] [PubMed] [Google Scholar]
- 40.Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, Xu QX, Davidson BP, Stice SL, Smith AK, Goldman SA, Reubinoff BE, Zhan M, Rao MS, Chesnut JD. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem Cells. 2007;25:1298–1306. doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- 41.Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, Munnich A, Lyonnet S, Vekemans M, Etchevers HC. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17:3411–3425. doi: 10.1093/hmg/ddn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu YF, Zhang ZJ, Sieber-Blum M. An epidermal neural crest stem cell (EPI-NCSC) molecular signature. Stem Cells. 2006;24:2692–2702. doi: 10.1634/stemcells.2006-0233. [DOI] [PubMed] [Google Scholar]
- 43.Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3:480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 46.Whitworth KM, Agca C, Kim JG, Patel RV, Springer GK, Bivens NJ, Forrester LJ, Mathialagan N, Green JA, Prather RS. Transcriptional profiling of pig embryogenesis by using a 15-K member unigene set specific for pig reproductive tissues and embryos. Biol Reprod. 2005;72:1437–1451. doi: 10.1095/biolreprod.104.037952. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M-T, Whitworth KM, Lin H, Zhang X, Isom SC, Dobbs KB, Bauer BK, Zhang Y, Prather RS. Porcine skin-derived progenitor (SKP) spheres and neurospheres: distinct “sternness” identified by microarray analysis. Cellular Reprogramming. 2010 doi: 10.1089/cell.2009.0116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunt DPJ, Jahoda C, Chandran S. Multipotent skin-derived precursors: from biology to clinical translation. Current Opinion in Biotechnology. 2009;20:522–530. doi: 10.1016/j.copbio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Belicchi M, Pisati F, Lopa R, Porretti L, Fortunato F, Sironi M, Scalamogna M, Parati EA, Bresolin N, Torrente Y. Human skin-derived stem cells migrate throughout Forebrain and differentiate into astrocytes after injection into adult mouse brain. Journal of Neuroscience Research. 2004;77:475–486. doi: 10.1002/jnr.20151. [DOI] [PubMed] [Google Scholar]
- 50.Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- 51.Gingras M, Champigny MF, Berthod F. Differentiation of human adult skin-derived neuronal precursors into mature neurons. J Cell Physiol. 2007;210:498–506. doi: 10.1002/jcp.20889. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD. Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol. 2006;201:32–48. doi: 10.1016/j.expneurol.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A, Parolini D, Draghi L, Fruguglietti ME, Gavina M, Porretti L, Cattaneo A, Battistelli M, Prelle A, Moggio M, Borsa S, Bello L, Spagnoli D, Gaini SM, Tanzi MC, Bresolin N, Grimoldi N, Torrente Y. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007;55:425–438. doi: 10.1002/glia.20470. [DOI] [PubMed] [Google Scholar]
- 54.Biernaskie J, Sparling JS, Liu J, Shannon CP, Plemel JR, Xie Y, Miller FD, Tetzlaff W. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKenzie IA, Biernaskie J, Toma JG, Midha R, Miller FD. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le LQ, Shipman T, Burns DK, Parada LF. Cell of Origin and Microenvironment Contribution for NF1-Associated Dermal Neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiu ZF, Miao CL, Li JX, Lei XH, Liu S, Guo WX, Cao YJ, Duan EK. Skeletal Myogenic Potential of Mouse Skin-Derived Precursors. Stem Cells and Development. 2010;19:259–268. doi: 10.1089/scd.2009.0058. [DOI] [PubMed] [Google Scholar]
- 58.Lavoie JF, Biernaskie JA, Chen Y, Bagli D, Alman B, Kaplan DR, Miller FD. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev. 2009;18:893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- 59.Kang EJ, Byun JH, Choi YJ, Maeng GH, Lee SL, Kang DH, Lee JS, Rho GJ, Park BW. In Vitro and In Vivo Osteogenesis of Porcine Skin-Derived Mesenchymal Stem Cell-like Cells with a Demineralized Bone and Fibrin Glue Scaffold. Tissue Engineering Part A. 2010;16:815–827. doi: 10.1089/ten.TEA.2009.0439. [DOI] [PubMed] [Google Scholar]
- 60.Guo W, Miao C, Liu S, Qiu Z, Li J, Duan E. Efficient differentiation of insulin-producing cells from skin-derived stem cells. Cell Prolif. 2009;42:49–62. doi: 10.1111/j.1365-2184.2008.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 62.Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, Lendahl U, Frisen J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 63.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 64.Liang LC, Bickenbach JR. Somatic epidermal stem cells can produce multiple cell lineages during development. Stem Cells. 2002;20:21–31. doi: 10.1634/stemcells.20-1-21. [DOI] [PubMed] [Google Scholar]
- 65.Lako M, Armstrong L, Cairns PM, Harris S, Hole N, Jahoda CAB. Hair follicle dermal cells repopulate the mouse haematopoietic system. Journal of Cell Science. 2002;115:3967–3974. doi: 10.1242/jcs.00060. [DOI] [PubMed] [Google Scholar]
- 66.Zhao M-T, Bennett MW, Zhang X, Spate L, Whitworth KM, Murphy CN, Rieke A, Zhang Y, Prather RS. Contribution to neural and mesodermal lineages by porcine skin-derived progenitors (SKPs) in vivo. Cell Cycle. 2010;9 doi: 10.4161/cc.9.10.11688. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]
- 68.Hao YH, Wax D, Zhong ZS, Murphy C, Ross JW, Rieke A, Samuel M, Spate L, Dyce P, Li JL, Sutovsky P, Prather RS. Porcine Skin-Derived Stem Cells Can Serve as Donor Cells for Nuclear Transfer. Cloning and Stem Cells. 2009;11:101–109. doi: 10.1089/clo.2008.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010 doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 71.Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, Dev A, Wulf G, Ehrmann IE, Elliott DJ, Okpanyi V, Zechner U, Haaf T, Meinhardt A, Engel W. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, 3rd, Boiani M, Scholer HR. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 73.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–U1050. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]


