Abstract
Objective
To assess salivary stress biomarkers (cortisol and alpha-amylase) and female fecundity.
Design
Prospective cohort design.
Setting
United Kingdom.
Patients
274 women aged 18–40 years attempting pregnancy were followed until pregnant or for six menstrual cycles. Women collected basal saliva samples on day 6 of each cycle, and used fertility monitors to identify ovulation and pregnancy test kits for pregnancy detection.
Main Outcome Measures
Exposures included salivary cortisol (μg/dL) and alpha-amylase (U/mL) concentrations. Fecundity was measured by time-to-pregnancy and the probability of pregnancy during the fertile window as estimated from discrete-time survival and Bayesian modeling techniques, respectively.
Results
Alpha-amylase but not cortisol concentrations were negatively associated with fecundity in the first cycle (fecundity odds ratio = 0.85; 95% confidence interval 0.67, 1.09) after adjusting for couples’ ages, intercourse frequency, and alcohol consumption. Significant reductions in the probability of conception across the fertile window during the first cycle attempting pregnancy were observed for women whose salivary concentrations of alpha-amylase were in the upper quartiles in comparison to women in the lower quartiles (HPD −0.284; 95% interval −0.540, −0.029).
Conclusions
Stress significantly reduced the probability of conception each day during the fertile window, possibly exerting its effect through the sympathetic medullar pathway.
Keywords: alpha amylase, conception, cortisol, fecundity, fertile window, stress
Introduction
Considerable speculation has arisen regarding the purported decline in human fecundity believed attributable to environmental factors including lifestyle (1). Available evidence suggests that obesity, cigarette smoking, and consumption of alcoholic beverages are associated with diminished female fecundity as measured by a longer time-to-pregnancy (TTP) among couples trying to become pregnant or undergoing assisted reproductive technologies (2–4). However at the population level, only maternal age, menstrual cycle length, parity, and oral contraceptive were retained in models and estimated to account for only 14% of the explained variation in TTP (5). Some authors argue that diminished fecundity is a function of couples intentionally delaying childbearing and, thereby, couples’ age-related effects (6).
Perceived psychosocial stress has been alleged to be detrimental for successful human reproduction, largely stemming from research reporting spontaneous conceptions among infertile couples following the adoption of a child (7,8) or improved in vitro fertilization outcomes among women enrolled in stress reduction interventions (9). Despite a plethora of literature reporting an adverse relation between stress and adverse pregnancy outcomes, its relation with fecundity has only been assessed in one epidemiologic study. Specifically, 430 couples planning pregnancy completed questionnaires regarding psychological distress until pregnant or through six cycles of follow up (10). The probability of conception in cycles with the highest distress scores was lower in comparison to cycles with lower distress scores, i.e., 12.8% and 16.5%, respectively. Despite the absence of empirical evidence supporting a relation between stress as measured by biomarkers and human fecundity, clinical guidance and folklore continue to advise women desiring pregnancy to relax while trying to become pregnant serving as an impetus for study.
Materials and Methods
Study design and population
We designed a prospective longitudinal stress component within the ongoing Oxford Conception Study (11) to assess the relation between salivary cortisol and alpha-amylase and female fecundity as measured by time-to-pregnancy (TTP) and the day-specific probability of conception during the fertile window while adjusting for purported determinants of couple fecundity. The study cohort comprised 374 women who were attempting to become pregnant as recruited through various media campaigns throughout the United Kingdom during 2005–2006. Inclusion criteria were: aged 18–40 years; menstrual cycle length between 21–39 days; and planning pregnancy or currently trying for <3 months. Exclusion criteria included: infertility history; currently breastfeeding; hormonal contraception within the past few menstrual cycles; or injectable contraceptives within the past year.
Data collection
Women completed daily diaries for menstruation, sexual intercourse in 12-hour intervals, and lifestyle behaviors (e.g., smoking, alcohol consumption), and tested their urine commencing on day 6 of their cycle for 20 days using the Clearblue Easy®Fertility Monitor (Unipath Ltd, UK). This monitor records urinary estrone-3-glucuronide (E3G) and luteinizing hormone (LH) levels during the cycle. Women were instructed to test their urine on the days after menses was expected using home pregnancy tests for the detection of human chorionic gonadotropin (hCG) to determine post-implantation pregnancies. Lastly, women were instructed to collect saliva samples upon waking (basal) on day six of each cycle using the Salivette® device (Sarstedt AG & Co., Germany). Specifically, women were instructed to remove the cotton roll from the Salivette® containing citric acid then lightly chew on it for 2–3 minutes or until the roll was saturated with saliva, to place it in a crush-proof tube, and return in the postage paid envelope. Saliva cortisol and alpha-amylase are stable at room temperature for several weeks (12). Upon receipt at the research office, samples were stored at −20°C. Institutional Review Board approval was given for this study, and all women were consented prior to enrollment.
Operational definitions
TTP was measured in menstrual cycles using the first day of bleeding exclusive of light spotting as recorded in daily diaries as day one of the cycle. We then matched E3G and LH measurements from the fertility monitor with diary calendar dates to define the estimated day of ovulation. Given the absence of ultrasonography as the gold standard for determination of ovulation, we relied upon a proxy using the detection of LH by the fertility monitor for all women. Ovulation was estimated to have occurred on day one of the LH surge as determined by the monitor. Pregnancy was defined by a positive home pregnancy test (n=186; 96%) as confirmed by the nurse or having exited the study for pregnancy (n=7; 4%).
Laboratory analysis
Salivary cortisol and alpha-amylase concentrations were quantified by a highly experienced laboratory (Salimetrics, LLC, State College, PA, USA) as markers of the hypothalamic-pituitary-adrenal (HPA) axis resulting in the secretion of glucocorticoids including cortisol into the circulatory system (12) and the sympathetic medullar system (SAM) resulting in the release of catecholamines (e.g., dopamine, epinephrine, norepinephrine) into the blood stream (13), respectively. Both cortisol and alpha-amylase have been demonstrated to be valid markers of perceived psychosocial stress (14,15), and the lack of correlation between these markers is indicative of their separate pathways (16).
From each salivary sample, cortisol (μg/dL) was quantified using the highly sensitive enzyme immunoassay (17), which is reported to be highly correlated with serum cortisol assays. Salivary α-amylase concentrations (U/mL) were determined using a commercially available kinetic reaction assay (18). Both techniques have good intra-inter-assay coefficients of variation.
Statistical analysis
Descriptive analyses were conducted to evaluate data completeness, the quality of the laboratory data, and comparing women by completion status. For a complete assessment of female fecundity, we assessed stress biomarkers by pregnancy outcome while under observation in the study: withdrew, no pregnancy within six months of trying, pregnancy loss, and live birth. Significance testing included Chi-square and t-tests for categorical and continuous data, respectively. We used discrete-time survival analysis modeling techniques to estimate the effect of cortisol and alpha-amylase concentrations and TTP (in menstrual cycles) after log-transforming the salivary measurements to achieve normality. We used a Gamma distribution with a mean of one for the frailty distribution; the variance and standard deviation are 0.335 and 0.856, respectively. We estimated fecundability odds ratios (FOR) and 95% confidence intervals (CIs) for salivary cortisol and alpha-amylase concentrations adjusting for partners’ ages (in years), frequency of sexual intercourse during the fertile window, and woman’s alcohol consumption standardized to a 28-day menstrual cycle to account for the variations in menstrual cycle lengths and time required for pregnancy across women. We conducted separate analyses for the first and all cycles under observation accounting for correlated cycles per woman.
We utilized Bayesian statistical techniques to identify the fertile window and to estimate day-specific probabilities of conception taking into account intercourse during the fertile window and inclusive of relevant covariates using the Dunson and Stanford’s adaptation of the Barrett and Marshall model (19,20). The appropriate point and interval estimates for the effects of covariates on day-specific conception model are the posterior mean and its corresponding 95% highest posterior density (HPD) interval, which is somewhat comparable to confidence intervals obtained via non-Bayesian approaches (21). We estimated the HPD interval using established procedures (22). Positive values of the posterior mean of the regression coefficients indicate a positive association, whereas a negative coefficient indicates a negative association with the day-specific conception probabilities. Also, HPD intervals excluding zero indicate a significant covariate effect on the day-specific probabilities. We also defined the fertile window using the Ogino-Knaus method (23,24) based upon calendar dates for menstrual cycles as captured in daily diaries assuming 14 days between ovulation and the onset of menstruation to assess the consistency of results. For conception cycles, we used the first day of pregnancy testing, given that women were instructed to test on the day they expected menstruation.
Results
The study cohort comprised 374 women of whom 274 (73%) women had salivary and covariate data for at least the first cycle along with an LH peak detected by the fertility monitor. Among the 100 excluded women, 28 had missing salivary data, 41 were missing daily journal data, 14 had non-consecutive cycles, and 17 had incomplete fertile windows. Two hundred (73%) women had complete cortisol and alpha-amylase data for all cycles under observation. We observed no significant differences between women who did or did not complete the study with regard to couples’ socioeconomic background including age and education nor by the detection of an LH peak in cycle 1 (data not shown). The salivary laboratory data were within established Westguard rules (25); no significant within-batch variation was observed. Salivary cortisol and alpha-amylase concentrations were not correlated (−0.06; p-value p=0.30).
Characteristics of the cohort varied by study outcome (Table 1). Women who withdrew contributed 135 cycles compared with 345 and 290 cycles contributed by women becoming pregnant or not, respectively. Overall, 175 (64%) of participants became pregnant. Higher mean female (31.8 ± 4.1) and male (34.4 ± 5.5) ages were significantly associated with no pregnancy in comparison to women who withdrew, experienced a pregnancy loss, or had a live birth. Women who did not become pregnant tended to have fewer pregnancies (gravidity) than women in other study outcome categories. However, no significant differences were observed for menstrual cycle characteristics or intercourse frequency during the fertile window. With regard to lifestyle, the highest mean caffeine consumption was reported by women experiencing pregnancy losses (65.4 ± 42.3 drinks per cycle) and the highest mean alcohol (23.7 ± 27.1 drinks per cycle) consumption for women not becoming pregnant. No significant differences were observed in mean concentrations of cortisol (μg/dL) or alpha-amylase (U/mL) by study outcome.
TABLE 1.
Comparison of cohort by study outcome (n=274)
| Characteristic | Withdrew (n=49) No. (%) | No Pregnancy (n=50) No. (%) | Pregnancy Loss (n=48) No. (%) | Live Birth (n=127) No. (%) |
|---|---|---|---|---|
| Female age (years): | ||||
| 20–24 | 10 (20) | 2 (4) | 8 (17) | 10 (8) |
| 25–29 | 19 (39) | 13 (26) | 15 (31) | 57 (45) |
| 30–34 | 14 (29) | 25 (50) | 16 (33) | 46 (36) |
| 35+ | 6 (12) | 10 (20) | 9 (19) | 14 (11) |
| Mean (±SD) | 28.7 (±4.8) | 31.8 (±4.1) | 29.3 (±5.1) | 29.5 (±3.9)* |
| Partner age (years): | ||||
| 20–24 | 5 (10) | -- | 5 (10) | 2 (2) |
| 25–29 | 13 (27) | 7 (14) | 15 (31) | 42 (33) |
| 30–34 | 19 (39) | 22 (44) | 13 (27) | 54 (43) |
| 35+ | 12 (25) | 21 (41) | 15 (31) | 29 (33) |
| Mean (±SD) | 31.4 (±5.6) | 34.4 (±5.5) | 31.8 (±6.1) | 31.5 (±4.3)* |
| Gravidity (# pregnancies): | ||||
| Nulligravida | 17 (35) | 21 (42) | 6 (13) | 33 (26) |
| 1 | 19 (39) | 22 (44) | 18 (38) | 43 (34) |
| 2 | 8 (16) | 3 (6) | 11 (23) | 22 (17) |
| 3+ | 5 (10) | 4 (8) | 13 (27) | 29 (23) |
| Mean (±SD) | 1.0 (±1.0) | 0.8 (±0.9) | 1.7 (±1.0) | 1.4 (±1.1)* |
| Basal Salivary Biomarkers: | ||||
| Cortisol (mg/dL) | 0.43 (±0.24) | 0.43(±0.16) | 0.44 (±0.19) | 0.47 (±0.20) |
| Alpha amylase (U/mL) | 10.75 (±22.61) | 8.01 (±6.73) | 7.11 (±5.30) | 7.68 (±7.79) |
| Menstrual cycle length (days):a | ||||
| <21 | -- | -- | 1 (2) | 2 (2) |
| 21–27 | 12 (20) | 13 (26) | 8 (17) | 26 (21) |
| 28 | 8 (16) | 10 (20) | 8 (17) | 11 (9) |
| 29–39 | 26 (53) | 27 (54) | 26 (54) | 81 (64) |
| >39 | 5 (10) | -- | 5 (10) | 7 (6) |
| Mean (±SD) | 32.2 (±8.4) | 29.5 (±2.9) | 31.3 (±5.0) | 31.2 (±5.0) |
| Bleeding duration (days):a | ||||
| 2–3 | 6 (12) | 5 (10) | 5 (10) | 13 (10) |
| 4–5 | 32 (65) | 33 (66) | 28 (58) | 84 (66) |
| ≥6 | 11 (22) | 12 (24) | 15 (31) | 30 (24) |
| Mean (±SD) | 4.8 (±1.2) | 4.8 (±1.3) | 5.1 (±1.3) | 4.8 (±1.0) |
| Cigarette smoking:b | ||||
| 0 | 40 (82) | 46 (92) | 39 (81) | 101 (80) |
| 1–28 | 4 (8) | 1 (2) | 1 (2) | 9 (7) |
| 29–99 | 2 (4) | -- | -- | 3 (2) |
| 100+ | 3 (6) | 3 (6) | 8 (17) | 14 (11) |
| Mean (±SD) | 15.9 (±52.3) | 14.1 (±57.4) | 30.8 (±77.0) | 36.5 (±108.3) |
| Alcohol consumption:c | ||||
| 0 | 5 (10) | 1 (2) | 8 (17) | 16 (13) |
| 1–28 | 36 (71) | 36 (74) | 34 (71) | 73 (58) |
| 29+ | 9 (18) | 12 (25) | 6 (13) | 38 (30) |
| Mean (±SD) | 18.1 (±17.4) | 23.7 (±27.1) | 13.8 (±12.4) | 19.7 (±19.5) |
| Caffeine consumption:b | ||||
| 0 | 4 (8) | 1 (2) | 2 (4) | 3 (2) |
| 1–28 | 14 (29) | 21 (42) | 9 (19) | 41 (32) |
| 29–199 | 27 (55) | 25 (50) | 27 (56) | 65 (51) |
| 100+ | 4 (8) | 3 (6) | 10 (21) | 18 (14) |
| Mean (±SD) | 44.8 (±35.6) | 40.9 (±32.1) | 65.4 (±42.3) | 51.2 (±38.2)* |
| Intercourse frequency:a | ||||
| 0 | 1 (2) | -- | -- | -- |
| 1–9 | 27 (55) | 26 (52) | 21 (44) | 72 (57) |
| 10–19 | 16 (33) | 23 (46) | 21 (44) | 46 (36) |
| 20+ | 5 (10) | 1 (2) | 6 (13) | 9 (7) |
| Mean (±SD) | 10.3 (±6.6) | 9.8 (±4.4) | 10.8 (±6.1) | 10.2 (±6.1) |
Averaged over all cycles.
Daily number standardized to a 28-day menstrual cycle.
Weekly number drinks standardized to a 28-day menstrual cycle.
SD, denotes standard deviation
p≤0.008; two-sided using Chi-square or t-test statistic.
An opposing pattern was observed for salivary stress biomarkers and fecundability in (un)adjusted Cox models using data for the first cycle attempting pregnancy while enrolled in the cohort (Table 2). Specifically, FORs were elevated for cortisol suggesting a shorter TTP (FOR=2.51, 95% CI 0.75–8.42), but were reduced for alpha-amylase indicative of a longer TTP (FOR= 0.09, 95% CI 0.71–1.13). As expected, FORs for age were below one and above one for frequency of intercourse. However, all confidence intervals included one. Similar patterns were seen when all cycles were considered for the adjusted cortisol (FOR=2.15, 95% CI 0.67–6.97) and alpha-amylase (FOR= 0.92, 0.72–1.17) models.
TABLE 2.
Stress biomarkers and fecundability in the first cycle (n=274)
| Cortisola | Alpha-Amylasea | ||||
|---|---|---|---|---|---|
| Model | FOR | 95% CI | Model | FOR | 95% CI |
| Unadjusted | |||||
| Cortisol | 1.74 | 0.46–6.60 | Alpha-amylase | 0.85 | 0.67–1.09 |
| Adjusted | |||||
| Cortisol | 2.51 | 0.75–8.42 | Alpha-amylase | 0.90 | 0.71–1.13 |
| Female age (years) | 0.98 | 0.93–1.02 | Female age (years) | 0.98 | 0.93–1.03 |
| Male age (years) | 0.98 | 0.94–1.02 | Male age (years) | 0.98 | 0.94–1.02 |
| Frequency intercourseb | 1.07 | 0.96–1.19 | Frequency intercourseb | 1.07 | 0.96–1.19 |
| Female alcohol consumptionc | 1.00 | 0.92–1.09 | Female alcohol Consumptionc | 1.00 | 0.92–1.09 |
Note: Results derived from discrete time survival analysis. FOR, denotes fecundability odds ratio; CI, denotes confidence interval.
On logarithm scale with 1 added.
Number of acts of sexual intercourse during the estimated fertile window.
Standardized to a 28-day menstrual cycle.
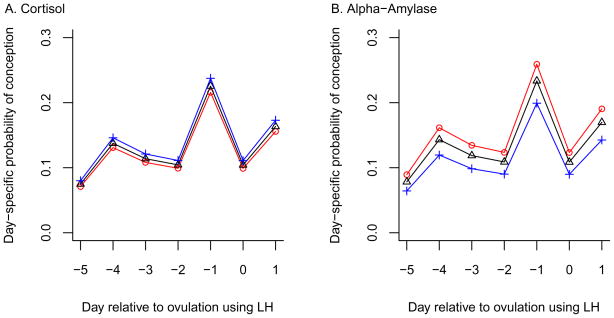
The fertile window was a priori defined as commencing five days before estimated ovulation through day one following ovulation. The results show that the mean effect of cortisol was positively associated with day-specific conception probabilities, while the alpha-amylase effects were negatively associated with day-specific conception probabilities (Table 3). The 95% HPD intervals indicate that alpha-amylase values were significant predictors of a decrease in day-specific conception probabilities for both the unadjusted and adjusted model for the first cycle. The day-specific conception probabilities were highest on the day prior to ovulation (day −1) followed by day one (day +1) after ovulation, and were lowest on day five (day −5) prior to ovulation for both cortisol and alpha-amylase (Figures 1a, 1b). While conception probabilities increased with increasing quartiles of cortisol for each day during the fertile window, the reverse was true for alpha-amylase as increasing quartiles reduced all day specific probabilities. A similar pattern was observed when using all cycles under observation in the cohort (data not shown). None of the lines crossed for alpha amylase irrespective of model.
TABLE 3.
Salivary biomarkers and overall daily probability of conception during the fertile window – Bayesian Analysis (n=274)
| Cycle | Model | Biomarker | Ogino-Knaus Method | Luteinizing Hormone Surge | ||
|---|---|---|---|---|---|---|
| Posterior Mean | 95% HPD Interval | Posterior Mean | 95% HPD Interval | |||
| First Cycle | Unadjusted | Cortisol | 0.141 | −0.094, 0.383 | 0.116 | −0.141, 0.375 |
| Alpha-amylase | −0.246 | −0.483, −0.008 | −0.284 | −0.540, −0.029 | ||
| Adjusted | Cortisol | 0.137 | −0.116, 0.387 | 0.100 | −0.167, 0.367 | |
| Alpha-amylase | −0.237 | −0.497, −0.005 | −0.269 | −0.534, −0.003 | ||
| All Cycles | Unadjusted | Cortisol | 0.068 | −0.173, 0.314 | 0.122 | −0.074, 0.319 |
| Alpha-amylase | −0.130 | −0.324, 0.067 | −0.101 | −0.311, 0.112 | ||
| Adjusted | Cortisol | 0.066 | −0.184, 0.312 | 0.123 | −0.077, 0.324 | |
| Alpha-amylase | −0.122 | −0.321, 0.075 | −0.083 | −0.301, 0.126 | ||
Note: Bayesian models were adjusted for female and male age (in years) and daily alcohol consumption
HPD, denotes highest posterior density, which is comparable to confidence intervals in Cox Discrete survival analysis.
Figure 1.
Stress biomarkers and day–specific probabilities of conception.
Discussion
To our knowledge, this is the first study to empirically demonstrate that stress is significantly associated with reduced female fecundity as measured by a lower probability of conception for each day during the fertile window as women’s salivary alpha-amylase concentrations rise. Moreover, the reduction in fecundability was mediated via the SAM pathway rather than through the HPA axis as evidenced by the opposing directions in FORs and the day-specific probabilities of conception for salivary alpha-amylase and cortisol, respectively. Irrespective of the day or frequency of sexual intercourse during the fertile window, women with higher concentrations of alpha amylase were less likely to conceive than women with lower concentrations underscoring the importance of a statistical model that is biologically responsive to the timing of intercourse relative to the fertile window and inclusive of other relevant covariates. While the findings were significant for alpha-amylase and the daily-specific conception probabilities in the first cycle, the results based on all cycles per woman were not. This most likely reflects a loss in statistical power with the most fecund women contributing one cycle coupled with the variability associated with alpha-amylase while trying.
Our findings do not support an earlier study involving 13 women prospectively followed that reported no differences in urinary adrenaline, noradrenaline, or cortisol concentrations between conception and non-conception cycles (26). Among nulliparous Chinese textile workers trying to conceive, perceived stress during the follicular phase was associated with dysmenorrhea, underscoring the importance of timing when assessing stress-related effects (27). While we did not measure dysmenorrhea in our study, all saliva samples were collected during the follicular phase. A recent cohort study of women undergoing their first in vitro fertilization/intra cytoplasmatic sperm injection cycle reported greater treatment success for women with lower urinary adrenaline concentrations at oocyte retrieval, and lower adrenaline and noradrenaline concentrations at embryo transfer in comparison to women with unsuccessful cycles (28). In addition, stress reduction behavioral therapies have been shown to improve IVF outcomes (9,29).
The mechanisms by which alpha-amylase may reduce fecundity are as yet unknown, but the reproductive tract has long been known to contain catecholamine receptors (30), which may alter blood flow through the fallopian tubes and gamete transportation (31). Alpha-amylase is the principal salivary protein whose secretion from the parotid gland is regulated by the SAM system in response to sympathetic stimuli (physical and/or emotional stressors) resulting in increased blood catecholamines. Since this biomarker is produced locally in the oral cavity, it is in relatively high concentrations compared to other salivary markers such as cortisol that are serum constituents produced elsewhere in the body and transported to saliva via ultrafiltration (32). To this end, alpha-amylase may be a novel biomarker for assessing psychosocial stressors and reproductive endpoints as mediated via the sympathetic nervous system. The opposing effects for stress biomarkers and fecundity observed in our study underscores the importance of measuring multiple stress biomarkers of stress-related systems such as the HPA and SAM.
While a promising biomarker, several important methodologic issues await further study to help interpret the findings. Our cohort was constructed within the Oxford Conception Study, a three-arm randomized trial to assess the efficacy of fertility monitor in helping women conceive. Our Bayesian models incorporated a woman-level random effect term to accommodate unobserved heterogeneity. The findings need to be interpreted within the context of utilizing the LH surge as a proxy for ovulation. Given the variability associated with ovulation, we have no reason to believe that the variation is nonrandom in this study cohort. Also, method of saliva collection including volume available for analysis may impact the measured concentrations (18). Timing of saliva collection is an important consideration in that stress biomarkers may be affected by circadian rhythms. Nater and colleagues (33) observed 76 participants who contributed 857 alpha-amylase measurements during the course of the day and reported a marked diurnal profile that was unrelated to perceived stress. However, a 4% increase in alpha-amylase was observed for every one point increase in the perceived psychosocial stress scale completed by participants. The authors also found that mean salivary cortisol concentrations did not predict alpha-amylase concentrations. Given the prospective nature our study, any biases arising from the salivary methodology should be non-differential driving the effects toward the null.
In sum, our findings support a reduction in the day-specific probability of conception among women with higher salivary alpha-amylase concentrations in comparison to women with lower concentrations. Our data support clinical and public health messages aimed at helping couples relax and minimize stressors when attempting to achieve pregnancy. This message becomes even more important when considering the maternal-fetal unit, given longstanding concern that stressors during pregnancy adversely affect fetal and infant well-being (34,35).
Acknowledgments
The authors thank Dr. Ronald Gray who assisted Dr. Cecilia Pyper with design and logistical aspects, and Ms. Karen Smith for assistance with reviewing the results.
Acknowledgement Financial Support:
Funding for this study was provided in part by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development for partial support of data collection and for complete analysis of the salivary biomarkers, the U.K. National Health Service Executive Primary Care Career Scientist and Service Research and Development Awards for covering the time of the study’s principal investigator (CP), the DLM Charitable Trust for funding the salaries of the research team, and the Unipath Corporation who provided fertility monitors and pregnancy tests and related technical assistance for the devices as used in the study.
Footnotes
None of the authors has a known conflict of interest with any aspect of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butler D. The fertility riddle. Nature. 2004;432:38–9. doi: 10.1038/432038a. [DOI] [PubMed] [Google Scholar]
- 2.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–92. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Jensen TK, Hjollund NH, Henriksen TB, Scheike T, Kolstad H, Giwercman A, et al. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. BMJ. 1998;317:505–10. doi: 10.1136/bmj.317.7157.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TIA, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–7. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 5.Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006;21:1279–84. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- 6.Dunson DB, Colombo B, Baird DD. Changes with age in the level and duration of fertility in the menstrual cycle. Hum Reprod. 2002;17:1399–1403. doi: 10.1093/humrep/17.5.1399. [DOI] [PubMed] [Google Scholar]
- 7.Rock J, Tietze C, McLaughlin HB. Effect of adoption on infertility. Fertil Steril. 1965;16:305–12. doi: 10.1016/s0015-0282(16)35583-2. [DOI] [PubMed] [Google Scholar]
- 8.Mai FM. Conception after adoption: an open question. Psychosom Med. 1971;33:509–14. doi: 10.1097/00006842-197111000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675–87. doi: 10.1016/s0015-0282(01)02008-8. [DOI] [PubMed] [Google Scholar]
- 10.Hjollund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, et al. Distress and reduced fertility: A follow-up study of first-pregnancy planners. Fertil Steril. 1999;72:47–53. doi: 10.1016/s0015-0282(99)00186-7. [DOI] [PubMed] [Google Scholar]
- 11.Pyper C, Bromhall L, Dummett S, Altman DG, Browbill P, Murphy M. The Oxford Conception Study design and recruitment experience. Paediatr Perinat Epidemiol. 2006;20 (supplement 1):51–59. doi: 10.1111/j.1365-3016.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 12.Kahn JP, Rubinow DR, Davis CL, Kling M, Post RM. Salivary cortisol: a practical method for evaluation of adrenal function. Biol Psychiatry. 1988;23:335–49. doi: 10.1016/0006-3223(88)90284-3. [DOI] [PubMed] [Google Scholar]
- 13.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–74. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 14.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 15.Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol. 2004;49:963–68. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, et al. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophyisology. 2005;55:333–42. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Raff H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clin Chem. 2003;49:203–4. doi: 10.1373/49.1.203. [DOI] [PubMed] [Google Scholar]
- 18.Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary α-Amylase in biobehavioral research: Recent developments and applications. Ann NY Acad Sci. 2007;1098:122–4. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- 19.Barrett JC, Marshall J. The risk of conception on different days of the menstrual cycle. Population Studies. 1969;23:455–61. doi: 10.1080/00324728.1969.10405297. [DOI] [PubMed] [Google Scholar]
- 20.Dunson DB, Stanford JB. Bayesian inference on predictors of conception probabilities. Biometrics. 2005;61:126–33. doi: 10.1111/j.0006-341X.2005.031231.x. [DOI] [PubMed] [Google Scholar]
- 21.Carlin BP, Louis TA. Bayesian Methods for Data Analysis. 3. Boca Raton: Chapman and Hall/CRC; 2008. [Google Scholar]
- 22.Chen MH, Shao QM. Monte Carlo estimation of Bayesian credible and HPD intervals. J Computat Graph Stat. 1999;8:69–92. [Google Scholar]
- 23.Knaus H. Eine neue Methods zur Bestimmung des Ovulationstermines. Zentralbl F Gynak. 1929;53:2193. [Google Scholar]
- 24.Ogino K. Ovulationstermin und Konzeptionstermin. Zentralbl F Gynak. 1930;54:464–79. [Google Scholar]
- 25.Harel O, Schisterman EF, Vexler A, Ruopp MD. Can we get better data: a statistical approach to monitor quality control? Epidemiology. 2008;19:621–7. doi: 10.1097/EDE.0b013e318176bfb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders KA, Bruce NW. A prospective study of psychosocial stress and fertility in women. Hum Reprod. 1997;12:2324–29. doi: 10.1093/humrep/12.10.2324. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Wang X, Wang W, Chen C, Ronnennberg AG, Guang A, et al. Stress and dysmenorrhoea: a population based prospective study. Occup Environ Med. 2004;61:1021–6. doi: 10.1136/oem.2003.012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smeenk JMJ, Verhaak CM, Vingerhoets AJJM, Sweep CG, Merkus JM, Willemsen SJ, et al. Stress and outcome success in IVF: the role of self-reports and endocrine variables. Hum Reprod. 2005;20:991–6. doi: 10.1093/humrep/deh739. [DOI] [PubMed] [Google Scholar]
- 29.de Liz TM, Strauss B. Differential efficacy of group and individual/couple psychotherapy with infertile patients. Hum Reprod. 2005;20:1324–32. doi: 10.1093/humrep/deh743. [DOI] [PubMed] [Google Scholar]
- 30.Moran NC. Adrenergic receptors. In: Blaschko H, Sayers G, Smith AD, editors. Handbook of Physiology, Section 7. Endocrinology, Vol. VI. Adrenal Gland. Baltimore, Maryland: Williams and Wilkins Company; 1975. pp. 117–125. [Google Scholar]
- 31.Schneker JG, Meirow D, Schenker E. Stress and human reproduction. Eur J Obstet Gynecol Reprod Biol. 1992;45:1–8. doi: 10.1016/0028-2243(92)90186-3. [DOI] [PubMed] [Google Scholar]
- 32.Harmon AG, Towe-Goodman NR, Fortunato CK, Granger DA. Differences in saliva collection location and disparities in baseline and diurnal rhythms of alpha-amylase: A preliminary note of caution. Horm Behav. 2008;54:592–6. doi: 10.1016/j.yhbeh.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 35.Cooper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins BA, Johnson F, Jones P, Meier AM. The preterm prediction study: maternal stress is associated with spontaneous preterm birth rates at less than thirty-five weeks’ gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gyencol. 1996;175:1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]