Abstract
Background
Neuroimaging studies reported cerebral perfusion abnormalities in individuals with alcohol use disorders. However, no longitudinal MRI studies of cerebral perfusion changes during abstinence from alcohol have been reported.
Methods
Arterial spin labeling MRI was used to evaluate cortical gray matter perfusion changes in short-term abstinent alcohol dependent individuals in treatment and to assess the impact of chronic cigarette smoking on perfusion changes during abstinence. Seventy-six patients were scanned at least once. Data from 19 non-smoking (17 males, 2 females) and 22 smoking (21 males, 1 female) patients scanned at one and five weeks of abstinence were used to assess perfusion changes over time. Twenty-eight age-equated healthy controls (25 males, 3 females) were scanned for cross-sectional comparison, 13 of them were scanned twice. Given the age range of the cohort (28 – 68 years), age was used as a covariate in the analyses. Mean perfusion was measured in voxels of at least 80% gray matter in the frontal and parietal lobes and related to neurocognitive and substance use measures.
Results
At one week of abstinence, frontal and parietal gray matter perfusion in smoking alcoholics was not significantly different from that in non-smoking alcoholics, but each group's perfusion values were significantly lower than in controls. After five weeks of abstinence, perfusion of frontal and parietal gray matter in non-smoking alcoholics was significantly higher than that at baseline. However, in smoking alcoholics, perfusion was not significantly different between the time-points in either region. The total number of cigarettes smoked per day was negatively correlated with frontal gray matter perfusion measured at five weeks of abstinence. Lobar perfusion measures did not correlate significantly with drinking severity or cognitive domain measures at either time-point.
Conclusion
Although cerebral perfusion in alcohol dependent individuals shows improvement with abstinence from alcohol, cigarette smoking appears to hinder perfusion improvement.
Keywords: magnetic resonance, perfusion, blood flow, smoking, alcohol use disorder
Introduction
Alcohol use disorders (i.e., alcohol abuse or dependence; AUD) are associated with brain tissue atrophy, enlargement of the ventricular system (Schroth et al., 1988; Zipursky et al., 1989) and widened sulci (Cala et al., 1978; Jernigan et al., 1991), especially in elderly alcohol dependent individuals (Pfefferbaum et al., 1992; Pfefferbaum et al., 1997). Brain metabolite abnormalities in AUD have also been reported (Durazzo et al., 2004; Jagannathan et al., 1996). The regions that appear particularly susceptible to the deleterious effects of AUD include the frontal and parietal cortices (Jernigan et al., 1991), the corpus callosum (Pfefferbaum et al., 1996) and the medial temporal lobe (Sullivan et al., 1995). Also, abnormalities of regional cerebral blood flow (rCBF) or perfusion in AUD have been reported by several studies using single photon emission computed tomography (SPECT) (Erbas et al., 1992; Harris et al., 1999; Melgaard et al., 1990; Nicolas et al., 1993; Rogers et al., 1983) and magnetic resonance imaging (MRI) (Gazdzinski et al., 2006). Cross sectional (Dupont et al., 1996) and longitudinal (Tutus et al., 1998) SPECT studies examined cerebral perfusion during short-term and long-term abstinence from alcohol and came to contradictory conclusions. Dupont et al. (1996) reported that both short-term (25 days) and long-term (7.7 years) abstinent alcohol dependents showed significantly decreased global rCBF compared to controls, with the short-term abstinent individuals demonstrating lower rCBF than their long-term abstinent counterparts. They concluded that cerebral blood flow abnormalities in abstinent alcohol dependents are persistent. Tutus et al. (1998) reported significantly reduced frontal lobe blood flow in recently detoxified alcoholics compared to blood flow levels after three weeks of abstinence from alcohol. Perfusion in the right parietal lobe was significantly lower compared to controls both at detoxification and three weeks later. The authors concluded that frontal lobe perfusion abnormalities may be transitory with abstinence.
One factor that may affect perfusion measurements in alcohol dependent samples is chronic cigarette smoking, which is exceedingly common in AUD (Hurt et al., 1994; Pomerleau et al., 1997; Romberger and Grant, 2004). Cigarette smoking is associated with cerebrovascular compromise and several studies have shown regional perfusion abnormalities in chronic smokers (Domino et al., 2004; Rose et al., 2003; Zubieta et al., 2001). In our previous MR perfusion study, we found that concomitant chronic cigarette smoking compounds rCBF abnormalities in recently detoxified alcoholics (Gazdzinski et al., 2006). In that report, cerebral perfusion in 1-week-abstinent alcohol dependent individuals in treatment (ALC) was 13% lower than in non-smoking light drinkers (nsLD) in frontal gray matter (fGM) and 8% lower in parietal gray matter (pGM). When divided into non-smoking (nsALC) and smoking (sALC) alcohol dependent individuals, perfusion in sALC was significantly lower than in nsLD and nsALC in both fGM and pGM; nsALC perfusion was not significantly different from nsLD in either region. These perfusion results and those from non-alcohol dependent cohorts suggest that previously reported brain blood flow abnormalities in AUD may be modulated by chronic cigarette smoking.
To our knowledge, there are no reports of longitudinal MRI studies of cerebral blood perfusion in ALC during early abstinence from alcohol. In a longitudinal MR spectroscopic imaging study, sALC demonstrated diminished recovery of regional brain metabolite concentrations relative to nsALC over approximately one month of abstinence (Durazzo et al., 2006). Over a similar interval, we also observed that sALC showed significantly lower recovery than nsALC of brain metabolite concentrations specifically in the medial temporal lobe; metabolite levels normalized in nsALC, but remained lower in sALC relative to controls (Gazdzinski et al., 2008). Therefore, we predicted that chronic cigarette smoking adversely affects recovery of perfusion in abstinent alcoholics.
The aims of this study were to investigate longitudinal changes of perfusion in fGM and pGM of alcohol dependent individuals over one month of abstinence, assess the effects of comorbid chronic cigarette smoking on perfusion recovery, and explore potential relationships of changes in fGM and pGM perfusion with neurocognition. Based on the findings of our other longitudinal neuroimaging studies, we hypothesized sALC exhibit significantly weaker perfusion recovery than nsALC.
Materials and Methods
Participants
Seventy-six ALC were recruited from the San Francisco Veterans Administration Medical Center Substance Abuse Day Hospital and the San Francisco Kaiser Permanente Chemical Dependence Recovery Program, whereas 28 (25 males, 3 females) non-smoking light drinkers (nsLD) were recruited from the San Francisco Bay Area through electronic poster boards. Fifty-eight ALC participants (23 nsALC, 35 sALC) were scanned at 6 ± 3 days after consumption of their last alcoholic drink (time-point 1 = TP1), and 59 (27 nsALC, 32 sALC) were scanned after 34 ± 12 days of abstinence (time-point 2 = TP2). Forty-one ALC participants (19 nsALC including 2 females, 22 sALC including 1 female) had scans at both time-points (‘longitudinal sample of ALC’). All 28 nsLD participants were scanned at baseline and 13 of them completed a second scan six months after the first scan (‘longitudinal sample of nsLD’). All participants were between the ages of 28 and 68 years at the time of enrollment. Table 1 shows the basic demographics and other variables of all groups and for both time-points of the ALC groups.
Table 1.
Demographics, participant characteristics and laboratory variables (mean ± standard deviation) at TP1 and TP2 (TP2 variables in parentheses). The p values are from ANOVA.
| Variable | nsLD | nsALC | sALC | Group comparison |
|---|---|---|---|---|
| N (incl. females) | 28 (3) | 19 (2) | 22 (1) | NA |
| Age (years) | 44.0 ± 8.2 | 52.1 ± 9.4 | 47.8 ± 9.2 | nsLD < sALC < nsALC, p = 0.035 |
| Education (years) | 16.9 ± 2.7 | 14.5 ± 2.3 | 13.2 ± 1.6 | sALC < nsALC < nsLD, p = 0.001 |
| Age of first alcoholic drink | 17.3 ± 2.4 | 17.2 ± 4.0 | 16.5 ± 2.8 | NS |
| 8 years average (drinks per month) | 13.2 ± 13.1 | 283.9 ± 139.9 | 352.4 ± 146.9 | sALC vs. nsALC, NS |
| 3 years average (drinks per month) | 13.6 ± 14.9 | 343.4 ± 167.2 | 383.5 ± 177.2 | sALC vs. nsALC, NS |
| 1 year average (drinks per month) | 13.6 ± 14.8 | 368.0 ± 186.2 | 418.1 ± 194.1 | sALC vs. nsALC, NS |
| Lifetime average (drinks per month) | 15.4 ± 12.6 | 178.3 ± 104.8 | 296.3 ± 149.8 | nsALC < sALC, p = 0.004 |
| Duration of regular drinking (years) | 26.2 ± 7.0 | 34.9 ± 9.8 | 31.4 ± 9.3 | NS |
| Age of onset of heavy drinking (years) | ……. | 28.7 ± 11.0 | 20.8 ± 6.1 | nsALC > sALC, p = 0.001 |
| Duration of heavy drinking (years) | ----- | 19.7 ± 9.1 | 23.7 ± 9.5 | nsALC < sALC, p = 0.022 |
| Total ethanol consumed over lifetime (kg) | 67.2 ± 57.8 | 1002.7 ± 658.3 | 1557.1 ± 997.4 | nsALC < sALC, p = 0.019 |
| Duration of Abstinence (days) | ------ | 5.0 ± 2.6 (35.3 ± 11.1) | 6.0 ± 2.6 (33.7 ± 11.0) | sALC vs. nsALC, NS (NS) |
| Fagerstrom total score | ------ | ------ | 5.5 ± 2.0 | N/A |
| Duration of smoking (years) | ----- | ------ | 27.4 ± 7.3 | N/A |
| Fagerstrom Pack-yrs | ----- | ------ | 23.4 ± 22.3 | N/A |
| Time since last caffeinated beverage (hrs) | 5.9 ± 2.7 | 7.5 ± 2.5 (5.8± 3.9) | 5.9 ± 3.8 (5.4 ± 4.4) | sALC vs. nsALC, NS (NS) |
| BDI | ------ | 14.7 ± 9.9 (6.7 ± 6.9) | 13.5 ± 8.9 (10.0 ± 8.2) | sALC vs. nsALC NS (NS) |
| STAI Y-2 | 31.7 ± 7.5 | 47.4 ± 11.8 (44.4 ± 13.5) | 47.3 ± 11.2 (41.9 ± 9.4) | sALC vs. nsALC, NS (NS) |
| AMNART | 118.8 ± 7.1 | 113.6 ± 9.7 (121.0 ± 8.1) | 111.8 ± 9.8 (114.7 ± 14.7) | sALC vs. nsALC, NS (NS) |
| Pre-albumin (g/dL) | 31.3 ± 5.0 | 28.9 ± 9.3 (29.5 ± 7.8) | 27.8 ± 5.6 (28.0 ± 5.3) | sALC vs. nsALC, NS (NS) |
| Albumin (g/dL) | 4.1 ± 0.3 | 3.9 ± 0.4 (4.0 ± 0.1) | 4.1 ± 0.3 (3.8 ± 0.3) | sALC vs. nsALC, NS (NS) |
| AST (IU) | 25.2 ± 6.7 | 43.9 ± 35.6 (30.0 ± 10.4) | 44.8 ± 33.0 (41.4 ± 21.7) | sALC vs. nsALC, NS (NS) |
| SGPT-ALT (IU) | 31.1 ± 14.9 | 55.7 ± 43.0 (32.5 ± 16.4) | 36.5 ± 20.7 (42.1 ± 21.9) | sALC vs. nsALC, NS (NS) |
| WBC | 6.7 ± 1.5 | 7.0 ± 1.3 (7.1 ± 1.9) | 7.2 ± 2.0 (7.9 ± 1.9) | sALC vs. nsALC, NS (NS) |
| RBC | 4.9 ± 0.3 | 4.5 ± 0.4 (4.7 ± 0.1) | 4.4 ± 0.5 (4.6 ± 0.2) | sALC vs. nsALC, NS (NS) |
| Hemoglobin | 15.1 ± 1.0 | 14.6 ± 1.4 (15.3 ± 0.1) | 14.2 ± 1.5 (14.1 ± 0.8) | sALC vs. nsALC, NS (NS) |
| Hematocrit | 43.9 ± 2.6 | 42.0 ± 3.8 (43.4 ± 0.5) | 41.6 ± 3.8 (41.4 ± 1.1) | sALC vs. nsALC, NS (NS) |
| CO2 | 28.6 ± 2.0 | 28.9 ± 3.1 (30.0 ± 2.8) | 28.7 ± 1.9 (29.3 ± 2.3) | sALC vs. nsALC, NS (NS) |
| BMI (lb/m2) | 26.0 ± 4.9 | 29.1 ± 4.6 (28.8 ± 4.7) | 24.3 ± 3.9 (24.2 ± 3.8) | sALC < nsALC, p = 0.001 (p = 0.001) |
| GGT (IU) | 24.0 ± 14.0 | 94.8 ± 62.5 (45.1 ± 28.4) | 64.8 ± 38.6 (46.1 ± 38.7) | sALC vs. nsALC, NS (NS) |
AMNART, American National Adult Reading Test; BDI, Beck Depression Inventory; CIWA-Ar, Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; 1-yr average, 3-yr average, 8-yr average: number of alcoholic drinks per month over 1, 3, and 8 years before study; lifetime average, number of drinks per month over lifetime; STAI Y-2, State Trait Anxiety Inventory – State; GGT, gamma-glutamylacidtransferase, local normal range 7-56 i.u.; albumin local normal range 3.3-5.2 g/dl; prealbumin local normal range 18-45 mg/dl; RBC, red blood cells; WBC, white blood cells; CO2, carbon dioxide; BMI, body mass index; NS, not significant; NA, not applicable
Ninety percent of the longitudinal ALC participants continued in outpatient substance abuse treatment programs at the San Francisco VA Medical Center or Kaiser Permanente over the assessment interval. They attended these programs 3-5 days per week and were given weekly drug screens. Breath alcohol levels were acquired randomly or in the case of suspected or obvious intoxication. Chart review confirmed that no ALC participant tested positive for illicit/non-prescribed substances or alcohol during continued outpatient treatment. No participant tested positive for breath alcohol nor had a positive urine test for THC, opiates, PCP, cocaine, or amphetamines at either time-point.
Primary inclusion criteria for ALC participants were: fluency in English, a Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV) diagnosis of alcohol abuse or dependence with physiological dependence and consumption prior to enrollment of at least 150 alcoholic drinks per month for at least 8 years (males), and more than 80 drinks per month for at least 6 years for female participants. A standard alcoholic drink is defined as containing 13.6 g of pure ethanol, equivalent to 12 oz of beer or 5 oz of wine or 1.5 oz of liquor. nsLD had no history of AUD. Participants were free of general medical, neurologic or psychiatric conditions that are known to influence brain morphology, cerebral tissue perfusion or neurocognition. However, unipolar mood disorders (i.e., major depression, dysthymia, substance-induced mood disorder with depressive features), medically controlled hypertension and hepatitis C were not exclusionary in ALC, given their very high prevalence in AUD (Gilman and Abraham, 2001; Stinson et al., 2005). All medical and psychiatric variables were obtained through structured interviews, as previously detailed (Durazzo et al., 2004). In summary, participants completed the Structured Clinical Interview for DSM-IV Axis I Disorder Patient Edition, Version 2.0 (First et al., 1998) and standardized questionnaires for alcohol withdrawal (CIWA-Ar; Addiction Research Foundation Clinical Institute of Withdrawal Assessment for Alcohol; (Sullivan et al., 1989)), depression (Beck Depression Inventory; (Beck, 1978)) and anxiety symptomatology (State-Trait Anxiety Inventory, Y-2, STAI; (Spielberger et al., 1977)) within one day of the MR study. Due to the length of our imaging, neurocognitive, psychiatric and alcohol/substance use assessment protocol, we were unable to screen participants for Axis II disorders. Alcohol consumption over lifetime was assessed with the lifetime drinking history (LDH), which gives quantitative/frequency information on alcohol consumption from the start of regular alcoholic drinking to present (Skinner and Sheu, 1982; Sobell and Sobell, 1990; Sobell et al., 1988), where regular drinking is defined as the intake of at least one standard drink per month. From the LDH we calculated average number of alcoholic drinks consumed per month over 1 year, 3 years, 8 years before enrollment and over lifetime; total amount of pure ethanol consumed over lifetime, number of years of regular drinking in lifetime, and age at onset of heavy drinking defined as consumption of more than 100 alcoholic drinks per month.
The Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991) was used to assess smoking characteristics in sALC. All sALC participants were actively smoking at the time of the study; and nsALC did not smoke for at least 1 year prior to the study. A brief neurocognitive battery was administered at TP1 and a comprehensive battery at TP2 (Durazzo et al., 2007).
To evaluate the nutritional status and alcohol-related or other hepatocellular injury in ALC individuals, laboratory tests for serum albumin, pre-albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyltransferase were obtained within 3 days of the MR scans (see Table 1).
Cross-sectional data on approximately two-thirds of the ALC and nsLD participants at TP1 were previously reported (Gazdzinski et al., 2006). All participants provided written informed consent prior to the study, and the Institutional Review Boards of the University of California at San Francisco and the San Francisco VA Medical Center approved all study procedures.
MRI Data Acquisition
MRI data were acquired at 1.5 Tesla (Vision, Siemens Medical, Inc., Iselin, NJ) with a circularly polarized transmit-receiver radio frequency head coil. Throughout the scan, participants laid supine, with their head immobilized within the coil with a padded sliding contact to minimize head motion. Before the perfusion image acquisitions, 3-D T1-weighted images (used for tissue segmentation and co-registration) were acquired with a Magnetization Prepared Rapid Gradient Echo imaging sequence with TR/TE/TI = 10/7/300 ms, a flip angle of 15°, 1.5 mm thick coronal slices oriented perpendicular to the long axes of the hippocampus with in-plane resolution of 1.0 × 1.0 mm 2. T2-weighted images (used for the creation of brain masks) were also acquired with a multi-slice double spin-echo imaging sequence with TR/TE1/TE2 = 2500/20/80 ms, 3 mm thick slices and 1.0 × 1.0 mm2 in-plane resolution. The T2-weighted images had the same orientation as the perfusion images.
Perfusion was measured with a Pulsed Arterial Spin Labeling (PASL) sequence called ‘double inversion with proximal labeling of both tagged and control images (DIPLOMA) (Jahng et al., 2003) (see Figure 1). This version of PASL sufficiently compensates for magnetization transfer, untagged blood modulation and arterial transit time effects, which usually limit the accuracy in measuring perfusion.
Fig. 1.
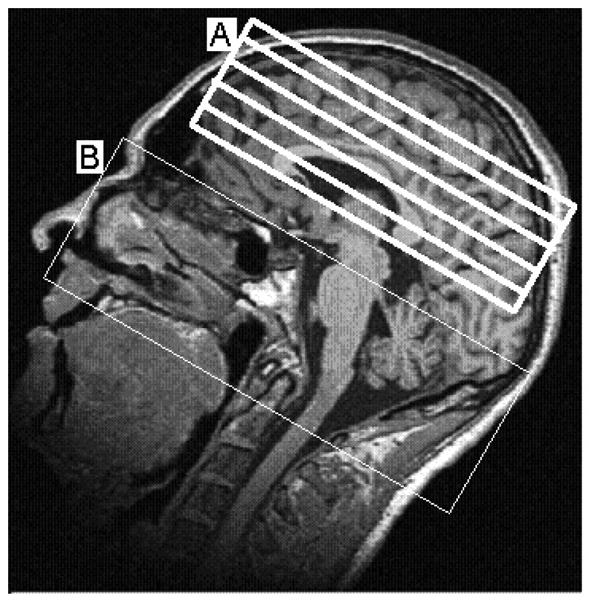
A T1-weighted MR image showing approximate locations of perfusion image slices (A) and blood tagging region (B)
The pulse sequence was a single-shot gradient-echo echo-planar imaging with five 8 mm thick slices oriented almost parallel (5° shallower) to the orbital-meatal angle and having 2 mm slice gaps. The following sequence parameters were used in all experiments: 225 × 300 mm2 FOV, 2.5 s TR, 15 ms TE and a 2.3 × 2.3 mm2 in-plane resolution. The time between the tagging pulses and the imaging pulses (i.e., post-labeling delay) was 1500 ms. The PASL acquisition time was approximately 5.5 minutes per study. The reliability and reproducibility of DIPLOMA were previously reported in a test-retest study on a different group of 13 healthy volunteers, who were scanned one hour apart (Jahng et al., 2005). In this sample, the intra-class correlation coefficient was 0.81 for global brain perfusion and 0.80 for gray matter and white matter perfusion measurements. We used this sequence in a previous report (Gazdzinski et al., 2006), where we obtained virtually identical results with post-labeling-delays of 1500ms and 1200ms. This indicates that our results are not affected by potential transit time differences that might exist between groups.
To avoid the effects of sleep and visual activation on perfusion, all participants were reminded to be awake, but with their eyes closed throughout the acquisition. All data were transferred as raw images to an off-line computer for processing and analyses.
Data Processing
Details of the processing steps were described previously (Gazdzinski et al., 2006). Briefly, the T1-weighted images were first segmented into probability maps for gray matter (GM), white matter (WM) and cerebro-spinal fluid (CSF). The major lobes were then outlined, partitioned into lobes, sub-cortical nuclei, brainstem and cerebellum (Cardenas et al., 2005) and overlaid on the segmented images. GM perfusion values from voxels with at least 80% GM were then averaged separately for the frontal and parietal lobes. Finally, the perfusion values considered were normalized to reflect 100% GM contribution, neglecting average WM contributions of about 5% in each of the groups. We excluded WM perfusion and its analysis here because the perfusion sequence was optimized for only GM perfusion imaging of the frontal and parietal lobes.
Data Analyses
Multivariate Analysis of Variance (MANOVA) was first run on the participants' demographics and other variables to examine for group differences on these factors. Cross-sectional and longitudinal repeated measures (generalized estimating equations) analyses employed the generalized linear model in SPSS v15. In cross-sectional analyses at TP1,, comparing nsLD and the entire ALC (sALC and nsALC combined), participant age, years of education and GM contribution to perfusion voxels were statistically different between groups, and were used as covariates (see Table 1). Also, for cross-sectional analyses, we used the normalized data to account for GM contribution differences between the groups. In longitudinal analyses examining for perfusion changes in nsALC and sALC over the TP1-TP2 interval, lifetime average alcohol consumption, the age at onset of heavy drinking, months of heavy drinking, the total amount of alcohol consumed over lifetime, and body mass index (BMI) were statistically different between nsALC and sALC, and were used as covariates (see Table 1). GM contribution to perfusion voxels was not significantly different between nsALC and sALC, so perfusion values for longitudinal analyses were not normalized to 100% GM to avoid the introduction of further noise into the data. The correlation analyses used Pearson correlations, but were also verified with the Spearman's method.
Results
Participants
nsLD and ALC differed in age (p = 0.016) and years of education (p < 0.001), whereas nsALC and sALC did not differ on these variables. The average amount of alcohol consumed per month over 1 year, 3 years and 8 years were not significantly different between nsALC and sALC. However, sALC began drinking at significantly younger age than nsALC (p = 0.001), had significantly more months of heavy drinking (p = 0.022), higher lifetime average of monthly alcohol consumption (p = 0.004), and consumed more alcohol over lifetime than nsALC (p = 0.019). nsALC had a higher body mass index (BMI) than sALC (p = 0.001). Thirty-nine percent of the combined ALC cohort (nsALC + sALC) had both a history of a comorbid medical (primarily hypertension or hepatitis C) and psychiatric (primarily unipolar mood disorders) conditions. Seventeen percent of the ALC cohort met lifetime criteria for substance abuse or dependence, but the period of dependence was more than 5 years prior to enrollment. The frequencies of the aforementioned comorbidities were equivalent between nsALC and sALC. Measures of self-reported depression, anxiety, withdrawal symptomatology and all laboratory variables at both time-points were not different between nsALC and sALC. Measures of withdrawal symptomatology at time of study for both groups were not clinically elevated. sALC smoked 22 ± 10 cigarettes per day (minimum, 5; maximum, 50) for 23 ± 14 years (minimum, 2; maximum, 44). Their cigarette pack-years was 24 ± 23 years (minimum, 2; maximum, 70); and their Fagerstrom score was 5.8 ± 1.9 (minimum, 2; maximum, 10), indicating medium-to-high levels of dependence on nicotine. Detailed demographics and other variables for the three groups are given in Table 1.
Table 2 shows lobar cortical perfusion values of the three groups (cross-sectional nsLD sample, nsALC and sALC from the ALC longitudinal sample) averaged over the left and right hemispheres.
Table 2.
Regional GM perfusion in institutional units (mean ± standard deviation) at one week; and at five weeks of abstinence from alcohol and % change over time.
| Region | Group | 1 week (TP1) | 5 weeks (TP2) | % change* |
|---|---|---|---|---|
| fGM | nsLD | 390.3 ± 90.7 | N/A | N/A |
| ALC (combined) | 350.8 ± 84.4 | 357.8 ± 91.8 | +2.0 | |
| nsALC | 349.5 ± 82.3 | 382.4 ± 99.3 | +9.4 | |
| sALC | 352.0 ± 89.2 | 336.6 ± 81.3 | -4.4 | |
| pGM | nsLD | 399.6 ± 87.3 | N/A | N/A |
| ALC (combined) | 358.5 ± 76.7 | 367.6 ± 89.5 | +2.5 | |
| nsALC | 352.0 ± 85.7 | 383.4 ± 103.7 | +8.9 | |
| sALC | 364.0 ± 84.2 | 354.0 ± 73.7 | -2.7 |
% change calculated as [(TP2-TP1)/TP1] × 100
Perfusion in nsLD
Analyses of the nsLD longitudinal data (n = 13) showed no significant differences in fGM and pGM perfusion between the two time points. Specifically, no significant differences were apparent between baseline fGM perfusion [371.3 ± 95.5 (mean ± standard deviation)] and follow-up (356.6 ± 55.0) after 6-months (-4.0%, p = 0.3). Similarly, no significant differences were observed between baseline pGM perfusion (382.0 ± 79.0) and follow-up (368.2 ± 64) in these participants (-3.6%, p = 0.28).
The average cross-sectional perfusion values of the nsLD longitudinal sample were not statistically different from the average values of the corresponding regions of the entire nsLD sample; therefore, group averages of the entire nsLD group (n = 28) as shown in Table 2 were used in our statistical analyses. The gray shaded regions in Figure 2a and b represent the mean ± standard error of the entire nsLD perfusion for fGM and pGM respectively.
Fig. 2.
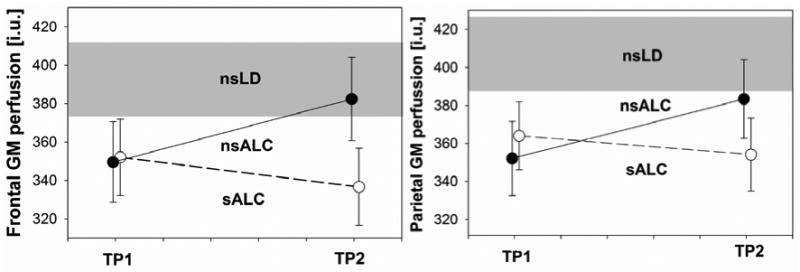
Cortical gray matter perfusion (institutional units, i.u., mean ± standard error) in non-smoking light drinkers (nsLD), non-smoking alcohol dependent (nsALC), and smoking alcohol dependent individuals (sALC) at one week of abstinence from alcohol (time-point 1, TP1) and at 5 weeks of abstinence (TP2). The gray shaded area represents mean ± standard error of the entire nsLD group. Left: frontal gray matter (GM), right: parietal GM. The group × time interaction for the alcoholic groups is significant for frontal GM (p = 0.049), but not for parietal GM (p = 0.077).
Longitudinal Perfusion Changes (nsALC vs sALC)
A 2 (group) × 2 (time-point) repeated measures analysis yielded a group-by-time-point interaction [(χ2(1) = 3.88, p = 0.049] for fGM perfusion (see Figure 2a), and a trend for a group-by-time interaction [(χ2(1) = 2.93, p = 0.077] for pGM perfusion (Figure 2b). There were no significant main effects for time-point or group in either fGM or pGM. nsALC showed a 9% increase in perfusion between time-points in both fGM (p = 0.049) and pGM (p = 0.036). In contrast, no significant perfusion changes were observed in sALC over the same time interval in either region [i.e., fGM: 4% decrease (p = 0.31), pGM: 3% decrease (p = 0.73)]. Controlling for potential effects of medical and psychiatric comorbidities or differences in alcohol consumption measures in the ALC groups (e.g., months of heavy drinking, average drinks/month over lifetime) did not alter these longitudinal findings in either group
Cross Sectional Group Comparisons
Entire nsLD group, nsALC, and sALC from longitudinal samples (see Figure 2)
fGM
At TP1, both sALC [-10.3%, p = 0.053)] and nsALC [-10.8%, p = 0.056], showed trends less than the entire nsLD. At TP2, fGM perfusion in sALC was 13.2% (p = 0.022) lower than nsLD TP1 perfusion and tended to be lower (-11.7% p = 0.054) compared to nsALC. However, at TP2, fGM perfusion in nsALC was not significantly different from the TP1 fGM perfusion in nsLD
pGM
At TP1, perfusion in both sALC and nsALC was lower by 11.1% (p = 0.015) and 13.3% (p = 0.025), respectively, relative to nsLD. At TP2, sALC showed 13.5% lower perfusion compared to TP1 pGM perfusion in nsLD (p = 0.013), and tended to be lower than in nsALC (-8% p = 0.12). However, at TP2, there were no significant differences in pGM perfusion between nsALC and TP1 pGM perfusion in nsLD. Cross sectional analyses of the larger cross sectional ALC sample (which included participants with data from only one time point) gave results for both cortical brain regions similar to those obtained with the smaller longitudinal sample.
Entire nsLD group and ALC from longitudinal sample
fGM
In the ALC group (nsALC + sALC), fGM perfusion at TP1 was 11% lower than in nsLD (p = 0.029). At TP2, ALC tended to have 8% lower fGM perfusion than nsLD TP1 fGM perfusion (p = 0.088).
pGM
At TP1, pGM perfusion in ALC was 12% lower than in nsLD (p = 0.008). At TP2, pGM perfusion in ALC was 10% lower than nsLD TP1 perfusion (p = 0.028).
Correlations
At TP2, we explored the relationships between fGM or pGM perfusion and the following cognitive domains: general intelligence, learning and memory, fine motor skills, ataxia/balance, processing speed, visuospatial skills, and working memory. We also examined the relationships between changes in perfusion and changes in the individual cognitive measures that constitute the neurocognitive domains (mentioned above) over the time between scans, as well as the relationships between perfusion measures and drinking or smoking severities.
Neither fGM nor pGM perfusion showed significant correlations with any of the neurocognitive domains at TP2 in either of the ALC groups. Also, there were no significant relationships between changes in perfusion and changes in the individual cognitive measures between the two time-points. However, the following individual cognitive measures showed modest correlations with fGM perfusion at TP2: Stroop color – word (r = 0.28, p = 0.02), a measure of response inhibition and processing speed (Golden, 1978); Ward-7 Full Scale IQ (r = 0.22, p = 0.05), a measure of general intelligence (Axelrod et al., 2001); and WAIS-III Digit Span (r = 0.26, p = 0.03), a measure of working memory (Wechsler, 1997).
There were no correlations between perfusion measures and any of the drinking severity measures at either time-point. However, the total number of cigarettes smoked per day by sALC related negatively with pGM perfusion at TP2 (r = -0.39, ρ = 0.036).
Discussion
To the best of our knowledge, this is the first magnetic resonance-based longitudinal report of cerebral perfusion in AUD; it also describes the effects of chronic smoking on cerebral blood flow recovery during short-term abstinence from alcohol. The major findings are as follows: 1) sALC demonstrated a significantly lower magnitude of recovery than nsALC over approximately five weeks of abstinence after controlling for group differences in severity of alcohol consumption and other potentially confounding variables; the perfusion abnormalities in sALC remained essentially unchanged over the four-week assessment interval. 2) At TP2 (at approximately five weeks of abstinence), fGM and pGM perfusion in nsALC recovered to nsLD levels, while sALC continued to manifest significantly lower perfusion levels than nsLD in both cortical regions, with trends for lower perfusion relative to nsALC. The overall pattern of results indicated that sALC, in contrast to nsALC, showed no appreciable recovery from fGM and pGM perfusion abnormalities over four weeks of abstinence from alcohol. Thus, chronic smoking in this alcohol dependent cohort appeared to have an adverse effect on the recovery from cortical gray matter perfusion abnormalities during abstinence from alcohol.
A SPECT study reported (Tutus et al., 1998) that fGM perfusion normalized, while pGM perfusion remained deficient in an AUD cohort after three weeks of abstinence from alcohol. In the present study, we also observed indications of fGM perfusion normalization, but continued lower pGM perfusion in the combined ALC group (i.e., nsALC + sALC) at TP2. However, both longitudinal increases in fGM and pGM perfusion and levels at TP2 were clearly driven by nsALC, highlighting the potential importance of considering smoking status in assessment of blood flow changes during alcohol abstinence.
Decreased cerebral blood flow in cigarette smokers has been attributed to the impact of smoking on the functional integrity of the cerebrovasculature (Domino et al., 2004; Rose et al., 2003; Zubieta et al., 2001), as smoking significantly increases the risk of atherosclerosis (Bolego et al., 2002) and nicotine affects the vascular endothelial function (Hawkins et al., 2002). These and other smoking effects may be long-lasting, and, thus, adversely affect perfusion recovery in sALC (for details on the mechanisms of the effects of cigarette smoking on the human brain, see (Durazzo et al., 2007)).
The differential recovery from perfusion deficits in nsALC and sALC with abstinence from alcohol may have implications for the interpretation of functional MRI (fMRI) studies. Hypoperfusion is accompanied by reduced oxygen supply, which can in turn reduce the blood oxygen level-dependent (BOLD) signal. Thus, in fMRI of alcohol dependent individuals, and particularly of smokers, care should be taken when interpreting low BOLD signal, as it may not necessarily reflect reduced neuronal activity only.
One interesting observation in this report is that perfusion increased by almost the same amount (9%) in each fGM and pGM in nsALC over one month of abstinence. Since cerebral blood flow and glucose metabolism are coupled (Kuschinsky, 1991; Leybaert, 2005), this suggests regionally similar increases of metabolic activity. However, Smith et al. (Smith et al., 2001), demonstrated partial normalization of glucose utilization in the parietal lobe and no normalization in the frontal lobe of alcohol preferring monkeys deprived of alcohol for two weeks (Smith et al., 2001). They argued that reductions in local cerebral glucose utilization and decreased neuronal activity due to chronic alcohol consumption are not likely secondary to hypercapnia, hypoglycemia or decreased cerebral blood flow, but rather to alterations in synaptic activity. If this argument is also true for humans, then despite the equal amount of perfusion recovery in fGM and pGM observed in this report for nsALC, different glucose utilization levels in the regions may still be apparent due to regional differences in synaptic activity. It is noteworthy that chronic cigarette smoking is associated with significant elevation of carbon monoxide (CO) levels (Deveci et al., 2004). This increase is associated with decreased effective hemoglobin concentrations, diminished oxygen carrying capacity of erythrocytes (Macdonald et al., 2004), as well as a diminished efficiency of the mitochondrial respiratory chain (Alonso et al., 2004). All these factors may work in concert to alter cellular bioenergetics, and, correspondingly synaptic activity. Reduced concentrations of N-acetyl aspartate, a marker of neuronal viability and mitochondrial function, in the frontal lobes of alcoholics, smoking and non-smoking (Durazzo et al. 2004), as well as differential recovery from these reduced metabolite concentrations (Durazzo et al. 2006) are consistent with our perfusion findings and their implications.
Finally, perfusion in both fGM and pGM were not related to any cognitive domain at either TP1 or TP2, and changes in perfusion between the two time-points did not relate to changes in the cognitive measures over the assessment interval. In our previous cross-sectional MR perfusion study (Gazdzinski et al., 2006) we also observed that perfusion was not correlated with neurocognition in alcoholics. However, in our previous MR longitudinal spectroscopy and neurocognition study (Durazzo et al., 2006), we found that at approximately one month of abstinence from alcohol, increased concentrations of N-acetyl aspartate and choline-containing metabolites in GM or WM of several brain regions of nsALC were associated with better neurocognition (though this was not observed in the sALC group). Thus, the lack of correlations between our perfusion measures and neurocognition may be due to the somewhat large within-subject variability of MRI perfusion measures. It may also reflect that the cognitive domains assessed in this study are likely sub-served by additional functional circuits than those imaged in the present study.
One limitation of this study is that we had no access to light drinkers who smoked at the same level as our alcoholic participants. Inclusion of such a group would have enabled us to assess potential main effects or interactions of cigarette smoking on perfusion. Also, the potential effects of gender on perfusion could not be evaluated due to the small number of women in the cohort, which was recruited at a Veterans Administration Medical Center. Furthermore, we did not formally screen participants for axis II disorders, such as subclinical levels of anti-social personality disorders known to be associated with AUD (Grant et al., 2004; Pridmore et al., 2005). Lastly, we could not account for potential unrecorded group differences in nutrition, exercise, overall physical health and genetic predispositions. Thus, prospective studies, which have a comparable number of females and also equate LD and ALC on measures of smoking severity, are necessary to evaluate comprehensively the effects of chronic smoking and gender on cerebral perfusion in ALC.
In conclusion, we demonstrate the utility of arterial spin labeling MR methods to measure regional cerebral perfusion changes in cigarette smoking and non-smoking short-term abstinent alcohol dependent individuals. In this cohort, regional cortical perfusion normalized in nsALC over five weeks of abstinence from alcohol, whereas perfusion abnormalities in sALC did not improve over the same time. This pattern of perfusion recovery in sALC and nsALC is consistent with our other longitudinal studies (Durazzo et al., 2006; Durazzo et al., 2007; Gazdzinski et al., 2008), in which sALC demonstrated significantly lower magnitudes of recovery of regional brain metabolite concentrations. It is uncertain whether these chronic cigarette smoking effects are related to the cumulative effects of many years of smoking or to continued smoking during abstinence from alcohol, or both. To fully understand the dynamics of cerebral perfusion changes and their functional relevance in abstinent alcoholics, extended longitudinal perfusion studies coupled with repeat neurocognitive assessments are necessary. Nevertheless, this perfusion study provides additional evidence that suggests that chronic smoking in alcohol dependence adversely affects neurobiological recovery during abstinence from alcohol.
Acknowledgments
This research was supported by AA10788 (DJM) and by the Radiology Research Service of the Veteran's Administration Medical Center.
Contributor Information
Anderson Mon, Department of Radiology, University of California, San Francisco and Center for Imaging of Neurodegenerative Diseases, Veterans Administration Medical Center San Francisco
Timothy C. Durazzo, Department of Radiology, University of California, San Francisco and Center for Imaging of Neurodegenerative Diseases, Veterans Administration Medical Center San Francisco
Stefan Gazdzinski, Center for Imaging of Neurodegenerative Diseases, Veterans Administration Medical Center San Francisco
Dieter J. Meyerhoff, Department of Radiology, University of California, San Francisco and Center for Imaging of Neurodegenerative Diseases, Veterans Administration Medical Center San Francisco
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Alonso JR, Cardellach F, Casademont J, Miro O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur Respir J. 2004;23(2):214–8. doi: 10.1183/09031936.03.00038203. [DOI] [PubMed] [Google Scholar]
- Axelrod BN, Ryan JJ, Ward LC. Evaluation of seven-subtest short forms of the Wechsler Adult Intelligence Scale-III in a referred sample. Arch Clin Neuropsychol. 2001;16(1):1–8. [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Center for Cognitive Therapy; Philadelphia: 1978. [Google Scholar]
- Bolego C, Poli A, Paoletti R. Smoking and gender. Cardiovasc Res. 2002;53(3):568–76. doi: 10.1016/s0008-6363(01)00520-x. [DOI] [PubMed] [Google Scholar]
- Cala LA, Jones B, Mastaglia FL, Wiley B. Brain atrophy and intellectual impairment in heavy drinkers--a clinical, psychometric and computerized tomography study. 1978;8:147–153. doi: 10.1111/j.1445-5994.1978.tb04502.x. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138(2):115–30. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Deveci S, Deveci F, Acik Y, Ozan A. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respiratory Medicine. 2004;98:551–56. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):319–27. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Rourke SB, Grant I, Lehr PP, Reed RJ, Challakere K, Lamoureux G, Halpern S. Single photon emission computed tomography with iodoamphetamine-123 and neuropsychological studies in long-term abstinent alcoholics. Psychiatry Res. 1996;67(2):99–111. doi: 10.1016/0925-4927(96)02769-2. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28(12):1849–60. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Brain metabolite concentrations and neurocognition during short-term recovery from alcohol dependence: Preliminary evidence of the effects of concurrent chronic cigarette smoking. Alc Clin Exp Research. 2006;30(3):539–51. doi: 10.1111/j.1530-0277.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol Clin Exp Res. 2007;31(7):1114–27. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Erbas B, Bekdik C, Erbengi G, Enunlu T, Aytac S, Kumbasar H, Dogan Y. Regional cerebral blood flow changes in chronic alcoholism using Tc-99m HMPAO SPECT. Comparison with CT parameters. Clin Nucl Med. 1992;17(2):123–7. doi: 10.1097/00003072-199202000-00012. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1991;69:763–5. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 revision) Biometrics Research Department; New York, NY: 1998. [Google Scholar]
- Gazdzinski S, Durazzo T, Jahng GH, Ezekiel F, Banys P, Meyerhoff D. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol Clin Exp Res. 2006;30(6):947–58. doi: 10.1111/j.1530-0277.2006.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162(2):133–45. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63(3):277–86. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test. Stoelting Company; Chicago, IL: 1978. [Google Scholar]
- Grant B, Stinson F, Dawson D, C P, Dufour M, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurance of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61:807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Oscar-Berman M, Gansler A, Streeter C, Lewis RF, Ahmed I, Achong D. Hypoperfusion of the cerebellum and aging effects on cerebral cortex blood flow in abstinent alcoholics: a SPECT study. Alcohol Clin Exp Res. 1999;23(7):1219–27. [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Brown RC, Davis TP. Smoking and ischemic stroke: a role for nicotine? Trends Pharmacol Sci. 2002;23(2):78–82. doi: 10.1016/s0165-6147(02)01893-x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res. 1994;18(4):867–72. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Jagannathan NR, Desai NG, Raghunathan P. Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magnetic Resonance Imaging. 1996;14(5):553–557. doi: 10.1016/0730-725x(96)00048-3. [DOI] [PubMed] [Google Scholar]
- Jahng GH, Song E, Zhu XP, Matson GB, Weiner MW, Schuff N. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234(3):909–16. doi: 10.1148/radiol.2343031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng GH, Zhu XP, Matson GB, Weiner MW, Schuff N. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49(2):307–14. doi: 10.1002/mrm.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15(3):418–27. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kuschinsky W. Coupling of function, metabolism, and blood flow in the brain. Neurosurg Rev. 1991;14(3):163–8. doi: 10.1007/BF00310651. [DOI] [PubMed] [Google Scholar]
- Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25(1):2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Kondor N, Yousefi V, Green A, Wong F, Aquino-Parsons C. Reduction of carboxyhaemoglobin levels in the venous blood of cigarette smokers following the administration of carbogen. Radiother Oncol. 2004;73(3):367–71. doi: 10.1016/j.radonc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, Danielsen UT, Sorensen H, Paulson OB. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82(2):87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, Herranz R, Monforte R, Cardenal C, Urbano-Marquez A. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34(9):1452–9. [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond J, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: An MRI study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16(6):1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21(3):521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Aubin HJ, Pomerleau OF. Self-reported alcohol use patterns in a sample of male and female heavy smokers. J Addict Dis. 1997;16(3):19–24. doi: 10.1300/J069v16n03_02. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Chambers A, McArthur M. Neuroimaging in psychopathy. Aust N Z J Psychiatry. 2005;39(10):856–65. doi: 10.1080/j.1440-1614.2005.01679.x. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, Shaw TG, Mortel KF. Reductions in regional cerebral blood flow associated with chronic consumption of alcohol. J Am Geriatr Soc. 1983;31:540–543. doi: 10.1111/j.1532-5415.1983.tb02198.x. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58(2):77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry. 2003;160(2):323–33. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Schroth G, Naegele T, Klose U, Mann K, Petersen D. Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology. 1988;30:385–389. doi: 10.1007/BF00404102. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43(11):1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Long-term effects of alcohol drinking on cerebral glucose utilization in alcohol-preferring rats. Pharmacol Biochem Behav. 2001;69(3-4):543–53. doi: 10.1016/s0091-3057(01)00553-6. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behavioral Assessment. 1990;12:77–90. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49(3):225–32. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Self-Evaluation Questionaire. 1977. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80(1):105–16. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assesment of alcohol withdrawal: the revised clinical institute withdrawl assesment for alcohol scale. Br J Addict. 1989;84:1353–57. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tutus A, Kugu N, Sofuoglu S, Nardali M, Simsek A, Karaaslan F, Gonul AS. Transient frontal hypoperfusion in Tc-99m hexamethylpropyleneamineoxime single photon emission computed tomography imaging during alcohol withdrawal. Biol Psychiatry. 1998;43(12):923–8. doi: 10.1016/s0006-3223(97)00322-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - III (WMS - III) The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Zipursky RB, Lim KC, Pfefferbaum A. MRI study of brain changes with short-term abstinence from alcohol. Alcohol Clin Exp Res. 1989;13:664–666. doi: 10.1111/j.1530-0277.1989.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, Ohl LE, Koeppe RA, Domino EF. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry. 2001;49(11):906–13. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]


