Abstract
To determine structural brain correlates of naming abilities in older adults, we tested 24 individuals aged 56 to 79 on two confrontation-naming tests (the Boston Naming Test (BNT) and the Action Naming Test (ANT)), then collected from these individuals structural Magnetic Resonance Imaging (MRI) and Diffusion Tensor Imaging (DTI) data. Overall, several regions showed that greater gray and white matter volume/integrity measures were associated with better task performance. Left peri-Sylvian language regions and their right-hemisphere counterparts, plus left mid-frontal gyrus correlated with accuracy and/or negatively with response time (RT) on the naming tests. Fractional anisotropy maps derived from DTI showed robust positive correlations with ANT accuracy bilaterally in the temporal lobe and in right middle frontal lobe, as well as negative correlations with BNT RT, bilaterally, in the white matter within middle and inferior temporal lobes. We conclude that those older adults with relatively better naming skills can rely on right-hemisphere peri-Sylvian and mid-frontal regions and pathways, in conjunction with left-hemisphere peri-Sylvian and mid-frontal regions, to achieve their success.
Introduction
While classical aphasiological research identified peri-Sylvian cortical regions in the left hemisphere as crucial to language, brain-imaging techniques have permitted appreciation of brain regions activated for language processes outside those areas in the left hemisphere—especially in more anterior areas-- as well as in the right hemisphere. Not only are posterior as well as anterior regions within peri-Sylvian cortex involved in lexical retrieval, (e.g., Graves et al., 2007; Okada and Hickok, 2006); naming tasks can also be shown to involve left-hemisphere anterior cingulate gyrus and mid-frontal gyrus (Brodmann areas 46 and 9) in addition to Broca’s area (Brodmann areas 44 and 45; Abrahams et al., 2003). Moreover, when Fridriksson et al. (2006) studied the intensity of activation in cortical areas in adults ranging in age from 20 to 82 performing a picture-naming task, they found activation in the right-hemisphere counterpart to Broca’s area in addition to the expected activation in the traditional Broca’s and Wernicke’s areas of the left hemisphere.
What is unclear in studies of elderly individuals like that of Fridriksson et al. is the extent to which these additional regions implicated in naming are compensating for the atrophy that increases with advancing age. Structural imaging has shown age-related degradation of gray and white matter which particularly affects frontal and temporal regions (e.g., Raz, 2000; Raz et al., 1998; Resnick et al., 2003; Sullivan and Pfefferbaum, 2006). However, it appears that language performance does not decline proportionally to this atrophy (Park et al., 2002). We must assume, then, that other brain regions are involved in compensating for it, and it is plausible that the left prefrontal regions and bilateral language-area regions are called on to compensate (e.g., Cabeza, 2002; Reuter-Lorenz, 2002).
To address this issue, Wierenga et al. (2008) studied overt naming of animals, tools, and vehicles in 20 younger (mean age 25.1) and 20 older (mean age 74.9) adults via event-related functional magnetic-resonance imaging (fMRI). Both groups performed at relatively high accuracy on this naming task. However the older adults recruited substantially more brain regions to perform the task. Most crucially, they showed the same regions for left-hemisphere activation that the younger adults showed. Their activation was greater in both medial and lateral frontal cortex bilaterally. As these older adults performed bimodally on the naming task, the authors then divided the group into ‘high’ (scoring above 90) and ‘low’(scoring below 87) older namers, and noted, further, that the better namers showed greater activation of the pars orbitalis region of the right inferior frontal gyrus.
Bilateral involvement has been evidenced for other naming tasks as well: Ellis et al. (2006) report bilateral frontal, parietal and mediotemporal activation during silent object naming (in young and middle-aged adults, aged 23–52), along with activation in left lingual and fusiform gyri. In their fMRI study of performance on a verb-generation task, Persson et al. (2004) reported that right inferior frontal gyrus was over-activated in older adults (aged 60–80), relative to younger ones (ages 18–30). In addition, Persson and colleagues observed underactivation of left frontal and inferior temporal gyri as well as anterior cingulate in their older participants, particularly for the task that required more challenges for selecting among possible responses, specifically among those older participants who performed more slowly on the task. They conclude that older adults recruit right-hemisphere areas and left-hemisphere areas beyond the traditional peri-Sylvian region to compensate for their increasing difficulties performing the task.
However, not all studies of language in older adults report right-hemisphere compensation; as Kan and Thompson-Schill (2004) point out, brain-region sites reported to activate in response to naming tasks are inconsistent across the studies. Such inconsistencies may arise from task differences of various types. Some authors deliberately choose high-frequency items for their naming task to assure participants name successfully; others deliberately choose lower frequency items in addition, or instead. Two naming tasks are frequently employed in imaging tests (though only one in a given study, as a rule): confrontation naming or list-generation. A third test, more similar to confrontation naming in that participants must come up with a single correct word, is that of responsive naming, in which a definition is read to participants who must then name the correct word. Tomaszeswki Farias, Harrington, Broomand, and Seyal (2005) report that those of their participants (aged 28 to around 50) who were tested on a confrontation naming task displayed activation only in left-hemisphere middle temporal gyrus, whereas activation for responsive naming was seen in superior and inferior temporal gyri as well. Moreover, the responsive naming task evoked anterior temporal activation in more participants (90%) than did confrontation naming (60%).
Such task-related differences in activation may be explained by a model that Wingfield and Grossman (2006) propose, albeit for the variability of findings for brain activation during sentence-comprehension tasks in aging. They posit ‘core’ regions involved in a language task and ‘support’ or ‘resource’ regions -- such as those subserving working memory in the case of sentence comprehension—that interact with them. The core network (they use the terms ‘region’ and ‘network’ interchangeably) is the one which brain-damage has revealed to be necessary for the task. The support regions, by contrast, are those additional regions that neuroimaging studies reveal in healthy adults that are outside the traditional language areas: various regions of frontal lobe and cingulate gyrus bilaterally, inferior temporal lobe on the left, etc. (e.g., Caplan et al., 2000; Friederici, 2002; Luke et al., 2002).
In a series of studies from the labs of Grossman and Wingfield, there is evidence of greater reliance on these support networks for tasks that involve sentence processing, including language-comprehension, with advancing age (e.g., Peelle et al., 2004; Cooke et al., 2002, 2006). This is of interest, they remind us, because, except in stressed situations such as listening to speeded speech or syntactically complex materials, older adults’ sentence comprehension may be spared (Nicholas et al., 1985; Wingfield et al., 2003, 2006; Goral et al., submitted). Situations in which listening conditions are stressful are, nevertheless, relatively common in daily life (e.g., listening to synthetic-speech choices when calling ‘service’ numbers, conversing in noise); a number of studies focus on age-related decline in comprehension in non-ideal conditions, even at the sentence level, rather than in the simpler conditions in which older adults’ comprehension remains at ceiling level. It is, then, all the more interesting to note the conclusions of Wingfield and Grossman, that even when older adults are performing well on comprehension tasks, they are employing more language areas than younger adults in doing so: right hemisphere counterparts to posterior lateral temporal cortex and dorsal portion of the left inferior frontal cortex areas and left hemisphere of dorsolateral prefrontal cortex.
In this study we ask a similar question concerning naming tasks: How precisely is better naming in older adults associated with broader (or different) areas than poorer naming? Naming problems, of course, are even more commonly complained of with advancing age –and at earlier ages-- than are comprehension problems. A substantial body of research has documented such naming problems via behavioral tasks, both confrontation naming ones (e.g., Au et al., 1995; Barresi et al., 2000; Burke et al., 1991; Connor et al., 2004, Goral et al., 2007; Kaplan et al., 1983; Nicholas et al., 1985, see Feyereisen, 1997, for a meta-analysis) and list-generation ones (Boone et al., 1990; Parkin & Walter, 1991; Pekkala et al., 2009; Troyer et al., 1997; Whelihan & Lesher, 1985). Most curious to us, in the series of studies from our Language in the Aging Brain Laboratory, is how some older participants, in their 70s and above, perform as well as some 30-year olds, even while peak performance on confrontation naming, for example, occurs in the late 30s, with accelerating decline into oldest adulthood. That there is increasing variability in performance on naming tasks with advancing age is not, in and of itself, surprising; a substantial literature documents increasing range of performance across all cognitive tasks. Rather, what remains of interest is determining what accounts for the age-related variability, since age per se is not a satisfactory explanation. In other studies we demonstrate the contributions of demographic factors such as education and gender to age-related naming performance (e.g., Goral et al., 2007) and of health-related factors such as hypertension (Albert et al., 2009; Spiro et al., 2008).
Asking about language and non-language regions recruited for naming is not sufficient, however. While there is substantial research on such “processing centers”, only recently have questions been asked concerning the connectivity among them that in fact drives language performance. For example, there are reports that additional bilateral and prefrontal recruitment predicts better performance in older adults on executive function tasks, but such evidence is inconsistent (see Langenecker and Nielson, 2003; Nielson et al., 2002). The fact that such compensatory mechanisms are not always effective may be explained by impaired communication between frontal regions and “core” peri-Sylvian language regions. In fact, Stamatakis and Tyler (2006) infer reduced fronto-temporal connectivity in functional networks among their elderly participants performing a morphological processing task.
In the current study, then, we asked what cortical regions and associated connective tracts were associated with better or worse naming in older adults. We employed structural magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) in order to address which pertinent brain areas and which connective tracts are available for processing to better older namers. Specifically, voxel-based morphometry provides local measures of white matter volume/density, whereas DTI-based fractional anisotropy (FA) is thought to reflect the amount of organization within the white matter. White matter density reduction is related to macroscopic changes in white matter, such as lesions and atrophy (Ge et al., 2002; Meier-Ruge et al., 1992). FA reduction has been linked to axonal demyelination and loss (Bartzokis, 2004). Although considered more sensitive than structural MRI-derived measures ( Hugenschmidt et al., 2008), FA alone is not a reliable indicator of white matter integrity: brain regions shared by major fiber tracts traveling in orthogonal directions will show dramatically reduced FA.
We employed tests of both nouns (Boston Naming Test –BNT, Kaplan, Goodglass & Weintraub, 1983) and verbs (Action Naming Test—ANT, Obler and Albert, 1986) because, while performance on these two tests is seen to decline in remarkably parallel fashion with advancing age (e.g., Goral et al., 2007), a substantial body of literature (e.g., Shapiro et al., 2006; Cappelletti et al., 2008) suggests differential engagement of frontal and temporal regions in processing them.
Methods
Participants
Our 24 participants were adults aged 56 to 79 who participated in our Language in the Aging Brain project. They volunteered for two days of language, cognitive, and health testing. These test sessions were conducted within six weeks of each other. From these sessions, the two tests discussed in this paper were administered on separate days: the BNT task during Visit 1 and the ANT task during Visit 2. Participants in the present study were then qualified to undergo an hour of MRI scanning on a third day, for additional pay. The imaging data were collected in one or two sessions within six months after the behavioral sessions, generally in a single day. Collection of the structural MRI scans required approximately ten minutes per scan (20 minutes total), and DTI data collection required approximately three minutes per scan (six minutes total). Of the 24 participants tested, eighteen had both DTI and MRI available. Three additional participants had only MRI but not DTI data available, and a different three had only DTI. Since in this study we do not perform any direct comparisons of DTI and MRI analyses results, we chose to report analyses of larger (N=21 for each of MRI and DTI datasets) samples rather than those of the full multimodal sample of 18. Demographically these samples were quite similar, as Table 1 demonstrates.
Table 1.
Demographics of the two samples
| MRI sample | DTI sample | |
|---|---|---|
| N | 21 | 21 |
| Age | 68(6.4) | 68(7.1) |
| Gender | 12 males | 12 males |
| Education | 16 (1.8) | 15 (2) |
Age and education in this table are provided as average (standard deviation).
Age, gender and education sample characteristics (averages and standard deviations) of the overlapping MRI and DTI groups are reported in Table 1. The participants studied were all native speakers of English with corrected vision (if necessary) who ranged in education level from 12 to 17+years. Written informed consent was obtained from all participants for behavioral and medical testing according to the IRBs of both Boston University School of Medicine and the Veterans Affairs Boston Healthcare System.
Lexical-Retrieval Tasks
Two lexical-retrieval tasks were employed to measure naming ability: the Boston Naming Test (BNT, Kaplan, Goodglass & Weintraub, 1983) and the Action Naming Test (ANT, Obler and Albert, 1986). We administered computerized versions of the 60-item BNT and the 55-item ANT using E-Prime software (Psychology Software Tools, Inc., 2001). High-resolution black-and-white drawings of the objects or activities were used as stimuli. Participants sat comfortably before a computer screen, where the pictures appeared one at a time, in standard administration order. A microphone was placed nearby but out of the line of sight of the computer screen. After several practice items, the task began. Participants were asked to name each picture as it appeared, using only one word, avoiding the use of words like “a” or “an,” and minimizing other noises (to collect accurate response times). Participants were given up to 20 seconds to make a response. If the participant did not respond or the response suggested a visual misperception, the examiner provided the standard semantic cue. Responses given immediately following a semantic cue were considered correct. If the response was otherwise incorrect or if the semantic cue was insufficient, the examiner offered a phonemic cue. For each trial, the participant received an item-level score (0 for incorrect, 1 for correct). For the purposes of this paper, only items correctly named before a phonemic cue were regarded as correct. Percentages correct were employed due to the different numbers of items in the two tests, and to correct for the rare administration error.
Response time (RT) was measured for correct responses only, and trimmed such that values greater (in the absolute value) than 2.5 SD from the individual mean were replaced by the individual mean plus or minus 2.5 SD, correspondingly.
Imaging Measures
Structural MRIs
The primary indices of gray matter volume, as well as of white matter density, were measured from T1-weighted MR images of the brain. Two high-resolution T1-weighted scans were collected from each participant using 3 Tesla Philips Intera (Philips Medical Systems, Cleveland, Ohio) with the following image parameters: TR = 7.3, TE = 3.2 or 3.0, flip angle = 7°, slice thickness = 1.3 mm, 128 slices, FOV = 256×256 mm. These scans were set up to optimize for high contrast between gray, white matter and cerebrospinal fluid. The two T1 scans were first motion corrected and averaged to create a single high signal, high contrast volume.
Gray matter volume analysis
The primary gray matter analysis procedures have been described in detail and applied and validated in a number of publications (Dale, Fischl, & Sereno, 1999; Fischl, et al., 2002; Fischl, et al., 2004; Fischl & Dale, 2000; Fischl, Liu, & Dale, 2001; Fischl, Sereno, & Dale, 1999; Salat, et al., 2004). In this study, the T1 images were analyzed using computerized reconstruction of the cortical surface (http://surfer.nmr.mgh.harvard.edu) to produce volume measurements for predefined cortical and subcortical regions. This analysis involves a multistep procedure that includes intensity normalization, stripping of non-brain tissue, and gray and white matter segmentation. The distance between the gray/white matter boundary and the outer cortical surface was used to calculate cortical thickness at each point across the cortical mantle (Rosas, et al., 2002; Salat, et al., 2004). Following cortical reconstruction and segmentation, an algorithm was implemented that automatically assigned a neuroanatomical label to each voxel in an MR volume based on a probabilistic atlas of class statistics derived from a manually labeled training set (Fischl, et al., 2002; Fischl, et al., 2004). This technique has been shown to be comparable in accuracy and reliability to manual labeling in normal brains (Fischl, et al., 2002) and has been used to study performance-structure correlates in aging brains (Fjell, et al., 2005). Volumetric measurements were automatically calculated from parcellated cortical regions that were labeled utilizing a predefined atlas (These included regions reported in Table (Summary of Significant Regression Coefficients) such as Mid-temporal gyrus, Angular gyrus,; for a complete list of regions see: Desikan, et al., 2006).
The volumetric measures obtained for each of the labeled cortical regions were used to construct a set of multiple linear regression models examining whether the volume of a region predicts performance on a naming task, while controlling for age, education and gender. In these regression models, measures of accuracy (ACC) and reaction time (RT; correct trials only) on each test, the ANT and the BNT, taken one at a time, served as the predicted task performance variables. Age, gender and education, by contrast, entered each model as a set of covariates. More specifically, we conducted 4 sets of regressions, one for each outcome (ACC or RT) for each test (ANT, BNT). In each set, we included age, gender, and education as covariates (regardless of their significance), and examined the impact of adding each brain region separately. For each outcome, we conducted 30 such regressions: 4 overall volumes (L & R hemisphere; white and gray matter) and 26 ROI’s (L & R hemispheres for 13 specific areas). To adjust in part for the large number of regressions run, we used p<0.01 as a cutoff. Linear regression coefficients (unstandardized) for predictors of interest were examined for statistical significance (indicated by p-value, see Table (Summary of Significant Regression Coefficients) where p values less than .01 are bolded) and the direction of effect (positive or negative sign of coefficient estimates. Note that, in Table, we report only on BNT RT and ANT accuracy, as that is where the significant findings arose).
White matter density analysis
White matter density analysis was conducted using an optimized voxel-based morphometry (VBM) protocol (Good et al., 2001) with a toolbox for SPM5 (Wellcome Department of Cognitive Neurology, 2005) by Christian Gaser (University of Jena, Department of Psychiatry; http://dbm.neuro.uni-jena.de/vbm/). T1 images were initially segmented into white matter, gray matter and cerebro-spinal fluid images, which were then averaged to create study-specific templates for the three tissue types. These templates were normalized to the standard MNI space and then used as a reference for spatial normalization (nonlinear registration) and as priors for final tissue segmentation. Additional morphological operators were applied to improve the quality of segmented images by removing non-connected parts of the segmentation.
The resulting 3D maps of local white matter density were used as predicted variables to create a set of multiple linear regression models similar to those described for the volumetric gray matter analysis. Naming task performance measures were used as a predictor, one at a time, in four sets of models, while age, gender and education entered the models as covariates. The resulting 3D maps of regression coefficients underwent statistical inference procedures implemented in SPM5, showing the clusters of voxels where white matter density was significantly correlated with behavioral measures while controlling for age, gender and education. Two p-values were chosen: cluster height threshold was set at .05, with no correction for multiple comparisons. In addition, cluster extent-based thresholding was employed, p<.05, with family-wise error (FWE) correction for multiple comparisons. The combination of these thresholding strategies is common in VBM studies to ensure a principled yet not overly conservative approach.
DTI measures
Diffusion-weighted images were collected during a separate imaging session with the following parameters: pulse sequence = Single Shot SE-EPI, TR=10646ms, TE =91ms, b-value = 1000sec/mm2, imaging resolution = 1.8 × 1.8 × 2.0mm3, number of diffusion directions = 15, number of axial slices = 73. Two scans with identical parameters were collected, then motion-corrected and averaged to create a single high signal, high contrast volume. Further preprocessing included skull removal and eddy current correction. Tensor estimates were reconstructed, and fractional anisotropy (FA) computed in every voxel using FMRIB’s Diffusion Toolbox v (FDT; http://www.fmrib.ox.ac.uk/fsl/fdt).
Tract-based spatial statistics
A whole brain assessment of individual differences in FA was performed using the tract-based spatial statistics approach (TBSS; Smith et al., 2006). TBSS uses nonlinear registration to project FA images from multiple subjects into an align-invariant tract representation (the “mean FA skeleton”), which improves the sensitivity of group analyses as well as spatial localization of effects. With this analysis, the search of effects is restricted to the brain areas of high average (across the participants) FA, which is usually observed along major fiber pathways. Individual FA data projected onto the mean FA skeleton were fitted with the same multiple linear regression model as described above for gray matter volumetric and white matter density data. Parameter estimation and the subsequent statistical inference were performed in SPM5. The resulting GLM parameter estimate maps were thresholded to achieve height p-value of .05 (no correction for multiple comparisons) plus cluster-wise statistical significance of .05 with FWE correction for multiple comparisons.
Results
Behavioral data: Naming performance and age
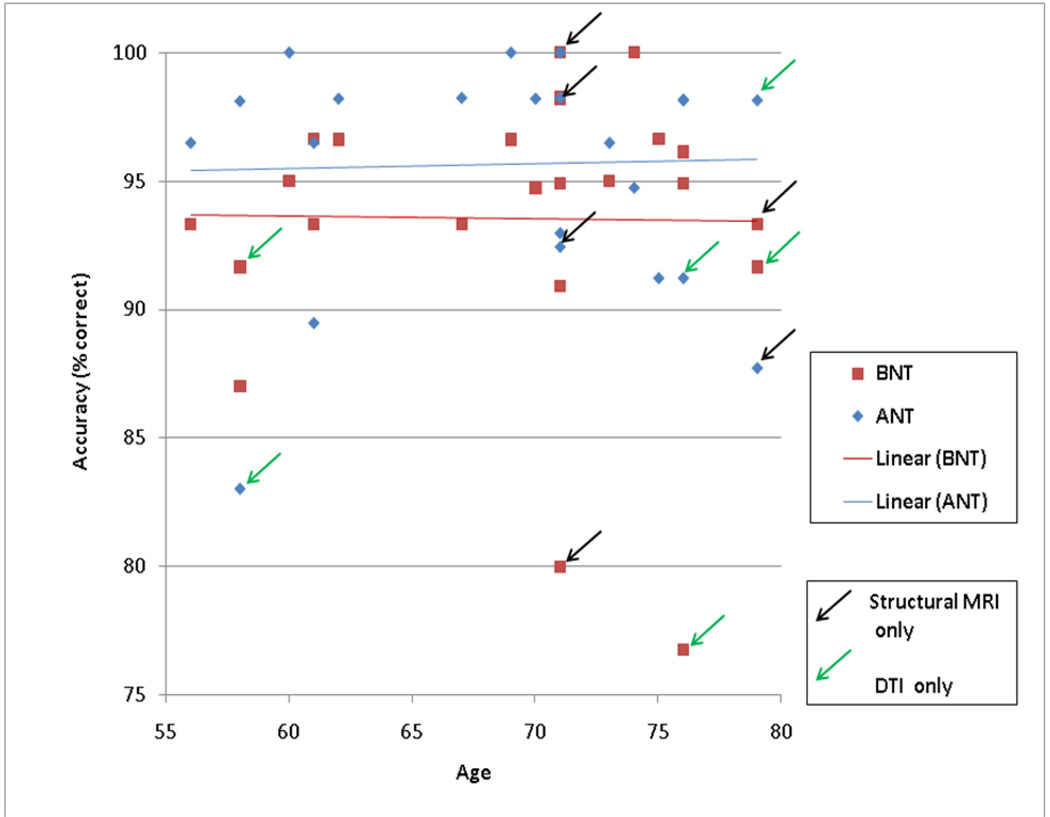
Accuracy and response time for the BNT and ANT tasks are presented in Table 2, also scatter plots of accuracy and response-time versus age for each of the two tasks are presented in Figure 1 and Figure 2. Note that, unlike in our prior studies that include younger adults, for this small group of older adults, a correlation between age and task performance is not evident (For BNT accuracy, BNT RT, ANT accuracy, and ANT RT we obtained correlation values (N=24) of −0.01 (p=.47), 0.13 (p=.27), 0.03 (p=.44), −0.03 (p=.44), respectively).
Table 2.
Response Time and Accuracy of BNT and ANT
| BNT RT (ms) |
BNT %correct |
ANT RT (ms) |
ANT %correct |
||
|---|---|---|---|---|---|
| DTI 21 | Mean | 1,211.67 | 94.52 | 1,271.95 | 96.36 |
| SD | 242.52 | 4.33 | 202.99 | 3.51 | |
| MRI 21 | Mean | 1,227.20 | 93.98 | 1,320.17 | 95.96 |
| SD | 218.01 | 4.85 | 245.83 | 4.16 |
Figure 1.
Accuracy and Age on Naming Tasks
Figure 2.
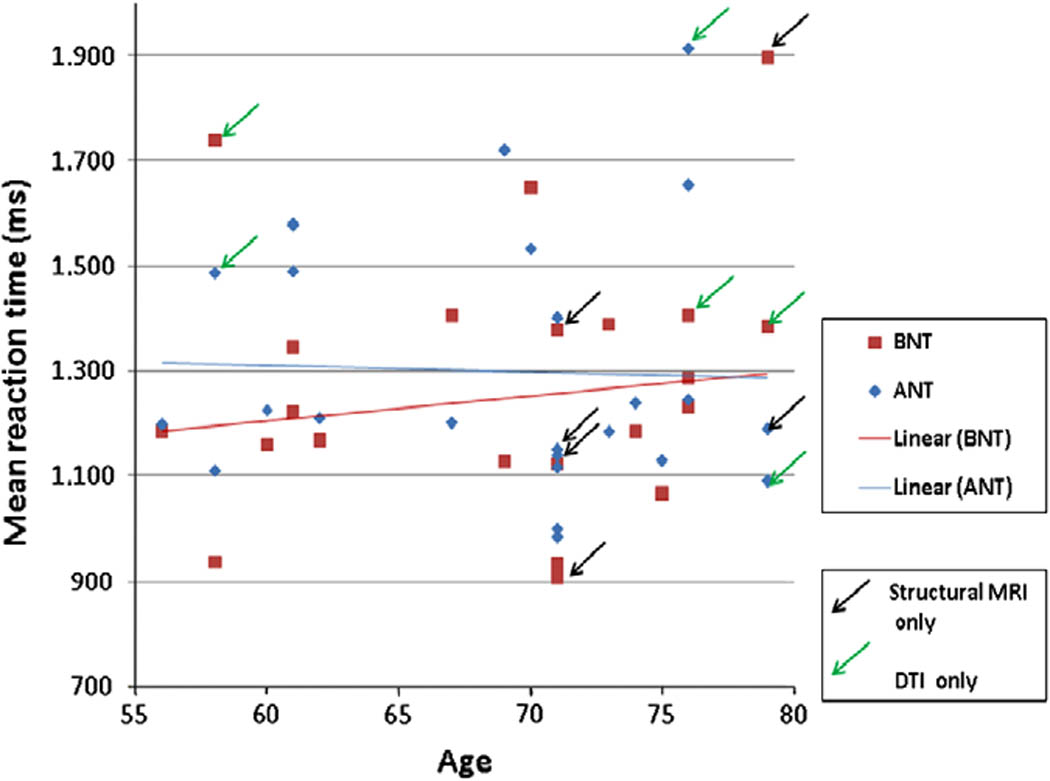
RT and Age on Naming Tasks
Gray matter volume
While there was not full overlap of cortical regions associated with good naming across the two tasks, inspection of Figure 3 and Figure 4 (which portray the information in Table 3) indicates that
-
-
volumes of the left mid-frontal gyrus, right angular gyrus, and right middle temporal gyrus are correlated with accuracy on the ANT.
-
-
volumes of the left mid-frontal gyrus and left planum temporale are negatively correlated with response time for correct trials on the BNT (i.e., those with more volume are, on average, faster).
Figure 3. Gray matter volumes correlated with BNT RT.
Flattened cortex visualization of the ROIs for which gray matter volume negatively correlated with BNT reaction times. Visualization was performed with FreeSurfer Software tools.
Blue - left middle frontal gyrus
Red - left planum temporale
Figure 4. Gray matter volumes correlated with ANT accuracy.
Flattened cortex visualization of the ROIs for which gray matter volume negatively correlated with ANT accuracy.
- Blue - middle frontal gyrus / Green - Inferior temporal gyrus
- Blue - middle frontal gyrus /Red - superior frontal gyrus /Green - angular gyrus / Yellow – opercularis / Teal - planum polare / Orange - middle temporal gyrus
Table 3.
Summary of Significant Regression Coefficients for Brain Region on Language Measure (shown bold for p < .01)
| Language measure |
Brain region (predictor) | Coefficient | Predictor p-value |
F statistic (5, 16 df) |
Model p-value |
R2 | |
|---|---|---|---|---|---|---|---|
| ANT ACC | Left Hemisphere |
MFG volume | 0.00272 | 0.00092 | 5.91 | .00406 | 0.596 |
| ITG volume | 0.00186 | 0.01372 | 3.23 | .04002 | 0.447 | ||
| Right Hemisphere |
IFG – opercularis volume | 0.00508 | 0.01424 | 3.20 | .04124 | 0.445 | |
| MFG volume | 0.00235 | 0.01410 | 3.21 | .04091 | 0.445 | ||
| SFG volume | 0.00098 | 0.03345 | 2.55 | .07994 | 0.389 | ||
| Angular gyrus volume | 0.00471 | 0.00208 | 5.00 | .00827 | 0.556 | ||
| MTG volume | 0.00258 | 0.00037 | 7.05 | .00180 | 0.638 | ||
| Planum polare volume | 0.00633 | 0.01126 | 3.40 | .03415 | 0.459 | ||
| BNT RT | Left Hemisphere |
MFG volume | −0.12977 | 0.01674 | 5.07 | .00782 | 0.559 |
| Planum temporale volume | −0.32403 | 0.00680 | 6.06 | .00365 | 0.602 | ||
(Note: We report only on BNT RT and ANT accuracy, as that is where the significant findings arose. The coefficients are unstandardized regression coefficients. Models are adjusted for age, years of education, and gender.)
White matter density
For each of four behavioral measures (ANT/BNT reaction time and accuracy) white matter density was correlated with better performance, that is, subjects with greater white matter density tend to have greater accuracy and faster reaction times. Clusters of voxels with significant regression coefficients, displayed in Figure 5, were found in temporal, frontal, parietal, occipital lobes and even brainstem. No voxels showed significantly greater white matter density in poorer compared to better namers.
Figure 5. White matter density and performance on naming tasks.
First row: ANT: Linear regression coefficients relating accuracy (left) and reaction time on correct trials (right) with local white matter density.
Second row: BNT: Linear regression coefficients relating accuracy (left) and reaction time on correct trials (right) with local white matter density.
Height threshold (uncorr.) and cluster extent threshold (FWE corrected) both at p=.05.
DTI results
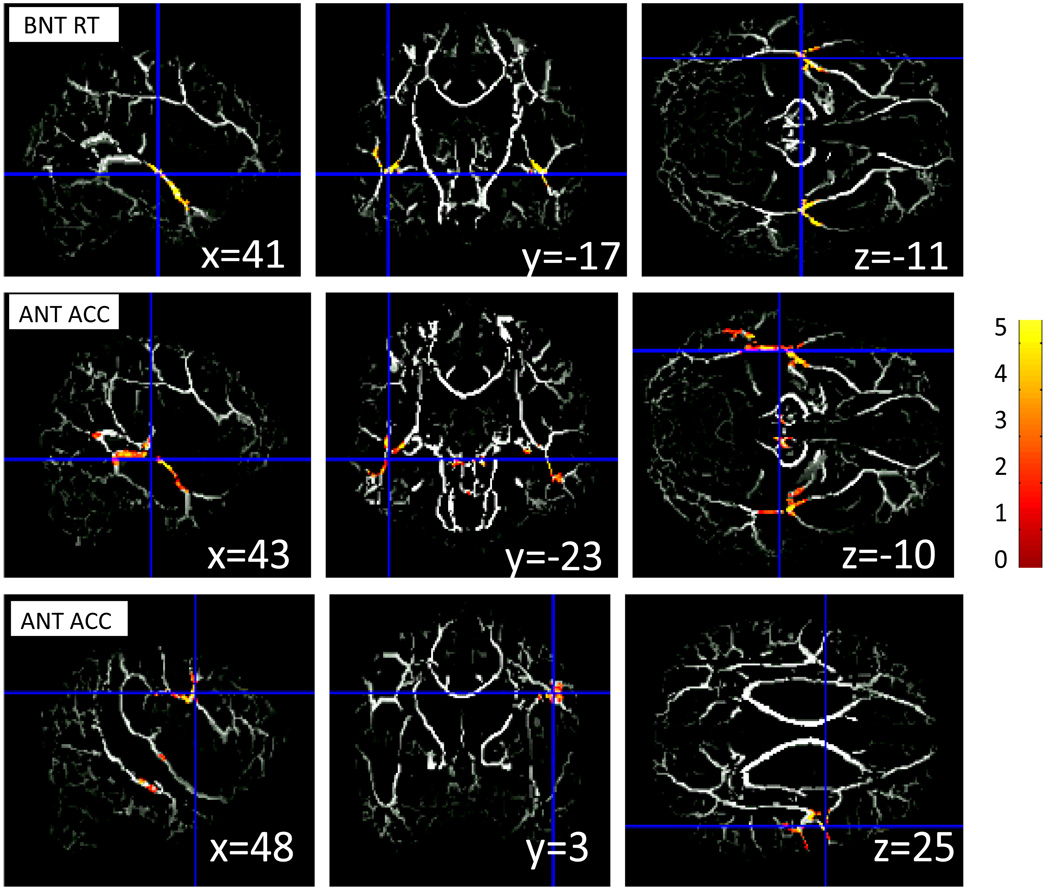
Several areas within the mean FA skeleton showed robust positive correlations with accuracy on ANT, as well as a negative correlation with reaction time on BNT (Table 4). For ANT accuracy, clusters of significant correlations were found in the white matter of middle temporal and inferior temporal gyri (bilaterally), cortico-cerebellar projections (bilaterally), right middle frontal gyrus, and the left posterior splenium of corpus callosum. For BNT reaction times, significant clusters were shown bilaterally in the white matter within middle temporal and inferior temporal gyri. These clusters projected onto the mean FA skeleton are displayed in Figure 6. Within the mean FA skeleton, there were no regions that showed an increase in FA related to poor performance on naming tasks.
Table 4. FA and performance on behavioral tasks: regions showing significant correlations.
Clusters of voxels showing significant correlation between good performing (high for accuracy, low for reaction time) and fractional anisotropy. STATISTICS: p-values adjusted for search volume.
| P-value corrected |
Cluster extent (voxels) |
Maxima x,y,z (mm) |
Anatomical label |
JHU White-Matter Tractography Atlas label (probability of observing given fiber group at the maxima) |
||
|---|---|---|---|---|---|---|
| ANT ACCURACY** | ||||||
| 0.001 | 1013 | 6 | −36 | −31 | Brainstem (bilaterally) |
18% Anterior thalamic radiation R, 3% Corticospinal tract R |
| −7 | −38 | −26 | 16% Anterior thalamic radiation L, 3% Corticospinal tract L | |||
| 0 | −26 | −19 | 3% Anterior thalamic radiation R, 3% Anterior thalamic radiation L | |||
| 0.001 | 495 | 49 | −2 | 23 | Right MFG | 29% Superior longitudinal fasciculus R, 3% Superior longitudinal fasciculus (temporal part) R |
| 0.015 | 352 | −27 | −66 | 14 | Splenium of CC |
37% Forceps major, 11% Inferior fronto-occipital fasciculus L, 8% Inferior longitudinal fasciculus L |
| 0.000 | 841 | 44 | −10 | −18 | Right MTG/ITG |
50% Inferior longitudinal fasciculus R |
| 0.000 | 1760 | −36 | −27 | −1 | Left MTG/ITG | 29% Inferior fronto-occipital fasciculus L, 3% Superior longitudinal fasciculus, temporal part L |
| BNT REACTION TIME* | ||||||
| 0.001 | 524 | 43 | −17 | −10 | Right MTG/ITG |
32% Inferior longitudinal fasciculus R, 8% Inferior fronto-occipital fasciculus R |
| 0.000 | 637 | −41 | −8 | −19 | Left MTG/ITG | 42% Inferior longitudinal fasciculus L, 17% Uncinate fasciculus L, 5% Inferior fronto-occipital fasciculus L |
Height threshold: T = 1.75, p = 0.049 (1.000) {p<0.05 (unc.)}
Extent threshold: k = 314 voxels, p = 0.000 (0.050)
Expected voxels per cluster, <k> = 10.955
Expected number of clusters, <c> = 0.05
Expected false discovery rate, <= 0.76
Degrees of freedom = [1.0, 15.0]
FWHM = 2.3 2.3 2.5 mm mm mm; 2.3 2.3 2.5 {voxels};
Volume: 206293; 206293 voxels; 131.1 resels
Voxel size: 1.0 1.0 1.0 mm mm mm; (resel = 12.99 voxels)
Height threshold: T = 1.75, p = 0.049 (1.000) {p<0.05 (unc.)}
Extent threshold: k = 301 voxels, p = 0.000 (0.050)
Expected voxels per cluster, <k> = 10.470
Expected number of clusters, <c> = 0.05
Expected false discovery rate, <= 0.75
Degrees of freedom = [1.0, 15.0]
FWHM = 2.2 2.3 2.4 mm mm mm; 2.2 2.3 2.4 {voxels};
Volume: 206293; 206293 voxels; 137.1 resels
Voxel size: 1.0 1.0 1.0 mm mm mm; (resel = 12.41 voxel)
Figure 6. FA (fractional anisotropy) for performance on naming tasks.
First row: Linear regression coefficient relating FA and reaction time (correct trials) on BNT, projected onto mean skeletonized FA image.
Height threshold T=1.75, p<.05
Cluster extent threshold=314 voxels, p=.05
MNI coordinates are [−41, −17, −11].
Second and third row: Linear regression coefficient relating FA and accuracy on ANT, projected onto mean skeletonized FA image.
Height threshold T=1.75, p<.05 (uncorr.)
Cluster extent threshold=301 voxels, p=.05 (FWE corrected)
Left and right maps show two clusters, MNI coordinates of [−43, −23, −10] and [48, 3, 25], correspondingly.
Discussion
Complementary findings were evident from structural MRI and DTI measures. Both sets of measures suggest that performance on ANT and BNT naming tasks relates somewhat differently to brain volume and connectivity in older adults. Both suggest right-hemisphere regions are available to support good naming. Furthermore, it appears that better older-adult namers, consistent with what Wierenga et al. (2008) and with the Wingfield and Grossman (2006) report for successful comprehenders, may employ prefrontal regions traditionally associated with executive function, anterior to classical frontal-lobe language areas, and right-hemisphere regions contralateral to left-hemisphere language ones in addition to left triangularis and superior temporal-lobe (including planum) regions. Our analyses were focused on location-specific properties of gray matter (volumetric measures) and white matter (fractional anisotropy estimates). In addition, voxel-based morphometry of white matter provided converging evidence of overall white matter density advantage in better namers. The precise localization of white matter density differences is limited here due to the challenges of intersubject alignment of corresponding white matter structures when performing whole-brain spatial normalization.
Regions compensating for age-related lexical-retrieval difficulties
Our findings vis-à-vis cortical regions available for naming success in older adults are, in sum, consistent with the literature suggesting additional recruitment of right-hemisphere regions contralateral to left-hemisphere language areas, involved in a variety of lexical-retrieval and comprehension tasks. Debate over the past two decades concerning whether degree of lateral dominance for language changes with advancing age would seem, thus, consistent with the HAROLD model of Cabeza (2002) in suggesting diminishing hemispheric asymmetry in older adults. Earlier findings of no differences in lateral dominance for strictly language tasks between younger and older adults (e.g., Obler et al., 1984) indicate, however, that rather than language representation itself changing in lateral dominance, it is the cognitive underpinnings that processing language engages that result in apparent changes in lateralization.
In addition to the prefrontal cortical areas available for recruitment in lexical retrieval of both nouns and verbs with advancing age, our data suggest that an intact system of white matter tracts is useful for successful naming, including strong intra-hemispheric connectivity between the classic left hemispheric anterior and posterior language areas via the left arcuate fasciculus and other white matter pathways, as well as corresponding contralateral connections.
It is worth considering how our findings from normal adults differ from those of individuals with aphasia. In such individuals, there have been reports of both peri-lesional activation during recovery from the aphasia and contralateral activation in the right homologues to language regions, especially to Broca’s area (see review in Saur et al., (2006). However Naeser and her colleagues have reported that repetitive transcranial magnetic stimulation (rTMS) sessions to right hemisphere pars triangularis (Naeser et al., 2005a and b) is linked to increased confrontation naming performance for nouns in individuals with chronic aphasia. They conclude that non-damaged right-hemisphere does not spontaneously compensate for difficulties the damaged left hemisphere has with naming, but, rather that the rTMS, perhaps in suppressing right-hemisphere Broca’s region, “ modulates” the bilateral prefrontal-temporoparietal networks involved in naming pictures. Recall that in our study, the right-hemisphere regions linked to robust naming in older adults are not exclusively in the homologue to Broca’s area, as they are in Fredriksson et al. (2006), though they are in the homologues and contiguous areas to other traditionally core language areas: most crucially they are in right mid-temporal and angular gyri and in planum temporale. All three frontal gyri are implicated, moreover: mid and superior along with inferior. It is conceivable that regions more robust in our better namers are not all required for their better naming. That is, some of those regions may be coincidentally better spared, rather than linked to the better naming. However if they are linked to the better naming, then it appears that non-aphasic older adults employ bilateral frontal regions outside the core language areas as well as substantial peri-Sylvian right-hemisphere areas to aid them in naming.
Such studies engage the interesting question of what, precisely, constitutes the ‘core’ areas for language processing debated hotly in the later 19th and early 20ths centuries and, recently among contemporary scholars like Thompson-Schill and her colleagues interested in the effects of task demands on language processes (e.g., Thompson-Schill et al., 1997; Hirshorn and Thompson-Schill, 2006; Spalek and Thompson-Schill, 2008; Schnur et al., 2009). For the purposes of this paper, we consider those areas that, when impaired, result in aphasia in most individuals –the peri-Sylvian region—to constitute the ‘core’ language areas, whereas regions beyond them in the frontal, temporal (or parietal) lobes of the left hemisphere lie outside the ‘core’ regions, as do all right-hemisphere regions. Scholars of right-hemisphere contributions to language –not only those who study pragmatic deficits associated with right-brain damage, but also those who studied the language abilities of the non-dominant hemisphere of split-brain patients - would beg to differ, of course, as would the ever-increasing numbers of scholars employing imaging techniques to understand brain activation during language processes. If we assume that the regions differentiating better namers, from poorer performers in this study are those providing supplementary contributions to core language areas, then we provide clear evidence that regions outside the core are available to subserve better naming.
Attempts at Converging Evidence
How do we explain the somewhat different patterns we report in the microstructure of white matter and white-matter density resulting from our two complimentary imaging modalities employed to search for converging evidence of brain regions recruited for successful naming? Granted, the two techniques, voxel-based morphometry (VBM) and diffusion tensor imaging (DTI), should provide complimentary data on the integrity and the microstructure of white matter. In healthy elderly, concurrent decreased FA and lower white matter volume are regularly reported in the literature. While the majority of FA reduction is predicted by white matter atrophy, lower FA values are still observed in normally-appearing white matter of healthy elderly, compared to young, subjects: most notably bilaterally in the inferior longitudinal fasciculus (Vernooij et al., 2008). Gray matter volume reduction is usually explained by atrophy/cortical thinning. It has been suggested that atrophy of white matter –rather than loss of grey-matter neurons -- dominates brain tissue loss in aging (Meier-Ruge, Ulrich, Brühlmann, & Meier, 1992). However there are studies that report a tight relationship between gray and white matter loss: e.g., hippocampal atrophy, accompanied with cingulum bundle disruption, is highly correlated to hypometabolism of the posterior cingulate cortex and temporoparietal associative cortex. In particular, studies employing GM (gray matter)-VBM and FA analysis, in healthy elderly subjects (Lehmbeck, Brassen, Weber-Fahr, Braus, 2006), as well as in Alzheimer patients (Ibrahim, et al., 2009), have shown a striking overlap in the results from corresponding gray and white matter areas, suggesting that these might reflect parts of the same network.
We plan to pursue accurate linking of performance-related differences in GM to the white matter microstructure in our research program. In our data, we can only speculate that the reported areas of FA reduction are located within the major tracts linking the gray matter regions. inferior longitudinal fasciculus, inferior fronto-occipital fasciculus and superior longitudinal fasciculus (see Table 4) are longitudinal tracts traveling in anterior-posterior direction and connecting temporal/occipital lobes to prefrontal cortex (see Figure 3–Figure 4).
Noun vs. Verb findings
That we find differences between the performance measures on ANT and BNT (Nicholas et al, 1985), as they are related to brain structure is not surprising. There is now substantial literature reporting differential brain engagement on tasks comparing performance on nouns and verbs (e.g., Caramazza and Hilles, 1991; Damasio and Tranel, 1993; Shapiro and Caramazza, 2003; Oliveri et al., 2004; see Cappelletti et al., 2008, for a recent review of this literature accompanying their report that transcranial magnetic stimulation to left mid-frontal gyrus results in disruption to verb—but not noun— production). Indeed, our findings are consistent with different regions being available for processing nouns from those available for verbs in that 1) RT correlated with regions spared in better object-namers whereas accuracy correlated with regions robust in better action namers, and 2) more right-hemisphere regions were implicated for better action-namers than were for better object-namers.
However, the thrust of many of these studies suggests additional pre-frontal involvement for verbs as compared to nouns, while we find prefrontal involvement for BOTH verbs and nouns in our better-naming older adults. The distinction between our report of pre-frontal involvement in lexical retrieval in successful naming for both nouns and verbs and others’ reports of more prefrontal involvement for verbs than for nouns may be accounted for by a number of explanations. First, task differences might account for whether similarities or differences are found between nouns and verbs, particularly with respect to involvement of pre-frontal regions. In some articles that show left prefrontal involvement for verbs but not for nouns, inflection is at issue. For example, K. Shapiro et al. (2006) and Cappelletti et al., (2008), cue inflected or uninflected real and pseudo words preceded by a stimulus phrase that includes a form of the word to be said in the response. Understandably the resources for lexical retrieval in such a task are less than for confrontation naming tasks such as those employed in the current study. By contrast, confrontation naming tasks for objects and actions require lexical retrieval but no manipulation of the words to be produced. When difficulties arise on the retrieval task, prefrontal regions might understandably be called upon to search for both verbs and nouns.
Perhaps the pre-frontal involvement we report for both nouns and verbs arises because the two tasks we employed, BNT and ANT, minimize the differences between nouns and verbs, since the two tasks were created to be as similar as possible. In Albert et al. (2009; see also Spiro et al., 2008), too, we report quite similar interactions for BNT and ANT performance vis-à-vis hypertension diagnoses in our older adults (ages 55–85). Each task requires viewing a line-drawing of an object or moment of action, respectively, and labeling it. Moreover, correct responses on the ANT are arguably noun forms of verbs (e.g., ‘swimming’, ‘knighting’) and thus less difference between the two types of substantives should be expected than would be found in discourse, or, perhaps, even with a verb-naming task that employed dynamic stimuli. Indeed, in Mackay et al. (2002), we report that when items from the BNT are matched with items on the ANT for the difficulty they pose for participants in their 50s, no differences between the two tasks are seen in the older adults, in their 60s and 70s.
Another potential difference between our study and the general literature distinguishing regions involved in processing nouns and verbs is they are generally based on studies of younger adults. One might posit that younger adults call upon somewhat different left-hemisphere regions for nouns and for verbs, but that older adults who attempt to do so are handicapped by the general difficulty with retrieving both types of words. Only those older adults who compensate for lexical retrieval problems by involving additional cortex are the better namers.
However, why the brain-regions we report here correlate with accuracy for the ANT and with RT for the BNT remains curious. We find no evidence in the literature of response time relating to one lexical category (nouns in our study) and accuracy to another (verbs in our study). One might expect that tasks on which accuracy was at ceiling performance would show variation only through RT, but there is no reason to believe that the BNT is easier than the ANT. In one study of normal adult native English-speakers aged 30–85, percentages correct on the BNT are somewhat higher than those on the ANT for middle-aged participants and somewhat lower than those on the ANT for older adults. (Connor et al., 2004). Goral et al. (2007) report no differences between ANT and BNT for the males in a similar population, but better performance on the ANT than the BNT for females.
Recall that Wierenga et al. (2008) found different regions associated with latency and accuracy on their fMRI study of naming; they did not, however, consider naming of verbs as well as nouns as we did. The areas they see linked to the nouns they tested, moreover, were near, but not the same as, those implicated in our study: we find bilateral mid-frontal gyrus volume associated with older adults’ better naming; they find BA 45 and pars orbitalis on the right associated with higher naming performance. They report subcortical areas on the left associated with their older adults’ naming, and superior temporal gyrus as well as insula bilaterally. Note that, in our study, for both ANT and BNT, left mid-frontal gyrus volume correlates with naming performance. In addition, left planum temporale correlates negatively with RT for BNT. Only one of these regions –the left planum temporale—is in what might be considered the ‘core’ peri-Sylvian language area. Thus, recalling the Wingfield and Grossman (2006) formulation, it appears that having core peri-Sylvian language areas available is less valuable to the older adult for performing a naming task than is having support areas available. These support areas, moreover, include those reported by some fMRI studies as useful for language tasks: prefrontal areas in the left hemisphere and language-area counterpart regions in the right hemisphere. In addition, our data indicate that strong temporal lobe tracts, which may serve for communication between core and support language areas, are more available to better namers than to poorer ones.
Acknowledgements
This project was supported in part by the Clinical Science Research and Development Service, US Department of Veterans Affairs, by Grant AG14345 (PI: Martin L. Albert) from the National Institute on Aging, and by a Merit Review to Avron Spiro by the Clinical Science Research and Development Service, US Department of Veterans Affairs. We thank all our participants and are particularly appreciative of the helpful suggestions made by two anonymous reviewers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams S, Goldstein LH, Simmons A. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Human Brain Mapping. 2003;20:29–40. doi: 10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Spiro R, Sayers K, Cohen J, Brady C, Goral M, Obler LK. Effects of health status on word finding in aging. Journal of the American Geriatrics Society. 2009;57:2300–2305. doi: 10.1111/j.1532-5415.2009.02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R, Joung P, Nicholas M, Obler LK, Kass R, Albert M. Naming ability across the adult lifespan. Aging and Cognition. 1995;2:300–311. [Google Scholar]
- Barresi BA, Nicholas M, Connor LT, Obler LK, Albert ML. Semantic degradation and lexical access in age-related naming failures. Aging, Neuropsychology, and Cognition. 2000;7:169–178. [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Boone KB, Miller BL, Lesser IM, Hill E, D’Elia LF. Performance on frontal lobe tests in healthy, older individuals. Developmental Neuropsychology. 1990;6:215–224. [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, Wade E. On the tip of the tongue: what causes word finding failure in young and older adults? Journal of Memory and Language. 1991;30:542–579. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters GS, Olivieri A. Activation of Broca’s area by syntactic processing under conditions of concurrent articulation. Human Brain Mapping. 2000;9:65–71. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Fregni F, Shapiro K, Pascual-Leone A, Caramazza A. Processing nouns and verbs in the left frontal cortex: a transcranial magnetic stimulation study. Journal of cognitive neuroscience. 2008;20:707–720. doi: 10.1162/jocn.2008.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. Lexical organization of nouns and verbs in the brain. Nature. 1991;349:788–790. doi: 10.1038/349788a0. [DOI] [PubMed] [Google Scholar]
- Connor LT, Obler LK, Albert ML, Spiro III A. Change in object naming ability during adulthood. Journal of Gerontology: Psychological Sciences. 2004;59:203–209. doi: 10.1093/geronb/59.5.p203. [DOI] [PubMed] [Google Scholar]
- Cooke A, Grossman M, DeVita C, Gonzalez-Atavales J, Moore P, Chen W, Gee JC, Detre J. Large-scale neural network for sentence processing. Brain and Language. 2006;96:14–36. doi: 10.1016/j.bandl.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M. The neural basis for sentence comprehension: grammatical and short-term memory components. Human Brain Mapping. 2002;15:80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I: segmentation and cortical surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire R, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ellis AW, Burani C, Izura C, Bromiley A, Venneri A. Traces of vocabulary acquisition in the brain: Evidence from covert object naming. NeuroImage. 2006;33:958–968. doi: 10.1016/j.neuroimage.2006.07.040. [DOI] [PubMed] [Google Scholar]
- E-Prime Software. Pittsburgh, PA: Psychology Software Tools, Inc; [Google Scholar]
- Feyereisen P. A meta-analytic procedure shows an age-related decline in picture naming: Comments on Goulet, Ska, and Kahn (1994) Journal of Speech, Language, and Hearing Research. 1997;40:1328–1333. doi: 10.1044/jslhr.4006.1328. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE transactions on medical imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically Parcellating the Human Cerebral Cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Dale AM, Quinn BT, Makris N, Fischl B. Age does not increase rate of forgetting over weeks: Neuroanatomical volumes and visual memory across the adult life-span. Journal of the International Neuropsychological Society. 2005;2:2–15. doi: 10.1017/S1355617705050046. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Morrow KL, Moser D. Age-Related Variability in Cortical Activity during Language Processing. Journal of Speech, Language and Hearing Research. 2006;49:690–697. doi: 10.1044/1092-4388(2006/050). [DOI] [PubMed] [Google Scholar]
- Friederici A. A neural basis of sentence processing. Trends in cognitive sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. American Journal of Neuroradiology. 2002;23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goral M, Spiro A, Albert ML, Obler LK, Connor L. Changes in lexical retrieval ability during adulthood. Mental Lexicon. 2007;2:215–238. [Google Scholar]
- Goral M, Clark-Cotton MR, Spiro AIII, Obler LK, Verkuilen J, Albert M. The contribution of set shifting and working memory to sentence processing in older adults. doi: 10.1080/0361073X.2011.619858. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: Distinguishing the effects of word frequency from familiarity and length in overt picture naming. Journal of Cognitive Neuroscience. 2007;19:617–631. doi: 10.1162/jocn.2007.19.4.617. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44:2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cerebral Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Ibrahim I, Horacek J, Bartos A, Hajek M, Ripova D, Brunovsky M, Tintera J. Combination of voxel based morphometry and diffusion tensor imaging in patients with Alzheimer's disease. Neuro Endocrinology Letters. 2009;30:39–45. [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cognitive, Affective & Behavioral Neuroscience. 2004;4:43–57. doi: 10.3758/cabn.4.1.43. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- Langenecker SA, Nielson KA. Frontal recruitment during response inhibition in older adults replicated with fMRI. NeuroImage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. Neuroreport. 2006;17:467–470. doi: 10.1097/01.wnr.0000209012.24341.7f. [DOI] [PubMed] [Google Scholar]
- Luke KK, Liu HL, Wai YY, Wan YL, Tan LH. Functional anatomy of syntactic and semantic processing in language comprehension. Human Brain Mapping. 2002;16:133–145. doi: 10.1002/hbm.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay AJ, Connor LT, Obler LK, Albert ML. Noun and verb retrieval in healthy aging. Journal of the International Neuropsychological Society. 2002;8:764–770. doi: 10.1017/s1355617702860040. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Brühlmann M, Meier E. Age-related white matter atrophy in the human brain. Annals of the New York Academy of Sciences. 1992;673:260–269. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient - Case Report. Neurocase. 2005a;11:182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain and Language. 2005b;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nicholas M, Obler L, Albert M, Goodglass H. Lexical retrieval in healthy aging. Cortex. 1985;21:595–606. doi: 10.1016/s0010-9452(58)80007-6. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan HP. Age differences in the functional neuroanatomy of inhibitory control across the adult lifespan. Psychology and Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Obler LK, Albert ML. Boston: VA Medical Centre; 1986. The Action Naming Test. Unpublished, available at http://www.bu.edu/lab/publications.htm. [Google Scholar]
- Obler LK, Woodward S, Albert ML. Changes in cerebral lateralization in Aging. Neuropsychologia. 1984;22:235–240. doi: 10.1016/0028-3932(84)90066-6. [DOI] [PubMed] [Google Scholar]
- Okada K, Hickok G. Left posterior auditory-related cortices participate both in speech perception and speech production: Neural overlap revealed by fMRI. Brain and Language. 2006;98:112–117. doi: 10.1016/j.bandl.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Finocchiaro C, Shapiro K, Gangitano M, Caramazza A, Pascual-Leone A. All talk and no action: A Transcranial Magnetic Stimulation study of motor cortex activation during action word production. Journal of Cognitive Neuroscience. 2004;16:374–381. doi: 10.1162/089892904322926719. 3. [DOI] [PubMed] [Google Scholar]
- Park DC, Davidson N, Lautenschlager G, Smith AD, Smith P, Hedden T. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Aging, short-term memory, and frontal dysfunction. Psychobiology. 1991;19:175–179. [Google Scholar]
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: evidence from fMRI. Brain and Language. 2004;91:315–325. doi: 10.1016/j.bandl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Pekkala S, Goral M, Hyun J, Obler LK, Erkinjuntti T, Albert ML. Semantic verbal fluency in two contrasting languages. Clinical Linguistics & Phonetics. 2009;23:431–445. doi: 10.1080/02699200902839800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Sylvester C-YC, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: Differential recruitment in older and younger adults. NeuroImage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Vol. 2. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. Journal of Neuroscience. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends in Cognitive Science. 2002;6:394–400. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca's area. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro KA, Moo LR, Caramazza A. Cortical signatures of noun and verb production. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1644–1649. doi: 10.1073/pnas.0504142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A. Grammatical processing of nouns and verbs in left frontal cortex? Neuropsychologia. 2003;41:1189–1198. doi: 10.1016/s0028-3932(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Spalek K, Thompson-Schill SL. Task-dependent semantic interference in language production: An fMRI study. Brain and Language. 2008;107:220–228. doi: 10.1016/j.bandl.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro AIII, Cohen JA, Albert ML, Brady CB, Clark-Cotton M, Goral M, Obler LK. Does hypertension affect naming in older adults?. The Language in the Aging Brain Project; Poster presented at the Cognitive Aging Conference; Atlanta, GA. 2008. [Google Scholar]
- Stamatakis EA, Tyler LK. Language lateralisation as a function of age. Journal of Cognitive Neuroscience. 2006;18 Suppl:A128. [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and biobehavioral reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski Farias S, Harrington G, Broomand C, Seyal M. Differences in functional MR imaging activation patterns associated with confrontation naming and responsive naming. American Journal of Neuroradiology. 2005;26:2492–2499. [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency; evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MMB. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. NeuroImage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Whelihan WM, Lesher EL. Neuropsychological changes in frontal function with aging. Developmental Neuropsychology. 1985;1:371–380. [Google Scholar]
- Wierenga CE, Benjamin M, Gopinath K, Perlstein W, Leonard CM, Gonzalez Rothi LJ, Conway T, Cato MA, Briggs R, Crosson B. Age-related changes in word retrieval: Role of bilateral frontal and subcortical networks. Neurobiology of Aging. 2008;29:436–451. doi: 10.1016/j.neurobiolaging.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Wingfield A, McCoy SL, Peelle JE, Tun PA, Cox LC. Effects of adult aging and hearing loss on comprehension of rapid speech varying in syntactic complexity. Journal of the American Academy of Audiology. 2006;17:487–497. doi: 10.3766/jaaa.17.7.4. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Peelle JE, Grossman M. Speech rate and syntactic complexity as multiplicative factors in speech comprehension by young and older adults. Journal of Aging, Neuropsychology and Cognition. 2003;10:310–322. [Google Scholar]