Abstract
Lipid biosynthesis and fatty acids composition of oleaginous zygomycetes, namely Cunninghamella bainieri 2A1, cultured in media with excess or limited nitrogen were quantitatively determined at different times of culture growth. Accumulation of lipids occurred even when the activity of NAD+-ICDH (β-Nicotinamide adenine dinucleotide-isocitrate dehydrogenase) was still detectable in both media. In C. bainieri 2A1, under nitrogen limitation, the ratio of lipids was around 35%, whereas in nitrogen excess medium (feeding media supplemented with ammonium tartarate), the lipid ratio decreased. The amount of this decrease depended on the level of ammonium tartarate in the media. The main findings in this paper were that C. bainieri 2A1 has the ability to accumulate lipid although nitrogen concentration detected inside the media and that NAD-ICDH was active in all culture periods. These results proved that the strain C. bainieri 2A1 has an alternative behavior in lipid biosynthesis that differs from yeast. According to the old hypotheses, yeasts could not accumulate lipid more than 10% when nitrogen was detected inside the media. Nitrogen-limited and excess media both contained the same fatty acids (palmitic acid, stearic acid, olic acid, linoleic acid and γ-linolenic acid), but at different concentrations. The C:N ratio was also studied and showed no effects on total lipid accumulation, but a significant effect on γ-linolenic acid concentration.
Keywords: Lipid biosynthesies, Nitrogen-excess media, Nitrogen-limited media, Gamma linolenic acid, NAD+-ICDH enzyme
Introduction
The biosynthesis of lipids such as triglycerides, phospholipids and glycolipids by oleaginous microorganisms is well documented and might represent an alternative source of fats and oils, a topic which has been widely studied (Fakas et al. 2006, 2008a, 2009; Ratledge 1982; Ratledge and Boulton 1985; Vani et al. 1988).
Specifically, Cunninghamella echinulata strains have been shown to accumulate lipid bodies rich in polyunsaturated fatty acids (PUFA) including γ-linolenic acid (GLA) (Gema et al. 2002; Chen and Chang 1996; Fakas et al. 2007, 2008b, 2008c; Tao and Zhang 2007). Other microorganisms producing oils containing a large amount of PUFA, including Mortierella alpina, Mortierella isabellina, Mucor circinelloides, Cryptococcus curvatus, Crypthecodinium cohnii and Yarrowia lipolytica, have been widely studied (Papanikolaou and Aggelis 2003; Papanikolaou et al. 2004a; Ratledge 2004; Szczesha-Antczak et al. 2006). Lipid accumulation in these microbes is triggered by cell exhausting nitrogen, but glucose continues to be assimilated as well as inhibition of ICDH activity within the mitochondrion. This leads to the accumulation of citrate, which is transported into the cytosol and cleaved to acetyl-CoA by ATP:citrate lyase (Papanikolaou et al. 2004b; Ratledge 2002). The biochemical mechanism of lipid accumulation in oleaginous fungus seems to be more complicated than that reported for yeasts ( Ratledge 1994; Wynn et al. 2001). The characteristics of NAD+-ICDH of oleaginous molds were shown to be distinct from those described for oleaginous yeasts, as fungal NAD+-ICDH was not absolutely dependent on cellular AMP for activation (Wynn et al. 2001). Synthesis of fatty acids in yeasts is induced by decreased activity of the isocitrate dehydrogenase enzyme under diminished nitrogen levels in culture (Evans and Ratledge 1984). The transition from biomass synthesis to lipogenesis was regulated by NAD+-ICDH (Makri et al. 2010)
The nature and concentration of the nitrogen source used in the medium is an essential factor for regulation of lipogenesis. Many reports have described various nitrogen sources employed for fungal fatty acid production (Certik et al. 1993, 1999), and have also suggested that the initial C:N ratio is important for lipid accumulation (Yong-Hong et al. 2006; Papanikolaou et al. 2004b).
In this study, the growth, lipid accumulation, types of fatty acids present, and activity of ICDH were studied in C. bainieri 2A1 cultured in nitrogen-limited (N-limited) or nitrogen-excess (N-excess) media. Different concentrations of glucose and different levels of nitrogen were used. The fatty acid composition of the cellular lipids was determined, and the N-limited and N-excess media were compared with respect to lipid yield, biomass yield, and γ-linolenic acid concentration.
Materials and methods
Microorganism and culture conditions
The fungus Cunninghamella bainieri 2A1 was maintained on potato dextrose agar (PDA) plates at 30°C for 7 days. The growth medium, Kendrick medium (Kendrick and Ratledge 1992) contained (g/L): glucose, 30; (NH4)2C4H4O6, 1; KH2PO4, 7; Na2HPO4, 2; MgSO4·H2O, 1.5; yeast extract, 1.5; CaCl2·2H2O, 0.1; FeCl3·6H2O, 0.008; ZnSO4·7H2O, 0.0001; CuSO4·5H2O, 0.001; Co(NO3)·6H2O, 0.0001 and MnSO4·5H2O, 0.0001. The PH was adjusted to 6. After sterilization (121°C for 15 min), the media were inoculated with the spore suspension at a final concentration of 1 x 106 spores/ml, which was produced by growing the strain on PDA for 7 days at 30°C. All cultivation experiments were performed in 500-ml Erlenmeyer flasks containing 200 ml of the above medium, incubated in a rotary shaker at 200 rpm and 30°C.
Analytical methods
Total biomass was harvested by vacuum filtration through a Whatman No. 1 filter, washed with distilled water, frozen at –20°C, freeze-dried for 24 h, and then gravimetrically determined. The dry biomass was disrupted and homogenized, and lipids were extracted with chloroform/methanol (2:1, v/v) using the method of Folch et al. (1957). Total lipids were determined gravimetrically. Lipid fractions were converted to methyl esters by using n-hexane to dissolve the oil and 1 M sodium methoxide to get methyl ester, and analyzed in a gas chromatograph shimadzu GC-2010 FID using packed column (DB-23) and flame ionization detector (FID) at 250°C. The fatty acids composition present in the sample was calculated based on the peak area of corresponding methyl ester against reference standard FAME mixture.
Preparation of cell–free extracts and assays for NAD+-ICDH
Harvested mycelia were washed using cold distilled water, and cell extracts for the determination of enzyme activities were prepared by suspending and disrupting mycelia in an extraction buffer (Wynn 1998). The disrupted cell suspension was centrifuged at 6,000g for 15 min at 4°C and the supernatant was filtered through a Whatman No.1 filter paper. The protein in the clear supernatant was measured according to the protocol of Bradford (1976), and NAD+-ICDH (EC 1.1.1.41) was assayed using the method described by Kornberg (1955); ΔA was measured at 340 nm and 30°C and changes were the result of reduction of NAD+.
Analysis of the culture supernatant
The glucose concentration in the culture medium was determined using a GOD test kit according to the manufacturer's instructions. The ammonium concentration in the culture filtrate was determined using the indophenol test (Chaney and Marbach 1962).
Reproducibility of data
All experiments were carried out at least three times, all data presented are reproducible. One-way ANOVA of triplicate data revealed a significant difference in lipid ratio between the first and third days, but no significant difference between the second and fourth days.
Results and discussion
Growth and accumulation of reserve lipid in N-limited media:
The kinetics of growth and lipid accumulation in C. bainieri 2A1 were investigated in N-limited batch cultures with different concentrations of glucose including 30 g/L (Fig. 1; Table 1). At the early stage of culture, the biomass accumulated as the culture period increased (Fig. 1). Lipid yield increased quickly from the first day of cultivation to the third day, where this might have been due to expenditure of nitrogen sources, resulting in the use of glucose to synthesize lipids. Other reasons for phenomena observed during this culture period include the closed nature of the batch culture system, the abundance of nutritional substrates, and limited metabolic materials at the initial stage of culture. C. bainieri 2A1 quickly entered the logarithmic phase after a short period of retention and accelerated growth, where it began to make use of nitrogen sources and other nutritional substrates to reproduce. During this period, a great amount of biomass accumulated (5.2 g/L). After the nitrogen sources were expended (at about 24 h after inoculation), carbon sources in the culture liquid were used. ICDH enzyme activity was present at all culture times from the first (129 nmol/min mg) until the fifth day (119 nmol/min mg), despite the depletion of nitrogen in the media.
Fig. 1.
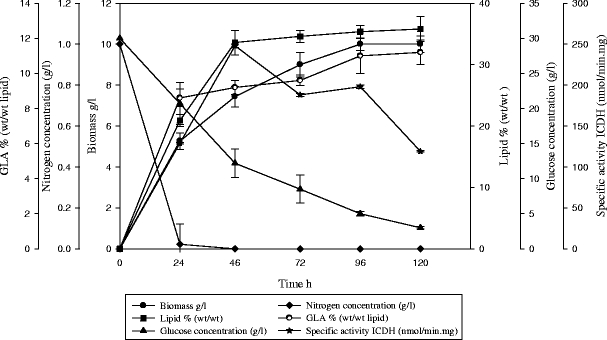
Evaluation of total biomass (g/L ♦), lipid in total biomass % (wt/wt ■), reduced glucose (g/L, ×), nitrogen (g/L, *), and γ-linoleic acid (GLA %) (wt/wt ●) levels as well as isocitrate dehydrogenase activity (ICDH nmol/min mg,) during growth of C. bainieri 2A1 on N-limited media
Table 1.
Growth of C. bainieri 2A1 in N-limited media
| C/N | Glc (g/L) | X (g/L) | Yx/Glc | YL/Glc | L/X (% wt/wt) | Pro X (mg/ml h) | Pro L (mg/ml h) |
|---|---|---|---|---|---|---|---|
| 42 | 30 | 9.4 | 0.37 | 0.13 | 35 | 0.09 | 0.035 |
| 69 | 50 | 10.6 | 0.28 | 0.097 | 34 | 0.14 | 0.05 |
| 97 | 70 | 11.6 | 0.23 | 0.085 | 36 | 0.12 | 0.043 |
Maximum L/X was achieved after 72–96 h. Culture conditions: initial pH 6 and temperature 30°C
X total biomass,Yx/Glc total biomass yield on glucose consumed, YL/Glcreserve lipid yield on glucose consumed, L/X maximum ratio of lipid accumulated, Pro X productivity of biomass, Pro L productivity of reserve lipid
Our findings with respect to the C. bainieri 2A1 growth curve were in agreement with those of Tao and Zhang (2007), who showed that Cunninghamella echinulata mainly used glucose to synthesize lipids, and accumulated lipids until they reached a maximum on the fourth day. The same study also showed that, in the growing stage, biomass and lipid yield accumulation was the fastest process. The GLA percentage did not increase. During the lipid accumulation stage, the lipid yield reached a maximum on the fourth day and then began to decrease with time. In contrast, Papanikolaou et al. (2004a) reported that the maximum lipid level was achieved after 310–400 h. Another study in 2002 showed that the maximum lipid level was achieved after 250 h, and that this strain C. echinulata cultivated in medium with a C:N ratio >100 accumulated more than 35% of cellular lipid with a GLA content greater than 11% (Gema et al. 2002), while our study achieved the same results with C:N ratio 42 and after 96 h of cultivation.
The hypothesis was that oleaginous microorganisms degrade AMP in low-nitrogen conditions in order to release nitrogen, thereby allowing synthesis of cellular materials. The 'energy charge' ratio should thus be increased inside the cell, resulting in inhibition of ICDH and accumulation of intracellular citric acid. Acetyl-CoA would then be produced by citric acid cleavage via the ATP:citrate lyase reaction (Botham and Ratledge 1979; Moreton 1988). Our results related to NAD+-ICDH show that ICDH in C. bainieri 2A1 appeared in high activity during the period of growth, and it appears that this enzyme was not implicated in lipid biosynthesis and that this activity may be due to the implication of NAD+-ICDH in different pathways, as, for example, it can be suggested to have a role in maintaining complete TCA cycle operation under conditions of sufficient isocitrate supply. Also, participation of two ICDH in mitochondria and one ICDH in cytosol in interconversions between isocitrate and oxoglutarate represents a flexible mechanism in which the ICDH enzymes are involved in the maintenance of the metabolic balance between reduced and oxidized pyridine nucleotides (Igamberdiev and Gardestrom 2003). Generally, NAD+-ICDH was inhibited by two key metabolites, citrate and the ratio of NAD+/NADH, which again illustrates the strategic position of this enzyme in cellular metabolism. Citrate is a weak inhibitor but, as it accumulates in the mitochondria, it could feed forward to increase its own production due to the equilibrium of aconites. NAD, on the other hand, is very strongly inhibitory. There are some indications that the catalysis by NAD+-ICDH is not reversible and that it is highly inhibitory with the ratio of NAD+/NADH. In contrast, the NADP-ICDH catalyzed reaction is easily reversible. This may be connected with different enzyme functions in isocitrate metabolism (Evans and Ratledge 1985). This enzyme was studied widely in yeast as such changes in Rhodosporidium toruloides CBS 14 would result in rapid inactivation of NAD+:ICDH, thus indicating why this enzyme occupies such a strategic position in the sequence of events leading to lipid accumulation (Evans and Ratledge 1985). The recent research on yeast by Makri et al. (2010) also proved that NAD+-ICDH activity was gradually decreased during transition from biomass production to lipogenic phase and further to citric acid production phase. In contrast, the activity of NADP+-ICDH that was essentially located in the cytoplasm, especially during both lipogenic and citric acid production phases, remained unchanged during the growth cycle. During biomass production phase, glycerol was essentially converted into fat-free cellular material, and when ammonium nitrogen was depleted, some quantities of storage lipid were then synthesized during the lipogenic phase. This data again suggest that transition from biomass synthesis to lipogenesis and then to citric acid synthesis was regulated by NAD+-ICDH. The stringent control of citrate metabolism at the level of ICDH therefore provides the necessary first step in the accumulation of lipid by oleaginous yeasts. This phenomena cannot be applied to our outcome as in C. bainieri 2A1 our data show high activity of ICDH in the growth phase. This activity may be due to the involvement of ICDH in other pathways or may be due to the build-up of lipid-free material which is produced until the stationary phase. The phenomena of C. bainieri 2A1 growth in Bach culture seems to be different from other species in the biomass production and lipogenic phases. The two phases come together at the same time and this result is in contrast to yeast and to other studies on fungi (Papanikolaou et al. 2004b), who demonstrated that the restriction of ICDH activity in the mycelium of C. echinulata and M. isabellina strains induced lipid biosynthesis. This was accompanied by a considerable decrease of respiration rate, and it is remarkable that, in C. echinulata, the activity of NAD-ICDH was maintained at high levels considerably later after nitrogen depletion in the medium, explaining the biosynthesis of lipid-free material biosynthesis for an extensive period of time and the simultaneous low reserve lipid accumulation inside the mycelia. These results in some way agreed with our results in cases of build-up of lipid-free material. Nitrogen, indispensable for lipid-free material synthesis, may be provided by AMP-desaminase reaction, while ICDH activity should be unaffected by the reduction of AMP. Indeed, it was found that NAD+-ICDH of some oleaginous zygomycetes was active even at high energy charge ratios (Certik et al. 1999; Wynn et al. 2001), whereas in the cases of oleaginous yeasts it was not (Botham and Ratledge 1979).
The effect of glucose concentrations on the growth of C. bainieri 2A1 and lipid accumulation in N-limited media were also investigated (Table 1) with specific respect to biomass, lipid content of biomass, ,Yx/Glc (total biomass yield on glucose consumed), YL/Glc (reserve lipid yield on glucose consumed) productivity for lipids, and productivity for biomass. Different C:N ratios were achieved by varying the concentration of glucose (30, 50, 70 g/L) while fixing the concentrations of the nitrogen sources, which consisted of yeast extract (1.5 g/L) and ammonium tartrate (1 g/L). Maximum lipid accumulation occurred at C:N 42, although it should be emphasized that yeast extracts containing significant quantities of N (8.9%) were included in the C:N ratio calculation, as were contributions from ammonium tartrate. Higher YX/Glc and YL/Glc values were also observed at C:N 42. There were no significant differences among different C:N ratio with respect to the lipid percentage.
Oleaginous organisms usually do not accumulate lipids to any great extent in media with initial C:N ratios <20; the optimum ratio for any organism will probably be between 30 and 80 (Moreton 1988). Previous studies showed that the lipid content of C. echinulata grown with various concentrations of the carbon source (soluble starch, 3–12%) decreased with increasing starch concentration and C:N ratio throughout the experimental range (Chen and Chang 1996). Maximum lipid accumulation and GLA yield both occurred at 10% soluble starch concentration, where this was equivalent to an initial C:N ratio of 35. The previous study was somewhat in agreement with our results showing no effect of carbon concentration on reserve lipids in total biomass. The maximum Yx/Glc and YL/Glc were observed at C:N ratios (42) of 0.37 and 0.13, respectively, while the best productivity was observed at C:N ratios (69) of 0.14 and 0.05, respectively. Another study in 2004 demonstrated that the initial C:N ratio is important for lipid accumulation (Papanikolaou et al. 2004b). Specifically, in C. echinulata cultivated in multiple limited media, when the C:N ratio was increased from 83.5 to 133.5, lipid content in C. echinulata subsequently increased from 36 to 47%. Papanikolaou et al. (2004b) also found that, at a high C:N ratio varying from 150 to 340, lipid productivity increased from 0.05 to 0.07 g/h.
The role of the C:N ratio in lipid accumulation has been shown to depend on the microorganism and the fermentation liquid medium (Vani et al. 1988). During growth-phase batch fermentation with an initial C:N ratio of 17, the lipid yield of the cells increased from 0.25 to 0.48 at the end of fed-batch mode, when the initial C:N was 30. When the C:N was further increased to 40 in fed-batch mode, the lipid yield decreased to 0.33. Another study of yeast reported that the optimum C:N ratio was 420, but at this ratio, biomass and lipid production was very low (Yong-Hong et al. 2006).
Growth and accumulation of reserve lipids in N-excess media
Growth and lipid accumulation were studied by feeding cultures with ammonium tartrate and by controlling the ammonium tartrate concentration (Fig. 2); cultures were fed thrice daily with ammonium tartrate (1 g/L).
Fig. 2.
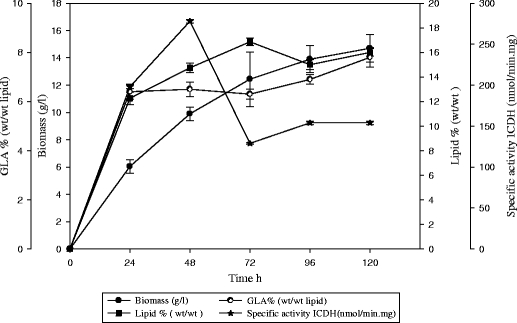
Evaluation of total biomass (g/L ♦), lipid in total biomass % (wt/wt ■), GLA% (wt/wt ●), and isocitrate dehydrogenase activity (NAD+-ICDH nmol/min mg,) during growth of C. bainieri 2A1 in N-excess media with thrice daily feeding with ammonium tartrate (1 g/L )
With three times daily feeding, biomass was (13 g/L) and lipid accumulation was (16%). Nitrogen concentration was between 0.4 and 0.6 g/L in the first days, and glucose gradually decreased (Fig. 3).
Fig. 3.
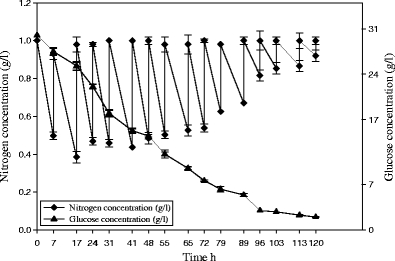
Evaluation of reduced glucose (g/L ♦) and nitrogen concentration before and after feeding with ammonium tartrate (g/L) during C. bainieri 2A1 growth in N-excess media with thrice daily feeding with ammonium tartrate (1 g/L)
Based on the above findings, we focused more on nitrogen level by controlling the nitrogen concentration (0.2–0.8, 0.5–1 and 0.8–1 g/L) for 2 days of culture (Table 2). There were significant differences among the three nitrogen concentrations with respect to biomass, lipid percentage, lipid yield, and biomass yield. N-excess media with different levels of nitrogen showed more biomass yield than N-limited media. However, the lipid yield in N-excess media with different levels of nitrogen was less than that observed in N-limited media. Despite the effects of excess nitrogen on microorganisms, the lipid yield when the nitrogen level was held between 0.5–1 g/L was around 15%. This finding disagrees with the old hypothesis suggesting that microorganisms can only accumulate lipids in N-limited media. However, to date, few studies have focused on N-concentration. Certik et al. (1999) used different concentrations of nitrogen (NaNO3) at the beginning of culture in modified Czapek–Dox media. On the second day of cultivation, the percent lipid accumulation was between 5 and 10% when using 1.2 g/L NaNO3 and around 15% when using 0.4 g/L NaNO3. On the fourth day of cultivation, the percent lipid accumulation was around 10% when using 1.2 g/L NaNO3 and around 20% when using 0.4 g/L. NaNO3 levels and NAD+ICDH activity increased concomitantly with NaNO3 concentration. This study thus indicated that lipid accumulation in C. echinulata is indirectly correlated with nitrogen concentration.
Table 2.
Growth of C. bainieri 2A1 in N-excess media
| Nitrogen level (g/L) | X (g/L) | Yx/Glc | YL/Glc | L/X (% wt/wt) | Pro X (mg/ml h) | Pro L (mg/ml h) |
|---|---|---|---|---|---|---|
| 0.2-0.8 | 8.3 | 0.55 | 0.12 | 21 | 0.17 | 0.039 |
| 0.5-1 | 9.3 | 0.6 | 0.09 | 15 | 0.19 | 0.03 |
| 0.8-1 | 10 | 0.7 | 0.08 | 11 | 0.2 | 0.02 |
X total biomass,Yx/Glc total biomass yield on glucose consumed, YL/Glcreserve lipid yield on glucose consumed, L/X maximum ratio of lipid accumulated, Pro X productivity of biomass, Pro L productivity of reserve lipid during 48 h of culture
In contrast, in C. echinulata cultivated on hydrolyzed tomato waste (Fakas et al. 2007), the lipid content increased to up to 25% of the biomass during the growth and lipogenic phases. Although approximately 0.4 g/L of organic nitrogen were measured in the growth environment, lipogenesis still commenced, indicating that this nitrogen could not be assimilated. Acid treatment for hydrolyzed tomato waste (TWH) oil yield reached around 40% at the highest nitrogen concentration utilized (Fakas et al. 2008b), the main trend observation being that increasing nitrogen concentration increased biomass production but decreased oil accumulation, except for growth on TWH. Our observation agreed with (Fakas et al. (2008b) in the case of increasing nitrogen concentration resulting in increased biomass production but decreased oil accumulation, the only difference being that, even when we controlled the level of nitrogen, the microbes still accumulate lipid but not the same as in limited media. The glucose concentration may divert the carbon source to either lipid-free cellular mass production or storage lipid synthesis. However, when the nitrogen source has no effect on glucose uptake, the C:N is an important factor affecting lipogenesis, which means that when carbon uptake rate was high, lipid accumulation occurred, even in the presence of relative high amounts of nitrogen in the growth medium (Fakas et al. 2008c). From this explanation, we can conclude that, when C. bainieri 2A1 is cultured in N-excess media, glucose uptake was high, so lipid accumulation occurred, even in the presence of nitrogen.
There have been insufficient previous studies of N-excess media to explain the phenomena observed in the present study since all previous studies have used N-limited media. The hypotheses for N-limited media were that oleaginous species could accumulate more than 10% lipids and that they would then degrade AMP in order to release additional nitrogen from ammonium. This would result in an increased energy charge ratio inside the cell, resulting in inhibition of ICDH and intra-cellular citric acid accumulation. Acetyl-CoA would then be produced by citric acid cleavage via the ATP:citrate lyase reaction (Holdsworth and Ratledge 1988; Holdsworth et al. 1988). However, in the present study, the microorganisms accumulated lipids to around 15% despite the presence of excess nitrogen (0.5–1 g/L). Thus, further studies are required to explain lipid production in N-excess media.
Fatty acid composition of total lipid produced by C. bainieri 2A1
In C. bainieri 2A1 cultivated on N-limited media at C:N 42, the principal cellular fatty acid was oleic acid (Δ9C18), the concentration of which remained constant during growth. In contrast, the concentrations of palmitic (C16:0) and stearic (C18:0) acids slightly decreased and that of linolenic (Δ9,12C18:3) acid slightly increased (Table 3). In the first phase of growth, γ-linolenic acid GLA content was elevated during lipid accumulation. Our results demonstrate the effect of the C:N ratio on fatty acid concentration. γ-linolenic acid GLA content reached 11.2% during lipid accumulation when using C:N 42, but only reached 6.5% and 7.0% when using C:N ratios of 69 and 97, respectively (Table 3). These findings indicate that the C:N ratio increases have effects contrary to those of GLA concentration. The fatty acid composition of N-excess media differed from that of N-limited media; palmitic acid (C16:0) remained almost constant during growth, and the γ-linolenic acid GLA content remained constant (Table 4 ). A negative relationship was observed between ammonium tartrate level and γ-linolenic acid concentration (Table 5).
Table 3.
Fatty acid composition of total lipid produced by C. bainieri 2A1 in N-limited media
| C:N | Culture time (h) | Fatty acid concentration (wt/wt) in total mycelium lipids | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ6,9,12C18:3 | C24:0 | ||
| 42 | 24 | 18.8 | 10.0 | 40.2 | 14.6 | 8.6 | 2.5 |
| 48 | 19.3 | 10.5 | 40.11 | 13.3 | 9.2 | 3.1 | |
| 72 | 17.8 | 10.4 | 39.6 | 15.0 | 9.6 | 3.2 | |
| 96 | 16.6 | 8.4 | 41.11 | 15.4 | 11.0 | 3.1 | |
| 120 | 16.3 | 8.3 | 40.4 | 16.1 | 11.2 | 3.2 | |
| 69 | 24 | 18 | 27.1 | 31.2 | 9.4 | 4.5 | 4.3 |
| 48 | 19.3 | 23.6 | 33.3 | 9.7 | 5 | 4.1 | |
| 72 | 18.1 | 23.6 | 33.8 | 9.7 | 5.5 | 4.3 | |
| 69 | 17.4 | 22.4 | 34.3 | 9.9 | 6.4 | 4.5 | |
| 120 | 18.9 | 18.7 | 36.4 | 10.4 | 6.5 | 4.0 | |
| 97 | 24 | 19.2 | 24.6 | 33.4 | 8.8 | 5.1 | 3.7 |
| 48 | 18.3 | 18.9 | 37.6 | 8.8 | 6.0 | 5.3 | |
| 72 | 19.2 | 16.2 | 38.6 | 10.9 | 6.9 | 3.4 | |
| 96 | 18.6 | 16.4 | 39.0 | 10.2 | 6.7 | 4.1 | |
| 120 | 18.4 | 15.2 | 39.6 | 10.2 | 7.0 | 4.3 | |
Culture conditions: initial pH 6, temperature 30°C and C:N ratios 42, 69 and 97
Table 4.
Fatty acid composition of total lipid produced by C. bainieri 2A1 in N-excess media
| Culture time (h) | Fatty acid concentration (wt/wt) in total mycelium lipid | |||||
|---|---|---|---|---|---|---|
| C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ6,9,12C18:3 | C24:0 | |
| 24 | 15.5 | 16.0 | 41.3 | 9.2 | 6.4 | 4.1 |
| 48 | 15.4 | 18.4 | 37.8 | 11.3 | 6.5 | 4.9 |
| 72 | 15.9 | 15.5 | 37.4 | 12.6 | 6.3 | 6.4 |
| 96 | 15.8 | 14.9 | 37.1 | 15.6 | 6.9 | 4.9 |
| 120 | 14.7 | 12.6 | 37.1 | 15.2 | 7.8 | 6.8 |
Culture conditions: initial pH 6, temperature 30°C, glucose, 30 g/L, and thrice daily feeding with ammonium tartrate (1 g/L)
Table 5.
Fatty acid composition of total lipid produced by C. bainieri 2A1 in N-excess media
| Ammonium tartrate (g/L) | Culture time (h) | Fatty acid concentration (wt/wt) in total mycelium lipid | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ6,9,12C18:3 | C24:0 | ||
| 0.2 -0.8 | 24 | 22.2 | 14.4 | 38.1 | 10.4 | 6.4 | 3 |
| 48 | 20.2 | 17.3 | 36.4 | 9.7 | 6.4 | 4 | |
| 0.5-1 | 24 | 22.3 | 15.6 | 34.9 | 10.4 | 5.5 | 3.4 |
| 48 | 20.7 | 18.5 | 35 | 9.2 | 5.2 | 5.2 | |
| 0.8-1 | 24 | 20.6 | 20.9 | 31.5 | 11.3 | 4.5 | 5.3 |
| 48 | 20.3 | 19.5 | 33.6 | 11.4 | 4.7 | 5.0 | |
Culture conditions: initial pH 6, temperature 30°C, glucose 30 g/L, and different levels of ammonium tartrate
A correlation between the quantity of GLA produced and the amount of lipid-free material was established in both media types. YGLA/Xf was determined to be 0.22 in N-limited media and 0.1 in N-excess media by linear regression analysis of GLA produced and units of lipid free biomass (Fig. 4).
Fig. 4.
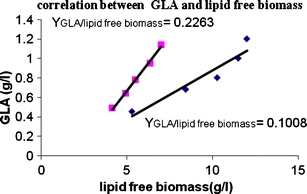
GLA (g/L) in the reserve lipid versus lipid-free materials determine (g/L) in N-limited media (■) and N-excess media (♦)
Conclusion
Lipid biosynthesis in C. bainieri 2A1 occurred in N-limited media at C:N ratios of 42, 69, and 93, and activity of NAD+- ICDH at C:N 42 appeared in high activity during the period of growth; this activity may be due to the build-up of lipid-free material which is produced until the stationary phase,. The lipid percentage was around 35% and GLA was around 11%. However, there were no significant differences among the three types of C:N ratio in total reserve lipids, whereas there were significant differences in GLA content. Statistical analysis of the lipid profile demonstrated a significant difference between the first day of culture and the third day, whereas there were no significant differences from the second to the fifth days. In N-excess media, lipid biosynthesis still occurred even when N was still detected in the media at levels of 0.2–0.8, 0.5–1, and 0.8–1 g/L, where the lipid percentages were 21, 15, and 11, respectively. NAD+- ICDH activity was strongly detected on all the 5 days of culture. Microbial growth and production of reserve lipids were not negatively affected by N-excess media and this may be due to the high uptake of glucose. Fatty acid composition was thus affected by the use of N-excess and N-limited media, by different C:N ratios, and by different levels of nitrogen. This result proved that the strain C. bainieri 2A1 has an alternative behavior in lipid biosynthesis that differs from yeast.
References
- Botham AP, Ratledge C. A biochemical explanation for lipid accumulation in candida 107 and other oleaginous micro organism. J Gen Microbiol. 1979;114:361–375. doi: 10.1099/00221287-114-2-361. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Arapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Certik M, Sajbidor J, Stredanska S. Effect of carbon and nitrogen sources on growth, lipid production and fatty acid composition of Mucormucedo F1384. Microbiol. 1993;74:7–15. [Google Scholar]
- Certik M, Megova J, Horenitzky R. Effect of nitrogen source on the activities of lipogenic enzymes in oleaginous fungus Cunninghamella echinulata. J Gen Appl Microbiol. 1999;45:289–293. doi: 10.2323/jgam.45.289. [DOI] [PubMed] [Google Scholar]
- Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonium. Clin Chem. 1962;8:130–132. [PubMed] [Google Scholar]
- Chen Hung-Chang, Chang Chi-Chia. Production of gamma-linoleic acid by the fungus Cunninghamella echinulata CCRC 31840. Biotechnol Prog. 1996;12:338–341. doi: 10.1021/bp960009y. [DOI] [Google Scholar]
- Evans TC, Ratledge C. Effect of nitrogen source on lipid accumulation in oleaginous yeasts. J Gen Microbiol. 1984;130:1693–1704. [Google Scholar]
- Evans TC, Ratledge C. The role of mitochondrial NAD+:isocitrate dehydrogenase in lipid accumulation by the oleaginous yeast Rhodosporidium toruloides CBS14. Can J Microbiol. 1985;31:845–850. doi: 10.1139/m85-157. [DOI] [Google Scholar]
- Fakas S, Papanikolaou S, Galiotou-Panayotou M, Komaitis M, Aggelis G. Lipid of Cunninghamella echinulata with emphasis of γ-linolenic acid distribution among lipid classes. Appl Microbiol Biotechnol. 2006;73:676–683. doi: 10.1007/s00253-006-0506-3. [DOI] [PubMed] [Google Scholar]
- Fakas S, Galiotoupanayotou M, Papanikolaou S, Komaitis M, Aggelis G (2007) Compositional shifts in lipid fraction during lipid turnover in Cunninghamella echinulata. Enzyme Microb Technol 1321–1327
- Fakas S, Papapostolou I, Papanikolaous S, Georgiou DC, Aggelis G. Susceptibilit to peroxidation of the major mycelia lipids of Cunninghamella echinulata. Eur J Lipid Sci Technol. 2008;110:1062–1067. doi: 10.1002/ejlt.200800025. [DOI] [Google Scholar]
- Fakas S, Certik M, Papanikolaou S, Aggelis G, Komaitis M, Galiotou-Panayotou M. γ-Linolenic acid production by Cunninghamella echinulata growing on complex organic nitrogen sources. Bioresour Technol. 2008;99:5986–5990. doi: 10.1016/j.biortech.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Fakas S, Papanikolaou S, Galiotou-Panayotou M, Komaitis M, Aggelis G. Organic nitrogen of tomato waste hydrolysate enhances glucose uptake and lipid accumulation in Cunninghamella echinulata. J Appl Microbiol. 2008;105:1364–5072. doi: 10.1111/j.1365-2672.2008.03839.x. [DOI] [PubMed] [Google Scholar]
- Fakas S, Makri A, Mavromati M, Tselepi M, Aggelis G. Fatty acid composition in lipid fraction lengthwise the mycelium of Mortierella isabellina and lipid production by solid state fermentation. Bioresour Technol. 2009;100:6118–6120. doi: 10.1016/j.biortech.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane–Stanley GH. Simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gema H, Kavadia A, Dimou D, Tsagou V, Komaitis M, Aggelis G. Production of γ- linolenic acid by Cunninghamella echinulata cultivated on glucose and orange peel. Appl Microbiol Biotechnol. 2002;58:303–307. doi: 10.1007/s00253-001-0910-7. [DOI] [PubMed] [Google Scholar]
- Holdsworth EJ, Ratledge C. Lipid turnover in oleaginous yeasts. J Gen Microbiol. 1988;134:339–346. doi: 10.1099/00221287-134-11-2907. [DOI] [PubMed] [Google Scholar]
- Holdsworth JE, Veenhuis M, Ratledge C. Enzyme activates in oleaginous yeasts accumulating and utilizing exogenous or endogenous lipids. J Gen Microbiol. 1988;134:2907–2915. doi: 10.1099/00221287-134-11-2907. [DOI] [PubMed] [Google Scholar]
- Igamberdiev UA, Gardestrom P. Regulation of NAD and NAD-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in mitochondria and cytosol of pea leaves. Biochim Biophys Acta: Bioenergetics. 2003;1606:117–125. doi: 10.1016/S0005-2728(03)00106-3. [DOI] [PubMed] [Google Scholar]
- Kendrich A, Ratiedge C. Desaturation of polyunsaturated fatty acid in mucorcircinelloides and the inevolvement of a novel membrane-bound malic enzyme. Eur J Microbiol. 1992;209:667–673. doi: 10.1111/j.1432-1033.1992.tb17334.x. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Isocitrate dehydrogenase of yeast. Meth Enzymol. 1955;1:705–7010. doi: 10.1016/0076-6879(55)01122-1. [DOI] [Google Scholar]
- Makri A, Fakas S, Aggelis G. Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repated bach cultures. Bioresour Technol. 2010;101:2351–2358. doi: 10.1016/j.biortech.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Moreton RS (1988) Physiology of lipid accumulation yeasts. In: Moreton RS (ed) Single cell oil. Longman, Essex, UK, pp 1–32
- Papanikolaou S, Aggelis G. Modeling lipid accumulation and degradation in Yarrowia lipolytic cultivated on industrial fats. Curr Microbiol. 2003;46:398–402. doi: 10.1007/s00284-002-3907-2. [DOI] [PubMed] [Google Scholar]
- Papanikolaou S, Komaitis M, Aggelis G. Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour Technol. 2004;95:287–291. doi: 10.1016/j.biortech.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Papanikolaou S, Sarantou S, Komaitis M, Aggelis G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J Appl Microbiol. 2004;97:867–875. doi: 10.1111/j.1365-2672.2004.02376.x. [DOI] [PubMed] [Google Scholar]
- Ratledge C. Microbial oils and fats: an assessment of their commercial potential. In: Ball JM, editor. Progress in industrial microbiology. Oxford: Elsevier; 1982. pp. 119–206. [Google Scholar]
- Ratledge C. Yeasts, moulds, algae and bacteria as sources of lipid. In: Kamel BS, Kakuda Y, editors. Technological advances in improved and alternative sources of lipids. London: Blackie; 1994. pp. 235–291. [Google Scholar]
- Ratledge C. Regulation of lipid accumulation in oleaginous microorganisms. Biochem Soc Trans. 2002;30:1047–1050. doi: 10.1042/BST0301047. [DOI] [PubMed] [Google Scholar]
- Ratledge C. Fatty acid biosynthesis in microorganism being used for single cell oil production. Biochimie. 2004;86:807–815. doi: 10.1016/j.biochi.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Ratledge C, Boulton AC. Fat and oil. In: Murray Moo–Young., editor. Comprehensive biotechnology. New York: Peramon; 1985. pp. 983–1003. [Google Scholar]
- Szczesha-Antczak M, Antczak T, Piotrowicz-Wasiak M, Rzyska M, Binkowska N, Bielecki S. Relationships between lipases and lipids mycelia of two mucor strains. Enzyme Microb Technol. 2006;39:1214–1222. doi: 10.1016/j.enzmictec.2006.03.008. [DOI] [Google Scholar]
- Tao J-YL, Zhang X-Y (2007) Growth low of gamma-inoleic acid production by Cunninghamella echinulata. J US-China Med Sci 1:55–60
- Vani S, Sharma CD, Bhagat SD, Saini VS, Adhikari DK. Lipid and fatty acid biosynthesis by Rhodotorula minuta. JAOCS. 1988;75:501–505. [Google Scholar]
- Wynn PJ. Microorganisms as sources of nutritionally important polyunsaturated fatty acid. Forum Appl Biotechnol. 1998;1:1215–1222. [Google Scholar]
- Wynn PJ, Hamid AA, Li Y, Ratledge C. Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpine. Microbiology. 2001;147:2857–2864. doi: 10.1099/00221287-147-10-2857. [DOI] [PubMed] [Google Scholar]
- Yong–Hong Li, BO Liu, ZHAO Zong–Bao, AB Feng Wa. Optimization of culture condition for lipid production by Rhodosporidium toruloides. Chin J Biotechnol. 2006;22:650–656. doi: 10.1016/S1872-2075(06)60050-2. [DOI] [PubMed] [Google Scholar]


