Abstract
It is well established that full activation of T cells requires the interaction of the TCR complex with the peptide–MHC complex (Signal 1) and additional signals (Signal 2). These second signals are generated by the interaction of costimulatory ligands expressed on antigen presenting cells with activating receptors on T cells. In addition, T cell responses are negatively regulated by inhibitory costimulatory pathways. Since professional antigen presenting cells (APC) harbour a plethora of stimulating and inhibitory surface molecules, the contribution of individual costimulatory molecules is difficult to assess on these cells. We have developed a system of stimulator cells that can give signal 1 to human T cells via a membrane bound anti-CD3 antibody fragment. By expressing human costimulatory ligands on these cells, their role in T cell activation processes can readily be analyzed. We demonstrate that T cell stimulator cells are excellent tools to study various aspects of human T cell costimulation, including the effects of immunomodulatory drugs or how costimulatory signals contribute to the in vitro expansion of T cells. T cell stimulator cells are especially suited for the functional evaluation of ligands that are implicated in costimulatory processes. In this study we have evaluated the role of the CD2 family member CD150 (SLAM) and the TNF family member TL1A (TNFSF15) in the activation of human T cells. Whereas our results do not point to a significant role of CD150 in T cell activation we found TL1A to potently costimulate human T cells. Taken together our results demonstrate that T cell stimulator cells are excellent tools to study various aspects of costimulatory processes.
Keywords: T cell activation, Costimulation, TL1A, CD150
1. Introduction
The two signal hypothesis of lymphocyte activation proposes that T cells that receive Signal 1 via their T cell receptor (TCR) complex depend on concomitant triggering of costimulatory receptors to achieve full activation (Greenwald et al., 2005; Watts, 2005). T cell activation is also modulated by inhibitory costimulatory receptors that are able to attenuate TCR-signals. By acting as potent regulators of host-protective as well as pathological processes, T cell costimulatory pathways play a pivotal role in immunity (Saunders et al., 2005; Keir et al., 2008; Nurieva et al., 2009). Consequently, such pathways are prime therapeutic targets in diseases that are associated with aberrant T cell responses (Ford and Larsen, 2009; Li et al., 2009). Likewise, tumor patients or individuals suffering from chronic viral infection might benefit from therapies that enhance costimulatory pathways or block inhibitory receptors (Blank and Mackensen, 2007). In this context it is evident that a more complete understanding regarding the function of human T cell costimulatory molecules is a prerequisite for the development of efficient therapeutic strategies.
Studies on costimulatory pathways on human cells are hampered by several circumstances. Antigen presenting cells (APC) harbour a plethora of activating and inhibitory ligands with overlapping and redundant functions, which complicate the assessment of the contribution of single molecules to T cell activation processes. Studies on individual costimulatory pathways often rely on the use of immobilized antibodies. Such antibodies might differ from the natural ligands regarding their binding site and affinity. Furthermore, the crosslinking of receptors by immobilized antibodies generates signals that might not accurately reflect the effects of interaction of costimulatory ligands with their receptors. However, there are numerous molecules that have been categorized as costimulatory based solely on their ability to generate a second signal when ligated with antibodies (Leitner et al., 2010). Recombinant proteins representing the extracellular domains of costimulatory ligands are valuable and widely used tools to study T cell activation processes. However, their generation is time consuming and costly and they might differ from their membrane resident natural counterparts regarding their capability to modulate T cell responses.
We have developed a simple cellular system to assess the role of costimulatory ligands in the activation of human T cells. This system, which we have designated T cell stimulator cells, is based on the murine thymoma cell line Bw5417 that expresses membrane-bound anti-human CD3 single chain antibody fragments at high or low densities. Upon retroviral expression of human costimulatory ligands on these cells their contribution to the activation of human T cells can readily be determined. In this study we describe this system in detail and demonstrate that T cell stimulator cells are an efficient and versatile tool to study various aspects of human T cell costimulatory processes.
2. Material and methods
2.1. Antibodies, cell culture and FACS staining
293T cells and the mouse thymoma cell line Bw5147 (short designation within this work Bw) were cultured as described (Pfistershammer et al., 2006, 2008). The ethical review board of the General Hospital and the Medical University of Vienna approved the human studies performed within this work and informed consent was obtained from the donors. PBMC were isolated from heparinised whole blood of healthy volunteer donors by standard density centrifugation with Ficoll-Paque (Amersham Bioscience, Roosendaal, Netherlands). Human T cells were obtained through depletion of CD11b, CD14, CD16, CD19, CD33 and MHC-class II bearing cells with the respective mAbs by MACS (Miltenyi Biotech, Bergisch Gladbach, Germany). The mAbs to CD11b (VIM12), CD14 (VIM13), CD33 (4D3), MHC-class II (1/47), CD80 (7-480), CD58 (1-456) and the non-binding control antibody VIAP (calf intestine alkaline phosphatase specific) were produced at our institute. The mAbs to CD14 (MEM-18) was purchased from An der Grub (Kaumberg, Austria), CD19 mAb (BU12) from Ancell (Bayport, MN), and 41BB-L and CD150/SLAM (A12) from Biolegend (San Diego, CA). Goat anti-human TL1A/TNFSF15 antibodies were obtained from R&D (Minneapolis, MN). FACS analysis was performed as described previously (Pfistershammer et al., 2006). Briefly, binding of primary antibodies was detected with PE-conjugated goat anti-mouse IgG-Fcγ specific Abs or donkey anti-goat IgG (H + L) (both Jackson ImmunoResearch, West Grove, PA). Expression of membrane-bound anti-CD3 antibody fragment was detected via APC-conjugated goat anti-mouse IgG (H + L) Abs, which reacts with the variable regions of murine antibodies (Jackson ImmunoResearch). Fluorescence intensity is shown on a standard logarithmic scale.
2.2. Double immunoflourescence of T cell and stimulator cell co-cultures
Human T cells were CFSE-labeled as described in detail (Kober et al., 2008). Irradiated T cell stimulator cells (2 × 106/ml) were incubated with 0.5 μM working solution of CellTracker™ Orange CMTMR (5-and 6 (4-chloromethyl-benzoyl-amino-tetramethylrhodamine) mixed isomers for 30 min at 37 °C in a CO2 incubator. The reaction was stopped by washing once with pre-warmed medium. For double-immunoflourescence CMTMR-labeled stimulator cells (8 × 104/well) and CFSE-labeled T cells (4 × 105/well) were co-cultured in a 24-well cell culture plate in phenolred-free cell culture medium for 24 h or 48 h. To visualize the stimulator cell–T cell interaction at a higher magnification, cells were co-cultured for 24 h, fixed in 4% paraformaldehyde and washed once with medium. Subsequently, cells were analyzed by laser scanning microscopy (LSM 410, ZEISS) (Kriehuber et al., 2001). CellTrace™ CFSE and CellTracker™ Orange CMTMR were both purchased from Molecular Probes (Eugene, OR).
2.3. Generation of expression constructs encoding membrane-bound anti-CD3 single chain fragments
cDNA derived from hybridoma cells producing the anti-human CD3 antibody OKT3 (ATCC, Manassas, VA) was subjected to PCR amplification using primer pairs specific for the variable regions of the heavy chain (VH-for 5′ GGAATTCGCTAGCCCAGGTCCAGCTGCAGCAGTCT 3′, VH-rev 5′ GGGGGATCCGGTGACCGTGGTGCCTTGGCCCCAGTA 3′) and light chain (VL-for 5 GGAATTCGAGCTCCCAAATTGTTCTCACCCAGTCTCCA 3′ and VL-rev 5 GGGATCCCCACCGCCCCGGTTTATTTCCAACTTTGT 3′). The resulting PCR products were digested with Nhe I plus BstE II (VH) and Sac I plus BamH I (VL) and joined via a Sac I to BstE II fragment encoding a (G4S)3-linker by ligation. Two distinct DNA-fragments were generated by employing additional PCR and ligation steps: CD5L-OKT3scFv-CD28 encoded the OKT3-single chain antibody fragment flanked by the CD5 leader sequence and a BamH I to Not I fragment encoding the transmembrane and intracellular domains of human CD28, which was amplified using the primer pair (5′ CGCGGGGGATCCCCCAAGTCCCCTATTTCCCGG 3′ and 5′ GCGCCCGCGGCCGCTTTAGGAGCGATAGGCTGCGAAGT 3′), whereas CD5L-OKT3-CD14 encoded the OKT3-single chain antibody fragment flanked by the CD5 leader peptide and the leaderless human CD14 molecule generated by fusing a CD14 BamH I to Nhe I fragment, which was amplified using the primer pair (5′ CGCGGGGGATCCCACCACGCCAGAACCTTGTGA 3′ and 5′ CCTTGAGGCGGGAGTACGCT 3′) to the Nhe I to Not I fragment of CD14 cDNA. Both constructs were cloned into the retroviral expression vector pMMP and the integrity of the synthetic expression constructs was confirmed by DNA-sequence analysis.
The nucleotide sequences encoding the surface expressed anti-CD3 antibody fragments have been submitted to GenBank: accession ns. HM208751 – CD5L-OKT3-scFv-CD28 (protein_id ADN42858); and HM208750 – CD5L-OKT3-scFv-CD14 (protein_id ADN42857).
2.4. Generation of T cell stimulators
Bw5147 cells were retrovirally transduced to express the CD5L-OKT3-scFv-CD28 or the CD5L-OKT3-scFv-CD14 constructs. Transduction with the OKT3::CD28 yielded Bw5147 cells expressing anti-CD3 antibodies at low density; the Bw-anti-CD3low stimulator cells. Transduction with the OKT3::CD14 construct resulted in Bw5147 cells expressing high levels of membrane-bound anti-CD3 antibody fragment on their surface and were thus termed Bw-anti-CD3high stimulator cells. Single cell clones were obtained from both Bw lines and cell clones expressing homogenous amounts of membrane-bound anti-CD3 antibodies were selected for further use. cDNAs encoding human CD80, CD58, CD54, CD150, TL1A, 41BB-L and ICOS-L were PCR amplified from a human dendritic cell library and cloned into the retroviral expression vector pCJK2 generated in our laboratory. Integrity of these expression plasmids was confirmed by DNA sequencing. Using retroviral transduction these molecules were expressed on the T cell stimulator cells as described (Steinberger et al., 2004). Control stimulator cell lines expressing no human molecule were generated by treating T cell stimulator cells with supernatants derived from retroviral producer cell lines transfected with empty vector DNA or a vector encoding GFP.
2.5. T cell proliferation assays
All T cell proliferation assays were done in triplicates, means and SD are shown. For T cell proliferation assays human T cells (1 × 105/well) were co-cultured with irradiated (6000 rad) T cell stimulator cells (2 × 104/well) for 72 h. In some experiments Adalimumab (Humira, Abbott Laboratories, Chicago, IL) or Beriglobin P as control (CSL Behring GmbH, Marburg, Germany), was added at a final concentration of 10 μg/ml at the onset of culture. To assess T cell proliferation methyl-3[H]-thymidine (final concentration: 0.025 mCi; Perkin Elmer/New England Nuclear Corporation, Wellesley, MA) was added for the last 18 h prior harvesting of the cells. Methyl-3[H]-thymidine uptake was measured as described (Pfistershammer et al., 2004).
2.6. In vitro expansion of human T cells
Purified human T cells (5 × 105/well) were co-cultured in 1 ml medium with 1.2 × 105 irradiated anti-CD3high T cell stimulator cells expressing human costimulatory molecules as indicated. Following 7 days of culture, T cells were harvested, counted and analyzed for CD8+ expression. 5 × 105 T cells were re-cultured with 1.2 × 105 irradiated stimulator cells as described above. Five rounds of stimulation were performed. For each round of stimulation the T cell expansion factor was calculated by dividing the starting cell number by the cell number obtained after 7 days of stimulation.
2.7. Cellular cytotoxicity assay
Cytotoxic activity of expanded T cells was measured using a europium release assay kit (Delfia, Perkin Elmer) following the manufacturer's protocol. Briefly, expanded T cells (1 × 105/well) were incubated with the labeled target cells (5 × 103/well; Bw-anti-CD3high cells or Bw cells not expressing anti-CD3 as control) for 2 h at 37 °C. For detection of cell lysis-associated europium release 20 μl of supernatant was transferred to a 96-well flat bottom plate and 200 μl enhancement solution was added. Fluorescence was measured using a time-resolved fluorometer (Victor; Perkin Elmer). The percentage of specific cytotoxicity was calculated as described using the formula: (experimental release-spontaneous release)/(maximum release-spontaneous release) × 100 (Pfistershammer et al., 2009).
2.8. Cytokine measurement
For cytokine measurement supernatants of T cell proliferation assays were collected after 48 h and pooled from triplicate wells. IFN-γ, IL-10 and IL-13 were measured in the supernatants using the Luminex System 100 (Luminex, Texas, USA).
2.9. Statistics
Two-tailed Student t test was used to assess significance. IMB® SPSS statistics software was used for Box plot and for analysis of variance (ANOVA) in Fig. 2.
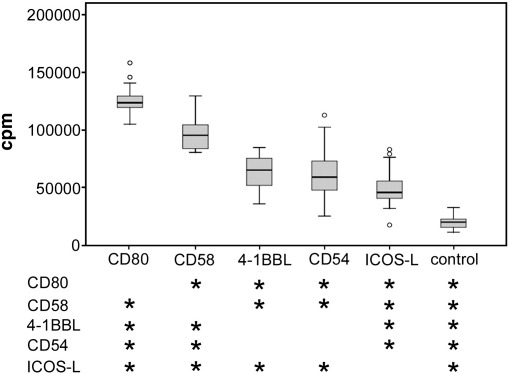
Fig. 2.
Comparison of different costimulatory ligands.
T cell stimulator lines expressing high levels of anti-CD3 antibodies and the indicated costimulatory ligands or no costimulatory molecule (control) were co-cultured with purified human T cells. 3[H]-thymidine uptake was assessed following 3 days of co-culture. Box plots show the results of 8 independent experiments with T cells from different donors. Circles indicate outliers. Stars indicate significant difference (p < 0.05, n = 8) from the stimulator cell type listed on the left.
3. Results
3.1. Generation of T cell stimulator cells
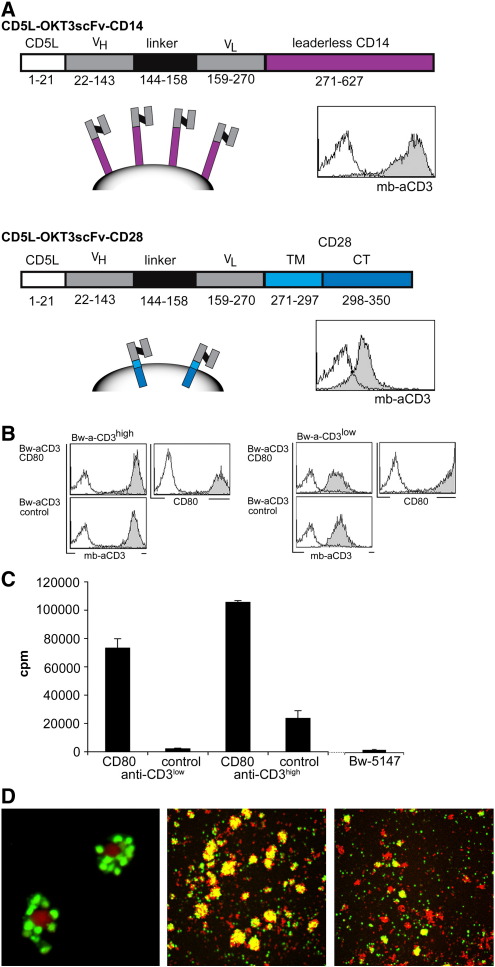
mAbs that trigger the T cell receptor complex by interacting with CD3 molecules are widely used to study the activation of T cells. We aimed to establish a cellular system that can give “Signal 1” to human T cells. In a first step we generated synthetic retroviral expression constructs that encode a CD5 leader peptide and a single chain antibody fragment of the anti-human CD3 antibody OKT3 fused to DNA sequences encoding the transmembrane and intracellular domains of human CD28 (CD5L-OKT3-scFv-CD28) or the leaderless human CD14 (CD5L-OKT3-scFv-CD14) molecule (Fig. 1A). These constructs were expressed on the murine thymoma line Bw5147. Their expression was assessed by flow cytometry using an anti-mouse IgG antibody that reacts with the variable regions of the anti-CD3 antibody. Whereas Bw cells expressing the CD5L-OKT3-scFv-CD14 construct displayed high levels of membrane-bound OKT3 antibody fragment on their surface (Bw-aCD3high), the CD5L-OKT3-scFv-CD28 molecule was expressed at a much lower density (Bw-aCD3low; Fig. 1A). Single cell clones that expressed homogeneous levels of membrane-bound anti-CD3 were established from both cell lines. Subsequently, both T cell stimulator cell lines were transduced to express human CD80 (Bw-aCD3high-CD80; Bw-aCD3low-CD80) or treated to express empty retroviral vector (Bw-aCD3high-control, Bw-aCD3low-control; Fig. 1B). In order to assess the T cell stimulatory capacity of these cell lines they were irradiated and co-cultured with purified human T cells. We found that T cell stimulator cells expressing low amounts of membrane-bound anti-CD3 antibody (mb-aCD3) and no human costimulatory molecules did not induce significant proliferation of purified human T cells. The low levels of cellular 3[H]-thymidine incorporation that were measured in these co-cultures are the result of residual uptake by the irradiated T cell stimulator cells since similar incorporation was observed in cultures of irradiated T cell stimulator cells where no human T cells were present. This indicates that the murine thymoma line Bw5147 that was used for the generation of our T cell stimulator cells does not harbour accessory molecules that can costimulate human T cells. By contrast T cell stimulator cells that co-express low levels of anti-CD3 antibody fragments and human CD80 elicited strong proliferative responses in human T cells. T cell stimulator cells expressing membrane-bound anti-CD3 antibodies at a high density induced moderate proliferation in human T cells even in the absence of human costimulatory molecules and as expected T cells activated with stimulator cells harbouring high levels of anti-CD3 in combination with human CD80 showed the highest proliferative response (Fig. 1C). To visualize the interaction of human T cells and stimulator cells, we performed co-culture experiments using CFSE-labeled T cells and CMTMR-labeled stimulator cells. Large clusters of T cells and stimulator cells expressing CD80 can be observed whereas much smaller clusters are formed when T cells were activated by stimulator cells expressing anti-CD3 but no human costimulatory molecule (Fig. 1D).
Fig. 1.
Generation and characterisation of T cell stimulator cells.
A) Schemes of expression constructs encoding membrane-bound anti-CD3 (mb-aCD3) single chain fragments. FACS analysis of Bw cells expressing the constructs is shown on the right. Open histograms: control cells; filled histograms: Bw cells expressing membrane-bound anti-CD3 single chain fragment. CD5L: CD5 leader; VH: variable domain of the heavy chain; VL: variable domain of the light chain; TM: transmembrane domain; CT: cytoplasmic domain.
B) Generation of T cell stimulator cells co-expressing anti-CD3 at high and low levels (Bw-anti-CD3high and Bw-anti-CD3low) and the costimulatory molecule CD80. Open histograms: control transduced cells; filled histograms: stimulator cells expressing CD80 or mock treated stimulator cells (control).
C) Human T cells were stimulated with T cell stimulator cells expressing anti-CD3high and anti-CD3low with and without CD80. 3[H]-thymidine uptake of irradiated Bw5147 cells in the absence of T cells is also shown (far right).
D) Confocal microscopy of CFSE-labeled T cells (green) co-cultured with CMTMR-labeled T-cell stimulator cells (red). Interaction of T cells and T cell stimulator cells (left). Cluster formation between T cells and stimulator cells expressing CD80 (middle) or stimulator cells expressing no costimulatory molecule (right).
3.2. Side by side comparison of different costimulatory molecules
T cell stimulator cells transduced to express different costimulatory molecules are excellent tools to compare these ligands regarding their capacity to activate human T cells. We have generated stimulator cell lines retrovirally expressing different costimulatory molecules at high levels (Fig. 2). The resultant cell lines were used to stimulate purified T cells isolated from different healthy donors and T cell proliferation was assessed. As shown in Fig. 2B stimulation of human T cells in the presence of the costimulatory molecules used in this study (CD80, ICOSL, CD58, CD54 and 4-1BBL) significantly enhanced T cell proliferation compared to T cells co-cultured with stimulator cells expressing no human costimulatory molecule. Furthermore, our data show that CD80 was the strongest costimulatory ligand tested in these experiments and demonstrate that among the other molecules analyzed CD58 is the most potent inducer of T cell proliferation.
3.3. The use of T cell stimulator cells to assess the role of immunomodulatory drugs in T cell activation
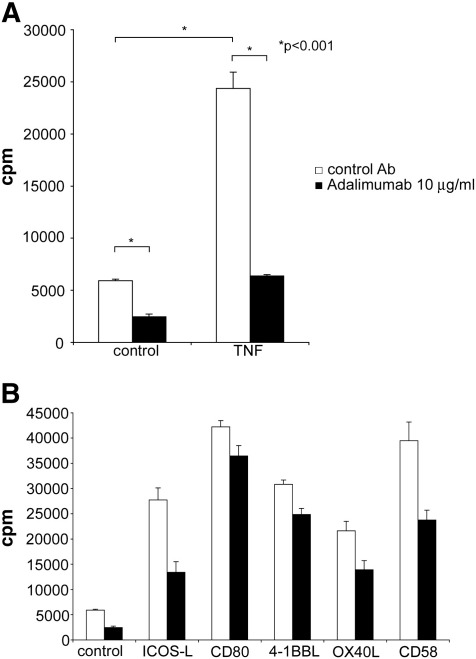
There is an increasing number of immunosuppressive and immunomodulatory drugs for treatment of patients suffering from autoimmune diseases and recipients of hematopoietic stem cells or solid organs. Many of these drugs target fast dividing cells whereas others specifically suppress T cells or counteract inflammatory processes. Antibodies or receptor fusion proteins that block the cytokine TNF-α are successfully used in patients suffering from psoriasis, rheumatoid arthritis and various other autoimmune diseases (Aringer and Smolen, 2008; Bosani et al., 2009; Taylor and Feldmann, 2009). TNF-α is a pleiotrophic cytokine and the beneficial effects of TNF-α blockade are mainly ascribed to its capacity to prevent and down-modulate proinflammatory processes. Whereas other members of the TNF-family have been shown to act as potent costimulatory molecules, few studies have addressed the ability of TNF-α to directly contribute to T cell activation processes. We found that expressing TNF-α on T cell stimulator cells enhances their ability to induce proliferation in purified human T cells (Fig. 3A). Furthermore, we show that Adalimumab, a humanized therapeutic antibody that targets TNF-α, reduced proliferation of T cells activated via different costimulatory molecules (Fig. 3B). Taken together these results indicate that TNF-α gives a costimulatory signal to human T cells and that TNF-α blockade reduces human T cell responses independent of accessory cells.
Fig. 3.
The therapeutic TNF-α blocking antibody Adalimumab down-modulates human T cell responses.
A) T cell stimulator cells expressing anti-CD3 antibodies at high levels (control) and T cell stimulator cells expressing anti-CD3 antibodies and human TNF-α were used to stimulate purified human T cells in the presence of control antibodies (control Ab) or the therapeutic anti-TNF-α antibody Adalimumab. B) T cell stimulator cells expressing the indicated costimulatory molecules were used to stimulate purified human T cells in the presence of control antibodies or Adalimumab (10 μg/ml). Data shown are representative for at least 5 independently performed experiments.
3.4. The use of T cell stimulator cells to assess the role of different costimulatory molecules in the in vitro expansion of human T cells
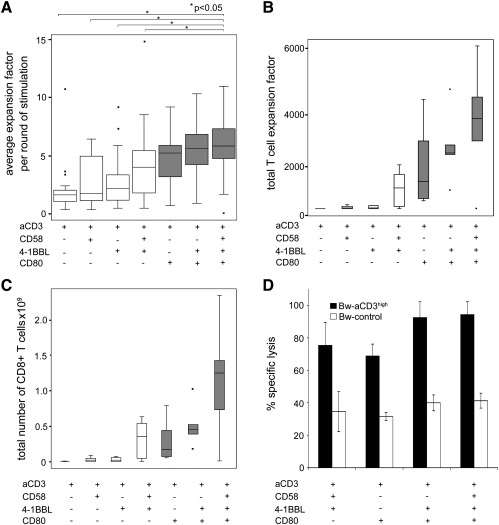
Adoptive T cell transfer is a promising therapeutic strategy in the treatment of malignancies, and to combat virus infections (Ho et al., 2002; June, 2007; Berger et al., 2009). Such approaches often depend on the efficient in vitro expansion of antigen specific T cells. We used T cell stimulator cells expressing individual costimulatory molecules or combinations thereof to assess their capacity to expand human T cells in vitro. In line with previous data we found that 4-1BB signals enhance the expansion of T cells costimulated via CD28 (Maus et al., 2002). Furthermore, our results demonstrate that costimulation via CD2 can also potently increase the expansion of human T cells. Stimulator cells co-expressing CD80, CD58 and 4-1BBL induced significantly stronger T cell expansion compared to stimulator cells not expressing CD80. This underlines the importance of CD28 signals and suggests that the combination of CD80, CD58 and 4-1BBL might be especially suited for the expansion of human T cells (Fig. 4). Importantly, we found that during 5 rounds of stimulation in the presence of these costimulatory ligands their effector function was retained as the expanded T cells were able to efficiently kill target cells expressing anti-CD3 antibodies as surrogate antigen (Fig. 4D).
Fig. 4.
Costimulatory signals and the in vitro expansion of human T cells.
T cells were subjected to five rounds of expansion using T cell stimulator cells expressing high levels of the indicated molecules. A) Box plots representing the average fold expansion per round of stimulation. T cell stimulator cells co-expressing CD80, CD58 and 4-1BBL induced significantly higher T cell expansion than the indicated T cell stimulator cells (p < 0.05). The total expansion of T cells during five rounds of stimulation (B) and the calculated CD8+ T cell number that would have been obtained from 5 × 105 T cells (C) is also shown. D) Effector function of T cells expanded for 5 rounds in the presence of the indicated costimulatory molecules was analyzed by a europium release assay. T cell stimulator cells expressing high levels of anti-CD3 (black bars) were used as target cells and Bw cells expressing no anti-CD3 served as control cells (white bars). Percentage of specific lysis is shown.
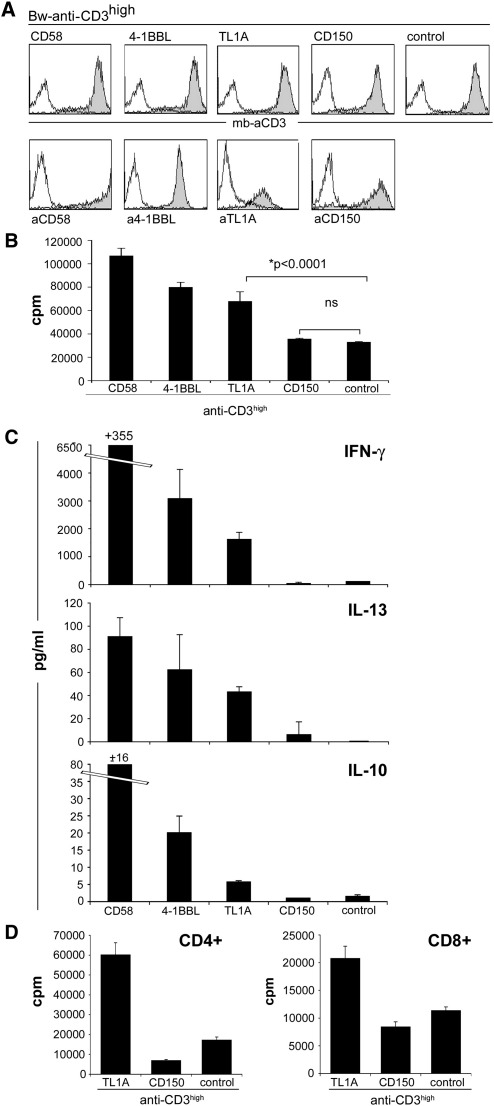
3.5. The use of T cell stimulator cells to assess the role of CD150 and TL1A in the activation of human T cells
There are a large number of human molecules that were described to costimulate T cell activation (Leitner et al., 2010). Although for several of these molecules such a role is well established, there are still some ligands where a limited number of studies have addressed their function in T cell stimulation. We have selected two such molecules, TL1A and CD150, to study their function in T cell activation using our system of stimulator cells (Fig. 5A). For comparison T cell stimulator cells expressing CD58, a member of the CD2 superfamily, and 4-1BBL, a member of the TNF-SF, which are well established costimulatory ligands were also used.
Fig. 5.
The role of CD150 and TL1A in the activation of human T cells.
A) Characterisation of T cell stimulator cells expressing anti-CD3high in conjunction with CD58, 4-1BBL, TL1A, CD150 or control stimulator cells expressing no costimulatory molecule by FACS. Upper panel filled histograms: expression of mb-anti-CD3 antibodies on stimulator cells; open histograms: control Bw cells. Lower panel: T cell stimulator cells expressing CD58, 4-1BBL, TL1A, and CD150 were probed with antibodies specific for these molecules (filled histograms). Open histograms: reactivity of the indicated antibodies with control Bw cells.
B) Human T cells were stimulated with stimulator cells expressing high levels of anti-CD3 in conjunction with CD58, 4-1BBL, TL1A, CD150 or control stimulator cells. Proliferation was assessed by measuring 3[H]-thymidine uptake. Presence of TL1A significantly enhanced T cell proliferation (p < 0.0001, n = 9), whereas CD150 had no effect on human T cells proliferation (ns, not significant). Data show +/− SD of triplicates from one experiment. The experiment shown is representative for the 9 independently performed.
C) Human T cells were stimulated with stimulator cells expressing high levels of anti-CD3 in conjunction with CD58, 4-1BBL, TL1A, CD150 or no costimulatory molecule (control). Culture supernatant was harvested after 72 h and subjected to multiplex cytokine measurement. The experiment shown is representative for the 3 independently performed.
D) Purified human CD4+ and CD8+ T cells were co-cultured with stimulator cells expressing anti-CD3high in conjunction with TL1A, CD150 or control. Data show +/− SD of triplicates from one experiment. The shown experiment was repeated with similar outcome.
TL1A (TNF-like molecule 1A), the newest member of the TNF-superfamily, is described to costimulate murine and human T cell proliferation via interaction with its receptor death receptor 3 (DR3, TRAMP) (Migone et al., 2002; Pappu et al., 2008; Zhan et al., 2009). In our experiments T cell stimulator cells expressing high levels of anti-CD3 and TL1A strongly enhanced the proliferation of human T cells (Fig. 5B). This costimulatory effect was observed with CD4+ and CD8+ T cells (Fig. 5D). In line with previous studies TL1A stimulation resulted in the induction of IFN-γ (Biener-Ramanujan et al., 2010). In addition, we obtained elevated levels of IL-10 and IL-13 in supernatants of TL1A stimulated T cell cultures (Fig. 5C).
CD150 (SLAM, signaling lymphocyte activating molecule) is a CD2 family member, expressed on activated human T cells. All previous studies that reported a costimulatory role of this molecule were based on the use of monoclonal antibodies to trigger the CD150 molecule on T cells (Cocks et al., 1995; Aversa et al., 1997; Howie et al., 2002). CD150 is a self-ligating molecule and no other binding partners have been described. Thus, we wanted to analyze whether the costimulatory effect was also observed upon engagement of T cell-expressed CD150 with its natural ligand. Therefore, we generated stimulator cells expressing CD150 in conjunction with anti-CD3. When co-culturing these stimulator cells with human T cells, no significant contribution of this interaction to T cell proliferation and cytokine production was observed (Fig. 5B,C). In some of our experiments reduced proliferation rates of human T cells were observed in the presence of human CD150 but additional experiments are required to confirm that CD150 can function as a negative regulator of T cell responses.
4. Disscussion
During APC–T cell interaction a complex interplay of numerous cell surface molecules modulates cellular immune responses by either enhancing or inhibiting T cell receptor complex signalling. Thus, assessing the function of individual costimulatory ligands using natural APC is a difficult task. With our T cell stimulator cells we have generated an experimental tool for studying individual costimulatory ligands in a cellular system, but detached from the context of numerous other molecules involved in the regulation of T cell activation that are expressed on professional APC. Whereas similar cellular systems that have been termed artificial APC (aAPC) use cells engineered to express Fc-γ receptors (CD32 or CD64) and depend on the addition of anti-CD3 antibodies (Thomas et al., 2002; Suhoski et al., 2007; Gong et al., 2008) we used cell lines that stably express membrane-bound anti-CD3 antibody fragments. Using different anti-CD3 expression constructs we have generated two cell clones that stably express different levels of anti-CD3 antibody fragments: A construct where the anti-CD3 antibody fragments are linked to the transmembrane domain of human CD28 molecules yielded Bw-aCD3low stimulator cells that give a weak signal 1 to human T cells, whereas a construct encoding anti-CD3 antibody fragments fused to the human CD14 molecule was used to generate cells expressing high levels of GPI-anchored anti-CD3 antibody fragments (Bw-aCD3high; Fig. 1). The GPI-anchored anti-CD3 antibody fragment is efficiently targeted to lipid rafts and has also successfully been used for the stimulation and manipulation of human T cells with immunosomes — virus-like particles decorated with TCR/CD3 ligands, costimulatory molecules and modified cytokines (Derdak et al., 2006; Kueng et al., 2007; Leb et al., 2009; Kueng et al., 2010). Another important difference between aAPC and stimulator cells is the cell type that is used as a scaffold: the former is based on the human K562 cell line and T cell stimulator cells are derived from the murine thymoma line Bw5147. Whereas K562 cells contain surface molecules that enhance T cell–APC interactions (Suhoski et al., 2007), Bw cells appear to be devoid of molecules that promote the proliferation of human T cells that receive a weak signal 1 (Fig. 1B). Thus, T cell stimulator cells are especially suited to study molecules that exert weak costimulatory effects. Furthermore, with this system it is also possible to compare different accessory molecules regarding their capacity to costimulate activation and proliferation of human T cells. Experiments where we have performed a side by side comparison of ligands belonging to different molecule families demonstrated a potent ability of CD58 to costimulate the activation of human T cells (Fig. 2).
In addition to the numerous different immunosuppressive drugs that are already used in the clinic to down-modulate T cell responses there are many additional compounds or biologics that are currently tested regarding their efficacy and safety for human use. Especially in the case of antibodies that often have limited or no reactivity with the non-human orthologues of their target antigens, extensive in vitro testing in human systems is highly warranted. Since costimulators govern the activation of T cells, their interplay with T cell suppressive antibodies and drugs is of great interest. Here, we have used our system of T cell stimulator cells to analyze the effect of Adalimumab, a therapeutic antibody to TNF-α, on T cell activation. We show that TNF-α has a costimulatory effect on human T cells and that TNF-α blockade reduces the proliferation of T cells, independent of accessory cells (Fig. 3). Adalimumab reduced T cell responses, regardless of the molecules used for their activation. However, we have observed that the capacity of some therapeutic antibodies and immunosuppressive drugs to diminish T cell proliferation and cytokine production is potently modulated by different costimulatory signals (our unpublished results).
The efficient in vitro expansion of antigen specific T cells crucially depends on appropriate costimulatory signals to ensure the generation of large amounts of potent effector cells. Different combinations of costimulatory ligands can be readily expressed on stimulator cells. The resultant stimulator cell lines can be tested in parallel to identify combinations of stimulatory molecules that potently drive expansion of human T cells in vitro. Our results indicate that concomitant stimulation via their CD28, CD2 and 4-1BB receptors leads to an efficient expansion of T cells, which retain their effector function during several rounds of stimulations (Fig. 4). These results, together with our findings summarized in Fig. 2, underline the potency and importance of the CD2–CD58 pathway for the activation of human T cells. CD2 was one of the first T cell costimulatory receptors identified (Meuer et al., 1984) and despite its importance this pathway is currently receiving limited attention. This is in part due to the fact that CD58 lacks a murine orthologue and demonstrates the current emphasis on mouse model systems to study the costimulatory pathways.
There are an ever growing number of ligands that have been implicated to play a role in T cell costimulatory processes and contradictory results have been reported for several of these molecules (Leitner et al., 2010). We believe that T cell stimulator cells are especially suited to assess the function of accessory molecules during T cell activation since they allow analyzing human T cell responses under conditions that only differ regarding the presence of the molecules of interest. We have recently used stimulator cells expressing PD-L2 and B7-H3, two members of the extended B7 family, to address their function during the activation of human T cells (Pfistershammer et al., 2006; Leitner et al., 2009). In these studies we could show that these molecules consistently inhibited T cell responses and our experiments did give any evidence for positive costimulatory functions for human PD-L2 and B7-H3. The CD2 superfamily member CD150 and the TNF-SF member TL1A have both been described to costimulate T cell activation. CD150 is a self-ligating receptor, whereas TL1A binds to DR3 a member of the TNFR-SF. However, few studies on these molecules have directly analyzed the consequences of the interaction of CD150 or TL1A with human T cells. In the present study we have generated T cell stimulator cell lines expressing CD150 and TL1A and used them to stimulate purified human T cells. Our results demonstrate that the presence of TL1A during T cell activation significantly costimulates their proliferation and production of cytokines, whereas T cells stimulated in the presence of stimulator cells expressing CD150 did not show enhanced proliferation and cytokine production. Previous studies that have described a positive costimulatory function for CD150 have used antibodies to crosslink the CD150 molecules on T cells (Cocks et al., 1995; Aversa et al., 1997). In contrast, we have used T cell stimulator cells expressing its natural ligand CD150, to assess the role of CD150–CD150 interaction in the activation of T cells. Our results, which suggest that CD150 does not function as a classical T cell costimulatory molecule, underline the importance of using natural ligands to study the functional consequences of receptor–ligand pairs implicated in T cell activation processes. The homophilic interaction of CD150 is of particular low affinity (Kd 200 mM; (Chattopadhyay et al., 2009)), which might explain the different outcome of our experiments compared to studies that used antibodies. It has previously been suggested that antibody-induced SLAM/CD150 activation may not fully mimic the physiological role of CD150, because mice deficient in this molecule exhibit essentially normal levels of IFN-γ, whereas the ligation of T cell-expressed CD150 by antibodies promotes strong IFN-γ secretion (Ostrakhovitch and Li, 2006). Further studies are required to address the physiological role of CD150 during human T cell activation. Since T cells that express costimulatory ligands can receive potent costimulatory signals (“autocostimulation”) it is also possible that homotypic interaction of CD150 in cis plays a role during human T cell activation (Stephan et al., 2007).
Taken together our results demonstrate that the system of T cell stimulator cells is a useful tool to assess the function of costimulatory ligands. In particular they are suited to compare the function of individual costimulatory molecules and analyze their effect on different T cell subsets and in context of a strong or weak signal 1. Since professional APC like DC harbour stimulatory as well as inhibitory ligands, the interplay of positive and negative signals determines the outcome of T cell responses. We have previously shown that combinations of costimulatory molecules can be expressed and analyzed on T cell stimulator cells (Kober et al., 2008). We are currently using our system of stimulator cells to analyze the interplay of defined costimulatory and coinhibitory molecules during the activation of human T cells. Studies on individual costimulatory pathways can complement investigations using experimental systems employing natural human APC or animal studies to get a better insight into the complex interplay of the numerous accessory surface molecules that govern human T cell responses.
Acknowledgements
We appreciate the excellent technical assistance of Christoph Klauser, Margarete Merio, Petra Cejka and Claus Wenhart. We thank Vera Kaiser, Graz University of Technology, for help with the statistical analyses. This study was supported by a grant from the Austrian Science Fund (FWF p21964-B20), a grant from the Austrian National Bank 12731 and in part by a grant from Abbott Austria. Judith Leitner is supported by a Doc fForte fellowship from the Austrian Academy of Science. WFP is supported by SFB grant 1816 from the Austrian Science Fund and by the Christian Doppler Society. The authors declare no conflict of interest.
References
- Aringer M., Smolen J.S. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin. Drug Saf. 2008;7:411. doi: 10.1517/14740338.7.4.411. [DOI] [PubMed] [Google Scholar]
- Aversa G., Chang C.C., Carballido J.M., Cocks B.G., de Vries J.E. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J. Immunol. 1997;158:4036. [PubMed] [Google Scholar]
- Berger C., Turtle C.J., Jensen M.C., Riddell S.R. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr. Opin. Immunol. 2009;21:224. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener-Ramanujan E., Gonsky R., Ko B., Targan S.R. Functional signaling of membrane-bound TL1A induces IFN-gamma expression. FEBS Lett. 2010;584:2376. doi: 10.1016/j.febslet.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Blank C., Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007;56:739. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosani M., Ardizzone S., Porro G.B. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chattopadhyay K., Lazar-Molnar E., Yan Q., Rubinstein R., Zhan C., Vigdorovich V., Ramagopal U.A., Bonanno J., Nathenson S.G., Almo S.C. Sequence, structure, function, immunity: structural genomics of costimulation. Immunol. Rev. 2009;229:356. doi: 10.1111/j.1600-065X.2009.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks B.G., Chang C.C., Carballido J.M., Yssel H., de Vries J.E., Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- Derdak S.V., Kueng H.J., Leb V.M., Neunkirchner A., Schmetterer K.G., Bielek E., Majdic O., Knapp W., Seed B., Pickl W.F. Direct stimulation of T lymphocytes by immunosomes: virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules. Proc. Natl. Acad. Sci. USA. 2006;103:13144. doi: 10.1073/pnas.0602283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M.L., Larsen C.P. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol. Rev. 2009;229:294. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W., Ji M., Cao Z., Wang L., Qian Y., Hu M., Qian L., Pan X. Establishment and characterization of a cell based artificial antigen-presenting cell for expansion and activation of CD8+ T cells ex vivo. Cell. Mol. Immunol. 2008;5:47. doi: 10.1038/cmi.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Ho W.Y., Yee C., Greenberg P.D. Adoptive therapy with CD8(+) T cells: it may get by with a little help from its friends. J. Clin. Invest. 2002;110:1415. doi: 10.1172/JCI17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie D., Simarro M., Sayos J., Guirado M., Sancho J., Terhorst C. Molecular dissection of the signaling and costimulatory functions of CD150 (SLAM): CD150/SAP binding and CD150-mediated costimulation. Blood. 2002;99:957. doi: 10.1182/blood.v99.3.957. [DOI] [PubMed] [Google Scholar]
- June C.H. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007;117:1466. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober J., Leitner J., Klauser C., Woitek R., Majdic O., Stockl J., Herndler-Brandstetter D., Grubeck-Loebenstein B., Reipert B.M., Pickl W.F., Pfistershammer K., Steinberger P. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur. J. Immunol. 2008;38:2678. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriehuber E., Breiteneder-Geleff S., Groeger M., Soleiman A., Schoppmann S.F., Stingl G., Kerjaschki D., Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001;194:797. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng H.J., Leb V.M., Haiderer D., Raposo G., Thery C., Derdak S.V., Schmetterer K.G., Neunkirchner A., Sillaber C., Seed B., Pickl W.F. General strategy for decoration of enveloped viruses with functionally active lipid-modified cytokines. J. Virol. 2007;81:8666. doi: 10.1128/JVI.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng H.J., Manta C., Haiderer D., Leb V.M., Schmetterer K.G., Neunkirchner A., Byrne R.A., Scheinecker C., Steinberger P., Seed B., Pickl W.F. Fluorosomes: a convenient new reagent to detect and block multivalent and complex receptor–ligand interactions. FASEB J. 2010;24:1572. doi: 10.1096/fj.09-137281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leb V.M., Jahn-Schmid B., Kueng H.J., Schmetterer K.G., Haiderer D., Neunkirchner A., Fischer G.F., Hartl A., Thalhamer J., Steinberger P., Bohle B., Seed B., Pickl W.F. Modulation of allergen-specific T-lymphocyte function by virus-like particles decorated with HLA class II molecules. J. Allergy Clin. Immunol. 2009;124:121. doi: 10.1016/j.jaci.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Leitner J., Klauser C., Pickl W.F., Stockl J., Majdic O., Bardet A.F., Kreil D.P., Dong C., Yamazaki T., Zlabinger G., Pfistershammer K., Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: no evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009;39:1754. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J., Grabmeier-Pfistershammer K., Steinberger P. Receptors and ligands implicated in human T cell costimulatory processes. Immunol. Lett. 2010;128:89. doi: 10.1016/j.imlet.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Li X.C., Rothstein D.M., Sayegh M.H. Costimulatory pathways in transplantation: challenges and new developments. Immunol. Rev. 2009;229:271. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- Maus M.V., Thomas A.K., Leonard D.G., Allman D., Addya K., Schlienger K., Riley J.L., June C.H. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002;20:143. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- Meuer S.C., Hussey R.E., Fabbi M., Fox D., Acuto O., Fitzgerald K.A., Hodgdon J.C., Protentis J.P., Schlossman S.F., Reinherz E.L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984;36:897. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Migone T.S., Zhang J., Luo X., Zhuang L., Chen C., Hu B., Hong J.S., Perry J.W., Chen S.F., Zhou J.X., Cho Y.H., Ullrich S., Kanakaraj P., Carrell J., Boyd E., Olsen H.S., Hu G., Pukac L., Liu D., Ni J., Kim S., Gentz R., Feng P., Moore P.A., Ruben S.M., Wei P. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16:479. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- Nurieva R.I., Liu X., Dong C. Yin–Yang of costimulation: crucial controls of immune tolerance and function. Immunol. Rev. 2009;229:88. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrakhovitch E.A., Li S.S. The role of SLAM family receptors in immune cell signaling. Biochem. Cell Biol. 2006;84:832. doi: 10.1139/o06-191. [DOI] [PubMed] [Google Scholar]
- Pappu B.P., Borodovsky A., Zheng T.S., Yang X., Wu P., Dong X., Weng S., Browning B., Scott M.L., Ma L., Su L., Tian Q., Schneider P., Flavell R.A., Dong C., Burkly L.C. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J. Exp. Med. 2008;205:1049. doi: 10.1084/jem.20071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfistershammer K., Majdic O., Stockl J., Zlabinger G., Kirchberger S., Steinberger P., Knapp W. CD63 as an activation-linked T cell costimulatory element. J. Immunol. 2004;173:6000. doi: 10.4049/jimmunol.173.10.6000. [DOI] [PubMed] [Google Scholar]
- Pfistershammer K., Klauser C., Pickl W.F., Stockl J., Leitner J., Zlabinger G., Majdic O., Steinberger P. No evidence for dualism in function and receptors: PD-L2/B7-DC is an inhibitory regulator of human T cell activation. Eur. J. Immunol. 2006;36:1104. doi: 10.1002/eji.200535344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfistershammer K., Klauser C., Leitner J., Stockl J., Majdic O., Weichhart T., Sobanov Y., Bochkov V., Saemann M., Zlabinger G., Steinberger P. Identification of the scavenger receptors SREC-I, Cla-1 (SR-BI), and SR-AI as cellular receptors for Tamm–Horsfall protein. J. Leukoc. Biol. 2008;83:131. doi: 10.1189/jlb.0407231. [DOI] [PubMed] [Google Scholar]
- Pfistershammer K., Lawitschka A., Klauser C., Leitner J., Weigl R., Heemskerk M.H., Pickl W.F., Majdic O., Bohmig G.A., Fischer G.F., Greinix H.T., Steinberger P. Allogeneic disparities in immunoglobulin-like transcript 5 induce potent antibody responses in hematopoietic stem cell transplant recipients. Blood. 2009;114:2323. doi: 10.1182/blood-2008-10-183814. [DOI] [PubMed] [Google Scholar]
- Saunders P.A., Hendrycks V.R., Lidinsky W.A., Woods M.L. PD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesion. Eur. J. Immunol. 2005;35:3561. doi: 10.1002/eji.200526347. [DOI] [PubMed] [Google Scholar]
- Steinberger P., Majdic O., Derdak S.V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W.F., Stockl J., Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 2004;172:2352. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- Stephan M.T., Ponomarev V., Brentjens R.J., Chang A.H., Dobrenkov K.V., Heller G., Sadelain M. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat. Med. 2007;13:1440. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- Suhoski M.M., Golovina T.N., Aqui N.A., Tai V.C., Varela-Rohena A., Milone M.C., Carroll R.G., Riley J.L., June C.H. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther. 2007;15:981. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.C., Feldmann M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5:578. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- Thomas A.K., Maus M.V., Shalaby W.S., June C.H., Riley J.L. A cell-based artificial antigen-presenting cell coated with anti-CD3 and CD28 antibodies enables rapid expansion and long-term growth of CD4 T lymphocytes. Clin. Immunol. 2002;105:259. doi: 10.1006/clim.2002.5277. [DOI] [PubMed] [Google Scholar]
- Watts T.H. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Zhan C., Yan Q., Patskovsky Y., Li Z., Toro R., Meyer A., Cheng H., Brenowitz M., Nathenson S.G., Almo S.C. Biochemical and structural characterization of the human TL1A ectodomain. Biochemistry. 2009;48:7636. doi: 10.1021/bi900031w. [DOI] [PMC free article] [PubMed] [Google Scholar]