Summary
The B7 family member programmed-death-1-ligand 2 (PD-L2/B7-DC) is a ligand for programmed-death-receptor 1 (PD-1), a receptor involved in negative regulation of T cell activation. Several independent studies have reported that PD-L2 can however also potently costimulate murine T cells via an additional yet unidentified receptor.
In this study we evaluated the contribution of PD-L2 to the activation of human T cells using a novel system of engineered T cell stimulators that expresses membrane-bound anti-CD3 antibodies. Analyzing early activation markers, cytokine production and proliferation we found PD-L2 to consistently inhibit T cell activation. PD-L2 inhibition affected CD4+ and CD8+ T cell and was not abrogated by costimulation via CD28. Blocking PD-1 reverted the inhibitory effect of PD-L2 demonstrating involvement of this pathway. In human T cells we found no evidence for any of the costimulatory effects described for PD-L2 in murine systems. In line with our functional data that do not point to stimulatory PD-L2-ligands, we show that binding of PD-L2-Ig to activated human T cells is abrogated by PD-1 antibodies. Our results demonstrate that PD-L2 negatively regulates human T cell activation and thus might be a candidate molecule for immunotherapeutic approaches aimed to attenuate pathological immune responses.
Keywords: T cells, costimulatory molecules, cellular immunology, immunoregulation
Introduction
Efficient T cell activation requires two different signals, provided by MHC-TCR interaction (signal 1) and additional stimuli (signal 2) {Mueller, 1989 #98}. The outcome of a T cell activation process is tightly regulated by these additional stimuli provided by both positive and negative accessory molecules. The primary costimulators, the B7 molecules B7.1 and B7.2 convey via CD28 stimulating signals to T cells {Linsley, 1991 #82}. Via CTLA-4, T cells can however also receive negative signals from B7.1 and B7.2 {Waterhouse, 1996 #181; Waterhouse, 1996 #181}. Recently additional members of the B7-family have been described and termed B7-homologs {Carreno, 2004 #15; Sharpe, 2002 #140}. These molecules interact with inducible ligands on T cells. For B7-H2/ICOS-L a costimulatory role is univocally established by numerous studies {Gonzalo, 2001 #39; Swallow, 1999 #169}. For PD-L1/B7-H1 and B7-H3 costimulatory as well as inhibitory functions have been described {Chapoval, 2001 #20; Dong, 1999 #31; Freeman, 2000 #35; Ling, 2003 #81; Selenko-Gebauer, 2003 #139; Steinberger, 2004 #148; Suh, 2003 #165}. B7-H4 (B7x, B7S1) was shown to have negative effects on the activation of murine T cells {Prasad, 2003 #122; Sica, 2003 #144; Zang, 2003 #188}. Studies on mice deficient in B7 homologs or their receptors show deregulation of immune processes and enhanced susceptibility to autoimmune phenomena accentuating the importance of these molecules {Dong, 2001 #28; Latchman, 2004 #78; Nishimura, 1999 #103; Suh, 2003 #165}.
In contrast to the other B7-homologs described up to date, PD-L2/B7-DC is mainly expressed on dendritic cells (DC) and activated macrophages {Tseng, 2001 #175}. PD-L2 has been described to interact, like PD-L1, with PD-1, which is known to have an inhibitory function in T cell activation {Freeman, 2000 #35}. In line with this, several studies on murine T cells describe a T cell inhibitory function for PD-L2 {Carter, 2002 #16; Latchman, 2001 #77}. However numerous reports show a positive role of PD-L2 in T cell activation and studies on PD-1 deficient mice show that PD-L2 can still interact with and convey costimulatory effects to PD-1(−/−) T cells, raising the hypothesis of a second, costimulatory receptor, which could explain the divergent results obtained in different studies and settings {Liu, 2003 #83; Shin, 2003 #141; Shin, 2005 #142; Tseng, 2001 #175; Wang, 2003 #178}.
Currently all functional data on human PD-L2 are based on the use of blocking mAbs. Inhibiting PD-L2 and PD-L1 with mAbs on DC or when activating PBMC in primary and second stages resulted in enhanced T cell activation and cytokine production suggesting an inhibitory role of PD-L2 on human T cells {Brown, 2003 #10; Cai, 2004 #11}. Furthermore blocking PD-L2 on endothelial cells was reported to enhance CD8+ T cell activation {Rodig, 2003 #129}.
In this study we wanted to directly evaluate the functional role of human PD-L2 in T cell activation. We therefore established a novel system based on engineered T cell stimulator cells that enables us to study the contribution of PD-L2 to T cell activation in a cellular system but detached from the context of numerous molecules regulating T cell activation that are present on cell membranes of professional APC. We found PD-L2 to inhibit T cell activation and cytokine production in primary as well as in pre-stimulated T cells. Furthermore antibodies to PD-1 completely blocked the interaction of PD-L2-Ig with human T cells, strongly suggesting that PD-1 is the sole PD-L2 receptor on these cells. From our data we conclude that PD-L2 is a negative regulator of human T cell activation and that there is no evidence for costimulatory PD-L2 receptors on human T cells.
Results
Generation of engineered T cell stimulators
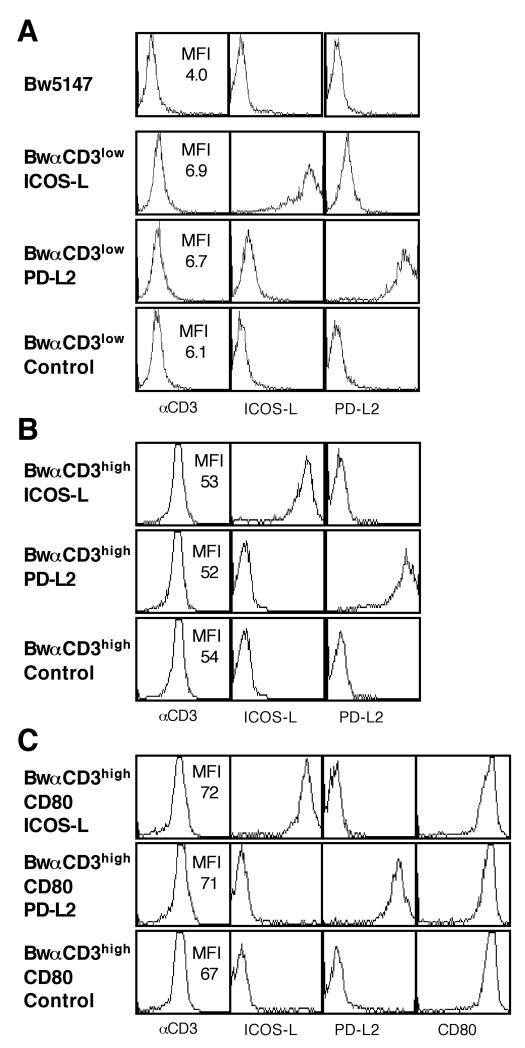
Cells engineered to express MHC molecules have been previously used to study the functional role of B7-family members on the activation of primary murine T cells {Latchman, 2001 #77; Carter, 2002 #16}. Such approaches however rely on TCR-transgenic animals and comparable cellular systems are thus not available to activate primary human T cells. We wanted to assess the role of human PD-L2/B7-DC in T cell activation in a cellular system that allows stimulating primary human T cells. To this end we generated T cell stimulators by transducing Bw cells with constructs encoding membrane-bound single chain antibodies (scFv) to human CD3 (mb-αCD3-scFv). When we used a construct where the anti-CD3-scFv is fused to human CD28 we obtained stimulator cells that expressed the mb-anti-CD3 antibody fragment at low levels (BwαCD3low stimulator cells, Fig. 1 A). Expression of a construct where the anti-CD3-scFv is fused to the human CD14 molecule led to higher expression of the mb-anti-CD3-scFv, yielding the BwαCD3high stimulator cells (Fig. 1 B). To study the function of PD-L2 in context of strong costimulatory signals, Bw cells were transduced to generate T cell stimulators that co-express the mb-anti-CD3-scFv with a strong costimulator (BwαCD3high/CD80 stimulator cells, Fig. 1 C). To assess the functional role of PD-L2 the different types of stimulator cell lines were then transduced to express human PD-L2 or for control purposes ICOS-L or with control vector (Fig. 1 A-C). The Bw5147 cell line does not express murine PD-L2 and low levels (MFI of 13) of murine PD-L1 (data not shown).
Figure 1.
Characterization of the T cell stimulator cell lines
To generate T cell stimulator cells the Bw5147 cell line was retrovirally transduced to express membrane-bound anti-CD3-antibody fragments at low density (BwαCD3low stimulator cells; A) or high denstiy (BwαCD3high stimulator cells; B). T cell stimulator cells expressing CD80 were also generated (BwαCD3high/CD80 stimulator cells, C). T cell stimulators were transduced to express PD-L2, ICOS-L or mock-transduced.
PD-L2 does not costimulate proliferation of human T cells
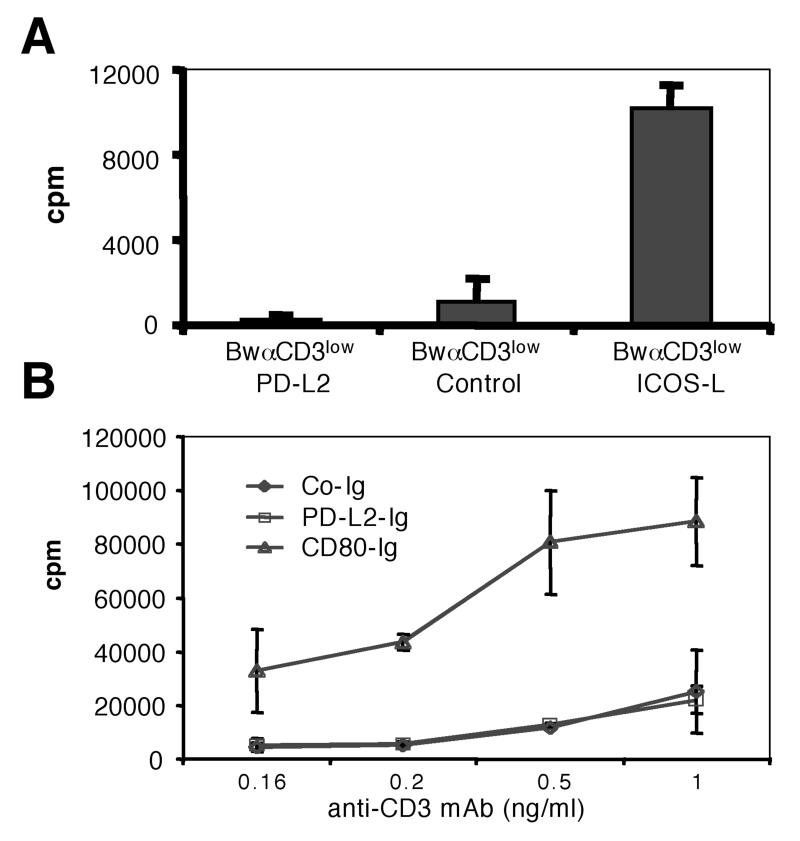
Murine T cells receiving suboptimal stimulation via immobilized CD3 antibodies were shown to be strongly costimulated by PD-L2 {Tseng, 2001 #175; Wang, 2003 #178}. Currently it has not been reported whether PD-L2 can also costimulate human T cells that receive weak stimuli through their TCR complex (Signal 1). To address this we co-cultured human T cells with irradiated BwαCD3low stimulator cells expressing PD-L2, ICOS-L or control stimulator cells expressing mb-αCD3 only. Upon co-culture with the control stimulator cells, T cells did not proliferate (Fig. 2 A). This indicates that the Bw cell line does not harbour significant amounts of molecules that can costimulate the weak signal 1. Expression of the costimulatory B7 family member ICOS-L on the BwαCD3low T cell stimulators however induced significant proliferation of human T cells. In contrast expression of high levels of PD-L2 on these stimulator cells did not induce T cell proliferation indicating that PD-L2 does not act costimulatory on human T cells receiving suboptimal signals via the TCR-complex (Fig. 2 A). We also tested the costimulatory potential of PD-L2 by using immobilized anti-CD3 mAb and PD-L2-Ig to stimulate human T cells. However in line with the results obtained with the stimulator cells using immobilized PD-L2-Ig in the same condition as Tseng and coworkers (0.1 μg/ml; {Tseng, 2001 #175}, we did again not obtain any costimulatory effect (data not shown). Since it is possible that higher densities of PD-L2 are required to exert costimulatory functions on human T cells, we also tested higher PD-L2-Ig concentrations (1 μg/ml). Even under these conditions PD-L2-Ig had no costimulatory activity, whereas CD80-Ig costimulated proliferation of human T cells (Fig. 2 B).
Figure 2.
PD-L2 does not costimulate proliferation of human T cells
A, Human T cells were co-cultured with stimulator cell lines expressing low amounts of membrane bound anti CD3 (BwαCD3low) and the indicated molecules for 72 hours.
B, Human T cells were cultured in the presence of immobilized anti-CD3 antibodies and indicated Ig-fusion proteins (coated at 1 μg/ml) for 72 hours. Mean and standard deviation (SD) are shown. Both experiments were repeated three times with similar outcome.
PD-L2 inhibits T cell proliferation
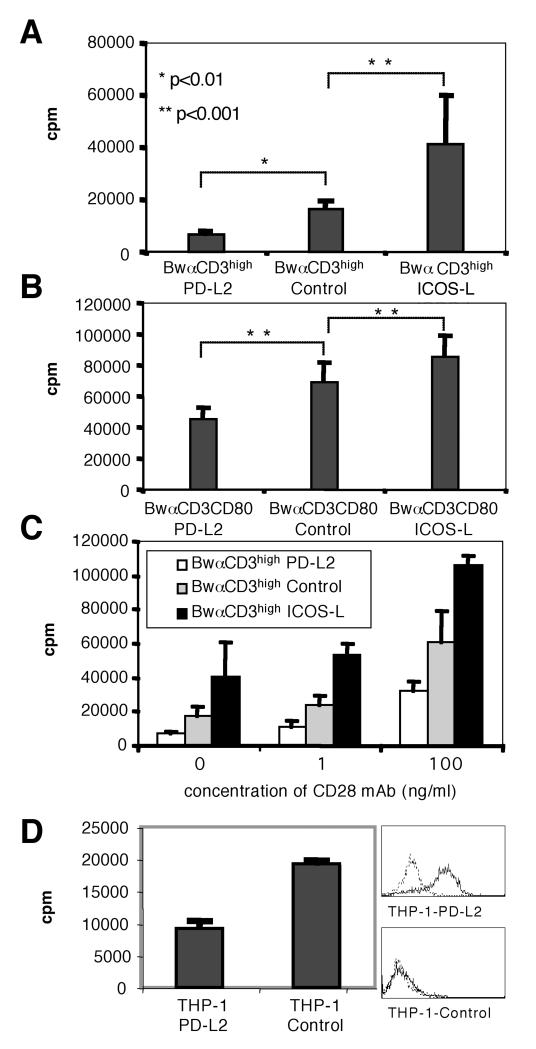
To further assess the functional role of human PD-L2, we used T cell stimulators expressing high levels of mb-αCD3 (Bw-αCD3high) to activate human T cells. These cells transfer a strong signal to the TCR-complex thereby inducing T cell proliferation in the absence of costimulatory signals (Fig. 3 A). In line with its well-established costimulatory role, presence of ICOS-L on these cells strongly enhanced these T cell responses. In contrast stimulator cells expressing PD-L2 induced significantly reduced T cell proliferation compared to stimulation with control stimulator cells expressing mb-α CD3 only (Fig. 3 A). Since PD-L2 is mainly expressed on professional APC like DC, we analysed whether costimulation provided by CD80 would abrogate the inhibitory effect of PD-L2. For this purpose we used stimulator cells co-expressing mb-αCD3 and human CD80 (BwαCD3high/CD80) to stimulate T cells as described above. These cells induced a much higher proliferation rate in human T cells and expression of ICOS-L on these stimulator cells further enhanced T cell proliferation. Presence of PD-L2 again inhibited the proliferation of T cells indicating that also strong costimulatory signals do not abrogate the inhibitory function of PD-L2 (Fig. 3 B). Similar results were obtained when adding anti-CD28 antibody to co-cultures of BwαCD3high cells at different concentrations to provide signal 2 (Fig. 3 C).
Figure 3.
PD-L2 inhibits T cell proliferation
A, T cells were co-cultured with stimulator cell lines expressing high levels of membrane-bound anti CD3 (BwαCD3high) and the indicated molecules. T cell proliferation induced with stimulator cells expressing PD-L2 was significantly reduced (p<0.01; n=8). Presence of ICOS-L on stimulator cells enhanced proliferation compared to control cells (p<0.001; n=8).
B, T cells were activated with stimulator cell lines co-expressing high levels of membrane-bound anti CD3 and human CD80 (BwαCD3high/CD80) and PD-L2, ICOS-L or control vector as indicated.
C, T cells were co-cultured with stimulator cell lines expressing high levels of membrane bound anti CD3 (BwαCD3high) and the indicated molecules. CD28 mAb was added to co-cultures in indicated concentrations.
In the presence of CD28 costimulation the reduction in T cell proliferation induced with stimulator cells expressing PD-L2 compared to control stimulators was statistically significant (p<0.001; n=8). Presence of ICOS-L on stimulator cells significantly enhanced proliferation compared to control stimulators when costimulation was provided (p<0.001; n=8).
D, Allogenic T cells were stimulated with the human cell line THP-1 transduced with PD-L2 or control vector. Expression of PD-L2 on the THP-1 transductant is shown on the right. This experiment was repeated with similar outcome.
All T cell proliferations were done in triplicates and mean and standard deviation (SD) are shown.
We also tested the influence of PD-L2 expression on allogenic T cell responses. For this purpose we transduced the myeloid cell line THP-1 to express human PD-L2 and used these cells to stimulate human T cells. Also in these experiments PD-L2 expressing cells induced reduced T cell proliferation compared to mock tranduced THP-1 cells (Fig. 3 D).
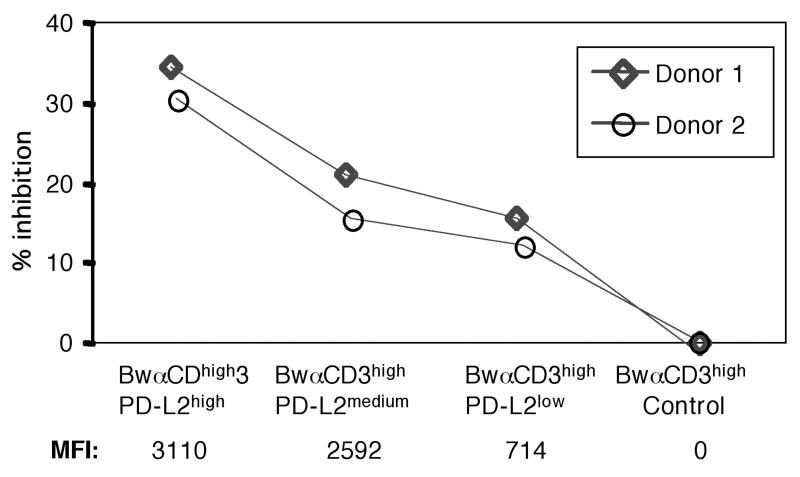
The inhibitory effect of PD-L2 is dose dependent
Different affinities of PD-L2 to its receptors could result in inhibitory or stimulatory effects when PD-L2 is expressed at high or low levels, respectively. We therefore investigated if different expression levels of PD-L2 would show different effects on T cell proliferation due to preponderance of one of the two described functions. We noticed a dose dependency of the negative effect of PD-L2 with relatively high expression levels required to obtain significant T cell inhibition (Fig. 4). Importantly, when we used T cell stimulators expressing PD-L2 at levels too low to obtain inhibitory effects, we still did not obtain any evidence for a costimulatory function of PD-L2 on human T cells (data not shown).
Figure 4.
The inhibitory effect of PD-L2 is dose dependent.
T cells were co-cultured with BwαCD3high stimulator cell lines expressing different levels of PD-L2 or control stimulator cells. PD-L2 expression levels of transductants are indicated as mean fluorescence intensity (MFI). This experiment was performed with T cells derived from four donors with similar outcome.
PD-L2 mediated inhibition is seen upon analysis of early events of activation of human T cell and affects CD4+ and CD8+ T cells
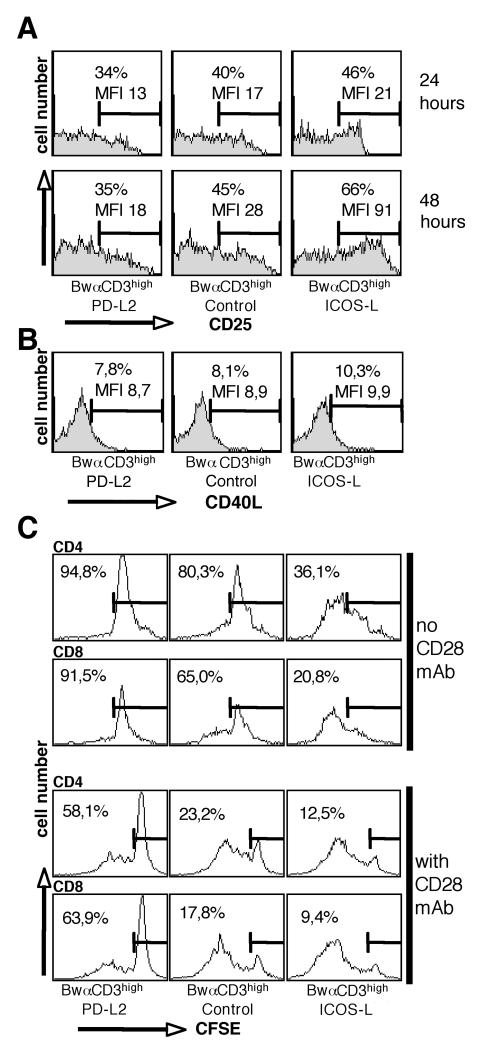
It has been suggested that PD-1 and putative costimulatory PD-Ligand receptors are expressed at different kinetics and that early up-regulation of non-PD-1 receptors results in costimulation of T cells via PD-L1 and 2 {Wang, 2003 #178}. To test this hypothesis on human T cells we analyzed the effect of PD-L2 by assessing early markers of T cell activation. However the inhibitory effect of PD-L2 was also evident in these experiments. CD25 and CD69 up-regulation was delayed and reduced on T cells stimulated by stimulator cells expressing PD-L2 (Fig. 5 A and data not shown). In murine T cells a very early and selective induction of CD40L expression was reported to be a specific feature of PD-L2 costimulation {Shin, 2003 #141}. In contrast to these results we found that PD-L2 did not cause early up-regulation of CD40L on human T cells (Fig. 5 B).
Figure 5.
PD-L2 inhibits early events in human T cell activation and affects both, CD4+ and CD8+ T cells.
A, PD-L2 inhibits up-regulation of CD25. T cells were co-cultured with stimulator cell lines expressing high levels of membrane-bound anti CD3 (BwαCD3high) co-expressing ICOS-L, PD-L2 or control stimulator cells. Expression of CD25 was analyzed after 24 and 48 hours of activation with the indicated stimulator cells. Presence of PD-L2 on the stimulator cells reduced the percentage of CD25 positive T cells in cocultures (p<0.05; n=4) whereas presence of ICOS-L significantly increased the percentage of CD25 positive T cells (p<0.05; n=4).
B, PD-L2 does not induce CD40L expression on human T cells. T cells co-cultured with stimulator cell lines expressing high levels of membrane-bound anti CD3 (BwαCD3high) co-expressing ICOS-L, PD-L2 and control cells were analyzed after 18 hours.
C, PD-L2 inhibits proliferation of CD4+ and CD8+ human T cells
T cells were CFSE labeled and co-cultured with stimulator cells lines expressing high levels of membrane bound anti CD3 (BwαCD3high) co-expressing PD-L2, ICOS-L or control stimulator cells for 6 days. mAb to CD28 (100 ng/ml) was added to the cultures as indicated. Cell cycling was analyzed for the CD4+ and CD8+ subset by counter-staining with CD4 and CD8 specific mAbs. All expreriments were at least repeated three times with similar outcome.
Also at very late time points (6 days) of T cell activation PD-L2 mediated inhibition was still detectable as revealed by monitoring the cell cycling profile of CFSE labelled T cells (Fig. 5 C). Presence of PD-L2 on BwαCD3high stimulators inhibited proliferation of CD4+ and CD8+T cells, while ICOS-L enhanced T cell proliferation in both subsets. CD28 mAb enhanced cell division activity in all subset but did not abrogate the inhibitory effect of PD-L2 (Fig 5 C).
PD-L2 inhibits cytokine production of human T cells
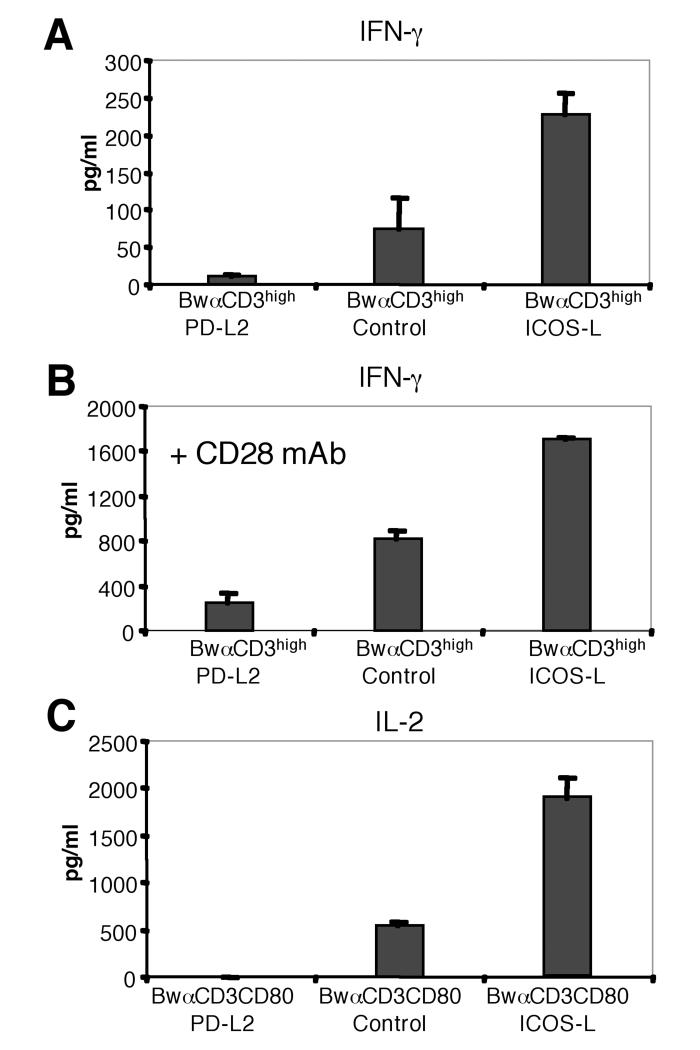
PD-L2 was described to be a potent inducer of IFN-γ production in murine T cells {Shin, 2005 #142; Tseng, 2001 #175} whereas Latchman and colleagues found PD-L2 to inhibit IFN-γ production {Latchman, 2001 #77}. We therefore measured IFN-γ production of human T cells co-cultured with PD-L2 expressing BwαCD3high stimulators or control stimulator cells expressing mb-αCD3 only. In strong contrast to results obtained by Tseng and coworkers with murine T cells, presence of PD-L2 led to a pronounced inhibition of IFN-γ production in human T cells (Fig. 6 A). PD-L2 also inhibited IFN-γ production when costimulation was provided to T cells (Fig. 6 B). Co-cultures of human T cells with BwαCD3high stimulator cells did not contain detectable amounts of IL-2 (data not shown). Only upon providing costimulation, IL-2 was detectable in culture supernatants. However when PD-L2 was expressed on the stimulator cells, the IL-2 content of the culture SN was below detection level (Fig. 6 C). This is in contrast with results obtained with murine T cells where CD80 and PD-L2 were reported to cooperate in IL-2 induction {Shin, 2003 #141}. PD-L2 also inhibited proliferation and cytokine production in pre-activated human T cells (data not shown).
Figure 6.
PD-L2 inhibits cytokine production in human T cells.
A, PD-L2 inhibits IFN-γ production in T cells. T cells were stimulated with stimulator cell lines expressing high levels of membrane-bound anti CD3 (BwαCD3high) co-expressing PD-L2, ICOS-L or control stimulator cells.
B, PD-L2 inhibits IFN-γ production in T cells costimulated via CD28. T cells were co-cultured with BwαCD3high stimulator cells expressing the indicated molecules in the presence of anti-CD28 mAb (100 ng/ml).
C, PD-L2 inhibits IL-2 production in T cells costimulated via CD28. T cells were activated with stimulator cell lines co-expressing high levels of mb-anti-CD3 and human CD80 (Bw-mbαCD3high/CD80) and PD-L2, ICOS-L control Bw-mbαCD3high/CD80) cells.
Mean and standard deviation (SD) are shown.
Blocking of PD-1 reveals no evidence for additional PD-L2 receptors on human T cells
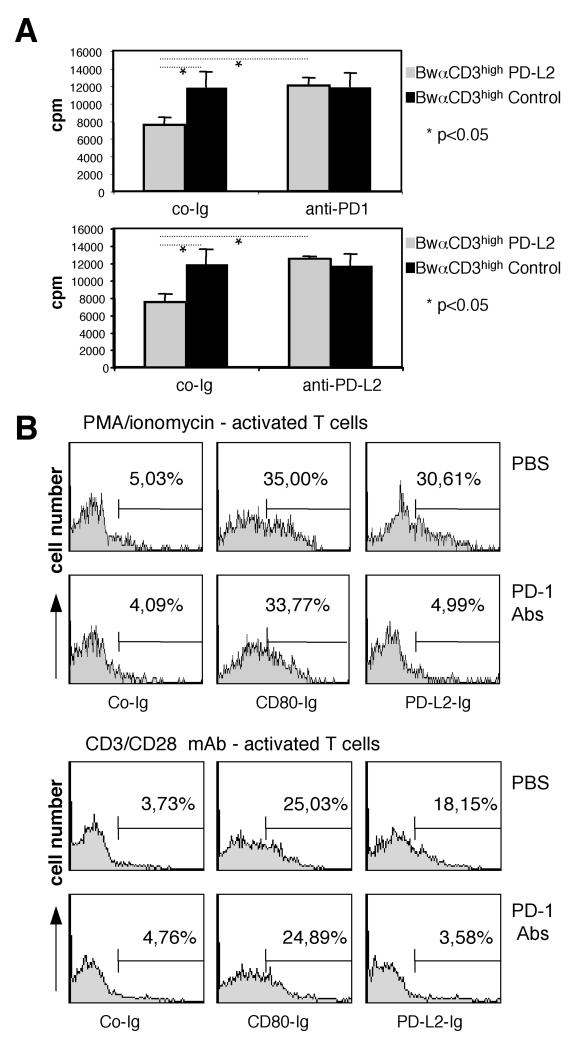
To assess the contribution of PD-1 to PD-L2 mediated T cell inhibition we blocked PD-L2/PD-1 interaction by polyclonal anti-PD-1 antibodies. In some experiments addition of these antibodies led to a slight increase in T cell proliferation. This effect was always well below 15%. Such an effect of PD-1 blocking was previously described to result from interference of PD-1 interaction with PD-L1 that is induced on T cells upon activation {Bennett, 2003 #7}. However we found in all experiments that addition of PD-1 antibodies resulted in a much stronger increase in T cell proliferation in co-cultures of T cells with PD-L2+ stimulator cells. Addition of anti-PD-1 Abs thus could revert the inhibitory effects of PD-L2, indicating that the PD-1 pathway is essentially involved in the observed PD-L2 mediated inhibition. Furthermore blocking this well-established inhibitory pathway did again not reveal any signs of a costimulatory role for human PD-L2 (Fig. 7 A). As expected blocking of PD-L2 with antibodies did also revert the inhibitory effects of the PD-L2 expressing stimulator cells (Fig. 7 A).
Figure 7.
Blocking of PD-1 reveals no evidence for additional PD-L2 receptors on human T cells.
A, The inhibitory effect of PD-L2 is PD-1-mediated.
T cells were stimulated with mb-αCD3high stimulator cells expressing PD-L2 or control stimulator cells expressing mb-αCD3 only for 3 days in presence of anti-human PD-1 antibodies (upper panel) or anti-human PD-L2 antibodies (lower panel) or isotype control antibodies. Mean and standard deviation (SD) of one experiment representative of 3 are shown. In the presence of control antibodies PD-L2 expressing αCD3 stimulator cells induced significantly less proliferation than control stimulator cells expressing αCD3 only (p<0.05; n=4). In the presence of PD-L2 or PD-1 antibodies PD-L2-expressing stimulator cells induced significantly more proliferation than in the presence of isotype control antibodies (p<0.05; n=4).
B, PD-1 antibodies completely blocks binding of PD-L2-Ig to human T cells.
Activated T cells were pre-incubated with PBS or anti-human PD-1 antibodies (Abs) and then stained with human PD-L2-Ig or for control purpose with a human CD80-Ig and a non-binding control-Ig. Percentages of reactive cells are indicated. The experiments shown are representative for three.
Whereas binding studies on murine cell provide evidence for a non PD-1 receptor, studies on the interaction of PD-L2 fusion proteins with human T cells have not been reported. We found that while PD-L2-Ig did not bind to resting human T cells (data not shown), PD-L2 ligands were strongly up-regulated upon stimulation of human T cells with PMA/Ionomycin or beads coated with mAb to CD3 and CD28 for 72 hours. Presence of antibodies to human PD-1 completely blocked binding of PD-L2-Ig to T cells. The blocking of PD-1 antibodies was specific for PD-1 since CD80-Ig interaction with human T cells was not affected (Fig 7 B). Together with our functional results these data strongly suggest that PD-1 is the sole receptor for PD-L2 on human T cells.
Discussion
During their interaction with APC T cells receive numerous stimulating but also negative signals, which will determine the quality and strength of immune responses. Besides TCR-MHC interaction and soluble factors an increasing number of accessory surface molecules are being recognized to be critically involved in this process. B7-like molecules have recently been subject of intense research in this context. Regarding their role in T cell activation contradictory results have been reported for B7-H3 but also for the PD-1 ligands PD-L1 and PD-L2. For PD-L1 and PD-L2 these discrepancies might be explained by receptors distinct from the inhibitory PD-1 molecule that was shown to convey negative signals to T cells upon interaction with its ligands. There is increasing evidence for the existence of such PD-L2 receptors on murine cells: (i) PD-L2-Ig binds to cells derived from PD-1(−/−) animals {Liu, 2003 #83}, (ii) engineered PD-L2-Ig molecules incapable of PD-1 binding still costimulate activation of T cells {Wang, 2003 #178}, (iii) several studies describe a costimulatory function of PD-L2 on murine T cells {Wang, 2003 #178; Tseng, 2001 #175; Shin, 2005 #142; Shin, 2003 #141; Liu, 2003 #83}.
Presently the issue of a putative costimulatory role of human PD-L2 has not been addressed. All functional data regarding the effects of PD-L2 on human T cells are based on the use of a blocking mAb {Wang, 2003 #178; Cai, 2004 #11; Brown, 2003 #10}. A recent study showed that the binding site of PD-1 on murine PD-L2 is distinct from the binding site with as of yet unknown costimulatory PD-L2-receptors {Wang, 2003 #178}. Thus the results obtained with a mAb to PD-L2 do not fully exclude the possibility that the mAb-PD-L2 interaction selectively blocks the inhibitory PD-L2-PD-1 pathway leaving a putative costimulatory pathway involving PD-L2 unaffected. In addition it has not been investigated whether human PD-L2 – like its murine orthologue {Wang, 2003 #178; Tseng, 2001 #175} – acts costimulatory on T cells that receive a weak signal 1.
To assess the functional role of this B7-homolog we developed a cellular system of T cell stimulators. We found that in contrast to ICOS-L, human PD-L2 does not costimulate the proliferation of human T cells that received weak signal 1. Presence of PD-L2 on stimulator cells that transferred a strong signal 1 to T cells consistently and significantly inhibited proliferation and cytokine production of primary but also pre-activated human T cells. We evaluated on one hand the consequences of PD-L2-T cell interaction at very early time points by measuring up-regulation of activation markers but on the other hand in CFSE-labeling experiments we monitored PD-L2 effects during T cell stimulation also for a prolonged period of time. All our results pointed to an inhibitory role of PD-L2 in the activation of human T cells and we never obtained any evidence for a costimulatory function. ICOS-L that was included in this study for control purposes, strongly and consistently costimulated activation of human T cells in all our experimental settings, demonstrating that our T cell stimulators are an excellent system to also reveal stimulating functions of accessory molecules involved in T cell-APC interaction. We consider the Bw5147 cell line that we used as a basis for our cellular T cell activation system to be especially suitable since these cells do not appear to provide significant costimulatory signals to human T cells and thus allows assessing PD-L2 function in a cellular system detached from accessory molecules that could potentially interfere with the effects of PD-L2. In addition, human cells that express PD-L2 also express the second PD-1 ligand, B7-H1, which further hampers functional studies of PD-L2 on these cells. Expression of PD-L2 is restricted to professional APC that harbor a plethora of costimulatory molecules. Therefore we also analysed the effects of PD-L2 on T cells that received strong costimulatory signals. We found PD-L2 to still inhibit T cell activation, indicating that it might retain its inhibitory properties on human APC. In line with our results blocking of PD-L2 on dendritic cells was previousely shown to enhance their T cells stimulatory capacity pointing also to an inhibitory role of PD-L2 {Brown, 2003 #10}. Thus our results complement the important study of Brown et al. in showing that expression of PD-L2 on stimulatory cells inhibits human T cell responses. While our results do not completely exclude the existence of costimulatory PD-L2 ligands on human T cells they clearly demonstrate that the net-effect of the interaction of PD-L2 with human T cells is inhibitory. Furthermore our binding studies with PD-L2-fusionproteins do not give any evidence for ligands on human T cells distinct from the inhibitory receptor PD-1.
As has been discussed previously B7 homologs have considerable therapeutic potential {Liang, 2002 #79; Latchman, 2001 #77; Brown, 2003 #10}. Expression of PD-L2 on murine tumor cells potently promotes CD8+T cell expansion in vitro and in vivo and enhances CD8+ T cell mediated rejection of tumor cells in mice {Liu, 2003 #83}. The authors discussed the expression of PD-L2 as a valuable approach in cancer immunotherapy. However currently there are no data that demonstrate stimulation of tumor-specific human CD8+ cells by PD-L2. In contrast the results presented in this study as well as data on the interaction of CD8+ T cells with endothelial cells from Rodig and coworkers indicate that PD-L2 inhibits human CD8+ T cells {Rodig, 2003 #129}. Thus from the data available up to now human PD-L2 clearly cannot be considered as a candidate molecule to promote tumor specific immunity. On the contrary, from the data on human PD-L2 function one could consider blocking PD-L2 on APC as a promising strategy to enhance tumor-specific T cell responses. On the other hand individuals suffering from autoimmune conditions or transplant recipients might benefit from therapeutic approaches aimed to express inhibitory molecules at target sites of pathological immune responses. Our data as well as all previous studies on human T cells suggest that PD-L2 might have therapeutic potential in this respect.
Material and Methods
Media and reagents
Cells were cultured in RPMI-1640 medium (Gibco Ltd., Paisley, Scotland) supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% of fetal calf serum (Sigma Aldrich Corporation, Europe). Propidium iodide (PI) was obtained from Sigma Chemie GmbH (Deisenhofen, Germany). CFSE (5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester) was purchased from Molecular Probes (Eugene, OR).
Antibodies and fusion-protein
The mAbs to CD4 (VIT4), CD8 (VIT8), CD25 (3G10), CD80 (7-480) and the non-binding control Ab VIAP were produced in our laboratory. mAbs to CD28 (M1650) was purchased from Sanquin (Amsterdam, Netherlands), CD3-mAb (OKT3) was from Ortho Pharmaceutical Corporation (Raritan, NJ), ICOS-L/B7-H2-mAb (2D3), CD69-mAb (FN50) and CD40L-mAb (TRAP1) were from BD Bioscience (Palo Alto, CA), PD-L2-mAb (MIH18) was from eBioscience (San Diego, CA). Goat anti human PD-1-Abs, Goat anti human PD-L2-Abs, Goat anti human LOX-1-Abs (used as control antibodies) and the human PD-L2-Ig-fusion protein were purchased from R&D (Minneapolis, MN). A control-Ig-fusion protein and a CD80-Ig-fusion protein were produced in our laboratory.
Generation of T cell stimulator cells
The single chain antibody (scFv) of the anti-human CD3ε antibody (clone OKT3, ATCC, Manassas, VA) that induces activation of the human TCR-complex was used as a template for the amplification of variable heavy chain (VH) and variable light chain (VL) gene segments using primers VH-for 5′-GGAATTCGCTAGCCCAGGTCCAGCTGCAGCAGTCT-3′, VH-rev 5′GGGGGATCCGGTGACCGTGGTGCCTTGGCCCCAGTA-3′ and VL-for 5′-GGAATTCGAGCTCCCAAATTGTTCTCACCCAGTCTCCA-3′, VL-rev 5′-GGGATCCCCACCGCCCCGGTTTATTTCCAACTTTGTCCC-3′. They were cloned together with a (G3S)4 encoding linker and a CD5 leader (CD5L) sequence to the leader-less human CD28 sequence into the retroviral pMMP-vector {Pickl, 2001 #119} to generate a CD5L-OKT3scFv-CD28 expression construct. Likewise a pMMP-CD5L-OKT3scFv-CD14 construct was generated leading to a gpi-anchored expression of the OKT3-scFv.
cDNAs of human ICOS-L/B7-H2, PD-L2/B7-DC and CD80 were PCR amplified from an expression library generated from human mdDC {Steinberger, 2004 #148}. PCR products were cloned into the retroviral expression vector pBMN {Kinoshita, 1997 #59}. The integrity of the resulting retroviral expression constructs was confirmed by DNA sequencing.
Bw 5147 (referred to as Bw cells), a murine thymoma cell line, was retrovirally transduced to express the constructs that encode membrane-bound scFv of the anti-CD3 antibody (mb-αCD3). Single cell clones that stably express these constructs at high or low density (T cell stimulator cells) were established. Cells derived from mb-αCD3+Bw5147 cell clones were then retrovirally transduced in parallel with pBMN plasmids encoding ICOS-L, PD-L2 and for control purposes with vector containing the lac Z gene (pBMN-Z). A single cell clone that stably co-expressed the mb-αCD3 and human CD80 was also transduced to express ICOS-L or PD-L2 or with the control vector (pBMN-Z). For retroviral transduction, 293T cells were co-transfected with the pEAK12-gag-pol vector {Steinberger, 2004 #148}. SN were supplemented with polybrene (5 μg/ml), sterile filtered and used to spin-infect the Bw5147 cell lines described above (700 × g, 1 h 30°C). To obtain high expression of ICOS-L or PD-L2 on the stimulator cells, low numbers of Bw5147 cells (1×104) were repeatedly transduced. In order to obtain T cell stimulator cells expressing PD-L2 at various levels, retrovirus-containing supernatants were diluted 10, 50 and 100-fold and applied to the stimulator cells.
To test the effects of PD-L2 on allogenic T cells responses the human cell line THP-1 was retrovirally transduced to express PD-L2 or mock transduced. Both parental cell lines do not express PD-L2 and very low levels (MFI ~13) of PD-L1.
T cell proliferation assays and cytokine measurement
T cell proliferation assays were performed in 96-well cell culture plates. All T cell proliferation assays were done in triplicates and mean and standard deviation (SD) are shown. For T cell stimulation the mb-αCD3 expressing Bw5147 transductants (T cell stimulators) co-expressing CD80, PD-L2, ICOS-L, or control T cell stimulator as indicated were irradiated (6000 rad) and added to flat bottom 96 well plates (2×104/well). Human T cells were prepared as described {Pfistershammer, 2004 #113}and were added (1 × 105/well) and co-cultured for 3 days. In some experiments CD28 mAb was added at the indicated concentrations. For the last 18 hours (methyl-3H)-TdR (ICN Pharmaceuticals Inc., Irvine, CA) was added. Cells were harvested and measured on a microplate scintillation counter (Packard, Topcount Instrument, Meriden, CT).
For blocking the PD-L2/PD-1 interaction, stimulator cells expressing the indicated molecules were co-cultured with T cells in the presence of either goat anti human PD-L2 antibodies (12 μg/ml); goat anti human PD-1 antibodies (12 μg/ml) or goat Ig control antibodies at the same concentration.
For allogenic T cell stimulation the human THP-1 cell line transduced with a PD-L2 containing plasmid or a control plasmid was used to stimulate human T cells for 5 days.
For proliferation assays with immobilized anti-CD3mAb and Ig-fusion proteins, 96-well-plates were coated with anti-CD3 mAb at indicated concentrations for 5 hours at 37°C, washed, and then coated with Ig-fusion proteins o/n at 4°C. After another washing step T cells (1×105/well) were added and proliferation was measured after 72 hours.
For cytokine measurement T cells were co-cultured with indicated stimulator cells for 48 hours and supernatants were used for ELISA measurement {Selenko-Gebauer, 2003 #139}. Mean and standard deviation (SD) of duplicate measurements are shown.
Fluorescence staining
For flow cytometric analysis, cells (1.5 × 104) were incubated with fluorochrome conjugated mAbs or unlabeled primary antibody (10 μg/ml) for 20 min on ice and washed. For indirect staining, PE conjugatedmouse Fcγ Fragment specific antibodies; Jackson Immuno Research, West Grove, PA) were used. For the detection of membrane-bound αCD3-scFv PE conjugated anti-Mouse IgG (Caltag, Burlingame, CA) that reacts with the V-regions of the murine OKT-3 antibody was used. Flow cytometric analysis was performed using a FACScalibur flow cytometer (Becton Dickinson Immunocytometry System, Palo Alto, CA) supported by CELLQUEST software (Becton Dickinson). In all histograms the fluorescence intensity is shown on a standard logarithmic scale. CFSE labeling experiments were performed as described {Pfistershammer, 2004 #113}.
Binding studies using human Ig-fusion proteins
Human T cells were activated for 72 hours with PMA and ionomycin (100nM each, both Sigma) or T Cell Expander (CD3/CD28 mAb coated supramagnetic beads; Dynal Biotech, Hamburg, Germany). Activated T cells (1 × 105) were incubated with human PD-L2-Ig, CD80-Ig or a control-Ig (at 50 μg/ml) for 30 minutes on ice. Binding was detected with PE conjugated anti human IgG (Jackson Immuno Research). For blocking experiments Goat anti human PD-1 antibodies (30 μg/ml) were used.
Statistics
Mann-Withney U-test was used to assess significances. Differences were considered significant at p<0.05.
Acknowledgments
We appreciate the excellent technical assistance of Petra Kohl, Margarete Merio, Alessandra Mathe and Claus Wenhardt. We thank Dr. Garry P. Nolan and colleagues for providing the retroviral vector pBMNZ.
The authors wish to acknowledge the support of Prof. Walter Knapp who died August 30, 2004.
This work was supported by a grant from the Austrian Science Fund (FWF; P17669-B13). PS is supported by the Children Cancer Research Institute (CCRI), grant 7003. WFP is supported by a grant of the Center for Molecular Medicine of the Austrian Academy of Sciences (CeMM #20030).
abbreviations used in this paper
- scFv
single-chain fragment of variable region
Footnotes
Note added in proof: While this manuscript was under revision another study that shows that on human T cells PD-L2 has only inhibitory functions was published [36]
References
- [1].Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- [2].Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Waterhouse P, Marengere LE, Mittrucker HW, Mak TW. CTLA-4, a negative regulator of T-lymphocyte activation. Immunol Rev. 1996;153:183–207. doi: 10.1111/j.1600-065x.1996.tb00925.x. [DOI] [PubMed] [Google Scholar]
- [4].Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2004;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- [5].Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- [6].Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Algarawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- [7].Swallow MM, Wallin JJ, Sha WC. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. [DOI] [PubMed] [Google Scholar]
- [8].Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- [9].Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- [10].Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, Collins M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–377. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- [12].Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J. B7-h1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- [13].Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- [14].Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- [15].Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- [16].Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- [17].Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- [19].Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- [21].Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [23].Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- [24].Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, Housseau F. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shin T, Yoshimura K, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- [29].Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4(+) T cells. Cell Immunol. 2004;230:89–98. doi: 10.1016/j.cellimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [30].Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- [31].Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- [32].Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–390. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- [33].Pickl WF, Pimentel-Muinos FX, Seed B. Lipid rafts and pseudotyping. J Virol. 2001;75:7175–7183. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- [35].Pfistershammer K, Majdic O, Stockl J, Zlabinger G, Kirchberger S, Steinberger P, Knapp W. CD63 as an activation-linked T cell costimulatory element. J Immunol. 2004;173:6000–6008. doi: 10.4049/jimmunol.173.10.6000. [DOI] [PubMed] [Google Scholar]
- [36].Saunders PA, Hendrycks VR, Lidinsky WA, Woods ML. PD-L2:PD-1 involvement in T cell proliferation, cytokine production, and integrin-mediated adhesion. Eur. J. Immunol. 2005;35:3561–9. doi: 10.1002/eji.200526347. [DOI] [PubMed] [Google Scholar]