Abstract
Introduction
We report responses to combination antiretroviral therapy (cART) in the TREAT Asia Pediatric HIV Observational Database.
Methods
Children included were those who had received cART (i.e., ≥3 antiretrovirals) at <18 years. The analysis was intention-to-treat by the first cART regimen. Median values are provided with interquartile ranges; hazard ratios (HR) with 95% confidence intervals.
Results
Of the 1655 children included, 50.4% were male, with a median age at cART of 7.0 (3.9, 9.8) years and CD4 of 8 (2.0, 15)%; 92.5% were started on an NNRTI; median duration of follow-up was 2.9 (1.4, 4.6) years. Loss-to-follow-up and death rates were 4.2 (3.7, 4.8) and 2.1 (1.7, 2.5) per 100 person-years, respectively. At 36 months, median CD4 was 26 (21, 31)%; 81% of those with viral load (N=302) were <400 copies/mL. Children who reached CD4 ≥25% within five years were more likely to be females (HR 1.4; 1.2, 1.7), start before 18 months old (HR 3.8; 2.4, 6.2), lack a history of mono/dual-therapy (HR 1.7; 1.4, 2.5), and have a higher baseline CD4 (per 10% increase: HR 2; 1.9, 2.2).
Conclusion
These data underscore the need for early diagnosis and cART initiation to preserve immune function.
Keywords: antiretroviral therapy, pediatric HIV, outcomes, Asia
Introduction
There were an estimated 4.7 million adults and children living with HIV in Asia in 2008, with approximately 30,000 children receiving combination antiretroviral therapy (cART).1, 2 This represents 53% regional treatment coverage by 2006 World Health Organization guidelines.2, 3 As has been demonstrated by randomized clinical trial, the early initiation of cART can result in immune recovery, and reduced morbidity and mortality.4 The goal of treating children into adulthood can be reached through the application of best practices that are feasible in resource-limited settings. Monitoring and evaluating pediatric HIV treatment outcomes can provide important guidance for informing the continued scale-up and maturation of ART programs.
The pediatric network of TREAT Asia (Therapeutic Research, Education, and AIDS Training in Asia) was established by amfAR in 2005 as the first, multicenter regional network of clinical, research, and social support programs caring for HIV-infected children in Asia. Twenty sites in seven Asian countries now participate in research and educational training activities. Selected sites contribute patient data to the TREAT Asia Pediatric HIV Observational Database (TApHOD) for regional and multiregional analyses through the US National Institutes of Health International Epidemiologic Databases to Evaluate AIDS (IeDEA) program.5 TApHOD facilitates basic research capacity for conducting standardized and systematic data collection to aid in patient care as well as research. We report the characteristics and outcomes after cART in HIV-infected children in TApHOD.
Methods
Study subjects and participating sites
Children included in this analysis were those who had received cART (i.e., ≥3 antiretrovirals) at some point in their treatment history, and were aged less than 18 years at first cART. Data were included from sites over the time period for which they had complete follow-up of all children. This required complete ascertainment of the treatment histories of children who were lost-to-follow-up (LTFU) or who had died during this period, thus creating a complete cohort, although not all additional clinical data variables may have been available for every patient (e.g., viral load). LTFU was defined as loss of contact with the site for ≥12 months. Clinical centers varied with regards to duration of ART availability and period of data collection.5, 6 Two centers representing 33% of the cohort provided retrospective data from 1991 and 1993 that described pre-ART clinical management practices; 65% of patient data were collected starting from 2002 to 2004.
TApHOD data collection
Data contributed to TApHOD were collected in the course of routine patient care. Sites can choose to submit data as entered into their own electronic databases (e.g., Excel files) or through a standardized Access database. All data are anonymized upon transfer. Variables include demographic data, clinical information (e.g., opportunistic infections, clinical staging, and hospitalizations), laboratory results, medication histories, and retention in program. The data are transferred twice yearly to the data management center at the National Centre in HIV Epidemiology and Clinical Research (NCHECR), Sydney, for quality control checks as well as statistical analysis.5 Institutional Review Board approval for study participation and data transfer was obtained at all participating sites and the data management (NCHECR) and coordinating centers (TREAT Asia/amfAR); informed consent was waived.
Growth references
For height-for-age z score (HAZ) and body mass index (BMI), the WHO 2006/2007 Child Growth Standards were used.7 WHO 1977 Standards were used for weight-for-age z scores (WAZ), to allow for scoring children >10 years of age.8 In assessing the applicability of the 1977 growth references, we compared WAZ from both the WHO 1977 and the WHO 2006/2007 reference curves in children <10 years, and found that the two WAZ standards gave similar results (data not shown).
Analysis
Baseline measurements were those closest to the date of cART initiation and within 91 days before and 30 days after. For the subsequent 6-monthly data points, clinical and laboratory data obtained within a window of plus or minus three months of the target visit date were included. The analyses were done on an intention-to-treat basis, according to the initial cART regimen, regardless of drug changes for toxicity or treatment failure.
Logistic regression was used to determine factors associated with HIV-RNA >400 copies/mL. Covariables included in this analysis were age at baseline, sex, infant antiretroviral exposure, maternal antiretroviral exposure, prior history of mono- or dual-therapy (yes/no), family status (both parents alive, one parent dead, both parents dead), and baseline WAZ, HAZ, WHO stage, CD4 percentage, and BMI.
Kaplan-Meier analysis was used to calculate time to CD4 recovery, defined as reaching a CD4 of 25%.9, 10 Cox proportional hazards regression was used to determine factors associated with time to CD4 recovery. Covariates included in this analysis were age at baseline, sex, infant antiretroviral exposure, maternal antiretroviral exposure, prior history of mono- or dual-therapy, family status, and baseline CD4, HIV-RNA, WAZ, HAZ, BMI, and WHO stage.
In all analyses, covariates with p <0.10 in univariate analyses were initially considered for inclusion in multivariate models. Multivariate models were built using forward stepwise methods, including variables that remained statistically significant at a two-sided level of <0.05. Goodness-of-fit of logistic models was assessed using the Pearson chi-square statistic. All statistical analyses were performed using Stata 10.0 software (StataCorp, College Station, TX).
Results
Eleven sites in Cambodia, India, Indonesia, Malaysia, and Thailand contributed data to this analysis with complete retrospective data collected as far back as 1991. Of the 1655 children meeting inclusion criteria, 50.4% were male and 73% were from a single country (Table 1). Of the 94.3% of children who acquired their infection through mother-to-child transmission, >50% did not have documentation of receiving perinatal antiretroviral interventions to prevent HIV transmission. Other reported modes of transmission included blood transfusion (0.7%), sexual transmission (0.1%), sexual abuse (0.2%), other (0.3%), or were unknown/unconfirmed (4.4%). At baseline, the median age was 7.0 [interquartile range (IQR) 3.9–9.8] years, and the median CD4 percentage was 8 (IQR 2–15) percent. For the 996 children who were >6 years of age at cART initiation, the median CD4 count at baseline was 100.5 (IQR 23–291) cells/mm3. There were 628 children that had baseline HIV-RNA measured, the median viral load being 5.3 (IQR 4.8–5.8) log10 copies/mL. At least 50.2% of the children were at WHO stage III or IV before initiation of cART. Most children had either lost one parent (30%) or both parents (24%).
Table 1.
Baseline characteristics of patients at initiation of cART (N=1655 unless specified)
| Characteristics | Total N (%) | Country A | Country B | Country C | Country D | Country E |
|---|---|---|---|---|---|---|
| N=1233 | N=112 | N=101 | N=135 | N=74 | ||
| Male | 834 (50.4) | 596 (48.3) | 58 (51.8) | 63 (62.4) | 80 (59.3) | 37 (50.0) |
| Median (IQR) age, years | 7.0 (3.9–9.8) | 7.7 (2.2–10.2) | 3.6 (1.8–6.6) | 7.9 (5.9–10.6) | 1.9 (1.0–3.6) | 6.4 (4.2–8.7) |
| <18 months | 162 (9.8) | 82 (6.7) | 26 (23.2) | 4 (3.9) | 50 (37.0) | 0 |
| 18–59 months | 361 (21.8) | 204 (16.5) | 44 (39.3) | 17 (16.8) | 69 (51.1) | 27 (36.5) |
| 60–155 months | 1038 (62.7) | 872 (70.7) | 42 (37.5) | 65 (64.4) | 16 (11.9) | 43 (58.1) |
| >156 months | 94 (5.7) | 75 (6.1) | 0 | 15 (14.9) | 0 | 4 (5.4) |
| Perinatal transmission | 1560 (94.3) | 1161 (94.2) | 100 (89.3) | 94 (93.1) | 134 (99.3) | 71 (95.9) |
| Infant antiretrovirals for PMTCT |
161 (9.7) | 88 (7.1) | 8 (7.1) | 0 | 2 (1.5) | 63 (85.1) |
| Unknown | 584 (35.3) | 472 (38.3) | 7 (6.3) | 94 (93.1) | 0 | 11 (14.9) |
| Maternal antiretrovirals for PMTCT |
135 (8.1) | 64 (5.2) | 5 (4.5) | 0 | 2 (1.5) | 63 (85.1) |
| Unknown | 574 (34.7) | 463 (37.6) | 9 (8.0) | 94 (93.1) | 0 | 9 (12.2) |
| WHO clinical stage | ||||||
| Stage I | 108 (6.5) | 90 (7.3) | 7 (6.2) | 0 | 5 (3.7) | 6 (8.1) |
| Stage II | 286 (17.3) | 209 (17.0) | 16 (14.3) | 10 (9.9) | 13 (9.6) | 38 (51.4) |
| Stage III | 470 (28.4) | 280 (22.7) | 35 (31.2) | 67 (66.3) | 63 (46.7) | 25 (33.8) |
| Stage IV | 361 (21.8) | 275 (22.3) | 20 (17.9) | 13 (12.9) | 48 (35.6) | 5 (6.7) |
| Unknown | 430 (26.0) | 379 (30.7) | 34 (30.4) | 11 (10.9) | 6 (4.4) | 0 |
| Median (IQR) BMI, kg/m2 (N=1151) |
14.7 (13.2– 15.7) |
14.6 (13.5– 15.9) |
13.5 (12.1– 14.4) |
13.0 (11.8– 13.9) |
13.2 (11.6– 14.7) |
14.7 (13.0– 15.4) |
| Median (IQR) weight-for- age z score (N=1189) |
−2.15 (−2.84 to −1.34) |
−2.1 (−3.1 to − 1.1) |
−3.1 (−4.0 to − 1.9) |
−2.8 (−3.7 to − 1.6) |
−2.7 (−3.9 to − 1.7) |
−2.4 (−3.2 to − 1.5) |
| Median (IQR) height-for- age z score (N=1189) |
−2.35 (−3.31 to −1.14) |
−2.3 (−3.3 to − 1.4) |
−2.4 (−3.3 to − 1.6) |
−1.5 (−3.0 to − 0.8) |
−2.8 (−3.9 to − 1.7) |
−3.0 (−3.9 to − 1.8) |
| Median (IQR) CD4 percentage |
8.0 (2.0–15.0) | 7.0 (2.0–14.2) | 9.3 (2.0–18.0) | 9.0 (6.0–16.0) | 9.0 (2.0–15) | 12 (6.0–19.0) |
| <10% | 735 (44.4) | 603 (48.9) | 22 (19.6) | 38 (37.6) | 49 (36.3) | 23 (31.1) |
| 10–14% | 230 (13.9) | 168 (13.6) | 7 (6.3) | 15 (14.9) | 17 (12.6) | 23 (31.1) |
| 15–24% | 241 (14.6) | 177 (14.4) | 9 (8.0) | 13 (12.9) | 21 (15.6) | 21 (28.4) |
| >25% | 96 (5.8) | 67 (5.4) | 5 (4.5) | 8 (7.9) | 10 (7.4) | 6 (8.1) |
| Unknown | 353 (21.3) | 218 (17.7) | 69 (61.6) | 27 (26.7) | 38 (28.1) | 1 (1.4) |
| Median log10 (IQR) HIV-RNA, copies/ml (N=628) |
5.3 (4.8–5.8) | 5.1 (4.7–5.6) | 5.3 (4.8–5.9) | 5.3 (5.1–5.9) | 5.9 (5.6–6.1) | 4.6 (3.9–5.0) |
| Most common cART regimens |
||||||
| NNRTI-based | 1531 (92.5) | 1125 (91.2) | 98 (87.5) | 99 (98.0) | 135 (100) | 74 (100) |
| d4T/3TC/NVP | 639 (38.6) | 524 (46.6) | 1 (1.0) | 51 (51.2) | 33 (24.4) | 30 (40.5) |
| AZT/3TC/NVP | 368 (22.2) | 197 (17.5) | 16 (16.3) | 22 (22.2) | 101 (74.8) | 32 (43.2) |
| AZT/3TC/EFV | 276 (16.7) | 196 (17.4) | 74 (75.5) | 3 (3.0) | 1 (0.7) | 2 (2.7) |
| d4T/3TC/EFV | 209 (12.6) | 173 (15.4) | 5 (5.1) | 22 (22.2) | 0 | 9 (12.2) |
| Median time on cART, years (IQR) |
2.9 (1.4–4.6) | 3.4 (1.8–4.9) | 1.9 (0.8–3.2) | 2.2 (0.8–3.4) | 1.7 (0.6–3.2) | 1.6 (0.9–2.2) |
Abbreviations: cART-combination antiretroviral therapy; PMTCT-prevention of mother-to-child transmission; IQR-interquartile range; NNRTI-non-nucleoside reverse transcriptase inhibitor; d4T–stavudine; 3TC-lamivudine; NVP-nevirapine; AZT-zidovudine; EFV-efavirenz
The first cART regimens were non-nucleoside reverse transcriptase inhibitors (NNRTI)-based (92.5%), and protease inhibitors (PI)-based (6%). The most common NNRTI-based regimens were stavudine (d4T) with lamivudine (3TC) and nevirapine (NVP), used in 38.6% of children, followed by zidovudine (AZT) with 3TC and NVP, used in 22.2% of children; 38.3% used fixed dose combinations in part or all of these regimens. Twelve percent had received mono- or dual-nucleoside reverse transcriptase inhibitor (NRTI) combinations before starting cART for a median duration of 2.1 (IQR 0.8–4.6) years.
The median duration of cART at the time of final data transfer was 2.9 (IQR 1.4–4.6) years, during which 121 (7.3%) children switched from an NNRTI- to PI-based regimen at the rate of 4.1 switches per 100 person-years (95% CI 3.4–4.8). The rate of change from PI-based regimens to NNRTI –based regimens was 3.8 switches per 100 person-years (95% CI 1.8–8.0).
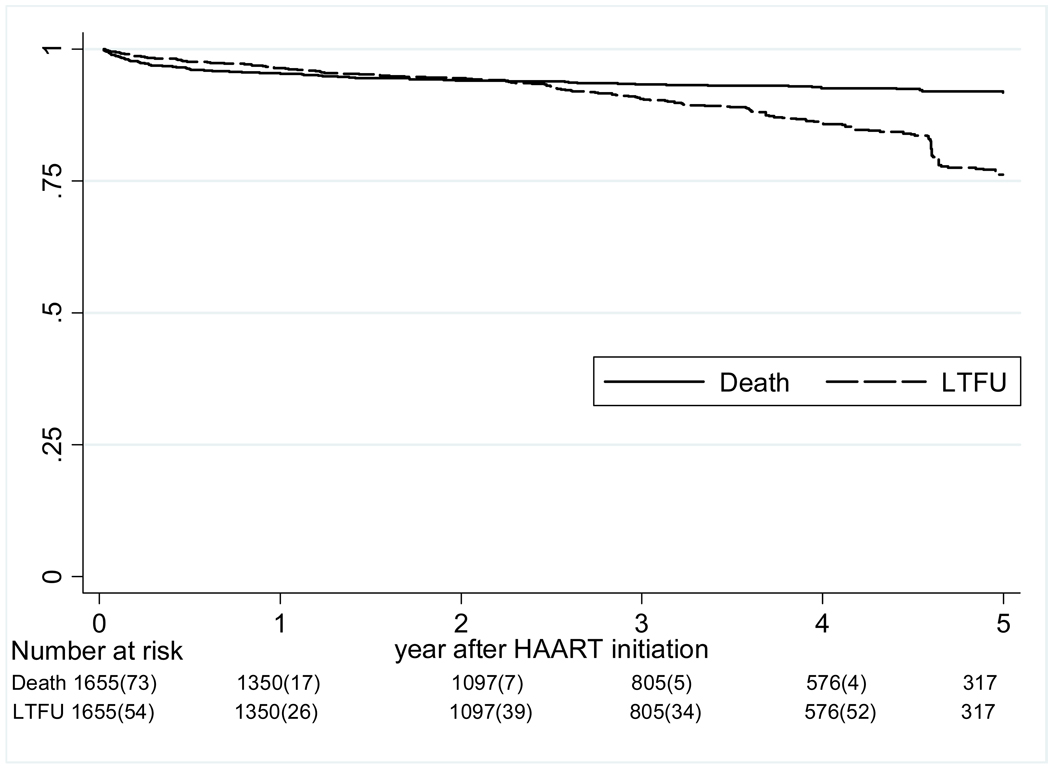
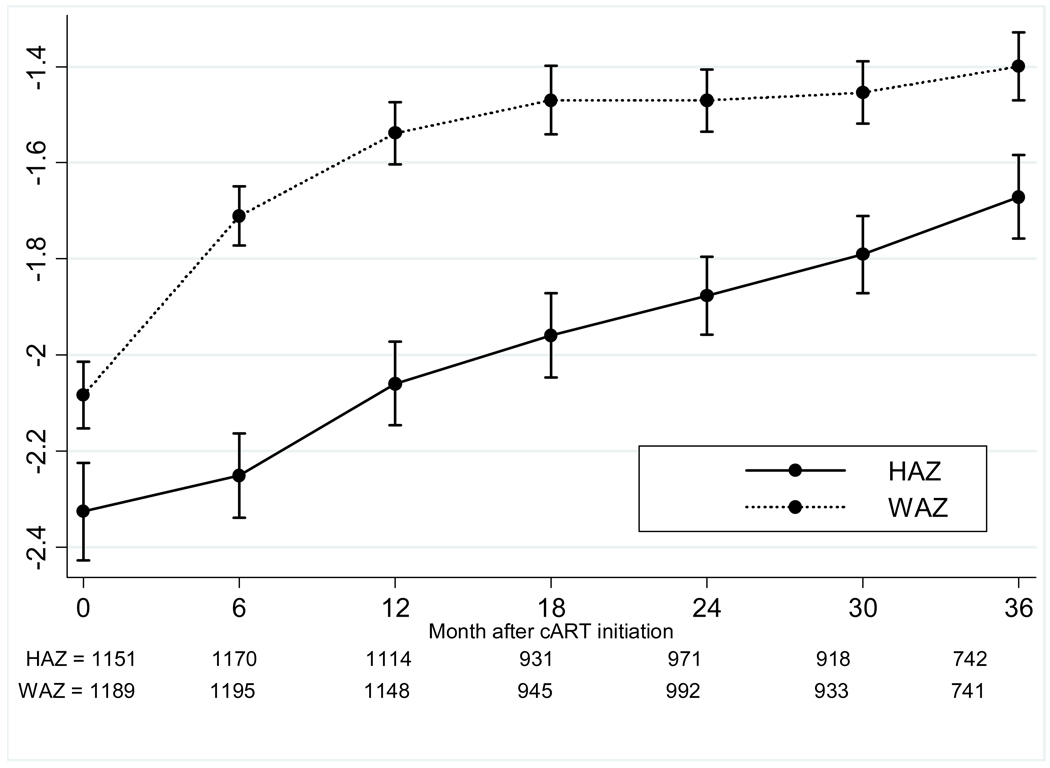
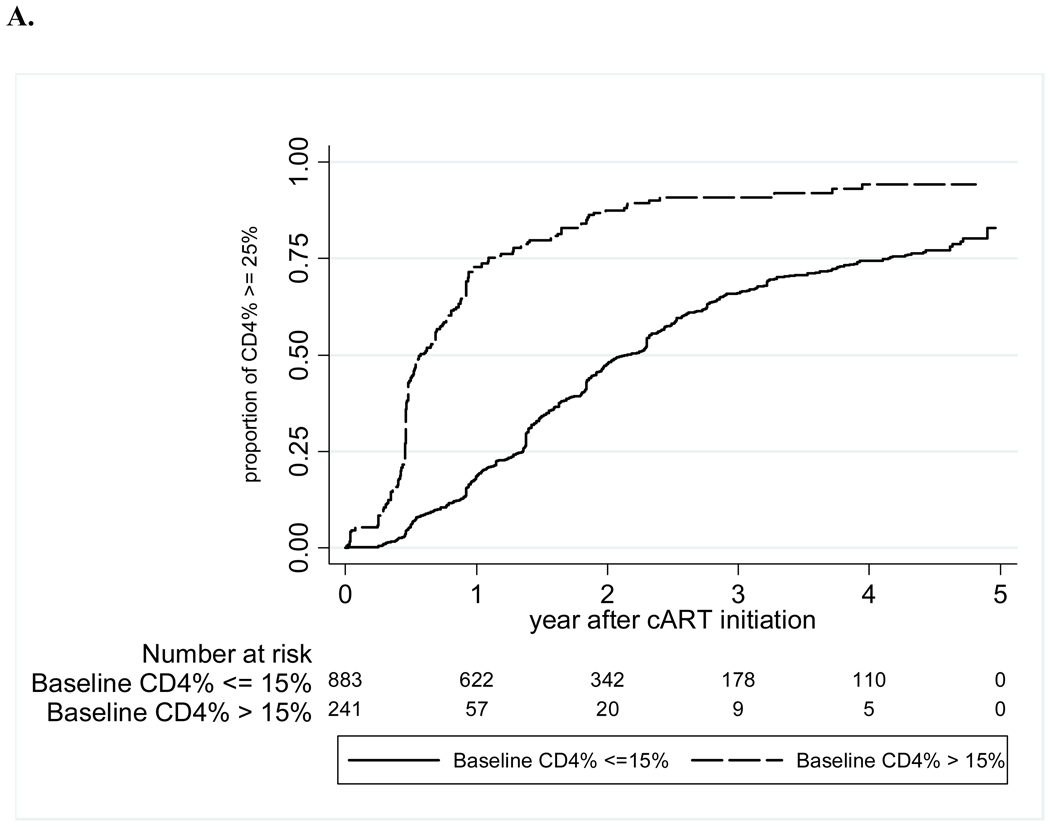
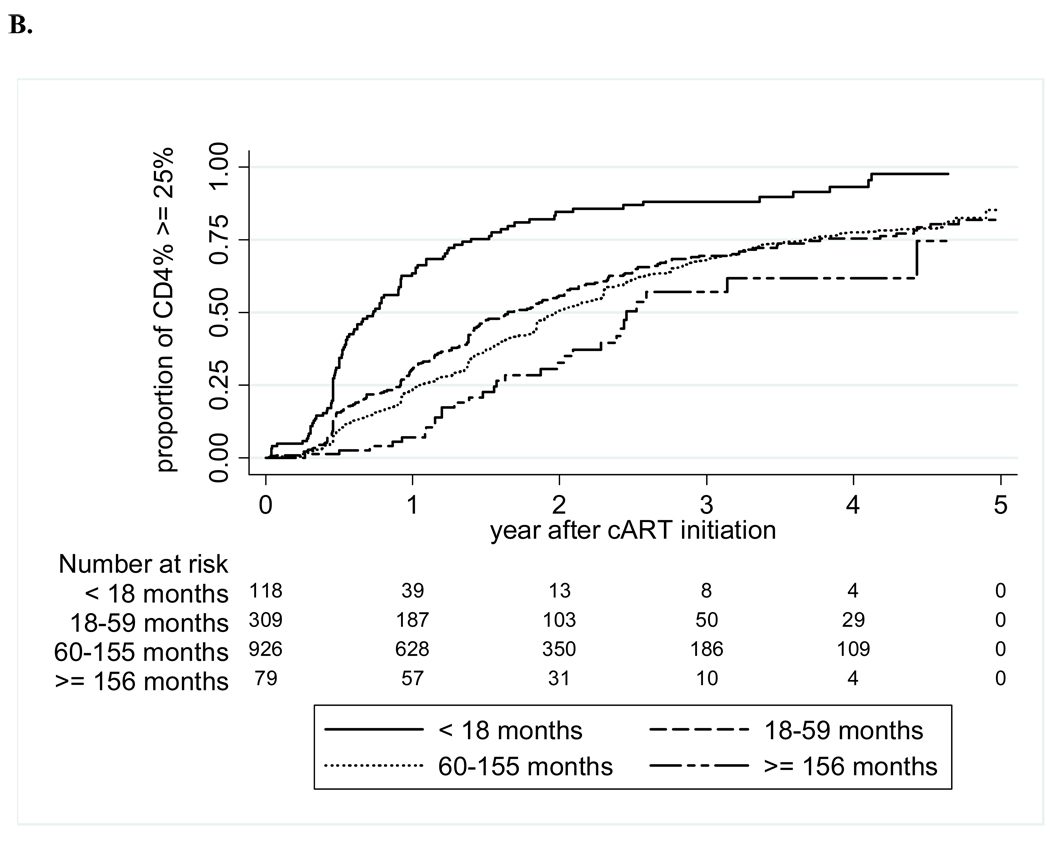
The crude mortality rate at one year of cART was 5%, and 8% at five years of cART, with a mortality rate of 2.1 (95% CI 1.7–2.5) per 100 person-years (Figure 1). LTFU increased from 4% at one year to 24% at five years, for an overall rate of 4.2 (3.7–4.8) per 100 person-years. The median WAZ gains after initiation of cART were 0.23 at one year, 0.3 at two years, and 0.43 at three years; HAZ gains were slower at 0.08 at one year and 0.32 at two years, but increased to 0.55 at three years (Figure 2). The median CD4 percentage gain from baseline increased from 11 percentage points at one year, to 15 at two years, and 18 percentage points at three years after cART. The probabilities of achieving immunologic recovery, defined as CD4 percentage ≥25%, were 28% at one year of cART, and increased to 77% by four years (Figure 3). The median duration from initiation of cART to CD4 recovery was 1.8 (IQR 0.9–3.5) years. In multivariate analysis, CD4 recovery to ≥25% was associated with starting cART at age <18 months, being female, lack of pre-cART mono- or dual-therapy exposure, and higher baseline CD4 percentage (Table 2).
Figure 1. Time to death and loss to follow-up after cART initiation.
Abbreviation: LTFU-loss to follow-up; cART-combination antiretroviral therapy.
Figure 2. Growth recovery after cART as assessed by median weight-for-age and height-for-age z scores.
Abbreviations: HAZ-height for age z score; WAZ-weight for age z score; cART-combination antiretroviral therapy.
Figure 3. Time to CD4 recovery to ≥25% after cART initiation, by baseline CD4% (A) and by age at cART initiation (B).
Abbreviation: cART-combination antiretroviral therapy.
Table 2.
Univariate and multivariate analyses for factors associated with time to CD4 recovery to ≥25%
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| ≥156 months of age | 1 | … | 1 | … |
| 60–155 months of age | 1.6 (1.1 – 2.3) | 0.013 | 2.1 (1.4 – 3.2) | 0.001 |
| 18–59 months of age | 1.8 (1.2 – 2.6) | 0.003 | 2.1 (1.3 – 3.3) | 0.001 |
| <18 months of age | 4.4 (2.9 – 6.6) | <0.001 | 3.8 (2.4 – 6.2) | <0.001 |
| Female | 1.3 (1.1 – 1.5) | <0.001 | 1.4 (1.2 – 1.7) | <0.001 |
| Infant ARV for PMTCT | 2.0 (1.6 – 2.6) | <0.001 | ||
| Maternal ARV for PMTCT | 1.7 (1.3 – 2.2) | <0.001 | ||
| No history of mono/dual | ||||
| ART | 1.3 (1.1 – 1.6) | 0.015 | 1.8 (1.4 – 2.3) | <0.001 |
| Single orphan | 0.8 (0.7 – 0.9) | 0.003 | ||
| Double orphan | 0.8 (0.7 – 1.0) | 0.029 | ||
| Weight for age z score1, per 5 unit change |
1.3 (0.9 – 1.7) | 0.157 | ||
| Height for age z score, per 5 | ||||
| unit change | 1.1 (0.9 – 1.2) | 0.509 | ||
| Baseline BMI, per 5 unit change |
1.0 (0.9 – 1.1) | 0.431 | ||
| WHO Stage | 0.021 | |||
| Stage I | 1 | |||
| Stage II | 0.9 (0.7 – 1.3) | 0.667 | ||
| Stage III | 0.8 (0.6 – 1.0) | 0.049 | ||
| Stage IV | 0.7 (0.5 – 1.0) | 0.029 | ||
| CD4% at baseline per 10% change |
2.0 (1.9 – 2.2) | <0.001 | 2.0 (1.9 – 2.2) | <0.001 |
using WHO 1977 growth standards
Abbreviations: HR-hazard ratio; CI-confidence interval; ARV-antiretroviral; ART-antiretroviral therapy; PMTCT-prevention of mother-to-child transmission; BMI-body mass index.
In 501 children who had HIV-RNA monitoring at 12 months of cART, factors independently associated with HIV-RNA >400 copies/mL at 12 months of cART included being male, pre-cART mono- or dual-therapy, and starting cART with NVP-based regimens as compared to EFV-based regimens (Table 3; Pearson chi-square for goodness-of-fit = 2.35, p=0.671). At 12 months of cART, 25% were >400 copies/ml. Of the 302 children with viral load at 36 months of cART, 19% were >400 copies/ml. Factors identified by univariate analysis to be associated with detectable viral load included starting cART with NVP-based regimens as compared to EFV-based regimens (OR 3.5; 95% CI 1.7–7.1, p= 0.001) and being a double orphan (OR 2.2; 95% CI 1.1–4.4, p=0.03). Multivariate analysis of detectable viral load at 36 months found that no variables were significantly associated after adjustment for NVP-based regimen.
Table 3.
Univariate and multivariate analyses for factors associated with HIV-RNA >400 copies/mL after 12 months of cART (N=501)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age <18 months of age | 1 | … | ||
| 18–59 months of age | 0.9 (0.4 – 2.1) | 0.833 | ||
| 60–155 months of age | 0.7 (0.4 – 1.6) | 0.446 | ||
| ≥156 months of age | 0.8 (0.2 – 2.7) | 0.705 | ||
| Male | 1.9 (1.3 – 2.9) | 0.002 | 1.9 (1.2 – 2.9) | 0.008 |
| Infant ARV for PMTCT | 0.9 (0.5 – 1.8) | 0.753 | ||
| Maternal ARV for PMTCT | 0.6 (0.3 – 1.3) | 0.214 | ||
| History of mono/dual ART | 4.5 (2.7 – 7.8) | <0.001 | 5.7 (3.2 – 10.4) | <0.001 |
| Initial cART regimen NVP-based | 1.6 (1.1–2.5) | 0.026 | 2 (1.2–3.2) | 0.005 |
| Single orphan | 1.7 (1.1 – 2.8) | 0.017 | ||
| Double orphan | 1.4 (0.8 – 2.3) | 0.272 | ||
| Weight for age z score1, per 5 unit | 1.9 (0.7 – 4.8) | 0.184 | ||
| Height for age z score, per 5 unit | 1.3 (0.5 – 3.0) | 0.571 | ||
| Baseline BMI, per 5 unit | 1.5 (0.9 – 2.4) | 0.103 | ||
| WHO Stage I | 1 | … | ||
| Stage II | 0.5 (0.2 – 1.3) | 0.168 | ||
| Stage III | 0.7 (0.3 – 1.9) | 0.545 | ||
| Stage IV | 0.7 (0.3 – 1.9) | 0.518 | ||
| CD4%, per 10% increase | 1.1 (0.8 – 1.4) | 0.659 | ||
| HIV RNA ≥400 copies/ml | 1.7 (0.2 – 14.4) | 0.624 | ||
Abbreviations: OR-odds ratio; CI-confidence interval; ARV-antiretroviral; cART-combination antiretroviral therapy; PMTCT-prevention of mother-to-child transmission; BMI-body mass index.
Discussion
Approximately 57,000 of the estimated 160,000 children in Asia with HIV need cART, of whom an estimated 53% were on treatment at the end of 2008.2 Those patients who had limited access to antiretrovirals early in the regional epidemic were frequently started on mono- or dual-therapy, which is reflected in the 12% of children in the cohort who report that history. With greater drug availability, all our participating clinical centers have begun initiating treatment with cART at the CD4 levels recommended by international guidelines.11 However, both the early history of suboptimal regimens and delays in identifying children with HIV early in their disease course have resulted in ongoing clinical challenges to optimal pediatric HIV management.
Children in our cohort started cART at a median of seven years of age, when their median CD4 levels were already at 8%; only 10% started treatment before 18 months of age. The older age may reflect the longer history of some of our site-level cohorts, where children entered into care before cART was widely available. The realities of early limitations in cART access have likely led to some bias towards inclusion of slower progressors who survived long enough to start cART when it became available. Younger children in our cohort were more likely to have received cART earlier as well (data not shown), which may further reflect a survivor bias and expanding access to cART in some sites. However, despite the delayed initiation of cART, the short- and long-term immunologic and growth responses in our cohort were similar or better than other studies in sub-Saharan Africa and in the US.12–15 Our finding that CD4 recovery to 25% was associated with earlier initiation of cART and higher CD4 levels is similar to other pediatric and adult studies,9, 10 and supports the implementation of recommendations to start cART earlier. The magnitude of weight and height gains was comparable to other studies in resource-limited settings.13, 16 That CD4 levels and growth continued to improve beyond three years of treatment was encouraging.
Approximately one-third of children in our cohort had HIV-RNA monitoring and 75% achieved virologic suppression to <400 copies/mL at 12 months of cART. This is comparable to the rate of 70% in a recent meta-analysis from studies in resource limited-settings17 and other studies in Europe and the US.18–20 However, the fact that virologic failure was associated with a history of receiving mono- or dual-NRTI treatment and NVP-based cART regimens reflects the consequences of using less potent regimens and raises concerns about the durability of NVP-based regimens in children. A study in Uganda and another in Thailand similarly found that NVP use was associated with virologic failure, relative to EFV.21, 22 Although the subset of children with available VL was small, we found a higher likelihood of virologic failure in those who starting with NVP-containing regimens up to 36 months of treatment. At a median of three years of cART, 7.3% of children had switched from an NNRTI to a PI-containing regimen – more than double the 3% of children in low-and middle-income countries on second-line reported to WHO in a 2009 survey.23 In addition, similar to studies on the risk factors for virologic failure at 12 months of treatment in Cambodia24 and Uganda,25 we found that being male was associated with both virologic failure and with a lower likelihood for CD4 recovery. At the same time, orphan status fell out of the final multivariate model, which may have been related to higher exposure to mono- or dual-NRTI therapy before starting cART, as these children were more likely to be older survivors. Identifying potent and durable regimens for use from infancy into childhood is critical to ensuring long-term immune recovery and treatment success.
Our study describes the largest pediatric HIV cohort reported from the Asian region and includes patients in primarily urban-based public hospitals. The LTFU rate of 4.7 per 100 person-years was lower than has been reported in sub-Saharan Africa.26 However, the heterogeneity of the data inherent in a multinational cohort and the types of clinical centers participating limit the generalizeability of these results. Although all centers were in low-and middle-income countries, each has had different experiences with HIV care and cART roll-out that can impact long-term outcomes. In addition, that some of the data were collected retrospectively is likely to have led to incompleteness in the clinical data. However, participating centers frequently are their nations’ referral hospitals for pediatric HIV care, and their patients have the highest levels of treatment access in their countries. Analyses were also adjusted for key individual patient surrogate markers (e.g., CD4) that controlled for variation between individual patients. Experiences of this cohort are likely to raise issues that will be faced by other children in less resourced areas of Asia in the future.
In conclusion, we have described the characteristics and outcomes of cART in HIV-infected children in a large regional network in Asia. The results of this study show that although children in the region started cART late and at low CD4 levels, their immune and virologic responses to cART were comparable to developed countries. The consequences of using of less potent regimens are already being reflected in the numbers of children on second-line regimens. Starting cART earlier and expanding access to a wider variety of antiretrovirals will be essential to sustaining the gains made in pediatric treatment scale-up.
Acknowledgments
TREAT Asia is a program of The Foundation for AIDS Research, amfAR. The TREAT Asia Pediatric HIV Observational Database (TApHOD) is supported in part by grants from the Austrian AIDS Life Association, the U.S. National Institutes of Health (National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development) through the International Epidemiologic Databases to Evaluate AIDS (Grant No.U01-AI069907). The National Centre in HIV Epidemiology and Clinical Research is funded by The Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The authors also wish to thank all the children and the staff in the participating centers who have given their time so generously during the course of this project.
The TREAT Asia Pediatric HIV Network
V Saphonn* and S Saramony, National Centre for HIV/AIDS Dermatology and STDs, Phnom Penh, Cambodia;
U Vibol* and S Sophan, National Pediatric Hospital, Phnom Penh, Cambodia;
J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia;
FJ Zhang and N Han, Beijing Ditan Hospital, Beijing, China;
N Kumarasamy* and S Saghayam, YR Gaitonde Centre for AIDS Research and Education, Chennai, India;1
N Kurniati* and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
SM Fong* and M Thien, Hospital Likas, Kota Kinabalu, Malaysia;
NK Nik Yusoff* and LC Hai, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia;
K Razali* and NF Abdul Rahman, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia;
R Nallusamy* and KC Chan, Penang Hospital, Penang, Malaysia;
V Sirisanthana* and L Aurpibul, Chiang Mai University, Chiang Mai, Thailand;
R Hansudewechakul*,† and A Khongponoi, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
P Lumbiganon* and P Kosalaraksa., Khon Kaen University, Khon Kaen, Thailand;
G Jourdain, Program for HIV Prevention and Treatment, Chiang Mai, Thailand;
J Ananworanich*,‡ and T Suwanlerk, The Netherlands, Australia, Thailand Research Collaboration (HIV-NAT), Bangkok, Thailand;
K Chokephaibulkit* and O Wittawatmongkol, Siriraj Hospital, Mahidol University;
HK Truong* and DAN Mai, Children’s Hospital 1, Ho Chi Minh City, Vietnam;
CV Do* and MT Ha, Children’s Hospital 2, Ho Chi Minh City, Vietnam;
HV Bui * and VL Nguyen, National Hospital of Pediatrics, Hanoi, Vietnam;
ON Le, Worldwide Orphans Foundation, Ho Chi Minh City, Vietnam;
AH Sohn*, L Messerschmidt, and J Pang, TREAT Asia, amfAR -- The Foundation for AIDS Research, Bangkok, Thailand;
DA Cooper, MG Law*, and A Kariminia, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia;
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented in part at the 5th Conference on HIV Pathogenesis and Treatment and Prevention (Cape Town, July 19–22, 2009) and the 1st International Workshop on HIV Pediatrics (Cape Town, July 17–18, 2009).
Funding to support the site in India is provided by the Austrian AIDS Life Association.
TApHOD Steering Committee member
Current Steering Committee Chair;
co-Chair
References
- 1.UNAIDS, WHO. Geneva: AIDS Epidemic Update: December 2009. 2009
- 2.UNICEF. Children and AIDS, Fourth stocktaking report. 2009
- 3.WHO. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: Towards universal access, Recommendations for a public-health approach. 2006 [PubMed]
- 4.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008 Nov 20;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort Profile: The TREAT Asia Pediatric HIV Observational Database. Int J Epidemiol. 2010 doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasitsuebsai W, Bowen AC, Pang J, Hesp C, Kariminia A, Sohn AH. Pediatric HIV clinical care resources and management practices in Asia: a regional survey of the TREAT Asia pediatric network. AIDS Patient Care STDS. 2009 Feb;24(2):127–131. doi: 10.1089/apc.2009.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. WHO growth chart 2006. http://www.who.int/childgrowth/en/.
- 8.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002 May;(246):1–190. [PubMed] [Google Scholar]
- 9.Puthanakit T, Kerr S, Ananworanich J, Bunupuradah T, Boonrak P, Sirisanthana V. Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy. Pediatr Infect Dis J. 2009 Jun;28(6):488–492. doi: 10.1097/inf.0b013e318194eea6. [DOI] [PubMed] [Google Scholar]
- 10.Soh CH, Oleske JM, Brady MT, et al. Long-term effects of protease-inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003 Dec 20;362(9401):2045–2051. doi: 10.1016/s0140-6736(03)15098-2. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Report of the WHO Technical Reference Group; Paediatric HIV/ART Care Guideline Group Meeting WHO Headquarters; 10–11 April 2008; Geneva, Switzerland. 2008. [Google Scholar]
- 12.O'Brien DP, Sauvageot D, Zachariah R, Humblet P. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. Aids. 2006 Oct 3;20(15):1955–1960. doi: 10.1097/01.aids.0000247117.66585.ce. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Haberer JE, Zhao Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: a 1-year analysis of clinical, immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2007 Dec 15;46(5):594–598. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]
- 14.McConnell MS, Byers RH, Frederick T, et al. Trends in antiretroviral therapy use and survival rates for a large cohort of HIV-infected children and adolescents in the United States 1989–2001. J Immune Defic Syndr. 2005 Apr 1;38(4):488–494. doi: 10.1097/01.qai.0000134744.72079.cc. [DOI] [PubMed] [Google Scholar]
- 15.Selik RM, Lindegren ML. Changes in deaths reported with human immunodeficiency virus infection among United States children less than thirteen years old, 1987 through 1999. Pediatr Infect Dis J. 2003 Jul;22(7):635–641. doi: 10.1097/01.inf.0000073241.01043.9c. [DOI] [PubMed] [Google Scholar]
- 16.Kline MW, Rugina S, Ilie M, et al. Long-term follow-up of 414 HIV-infected Romanian children and adolescents receiving lopinavir/ritonavir-containing highly active antiretroviral therapy. Pediatrics. 2007;119(5):e1116–e1120. doi: 10.1542/peds.2006-2802. [DOI] [PubMed] [Google Scholar]
- 17.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009 Dec 15;49(12):1915–1927. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaspan HB, Berrisford AE, Boulle AM. Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J. 2008 Nov;27(11):993–998. doi: 10.1097/INF.0b013e31817acf7b. [DOI] [PubMed] [Google Scholar]
- 19.Lapphra K, Vanprapar N, Chearskul S, et al. Efficacy and tolerability of nevirapine- versus efavirenz-containing regimens in HIV-infected Thai children. Int J Infect Dis. 2008 Nov;12(6):e33–e38. doi: 10.1016/j.ijid.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin DK, Jr, Miller WC, Benjamin DK, et al. A comparison of height and weight velocity as a part of the composite endpoint in pediatric HIV. AIDS. 2003 Nov 7;17(16):2331–2336. doi: 10.1097/00002030-200311070-00007. [DOI] [PubMed] [Google Scholar]
- 21.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Sustained immunologic and virologic efficacy after four years of highly active antiretroviral therapy in human immunodeficiency virus infected children in Thailand. Pediatr Infect Dis J. 2007 Oct;26(10):953–956. doi: 10.1097/INF.0b013e318125720a. [DOI] [PubMed] [Google Scholar]
- 22.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008 Aug;8(8):477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 23.WHO, UNAIDS, UNICEF. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector, progress report 2009. 2009
- 24.Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007 Nov;120(5):e1134–e1140. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 25.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 26.KIDS-ART-LINC. Low Risk of Death, but Substantial Program Attrition, in Pediatric HIV Treatment Cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008 Nov 4; doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]