Abstract
Background. This study investigated the association between betel nut chewing and subclinical ischemic heart disease (IHD) in Taiwanese type 2 diabetic patients. Methods. A total of 394 male patients aging ≥45 years and without previous heart disease were studied. Among them 349 had no habit of chewing betel nut and 45 possessed the habit for ≥5 years. Subclinical IHD was diagnosed by a Minnesota-coded resting electrocardiogram and was present in 71 cases. Statistical analyses were performed considering confounding effects of age, diabetic duration, smoking, body mass index, blood pressure, dyslipidemia, and metabolic control status. Results. Betel nut chewers were younger and had higher prevalence of smoking (86.7% versus 60.5%), higher body mass index, poorer glycemic control, and higher prevalence of subclinical IHD (28.9% versus 16.6%). Patients with subclinical IHD were older and had higher prevalence of betel nut chewing (18.0% versus 9.9%). The multivariate-adjusted odds ratio for subclinical IHD for chewers versus nonchewers was 4.640 (1.958–10.999). The adjusted odds ratios in younger or older patients divided by the median age of 63 years were similar: 4.724 (1.346–16.581) and 4.666 (1.278–17.028), respectively. Conclusions. Betel nut chewing is significantly associated with increased risk of subclinical IHD.
1. Introduction
Areca nut is the seed of the palm tree Areca catechu, which is the fourth most commonly used psychoactive substance, after caffeine, nicotine, and alcohol [1]. Because areca nut is always consumed with the leaf of Piper betle, chewing of areca nut has always been referred to as “betel nut chewing” in the English literature [2]. There is an estimated 600 million people chewing betel nut worldwide [3].It is a common habit and is a means of social interaction in Asia, particularly the South Pacific islands, Southeast Asia, Papua New Guinea, Bangladesh, Pakistan, and India [1–4]. Chewing of betel nut was forbidden in Taiwan during the Japanese reign more than 60 years ago [4]. But this habit has become popular in Taiwan during the past two to three decades, and it has been estimated that about 2.4 millions, or 11.4%, of the total population are chewing betel nut [5]. The chewing population in Taiwan keeps on increasing, especially in the male sex of the younger generation [6, 7]. In Taiwan, unripe areca nut is commonly chewed with a mixture of lime and the leaf or flower of the Piper betle, but without tobacco [4].
Betel nut chewing has been linked to a variety of health problems including oral lesions of leukoplakia, submucosal fibrosis, squamous cell carcinoma and periodontal disease [8, 9], albuminuria in diabetic patients [10], disruption of gastric mucosal barriers [11], aggravation of asthma [12], induction of extrapyramidal syndrome [13], milk-alkali syndrome (in a case report) [14], induction of uterine cervical dysplasia [15], cancers of the esophagus [16] and liver [17], and low birth weight of babies born to mothers chewing betel nut [18]. In more recent population-based studies in Taiwan, betel nut chewing is also associated with a higher risk of type 2 diabetes mellitus (T2DM) [19], hypertension [20], and total and cerebrovascular deaths [21].
Studies on the cardiovascular effects of betel nut chewing are rare. Hemodynamic changes have been observed during betel nut chewing [7]. However, whether the prolonged chewing of betel nut could exert an effect on the heart has not been previously studied. Therefore, the purpose of this study was to evaluate whether betel nut chewing could be associated with the prevalence of subclinical IHD in a subgroup of patients with T2DM recruited as a long-term follow-up cohort in Taiwan.
2. Methods
2.1. Study Subjects
The study was approved by an ethics committee of the Department of Health, Taiwan, and the subjects voluntarily participated in the study. More than 96% of the population of Taiwan is covered by a compulsory National Health Insurance program. A total of 256,036 patients using this health insurance program were assembled from 1995 to 1998 [22–24]. Baseline data was collected by questionnaires on the onset symptoms and confirmation of diabetic diagnosis from 93,484 patients of the original cohort [22–24]. At random, 4,164 patients were selected from the main cluster of 93,484 patients and invited to participate in a health examination. A total of 1,441 patients participated in the health examination from March 1998 to September 2002. After excluding 21 patients with type 1 diabetes mellitus (T1DM), there were a total of 1420 patients diagnosed as T2DM. The patients with T2DM did not show a history of diabetic ketoacidosis at the onset of diabetes and were being treated with either oral antidiabetic drugs or insulin at the time of recruitment. For those under insulin treatment, none received such treatment within one year of diagnosis of diabetes mellitus.
Patients with T1DM were excluded because of the small number of cases who might also have different pathogenesis of IHD. Women with T2DM were further excluded because the habit of betel nut chewing is very uncommon in women in Taiwan [6, 7, 25]. Taking into account the possible requirement of prolonged chewing for the manifestations of clinical outcomes, this study recruited only adult male patients aging ≥45 years, and chewers must be current chewers and have retained the habit for ≥5 years at the time of recruitment. Patients with a clinical history of heart disease including angina pectoris, myocardial infarction, congestive heart failure or under treatment for such were also excluded because of the impossibility to clarify the correctness of temporality between cause and effects and because of the potential confounding effect caused by treatments. As a result, a total of 394 men with T2DM were included in this study. They were divided into two groups: one with no habit of chewing betel nut and the other should have persistently chewed betel nut for more than 5 years at the time of recruitment.
2.2. Diagnosis of Subclinical Ischemic Heart Disease
Resting electrocardiogram was performed in each subject, and the Minnesota codes were used to code the electrocardiograms. The coder was blind to the history or the biochemical data of the subjects. Subclinical IHD was defined by Minnesota codes of coronary probable (1.1, 1.2, 7.1) and coronary possible (1.3, 4.1–4.3, 5.1–5.3) [26].
2.3. Measurements of Blood Biochemistry and Other Covariates
Blood samples were collected in the early morning after fasting for at least 12 hours. Fasting plasma glucose (FPG), serum total cholesterol (TC), and triglyceride (TG) were measured by an automatic biochemistry analyzer (Cobas Mira S, Roche Diagnostica, Basel, Switzerland) with reagents obtained from Randox Laboratories Ltd. (Antrium, UK) [27].
The patients' age, duration of diabetes, body mass index (BMI), smoking status, and systolic (SBP) and diastolic blood pressure (DBP) were recorded or measured. Duration of diabetes was defined as the time period in years between the time being recruited into the study and the time diabetes was diagnosed. Blood pressure was measured on the right arm after 20 minutes rest in a sitting position with a mercury sphygmomanometer by the auscultatory method. Body height (in centimeters) was measured by having the subjects stand with their heals, buttocks, and heads against a wall. A flat object was placed on top of the subjects' head, and their height was marked on a tape measure affixed to the wall. Body weight was measured in kilograms with a standard portable scale. Body height and body weight were measured with the patient wearing light clothes and without socks and shoes. BMI was calculated as body weight in kilograms divided by the square of the body height in meters. Hypertension was defined by a positive history with the use of antihypertensive agents or by SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg. Dyslipidemia was diagnosed by the use of lipid-lowering agents and/or a TC level ≥ 200 mg/dL and/or TG ≥ 150 mg/dL.
2.4. Statistical Analyses
Data were expressed as mean (SD) or percentage. A P < .05 was considered statistically significant, while .05 < P < .1 was borderline significant. Age was divided into two groups by the median and the prevalences of subclinical IHD between chewers and nonchewers were compared by Chi-square test in the respective age groups. The baseline characteristics between chewers and nonchewers and between patients with subclinical IHD and those without subclinical IHD were compared by Student's t-test for continuous variables and by Chi-square test for categorical variables. Logistic regression models estimating the odds ratios for subclinical IHD were created for all patients and for patients in the respective two age groups divided by the median of age. These regression models were generated in the following 3 ways: (1) unadjusted; (2) adjusted for variables found to be different between chewers and non-chewers, or between patients with and without subclinical IHD with P values < .1; (3) adjusted for all potential covariates (i.e., age, diabetic duration, body mass index, smoking, hypertension, dyslipidemia, FPG, SBP, DBP, TC, and TG).
3. Results
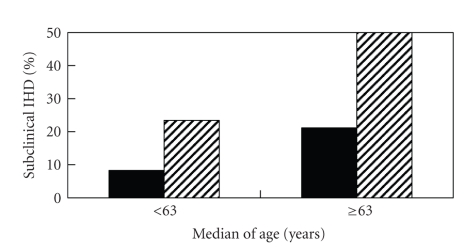
Figure 1 shows the prevalences of subclinical IHD in chewers and non-chewers in the respective age groups divided by the median of 63 years old.The prevalences of subclinical IHD differed significantly between chewers and non-chewers in either the younger or the older age groups (P < .05).
Figure 1.
Prevalence of subclinical ischemic heart disease (IHD) between betel nut chewers (shaded column) and non-chewers (black column) by median of age (P < .05 for each subgroup of age).
Table 1 compares the baseline characteristics between chewers and nonchewers and between patients with and without subclinical IHD. Chewers were significantly younger in age, more prevalent in smoking and subclinical IHD, and had higher BMI and poorer glycemic control. Patients with subclinical IHD were significantly older and more prevalent in betel nut chewing.
Table 1.
Comparisons between patients chewing and not chewing betel nut and having and not having subclinical ischemic heart disease (IHD).
| Betel nut chewing | Subclinical IHD | |||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| n | 349 | 45 | 323 | 71 |
| Age, years | 63.9 (9.3) | 56.3 (8.6)** | 62.0 (9.4) | 67.6 (9.1)** |
| Diabetic duration, years | 10.4 (7.6) | 10.3 (8.8) | 10.4 (7.6) | 10.7 (8.5) |
| Body mass index, kg/m2 | 24.5 (3.0) | 25.9 (3.7)** | 24.6 (3.2) | 24.8 (2.9) |
| Smoking, % | 60.5 | 86.7** | 63.2 | 64.8 |
| Fasting plasma glucose, mg/dL | 159.4 (57.3) | 178.6 (74.8)* | 162.2 (56.5) | 159.1 (72.9) |
| Hypertension, % | 52.4 | 60.0 | 52.3 | 57.8 |
| Systolic blood pressure, mmHg | 132.8 (15.8) | 131.9 (16.1) | 132.1 (15.4) | 135.2 (17.5) |
| Diastolic blood pressure, mmHg | 83.2 (8.8) | 84.8 (7.0) | 83.4 (8.4) | 83.1 (9.6) |
| Dyslipidemia, % | 56.0 | 68.9 | 56.5 | 62.0 |
| Total cholesterol, mg/dL | 199.8 (45.9) | 196.9 (38.2) | 199.1 (45.1) | 201.3 (44.9) |
| Triglyceride, mg/dL | 168.7 (193.3) | 180.0 (83.1) | 168.9 (196.2) | 174.9 (114.6) |
| Ischemic heart disease, % | 16.6 | 28.9* | — | — |
| Betel nut chewing, % | — | — | 9.9 | 18.0* |
*P < .05; **P < .01.
Table 2 shows the odds ratios for subclinical IHD. Chewers consistently showed a significantly higher risk of subclinical IHD in either the older or the younger age group in all of the models.
Table 2.
Odds ratios for subclinical ischemic heart disease comparing chewers versus nonchewers of betel nut.
| Odds ratio (95% confidence interval) | |||
|---|---|---|---|
| <63 years old | ≥63 years old | All ages | |
| Unadjusted | 2.982 (1.087–8.182)* | 3.267 (1.004–10.629)* | 2.038 (1.008–4.118)* |
| Adjusted for age, BMI, smoking, and FPG | 4.153 (1.280–13.471)* | 4.183 (1.170–14.955)* | 4.269 (1.837–9.920)** |
| Adjusted for all covariates (age, diabetic duration, BMI, smoking, hypertension, dyslipidemia, FPG, SBP, DBP, TC, and TG) | 4.724 (1.346–16.581)* | 4.666 (1.278–17.028)* | 4.640 (1.958–10.999)** |
*P < .05; **P < .01.
4. Discussion
The findings of this study clearly demonstrated a higher risk of subclinical IHD in T2DM patients without a previous history of heart disease (Tables 1 and 2; Figure 1). This association was independent of traditional risk factors, and could be demonstrated in either the younger or the older patients (Figure 1; Table 2). To the best of our knowledge, this was the first study demonstrating a link between betel nut chewing and subclinical IHD. The association was consistent, and the magnitude of the odds ratios was large.
Although the real pathogenetic mechanism(s) remains unknown, some of the biological effects associated with the ingredients of areca nut or the compounds formed during chewing might explain some of the possibilities. The common preparations of the betel nut quid in Taiwan consist of three major components: the nut of Areca catechu, quicklime, and the leaf or the flower of Piper betle [4]. Areca nut contains arecoline which has a cholinergic action at the muscarinic and nicotinic receptors [28]. This cholinergic action on the central nervous system could possibly produce the cortical arousal and alertness which is always claimed as one of the merits experienced by the betel nut chewers. On the other hand, arecoline might stimulate the hypothalamic-pituitary-adrenal axis through a centrally mediated corticotrophin-releasing hormone-dependent mechanism in rats [29]. In the presence of lime, arecoline is hydrolyzed to arecaidine, which lacks the parasympathomimetic effects of arecoline [4] and exerts sympathetic effect by inhibition of γ-aminobutyric acid (GABA) uptake [30]. However, a later study suggested that arecaidine may not cross the blood-brain barrier and the central effects may involve transmitters other than GABA [31]. The aromatic substances (e.g., eugenol, isoeugenol, and hydroxychavicol) in the flower or leaf of Piper betle can stimulate the release of catecholamines from chromaffin cells in vitro [32], and circulating norepinephrine and epinephrine levels are elevated following betel nut chewing [33]. However, these sympathomimetic effects of arecoline might be mediated by central cholinergic mechanisms [34]. Therefore, both areca nut and Piper betle flower may exert sympathomimetic effects. Whether these sympathomimetic effects may be responsible for the subclinical IHD observed in the present study awaits further investigations. Reactive oxygen species and N-nitroso compounds can also be formed in the oral cavity during chewing of betel nut [35, 36]. In vitro studies also demonstrated that betel nut components increased the release of inflammatory mediators such as prostanoids, interleukin-6, and tumor necrosis factor-α [37, 38]. The production of these chemical agents has always been regarded as the mediators of carcinogenicity and diabetogenicity associated with betel nut chewing. Whether they can also be responsible for a hemodynamic or structural change in the coronary vascular system leading to subclinical IHD is an issue worthy of further investigation.
Some limitations deserve mentioning. This study was conducted in the diabetic patients, and it is not known whether similar effects can be extended to the general population without diabetes. Future studies should be aimed at a dose-response relationship and taking the duration of betel nut chewing into consideration. Longitudinal prospective studies are required to clarify the cause/effect relationship between betel nut chewing and subclinical IHD.
In conclusions, betel nut chewing in Taiwanese patients with T2DM is associated with subclinical IHD. While the chewing of betel nut is decreasing in some countries like Thailand [39], the prevalence keeps on increasing in Taiwan, especially in the younger generation. It is urgent for policy makers to implement programs of health education to the younger generation to curb the increasing prevalence of betel nut chewing and its associated health problems.
Acknowledgments
The author thanks the following institutes in Taiwan for their continuous support on the epidemiologic studies of diabetes and arsenic-related health hazards: the New Century Health Care Promotion Foundation; the National Genotyping Center of National Research Program for Genomic Medicine, National Science Council; the Department of Health (DOH89-TD-1035; DOH97-TD-D-113-97009); the National Taiwan University Hospital Yun-Lin Branch (NTUHYL96.G001); the National Science Council (NSC-86-2314-B-002-326, NSC-87-2314-B-002-245, NSC88-2621-B-002-030, NSC89-2320-B002-125, NSC-90-2320-B-002-197, NSC-92-2320-B-002-156, NSC-93-2320-B-002-071, NSC-94-2314-B-002-142, NSC-95-2314-B-002-311, and NSC-96-2314-B-002-061-MY2).
References
- 1.Gupta PC, Ray CS. Epidemiology of betel quid usage. Annals of the Academy of Medicine Singapore. 2004;33(4) [PubMed] [Google Scholar]
- 2.Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addiction Biology. 2002;7(1):77–83. doi: 10.1080/13556210020091437. [DOI] [PubMed] [Google Scholar]
- 3.Nelson BS, Heischober B. Betel nut: a common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Annals of Emergency Medicine. 1999;34(2):238–243. doi: 10.1016/s0196-0644(99)70239-8. [DOI] [PubMed] [Google Scholar]
- 4.Chu N-S. Effects of Betel chewing on the central and autonomic nervous systems. Journal of Biomedical Science. 2001;8(3):229–236. doi: 10.1007/BF02256596. [DOI] [PubMed] [Google Scholar]
- 5.Huang A. Betel nuts, better not. Free China Review. 1997;47:18–27. [Google Scholar]
- 6.Yang M-S, Su I-H, Wen J-K, Ko Y-C. Prevalence and related risk factors of betel quid chewing by adolescent students in southern Taiwan. Journal of Oral Pathology and Medicine. 1996;25(2):69–71. doi: 10.1111/j.1600-0714.1996.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin S-K, Chang Y-J, Ryu S-J, Chu N-S. Cerebral hemodynamic responses to betel chewing: a doppler study. Clinical Neuropharmacology. 2002;25(5):244–250. doi: 10.1097/00002826-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addiction Biology. 2002;7(1):115–125. doi: 10.1080/13556210120091482. [DOI] [PubMed] [Google Scholar]
- 9.Mehta FS, Sanjana MK, Baretto MA. Relation of betel leaf chewing to periodontal disease. The Journal of the American Dental Association. 1955;50:531–536. doi: 10.14219/jada.archive.1955.0098. [DOI] [PubMed] [Google Scholar]
- 10.Tseng C-H. Betel nut chewing is independently associated with urinary albumin excretion rate in type 2 diabetic patients. Diabetes Care. 2006;29(2):462–463. doi: 10.2337/diacare.29.02.06.dc05-2186. [DOI] [PubMed] [Google Scholar]
- 11.Hung C-R, Cheng J-T. Betel quid chewing damaged gastric mucosa: protective effects of cimetidine and sodium bicarbonate. Chinese Journal of Physiology. 1994;37(4):213–218. [PubMed] [Google Scholar]
- 12.Taylor RFH, Al-Jarad N, John LME, Conroy DM, Barnes NC. Betel-nut chewing and asthma. The Lancet. 1992;339(8802):1134–1136. doi: 10.1016/0140-6736(92)90732-i. [DOI] [PubMed] [Google Scholar]
- 13.Deahl M. Betel nut-induced extrapyramidal syndrome: an unusual drug interaction. Movement Disorders. 1989;4(4):330–333. doi: 10.1002/mds.870040406. [DOI] [PubMed] [Google Scholar]
- 14.Lin S-H, Lin Y-F, Cheema-Dhadli S, Davids MR, Halperin ML. Hypercalcaemia and metabolic alkalosis with betel nut chewing: emphasis on its integrative pathophysiology. Nephrology Dialysis Transplantation. 2002;17(5):708–714. doi: 10.1093/ndt/17.5.708. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti RN, Dutta K, Ghosh K, Sikdar S. Uterine cervical dysplasia with reference to the betel quid chewing habit. European Journal of Gynaecological Oncology. 1990;11(1):57–59. [PubMed] [Google Scholar]
- 16.Wu M-T, Wu D-C, Hsu H-K, Kao E-L, Lee J-M. Relationship between site of oesophageal cancer and areca chewing and smoking in Taiwan. British Journal of Cancer. 2003;89(7):1202–1204. doi: 10.1038/sj.bjc.6601251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun C-A, Wu D-M, Lin C-C, et al. Incidence and cofactors of hepatitis C virus-related hepatocellular carcinoma: a prospective study of 12,008 men in Taiwan. American Journal of Epidemiology. 2003;157(8):674–682. doi: 10.1093/aje/kwg041. [DOI] [PubMed] [Google Scholar]
- 18.de Costa C, Griew AR. Effects of betal chewing on pregnancy outcome. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1982;22(1):22–24. doi: 10.1111/j.1479-828x.1982.tb01392.x. [DOI] [PubMed] [Google Scholar]
- 19.Tseng C-H. Betel nut chewing and incidence of newly diagnosed type 2 diabetes mellitus in Taiwan. BMC Research Notes. 2010;3, article 228 doi: 10.1186/1756-0500-3-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng C-H. Betel nut chewing is associated with hypertension in Taiwanese type 2 diabetic patients. Hypertension Research. 2008;31(3):417–423. doi: 10.1291/hypres.31.417. [DOI] [PubMed] [Google Scholar]
- 21.Lan T-Y, Chang W-C, Tsai Y-J, Chuang Y-L, Lin H-S, Tai T-Y. Areca nut chewing and mortality in an elderly cohort study. American Journal of Epidemiology. 2007;165(6):677–683. doi: 10.1093/aje/kwk056. [DOI] [PubMed] [Google Scholar]
- 22.Tseng C-H, Tseng C-P, Chong C-K, et al. Increasing incidence of diagnosed type 2 diabetes in Taiwan: analysis of data from a national cohort. Diabetologia. 2006;49(8):1755–1760. doi: 10.1007/s00125-006-0314-4. [DOI] [PubMed] [Google Scholar]
- 23.Tseng C-H, Chong C-K, Tai T-Y. Secular trend for mortality from breast cancer and the association between diabetes and breast cancer in Taiwan between 1995 and 2006. Diabetologia. 2009;52(2):240–246. doi: 10.1007/s00125-008-1204-8. [DOI] [PubMed] [Google Scholar]
- 24.Tseng C-H, Chong C-K, Tseng C-P, Chan T-T. Age-related risk of mortality from bladder cancer in diabetic patients: a 12-year follow-up of a national cohort in Taiwan. Annals of Medicine. 2009;41(5):371–379. doi: 10.1080/07853890902729778. [DOI] [PubMed] [Google Scholar]
- 25.Tseng C-H, Chong C-K, Chan T-T, et al. Optimal anthropometric factor cutoffs for hyperglycemia, hypertension and dyslipidemia for the Taiwanese population. Atherosclerosis. 2010;210(2):585–589. doi: 10.1016/j.atherosclerosis.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Tseng C-H. Waist-to-height ratio and coronary artery disease in Taiwanese type 2 diabetic patients. Obesity. 2008;16(12):2754–2759. doi: 10.1038/oby.2008.430. [DOI] [PubMed] [Google Scholar]
- 27.Tseng C-H, Tseng C-P, Chong C-K. Joint effects of hypertension, smoking, dyslipidemia and obesity and angiotensin-converting enzyme DD genotype on albuminuria in Taiwanese patients with type 2 diabetes mellitus. Clinical Biochemistry. 2010;43(7-8):629–634. doi: 10.1016/j.clinbiochem.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Nieschulz O. Pharmacology of the active substances in betel. 3. Experiments with arecaidine. Arzneimittel-Forschung. 1970;20(2):218–229. (Ger). [PubMed] [Google Scholar]
- 29.Calogero AE, Kamilaris TC, Gomez MT, et al. The muscarinic cholinergic agonist arecoline stimulates the rat hypothalamic-pituitary-adrenal axis through a centrally-mediated corticotropin-releasing hormone-dependent mechanism. Endocrinology. 1989;125(5):2445–2453. doi: 10.1210/endo-125-5-2445. [DOI] [PubMed] [Google Scholar]
- 30.Johnston GAR, Krogsgaard-Larsen P, Stephanson A. Betel nut constituents as inhibitors of γ aminobutyric acid uptake. Nature. 1975;258(5536):627–628. doi: 10.1038/258627a0. [DOI] [PubMed] [Google Scholar]
- 31.Lodge D, Johnston GAR, Curtis DR, Brand SJ. Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system. Brain Research. 1977;136(3):513–522. doi: 10.1016/0006-8993(77)90075-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang CK, Hwang LS. Effect of betel quid on catecholamine secretion from adrenal chromaffin cells. Proceedings of the National Science Council, Republic of China. Part B. 1997;21(4):129–136. [PubMed] [Google Scholar]
- 33.Chu N-S. Sympathetic response to betel chewing. Journal of Psychoactive Drugs. 1995;27(2):183–186. doi: 10.1080/02791072.1995.10471690. [DOI] [PubMed] [Google Scholar]
- 34.Nurnberger JI, Jr., Jimerson DC, Simmons-Alling S. Behavioral, physiological, and neuroendocrine responses to arecoline in normal twins and 'Well State' bipolar patients. Psychiatry Research. 1983;9(3):191–200. doi: 10.1016/0165-1781(83)90043-4. [DOI] [PubMed] [Google Scholar]
- 35.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19(4):251–262. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 36.Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addiction Biology. 2002;7(1):103–110. doi: 10.1080/13556210120091464. [DOI] [PubMed] [Google Scholar]
- 37.Peng J-P, Su C-Y, Chang H-C, Chai C-Y, Hung W-C. Overexpression of cyclo-oxygenase 2 in squamous cell carcinoma of the hypopharynx. Human Pathology. 2002;33(1):100–104. doi: 10.1053/hupa.2002.30187. [DOI] [PubMed] [Google Scholar]
- 38.Jeng J-H, Wang Y-J, Chiang B-L, et al. Roles of keratinocyte inflammation in oral cancer: regulating the prostaglandin E2, interleukin-6 and TNF-α production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis. 2003;24(8):1301–1315. doi: 10.1093/carcin/bgg083. [DOI] [PubMed] [Google Scholar]
- 39.Reichart PA. Oral cancer and precancer related to betel and miang chewing in Thailand: a review. Journal of Oral Pathology and Medicine. 1995;24(6):241–243. doi: 10.1111/j.1600-0714.1995.tb01175.x. [DOI] [PubMed] [Google Scholar]