Abstract
Objective. To determine the prevalence of left ventricular hypertrophy (LVH) and its associated risk factors in lupus nephritis (LN) patients. Methods. 287 LN patients (age: 38.54 ± 13.31, 262 female) were recruited. Echocardiography and serum high-sensitivity C-reactive protein (hs-CRP) were measured. Their relationship was evaluated by univariate correlation analysis and multivariate regression analysis. Results. The prevalence of LVH in this cohort was 21.25% (n = 61). Serum hs-CRP level was significantly elevated in patients with LVH compared to those without (8.03 (3.22–30.95) versus 3.93 (1.48–9.48) mg/L, P < .01), and correlated with left ventricular mass index (LVMI) (r = 0.314, P = .001). Multivariate regression analysis further confirmed that hs-CRP was an independent risk factor (β = 0.338, P = .002) for LVH in patients with LN. Conclusions. Our findings demonstrated that serum hs-CRP level is independently correlated with LVMI and suggested that measurement of hs-CRP may provide important clinical information to investigate LVH in LN patients.
1. Introduction
Alterations in left ventricular structure and function have been reported among the cardiac manifestations of systemic lupus erythematosus (SLE), especially in those who have renal complications. These alterations include echocardiographic evidence of increases in LV wall thicknesses and mass, a decrease in LV ejection fraction, and impaired diastolic filling [1–3]. However, it is currently uncertain whether these abnormalities are disease-related effects or a result of other predisposing conditions, such as inflammation, hypertension, anemia, and disorder of mineral metabolism.
In recent years, there has been a growing interest in the hypothesis that atherosclerosis may be an inflammatory disease. It has been noted that C-reactive protein (CRP), a marker of the reactant plasma protein component of the inflammatory response, is a major predictor of cardiovascular disease (CVD) in apparently healthy subjects [4–6]. Previous reports have found the association between CRP and left ventricular hypertrophy (LVH) in several pathologic states such as hypertension, insulin resistance, and chronic kidney disease (CKD) [7–9]. In this study, we investigated the potential interrelationships among hs-CRPs, a more sensitive marker of systemic inflammation and LV mass index (LVMI) in patients with lupus nephritis (LN) by using the clinical cutoff levels of CRP.
2. Methods
2.1. Study Subjects
A total of 287 incipient LN patients were consecutively enrolled from January 2005 to December 2008. All participants met the diagnostic criteria of the American College of Rheumatology [10, 11]. Exclusion criteria included ischemic heart disease, acute coronary syndrome, congestive heart failure (CHF) (New York Heart Association (NYHA) class II or greater), old cerebral infarction, history of transient ischemic attack, secondary hypertension, receipt of any immunosuppressant and/or an anti-inflammatory drug (aspirin or nonsteroidal anti-inflammatory drug (NSAID)), chronic infection, cancer, and pregnancy. Participants with moderate or severe aortic or mitral regurgitation were also excluded. The study protocol was approved by the local ethics committee, and all participants gave their written informed consent to participate in this study.
2.2. Baseline Clinical Characteristics
After fasting overnight, BP was measured with an appropriate arm cuff and a mercury column sphygmomanometer on the left arm after a resting period of at least 10 min in the supine position. After BP measurement, venous blood sampling was performed in all subjects. Height and body weight were measured, and body mass index was calculated. The following parameters were also determined: serum creatinine, serum lipids including cholesterol, triglyceride, and lipoprotein(a), measurement of serum complement C3 and C4, high-sensitivity C-reactive protein, and antibody testing. Estimated glomerular filtration rate (eGFR) was calculated by MDRD formula. High-sensitivity CRP (hs-CRP) was measured by autoimmune scattering rate nephelometry (BNP nephelometer, Dade Behring). If hs-CRP level was >10 mg/L, the test was repeated. Antinuclear antibodies (ANA) were detected by indirect immunofluorescence (IIF). Double-stranded DNA (ds-DNA) was detected by FARR assay (EUROIMMUN AG, Germany), and antiphospholipid antibodies (ACL) were measured by Enzyme-Linked Immunosorbent Assay (ELISA) (EUROIMMUN AG, Germany).
2.3. Echocardiographic Methods and Calculation of Derived Variables
Echocardiography was performed by an experienced research technician using standard techniques who was unaware of the clinical characteristics of the patients. Studies were performed using phased-array echocardiography with M-mode, 2-dimensional, pulsed, and color-flow Doppler capabilities. LV mass (LVM) was calculated using the following formula: LVM = 0.8 (1.04 (LVST+LVPWT+LVDd)3−LVDd3)+ 0.6, where LVST is LV septal wall thickness, and LVPWT is LV posterior wall thickness, LVDd is LV diastolic diameter. LVMI was indexed for body surface area (BSA), and LVH was defined by an LVMI of over 110 g/m2 in women and 125 g/m2 in men [12].
2.4. Statistical Analysis
Data were described as means ± SDs for those with normal distribution and as medians and interquartile ranges for asymmetrical distribution. Comparisons between patients divided by CRP cutoff level and with or without LVH were performed by unpaired t-tests in normally distributed data and by nonparametric Mann-Whitney test in asymmetrically distributed data, or by X2 test in categorical data. The cut-off level of hs-CRP was defined according to the AHA/CDC recommendations [13], in which CRP levels ≥3 mg/L were defined as average- and high-risk groups for CVD. Bivariate relationships with LV mass were assessed using the Spearman correlation coefficient. All variables that had significant relations were evaluated for inclusion in a model predicting LV mass using multivariable regression analysis; unstandardized regression coefficients (B) with their 95% confidence intervals were reported. All of the statistics were performed by SPSS version 13.0, and a 2-tailed P < .05 was considered to indicate statistical significance.
3. Results
3.1. Description of LN Patients
The 287 subjects were predominantly female (91.29%), with a mean age of 38.5 ± 13.3 at their entry. Totally 223/239 patients (93.30%) showed positive ANA, 177/200 patients (88.50%) had positive ds-DNA, and 29/153 patients (18.95%) showed ACL antibodies. Renal biopsy was obtained from 135 (47.04%) patients, which showed minimal mesangial LN (class I) in 4 (3.0%), mesangial proliferative LN (class II) in 7 (5.2%), focal LN (class III) in 16 (11.9%), diffuse LN (class IV) in 77 (57.0%), membranous LN (class V) in 28 (20.7%), and advanced sclerotic LN (class VI) in 3 (2.2%) patients according to International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification [14].
3.2. Prevalence of LVH in LN Patients
LVH was diagnosed in 61 LN patients (21.25%). We compared the baseline characteristics of patients with and without LVH, as showed in Table 1. Patients with LVH were much older, had significantly elevated hs-CRP level and higher uric acid level, lower hemoglobin level, and eGFR. However, BMI, blood pressure, and serum lipids were not significantly different between the two groups. Meanwhile, autoantibody parameter positive incidence including ds-DNA, ANA, and ACL did not differ between patients with and without LVH.
Table 1.
Baseline characteristics of LN patients with and without echocardiographic LVH.
| Baseline characteristics | Echo-LVH (n = 61) | Normal LVMI (n = 226) |
|---|---|---|
| Gender, male, % | 8.85 | 8.20 |
| Age, y | 40.92 ± 14.16* | 35.43 ± 11.88 |
| BMI, kg/m2 | 21.48 ± 3.10 | 22.10 ± 3.63 |
| Smoke history, % | 17.69 | 18.03 |
| SBP, mmHg | 140.00 (120.00–155.00) | 130.00 (110.00–140.00) |
| DBP, mmHg | 87.00 (80.00–91.60) | 85.00 (75.00–90.00) |
| MABP, mmHg | 103.33 (93.33–113.97) | 98.33 (88.33–110.00) |
| Hemoglobin level, g/L | 90.62 ± 22.99** | 105.95 ± 22.43 |
| ESR, mm/h | 57.00 (26.00–85.00) | 43.00 (21.00–73.00) |
| hs-CRP, mg/L | 8.03 (3.22–30.95)** | 3.93 (1.48–9.48) |
| Serum albumin, g/L | 30.03 ± 6.34 | 33.26 ± 6.06 |
| Triglyceride, mmol/L | 2.25 ± 1.16 | 2.42 ± 1.10 |
| Cholesterol, mmol/L | 5.47 ± 1.99 | 5.64 ± 2.12 |
| Lipoprotein(a), mg/L | 328.90 ± 45.62 | 276.14 ± 21.66 |
| GFR, ml/min/1.73 m2 | 66.05 ± 4.68* | 90.24 ± 4.52 |
| Uric acid, mmol/L | 491.78 ± 29.35* | 402.44 ± 17.03 |
| Calcium, mmol/L | 2.07 ± 0.22 | 2.07 ± 0.20 |
| Phosphate, mmol/L | 1.59 ± 0.64 | 1.43 ± 0.36 |
| 24 hours urine protein, g/24 h | 3.37 ± 2.27 | 3.00 ± 2.62 |
| ds-DNA (%) | 32 (82.05) | 145 (90.06) |
| ANA (%) | 43 (91.49) | 180 (93.75) |
| ACL (%) | 6 (26.08) | 23 (17.69) |
| Complement C3, g/L | 0.54 (0.38–0.83) | 0.48 (0.37–0.65) |
| Fibrinogen, g/L | 3.77 ± 1.47 | 3.87 ± 1.40 |
Echo-LVH: echocardiographic LVH; SBP: systolic blood pressure; DBP: diastolic blood pressure; MABP: mean arterial blood pressure; ESR: erythrocyte sedimentation rate; GFR: glomerular filtration rate; ANA: antinuclear antibody; ACL antiphospholipid antibody. Case number and positive incidence of ds-DNA, ANA, and ACL was presented here in the table. Compared with normal LVMI, *P < .05, **P < .01.
3.3. Association between hs-CRP and LVH
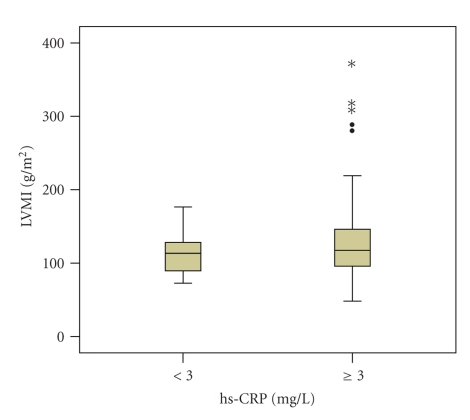
To further explore the extent to which inflammation augment LVH, the patients were subdivided into low- and average-to-high risk groups according to hs-CRP cutoff level. Among those who had higher hs-CRP levels (≥3 mg/L), LVMI was significantly increased (132.68 ± 57.84 versus 113.67 ± 29.17, P = .018) (Figure 1). In addition, these patients had elder age (39.37 ± 14.09 versus 34.97 ± 11.55, P = .02), lower hemoglobin level (93.04 ± 24.91 versus 106.57 ± 23.65, P < .001), lower cholesterol level (5.60 ± 1.89 versus 6.41 ± 2.76, P = .018), higher ESR (56.00 (31.50–83.00) versus 32.00 (19.75–58.50), P < .001) and higher serum fibrinogen level (4.17 ± 1.59 versus 3.54 ± 1.12, P = .004) (Table 2).
Figure 1.
Comparison of LVMI in two groups divided by hs-CRP cutoff level.
Table 2.
Laboratory parameters of LN patients grouped by hs-CRP level.
| Baseline characteristics | CRP ≥ 3 mg/dl (n = 198) | CRP < 3 mg/dl (n = 89) |
|---|---|---|
| Gender, male, % | 8.59 | 8.98 |
| Age, y | 39.37 ± 14.09* | 34.97 ± 11.56 |
| BMI, kg/m2 | 22.29 ± 3.18 | 21.67 ± 3.07 |
| Smoke history, % | 18.18 | 16.86 |
| SBP, mmHg | 132.90 (120.00–148.10) | 130.00 (117.50–150.00) |
| DBP, mmHg | 86.00 (79.50–94.40) | 83.50 (73.75–90.00) |
| MABP, mmHg | 103.33 (90.00–110.64) | 97.5 (89.08–110.83) |
| Hemoglobin level, g/L | 93.04 ± 24.91** | 106.57 ± 23.65 |
| ESR, mm/h | 56.00 (31.50–83.00)** | 32.00 (19.75–58.50) |
| Serum albumin, g/L | 27.70 ± 7.03 | 33.09 ± 6.95 |
| Triglyceride, mmol/L | 2.48 ± 1.32 | 2.46 ± 1.06 |
| Cholesterol, mmol/L | 5.60 ± 1.89* | 6.41 ± 2.76 |
| Lipoprotein(a), mg/L | 275.99 ± 28.90 | 281.15 ± 31.16 |
| GFR, ml/min/1.73 m2 | 73.45 ± 4.62 | 82.11 ± 5.75 |
| Uric acid, mmol/L | 463.96 ± 17.85 | 421.40 ± 19.48 |
| Calcium, mmol/L | 2.04 ± 0.22 | 2.12 ± 0.16 |
| Phosphate, mmol/L | 1.68 ± 0.80 | 1.53 ± 0.29 |
| 24 hours urine protein, g/24 h | 3.00 ± 2.62 | 3.37 ± 2.27 |
| ds-DNA (%) | 124 (88.57) | 53 (88.33) |
| ANA (%) | 148 (91.36) | 73 (94.81) |
| ACL (%) | 20 (20.00) | 9 (16.98) |
| Complement C3, g/L | 0.44 (0.34–0.76) | 0.43 (0.32–0.64) |
| Fibrinogen, g/L | 4.17 ± 1.60** | 3.54 ± 1.12 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; MABP: mean arterial blood pressure; ESR: erythrocyte sedimentation rate; GFR: glomerular filtration rate; ANA: antinuclear antibody; ACL: antiphospholipid antibody. Case number and positive incidence of ds-DNA, ANA and ACL was presented here in the table. Compared with hs-CRP ≥ 3 mg/L, *P < .05, **P < .01.
In univariate analysis involving the entire sample (Table 3), significant correlates of LVMI included age, body mass index, blood pressure, hemoglobin level, hs-CRP, uric acid level, and eGFR. After introducing all these significant variables into multivariate regression analysis, hs-CRP (β = 0.228, P = .009), along with uric acid (β = 0.382, P < .001), was further confirmed to have positive associations with LVMI.
Table 3.
Individual correlates of LVMI among LN patients.
| r | P | |
|---|---|---|
| Age | 0.154 | .048 |
| BMI | −0.171 | .030 |
| SBP | 0.214 | .006 |
| DBP | 0.156 | .045 |
| MABP | 0.183 | .018 |
| Hemoglobin | −0.304 | <.001 |
| ESR | 0.081 | .314 |
| Hs-CRP | 0.225 | .014 |
| Serum albumin | −0.107 | .177 |
| Triglyceride | −0.035 | .666 |
| Cholesterol | −0.015 | .850 |
| Lipoprotein(a) | 0.043 | .607 |
| GFR | −0.292 | <.001 |
| Uric acid | 0.202 | .011 |
| Calcium | 0.097 | .451 |
| Phosphate | 0.065 | .614 |
| 24 hours urine protein | 0.091 | .263 |
| Ds-DNA | −0.086 | .351 |
| ANA | −0.099 | .260 |
| ACL | 0.139 | .232 |
| Complement C3 | −0.07 | .394 |
| Fibrinogen | −0.04 | .646 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; MABP: mean arterial blood pressure; ESR: erythrocyte sedimentation rate; GFR: glomerular filtration rate; ANA: antinuclear antibody; ACL: antiphospholipid antibody.
4. Discussion
Our cross-sectional study revealed a linear relationship between low-grade chronic inflammation estimated by high-sensitivity CRP levels and LVMI, independent of several other important covariates, such as adipose tissue distribution BMI, BP levels, serum lipids, renal function, age, and gender. The observation of this independent association between hs-CRP level and LVMI is consistent with previous findings [15, 16], and the present study extended to LN patients. As far as we know, these findings are new and potentially important for refining CVD risk stratification in this population.
At the initial stage of the atherosclerotic process, systemic inflammation would appear most importantly associated with subclinical cardiovascular disease development, such as LVH occurrence. A raised baseline CRP value has been associated with inflammation, endothelial dysfunction, obesity [17], the metabolic syndrome [18, 19], diabetes mellitus [20], insulin resistance [7], and severity of hypertension [21], and thus, various metabolic disorders may occur by increasing CRP level and simultaneously promote an increase in LV mass. On the other hand, local CRP synthesis and secretion by smooth muscle cells, including those of the human coronary artery, have been suggested to play an important role as well [22]. It is possible to speculate that CRP may play a direct role in promoting LVH through these mechanisms, including (1) increasing phosphatidylinositol3- kinase activity [23];(2) upregulating inducible nitric oxide synthase, certain cell signal transduction pathways including the mitogen-activated protein kinase pathway, and nuclear factor κ-B; (3) upregulating angiotensin II type 1 receptor in vascular smooth muscle cells, and directly quenching the production of nitric oxide by endothelial cells [24, 25], resulting in increased production of endothelin-1 [26]; (4) elevation of von Willebrand factor [27], which is known to be associated with endothelial dysfunction. Thus, cardiac hypertrophy may be, at least in part, attributable to an increase in CRP itself, via activated transcriptional regulatory mechanisms, proinflammatory and proatherogenic effects, and stimulation of endothelial dysfunction.
Some limitations of this study are important to note. First, it is not possible to conclude from this observational research whether CRP stimulates higher LVMI or whether CRP is increased before the development of LVH. The cross-sectional design prevents the demonstration of the mechanisms by which LVH is related to inflammation. These speculations should be addressed in future prospective longitudinal studies. Second, it may be better to introduce SLEDAI score into multivariate regression analysis to further estimate the effect of disease itself on LVH, and inflammation status as well. Third, it is very regrettable that some of our patients' autoantibodies data were missing and incomplete. This cohort will be followed and expanded to further observe the prevalence and correlative factors of LVH, especially after intervention therapy.
5. Conclusions
In conclusion, in LN subjects initially free of CVD, hs-CRP showed a significant association with LVMI, which suggested that assessment of hs-CRP level may help to refine CVD risk stratification in this population.
References
- 1.Omdal R, Lunde P, Rasmussen K, Mellgren SI, Husby G. Transesophageal and transthoracic echocardiography and Doppler-examinations in systemic lupus erythematosus. Scandinavian Journal of Rheumatology. 2001;30(5):275–281. doi: 10.1080/030097401753180354. [DOI] [PubMed] [Google Scholar]
- 2.Pieretti J, Roman MJ, Devereux RB, et al. Systemic lupus erythematosus predicts increased left ventricular mass. Circulation. 2007;116(4):419–426. doi: 10.1161/CIRCULATIONAHA.106.673319. [DOI] [PubMed] [Google Scholar]
- 3.Naarendorp M, Kerr LD, Khan AS, Ornstein MH. Dramatic improvement of left ventricular function after cytotoxic therapy in lupus patients with acute cardiomyopathy: report of 6 cases. Journal of Rheumatology. 1999;26(10):2257–2260. [PubMed] [Google Scholar]
- 4.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 5.Rost NS, Wolf PA, Kase CS, et al. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham Study. Stroke. 2001;32(11):2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 6.Koenig W, Sund M, Fröhlich M, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (monitoring trends and determinants in cardiovascular disease) Augsburg cohort study, 1984 to 1992. Circulation. 1999;99(2):237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 7.Festa A, D’Agostino R, Jr., Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 8.Iwashima Y, Horio T, Kamide K, Rakugi H, Ogihara T, Kawano Y. C-reactive protein, left ventricular mass index and risk of cardiovascular disease in essential hypertension. Hypertension Research. 2007;30(12):1177–1185. doi: 10.1291/hypres.30.1177. [DOI] [PubMed] [Google Scholar]
- 9.Kim B-S, Doo SJ, Mi JS, et al. Persistent elevation of C-reactive protein may predict cardiac hypertrophy and dysfunction in patients maintained on hemodialysis. American Journal of Nephrology. 2005;25(3):189–195. doi: 10.1159/000085585. [DOI] [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF. The 1982 revised criteria for the classification of systemic lupus erythrematosus. Arthritis and Rheumatism. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40(9):p. 1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. American Journal of Kidney Diseases. 1996;27(3):347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 13.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 14.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney International. 2004;65(2):521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim B-S, Doo SJ, Mi JS, et al. Persistent elevation of C-reactive protein may predict cardiac hypertrophy and dysfunction in patients maintained on hemodialysis. American Journal of Nephrology. 2005;25(3):189–195. doi: 10.1159/000085585. [DOI] [PubMed] [Google Scholar]
- 16.Yasunari K, Maeda K, Watanabe T, Nakamura M, Yoshikawa J, Asada A. Comparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophy. Journal of the American College of Cardiology. 2004;43(11):2116–2123. doi: 10.1016/j.jacc.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 17.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(4):972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 18.Saijo Y, Yoshioka E, Fukui T, Kawaharada M, Kishi R. Metabolic syndrome, C-reactive protein and increased arterial stiffness in Japanese subjects. Hypertension Research. 2006;29(8):589–596. doi: 10.1291/hypres.29.589. [DOI] [PubMed] [Google Scholar]
- 19.Frohlich M, Imhof A, Berg G, et al. Association between Creactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. Adults. Diabetes Care. 1999;22(12):1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 21.Blake GJ, Rifai N, Buring JE, Ridker PM. Blood Pressure, C-Reactive Protein, and Risk of Future Cardiovascular Events. Circulation. 2003;108(24):2993–2999. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- 22.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44(1):6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 23.Zhong W, Zen Q, Tebo J, Schlottmann K, Coggeshall M, Mortensen RF. Effect of human C-reactive protein on chemokine and chemotactic factor- induced neutrophil chemotaxis and signaling. Journal of Immunology. 1998;161(5):2533–2540. [PubMed] [Google Scholar]
- 24.Verma S, Wang C-H, Li S-H, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 25.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 26.Mazer SP, Rabbani LE. Evidence for C-reactive protein’s role in (CRP) vascular disease: atherothrombosis, immuno-regulation and CRP. Journal of Thrombosis and Thrombolysis. 2004;17(2):95–105. doi: 10.1023/B:THRO.0000037664.77460.d8. [DOI] [PubMed] [Google Scholar]
- 27.Bisoendial RJ, Kastelein JJP, Levels JHM, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circulation Research. 2005;96(7):714–716. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]