Abstract
Transient elastography (TE) is a new non-invasive tool for assessing liver stiffness, which is correlated with the histologic stage of liver fibrosis. Many studies have reported a good accuracy of TE in predicting significant fibrosis and an optimal accuracy in predicting cirrhosis. Furthermore, the potential role of TE in screening the general population has also been proven. TE thus helps physicians to decide treatment strategies, predict prognosis, and monitor disease progression in patients with chronic liver disease and to screen the general population to identify high risk patients with potential liver disease. However, most data on the clinical roles of TE have been gathered in European patients with chronic hepatitis C (CHC), because TE was first developed in France. Accordingly, much data on the usefulness of TE in patients with CHC has accumulated. Recently, however, vigorous efforts have been made to apply TE to patients with chronic hepatitis B (CHB), and TE has also proved to have acceptable accuracy in diagnosing liver fibrosis and cirrhosis in these patients. Thus, we focused on TE in the Asian population with CHB in comparison with the European population with CHC and found that the diagnostic performance and cutoff values were different between the 2 populations possibly as a result of several different confounders between Asian and European populations (the etiology of chronic liver disease, histologic features, major fluctuation in alanine aminotransferase levels, and the prevalence of high body mass index and metabolic syndrome). Therefore, further studies tailored to the Asian population with CHB should be performed before the widespread application of TE in Asian populations with CHB.
Keywords: Asia, Chronic hepatitis B, Fibroscan, Hepatitis B virus, Liver stiffness measurement, Transient elastography
INTRODUCTION
Transient elastography (TE) using FibroScan® (EchoSens, Paris, France) is a newly introduced non-invasive tool, that generates an elastic wave using a vibrator applied to the thoracic wall at the level of the right lobe of the liver and measures the propagation velocity of the wave, which is directly associated with liver stiffness (LS)[1]. To date, the clinical utility of TE has been widely reviewed, and it is regarded as having considerable accuracy for predicting liver cirrhosis in patients with chronic liver disease (CLD) of diverse etiologies[2-5]. TE is quick, highly reproducible, and completely harmless to patients. In addition, it can be learned and performed easily after a short training period (by nurses or technicians) without consuming the time of clinicians. For these reasons, TE has become popular in clinical practice as a tool for aiding in the diagnosis and follow-up of liver disease.
Because TE was first developed in France, most studies on its benefits have been explored in European countries where chronic hepatitis C (CHC) is prevalent. Accordingly, extensive data on the clinical roles of TE in assessing liver fibrosis in patients with CHC have been gathered. Recently, several meta-analysis studies reported that TE is a reliable non-invasive tool to detect advanced liver fibrosis and liver cirrhosis[6-8]. However, most studies included in the meta-analysis investigated European populations with CHC. Data from Europe cannot be extrapolated to the Asian population, as subsequent trials of TE in Asian countries where hepatitis B virus (HBV) infection is more prevalent than hepatitis C virus (HCV), displayed divergent results. In this Topic Highlight, we will focus on TE in the Asian population with chronic hepatitis B (CHB).
METHODOLOGICAL CONSIDERATION OF TE IN THE ASIAN POPULATION
LS values are defined as the median of 10 valid measurements and at least 60% of TE shots should be successful for each examination according to the manufacturer’s recommendations. Accordingly, to date, only the results of TE examinations satisfying the above criteria have been considered as reliable and have been included for the analysis in reported studies.
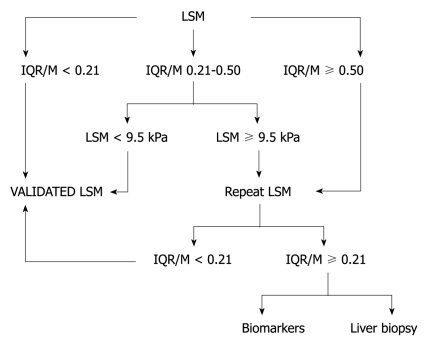
Another important parameter for confirming the validity of LS values is the interquartile range (IQR), which is defined as the values between which 50% of observations fall. The lower boundary is the 25th percentile (lower quartile), the upper boundary is the 75th percentile (upper quartile). Generally, the IQR/median LS value ratio (IQR/M) should not exceed 30% if the reliability of LS values is to be preserved. However, recently, Lucidarme et al[9] proposed new criteria for reliable LS values, using the IQR/M based on the data from more than 250 French patients with CHC. They found, in a multivariate analysis, the fibrosis stage (F0-2 vs F3-4) and IQR/M were significantly associated with significant discordances between TE and liver biopsy (LB), with an optimal discriminant cutoff value of 0.21. They thus concluded that the IQR/M can overestimate liver fibrosis using TE, irrespective of success rate, when the significance discordance was defined as a discordance of at least 2 stages of fibrosis between LS values by TE examination and the METAVIR scoring system. From these results, they suggested a novel algorithm for clinical practice as first-line assessment of liver fibrosis in patients with CHC (Figure 1).
Figure 1.
Suggested algorithm for clinical practice as first-line assessment of hepatic fibrosis in patients with chronic hepatitis C. IQR/M: Interquartile range/median liver stiffness value ratio; LSM: Liver stiffness measurement.
Because the IQR/M was confirmed in only one European study with CHC showing relatively high body mass index (BMI) (25 ± 4.1 kg/m2), its application to the Asian population with CHB (who generally show a lower BMI) is difficult and requires further validation. Although the data are preliminary, we have attempted to validate the IQR/M for our cohort of 156 patients with CHB (mean age 38.8 years, male 75.0%, mean BMI 23.2 kg/m2) who underwent LB and TE prior to starting antiviral treatment. In this study, we selected the cutoff LS values of Marcellin et al[10] (7.2 kPa for ≥ F2, 8.1 kPa ≥ F3, and 11.0 kPa for F4) as the reference values between 2 external studies that investigated the performance of TE and then set up the optimal cutoff LS values for each fibrosis stage in patients with CHB[10,11]. We identified 28 (18.0%) patients showing significant discordance between TE and LB. However, the IQR/M was not significantly different between patients with discordance and those with non-discordance (0.125 ± 0.083 vs 0.129 ± 0.079, P = 0.831), and the success rate also failed to show a significant difference.
Interestingly, the mean IQR/M of our study population was greatly reduced compared to that of subjects in Lucidarme et al[9] (0.30 in patients with discordance and 0.22 in those with non-discordance) and the proportion of patients with an IQR/M > 0.03 (n = 7, 4.5%) was very small. The reasons are not clear why the IQR/M in our study was reduced in comparison with that in the previous study, and was not selected as a discriminant factor to predict discordance. However, we think that the optimal cutoff value of the IQR/M will be lower than that from patients with CHC even if the IQR/M can be validated in the future for the Asian population with CHB, or that the IQR/M is not a predictor of discordance in the Asian population with CHB due to other potential confounders that overwhelm the influence of the IQR/M, such as the inhomogeneous histological features of CHB which often makes liver texture inherently macronodular. This would result in LS values that vary depending on the area of the liver and necroinflammatory activity, which has been demonstrated to raise LS values. Therefore, the validation of the IQR/M in the Asian population with CHB requires further studies using increased sample sizes to allow sufficient cases with a spectrum of IQR/M values.
Several issues still remain to be resolved regarding the Asian population with CHB: How many valid measurements do we need to maintain the performance of TE[12]? What is the optimal success rate of the TE measurement to accurately exclude unreliable LS values? Can the IQR/M alone be applied to increase the performance of TE without a sufficient number of valid measurements and an adequate success rate?
WHAT ARE THE NORMAL LS VALUES FOR THE ASIAN POPULATION
Before discussing data on TE in the Asian population with CHB, it is important to first confirm that TE can reliably identify patients with CLD from the normal population. If TE cannot, the clinical meaning of further analysis on the performance of TE in grading the severity of CLD would be significantly lessened. Despite the importance of this, to date, most studies on TE have focused on patients with CLD.
The concept of “what are normal LS values assessed by TE?” is a critical issue that should be addressed to determine whether TE can be used for screening the general population and for subsequent selection of high-risk patients who require periodic follow-up and adequate treatment measures. Indeed, concerns regarding normal LS values are increasing, and several studies (including 2 Asian studies) have proposed normal LS values despite variations in study design[13-19].
In a study by Corpechot et al[15], the authors found that the normal LS values in the healthy population were significantly higher in men than in women and that the median LS value was 4.8 kPa (range, 2.5-6.9 kPa). Roulot et al[13] examined a large cohort of 429 apparently healthy subjects to establish normal LS values [5.81 ± 1.54 kPa (range, 3.8-8.0 kPa) in men vs 5.23 ± 1.59 kPa (range, 3.3-7.8 kPa) in women, P < 0.01]. Another study conducted in Romania demonstrated that the mean values of TE in 144 normal subjects were 4.8 ± 1.3 kPa (range, 2.3-8.8 kPa) and that the normal LS values were significantly different between genders[16]. Colombo et al[17] also assessed the LS values of 327 voluntary blood donors and found that the mean LS value was 4.9 kPa (95% CI: 4.6-5.1 kPa) and the normal LS values showed no significant differences between genders. However, Colombo et al[18] in their follow-up study that enrolled more than 1000 healthy blood donors reported that the normal LS value was 4.4 kPa (95th percentile, 6.7 kPa) and that the male gender presents with increased LS values.
All the aforementioned studies were conducted in Europe. However, normal LS values in the Asian population are now available. Fung et al[19] reported a mean LS value in 28 healthy living-related liver donors of 4.6 kPa (range, 2.0-7.1 kPa), and all subjects had LS values of < 7.2 kPa, which indicated that they had no significant fibrosis. We have previously reported that the normal range of LS values was 3.9-5.3 kPa, which was calculated from 69 strictly selected living liver and kidney donors[14].
Table 1 indicates the results of all studies on normal LS values. The upper normal range of LS values was consistently lower than the range generally used for identifying significant liver disease (7-8 kPa) in the majority of previous studies[20-22]. These data demonstrated that TE can perform reliably in identifying high-risk subpopulations without an overlap between the normal and abnormal ranges of LS values. Interestingly, the mean LS values in Asian studies appear lower in comparison with European studies, and the effects of gender on the performance of TE are variable among studies. The discrepancy cannot be fully explained, because the complete histological data of the normal subjects were largely unavailable except a small number of liver donors in the studies by our group[14] and Fung et al[19]. Lower mean BMI, younger age, and lower prevalence of metabolic syndrome in the Asian studies can explain, at least in part, the lower mean LS values observed. Another reasons are that other confounders of TE performance, such as high alanine aminotransferase (ALT) levels and fatty liver, were not sufficiently excluded[17,18] and that the hepatic imaging studies and/or cardiologic evaluation were not fully completed in European studies[13,16]. These reasons may have increased the normal range of LS values in the European studies.
Table 1.
Normal liver stiffness values of transient elastography
|
Asian study |
European study |
||||||
| Kim et al[14] | Fung et al[19] | Corpechot et al[15] | Roulot et al[13] | Sirli et al[16] | Colombo et al[17] | Colombo et al[18] | |
| Type | Full article | Full article | Letter | Full article | Full article | Abstract | Abstract |
| No. of subjects | 69 | 28 | 71 | 429 | 144 | 327 | 746 |
| Population | Liver and kidney donors | Liver donors | Healthy volunteers | Medical check-up | Healthy volunteers | Blood donors | Blood donors |
| Liver stiffness (kPa) | 4.6 (mean) | 4.6 (median) | 4.8 (median) | 5.5 (mean) | 4.8 (mean) | 4.9 (mean) | 4.4 (median) |
| 95th percentile (kPa) | 4.7 | - | - | 8.6 | - | 7.8 | 6.7 |
| BMI (kg/m2) | 22.6 (mean) | - | 22.5 (median) | 25.8 (mean) | - | - | - |
| Effects on TE | |||||||
| Age | No | - | No | No | No | No | No |
| Gender | M = F | - | M > F | M > F | M > F | M = F | M > F |
| High BMI | No | - | No | Increased TE values | No | Increased TE values | No |
| Metabolic syndrome | - | - | - | Increased TE values | - | - | - |
| Fatty liver | - | - | - | - | - | Increased TE values | Increased TE values |
BMI: Body mass index; TE: Transient elastography.
More carefully designed studies with a large number of subjects are thus still required to fully assess the normal LS values for both Western and Asian populations. However, it should be remembered that the actual normal range of LS values cannot be precisely defined without a definition of a “normal liver”, sufficient histological evaluation of normal subjects, and further stratified analysis with lifestyle factors between genders (such as alcohol consumption). If these issues are resolved, we can identify the true normal LS value and use this as a reference for future studies.
INFLUENCE OF BMI ON THE PERFORMANCE OF TE IN THE ASIAN POPULATION
Previous work has demonstrated that BMI can affect the performance of TE. This can be explained in 2 ways. First, BMI influences the failure rate of TE, and second, BMI acts to increase LS values. Although previous studies have reported that the success rate of TE decreases in subjects displaying higher BMI[13,23], TE failure may not be significant, because these patients were largely excluded from the analysis. However, in another point of view, this effect of high BMI on TE failure can be a significant pitfall of TE, as the majority of patients with high BMI have an increased chance of suffering from fatty liver and, if TE examination fails, they miss the opportunity to receive rapid and non-invasive methods to exclude significant fibrosis. From the knowledge of the varying failure rates between Asian and European study populations, we can discern the possible influence of BMI on TE failure rate according to the study population.
Table 2 summarizes the failure rates of previous studies conducted in Asian and European populations. Overall, TE failure rates and BMI both appear to be lower in Asian compared to European studies, indicating that the lower BMI found in Asian populations is possibly associated with a lower TE failure rate.
Table 2.
Failure rate of transient elastography measurement
|
Asian study |
European study |
||||||
| Kim et al[14] | Chan et al[24] | Masuzaki et al[25] | Wong et al[26] | Ziol et al[2] | Marcellin et al[10] | Maimone et al[27] | |
| Etiology | HBV | HBV | HCV | HBV | HCV | HBV | HBV |
| Total patients (n) | 74 | 1136 | 876 | 487 | 327 | 202 | 230 |
| Excluded patients (n) | 5 | 30 | 10 | 34 | 76 | 29 | 10 |
| Excluded patients due to TE failure (n) | 1 | 30 | 10 | 17 | 23 | 14 | 10 |
| TE failure rate (%) | 1.4 | 2.6 | 1.1 | 3.5 | 7 | 6.9 | 4.3 |
| Enrolled patients (n) | 69 | 1106 | 866 | 453 | 251 | 173 | 220 |
| Age (yr) | 38.9 (mean) | 47 (mean) | 66.2 (mean) | 37 (median) | 47.5 (mean) | 40.1 (mean) | 45.2 (mean) |
| Male, n (%) | 35 (50.7) | - | 398 (46.0) | 270 (60.0) | 155 (61.8) | 115 (66.5) | 99 (45.0) |
| BMI (kg/m2) | 22.6 (mean) | - | 22.5 (mean) | 22.4 (median) | 23.9 (mean) | 24.5 (mean) | - |
| Failure criteria | |||||||
| Success rate | < 60% | < 60% | < 60% | < 60% | < 60% | < 50% | < 60% |
| The number of VMs | < 10 VMs | < 10 VMs | < 8 VMs | < 10 VMs | < 10 VMs | < 7 VMs | < 10 VMs |
HBV: Hepatitis B virus; HCV: Hepatitis C virus; TE: Transient elastography; BMI: Body mass index; VM: Valid measurement.
In spite of debate, several European studies have reported that a high BMI (which might cause hepatic steatosis) could potentially increase LS values[13,17], although research to date has not determined whether steatosis itself increases LS value in patients with chronic viral hepatitis[28-30]. For the Asian population, the effects of high BMI on TE performance have not been fully validated, because most Asian studies on TE lack sufficient numbers of patients with high BMI (> 30 kg/m2). In our previous study, BMI did not influence LS values, although our study population was younger and showed lower BMI values than European studies[14]. This potential effect of high BMI on TE performance should therefore be further investigated for the Asian population.
INFLUENCE OF METABOLIC SYNDROME ON THE PERFORMANCE OF TE IN THE ASIAN POPULATION
Recently, the effects of metabolic syndrome (MS) on TE have been examined in a French study[13]. Here, BMI > 30 kg/m2 was more frequent among subjects with MS than among normal subjects (49.1% vs 8.9%, respectively, P < 0.001). The mean LS value was higher in subjects with MS compared to controls (6.51 ± 1.64 kPa vs 5.33 ± 1.51 kPa, P < 0.001). In a multivariate analysis, LS values were still significantly different between subjects with and without MS, irrespective of BMI and other variables. Because MS has been demonstrated to increase LS values significantly, and hepatic steatosis which can result in histological progression to cirrhosis and hepatocellular carcinoma is strongly associated with MS[31-33], a potential role of TE in detecting MS was proposed. However, 88% of the subjects with MS had LS values within the defined normal range. This result indicated that not only could TE not precisely perform in the diagnosis of MS, but also that hepatic steatosis differentially affected by MS may have influenced LS values differently for different individuals. One drawback of the study[13] was a lack of ultrasonographic evaluation of hepatic steatosis. It is thus unclear whether the increased LS values in the subjects with MS were dependent on MS itself or on hepatic steatosis associated with MS. Other studies in Italy[17,18] attempted to overcome this drawback by including ultrasonographic evaluation of the healthy blood donors and concluded that the severity of hepatic steatosis was independently related to LS values.
The association between MS or hepatic steatosis and LS values was investigated primarily in healthy subjects, because the effects of MS or hepatic steatosis on TE can be attenuated if the study population presented with background fibrotic liver (the most significant factor for high LS values). Therefore, it is difficult to identify the main factors that increase LS values among MS, hepatic steatosis, associated steatofibrosis, and background fibrosis, if the study enrolls patients already presenting with CLD.
As mentioned above, only one published European study reporting the effects of MS on TE in healthy individuals is currently available. Furthermore, no available European and Asian data address the effects of MS on TE performance in patients with chronic viral hepatitis. Considering the different clinicopathological course of hepatic steatosis in patients with CHC and CHB and the varied distribution of body fat according to race, further studies on the effects of MS and hepatic steatosis should be performed with the Asian populations.
INFLUENCE OF ALT ON THE PERFORMANCE OF TE IN THE ASIAN POPULATION WITH CHB
The most important confounding factor of TE is serum ALT levels. Hepatic inflammation, as reflected by higher ALT levels, tends to increase LS values[11,20,34]. Even minor changes in ALT levels have been shown to influence LS values[35]. Because HBV, unlike HCV, frequently exhibits acute ALT flares[36,37], the interpretation of LS values becomes increasingly difficult in the setting of CHB when major fluctuations of necrosis and inflammatory activity occur[24,38]. It thus becomes more relevant to consider ALT levels when examining TE in the Asian population with CHB.
Considering the significant effects of high ALT levels, several Asian studies with CHB patients attempted to establish varying cutoff LS values according to ALT levels. Chan et al[11] proposed ALT-based algorithms when interpreting LS results, and established different optimal cutoff values according to ALT levels (one group with normal ALT vs the other group with ALT > upper limit of normal (ULN) and ≤ 5 × ULN). In our previous study, we also stratified the study population according to ALT levels and calculated the optimal cutoff values for each group[34]. For patients with normal ALT levels, 6.0, 7.5 and 10.1 kPa were selected as the optimal cutoff values for ≥ F2, ≥ F3, and F4, respectively, whereas 8.9, 11.0, and 15.5 kPa were selected in those with ALT > ULN and ≤ 2 × ULN. Considering this unreliability of TE performance in patients with high ALT levels, more recent studies began to exclude patients displaying ALT > 5 × ULN for analysis[24]. In addition, the optimal time interval for TE to recover its reliability in patients who experience acute exacerbation of CHB was recently reported[38].
Although it seems reasonable to use different cutoff values according to ALT levels, large-scale validation of these LS values has not been performed for Asian patients with CHB. Furthermore, some issues still remain unresolved, such as how we stratify patients with CHB according to ALT levels to establish the cutoff values, and who should be excluded for TE examination because of unreliability of TE among patients with high ALT levels.
DIAGNOSTIC PERFORMANCE OF TE IN THE ASIAN POPULATION WITH CHB
Few studies have investigated the performance of TE in the Asian population with CHB[4,11,39-43]. The characteristics of studies and the identified performance of TE to predict significant fibrosis and cirrhosis are summarized in Tables 3 and 4 (Marcellin et al[10] is listed for comparison with other Asian studies). The listed Asian studies were selected if they evaluated TE in Asian populations with CHB, they used LB as a reference standard, and they assessed the diagnostic accuracy of TE [using area under the receiver operating characteristic curve (AUROC)] for F ≥ 2 or F = 4 fibrosis stage and/or diagnostic indexes (sensitivity, specificity, positive predictive value, or negative predictive value based on some cutoff LS values). Most Asian studies were conducted in Korea and China (Hong Kong) and some studies were available in abstract form from Korea, Singapore, and Thailand.
Table 3.
Characteristics of studies evaluating the performance of transient elastography for the diagnosis of liver fibrosis in patients with chronic hepatitis B
| Type | Country | Total sample (n) | Samplesize (n) |
Excluded due to failure |
Age (yr) | Male (%) | BMI (kg/m2) | LB length (mm) | Staging | ||
| TE (reason) | LB (reason) | ||||||||||
| Kim et al[4] | Original | Korea | 194 | 103 | 0 (SR < 60%, < 10 VMs) | 4 (< 10 mm, < 10 PTs) | 40.0 | 80.2 | 23.8 | 16.7 | METAVIR |
| Chan et al[11] | Original | China | 186 | 161 | 1 (SR < 60%, < 10 VMs) | 22 (< 15 mm, < 6 PTs) | 45.0 | 76.0 | 24.0 | 19.0 | METAVIR |
| Kim et al[36] | Original | Korea | 130 | 130 | 0 (SR < 60%, < 10 VMs) | 0 (< 10 mm, < 6 PTs) | 42.5 | 79.2 | 25.3 | 14.5 | METAVIR |
| Marcellin et al[10] | Original | France | 202 | 173 | 14 (SR < 50%, < 7 VMs) | 15 (< 10 PTs) | 40.1 | 66.5 | 24.5 | 16.6 | METAVIR |
| Chang et al[40] | Abstract | Singapore | 35 | 33 | 2 (obesity, narrow ICS) | 0 (-) | 43.0 | - | 25.6 | - | Ishak |
| Tanwandee et al[41] | Abstract | Thailand | 104 | 104 | 0 (-) | 0 (-) | 44.0 | 63.0 | 23.6 | - | METAVIR |
| Choi et al[42] | Abstract | Korea | 48 | 48 | 0 (-) | 0 (-) | 41.7 | 58.3 | 23.3 | - | - |
| Chang et al[43] | Abstract | Singapore | 88 | 84 | 3 (-) | 1 (-) | 49.0 | 71.6 | - | - | - |
TE: Transient elastography; VM: Valid measurement; LB: Liver biopsy; BMI: Body mass index; SR: Success rate; PT: Portal tract; ICS: Intercostal space.
Table 4.
Histologic distribution and the performance of transient elastography for the diagnosis of liver fibrosis in patients with chronic hepatitis B
|
n (%) |
METAVIR and other scoring system (≥ F2/F4) |
||||||||||||
| F0 | F1 | F2 | F3 | F4 | AUROC | Cutoff (kPa) | Se (%) | Sp (%) | PPV (%) | NPV (%) | LR (+) | LR (-) | |
| Kim et al[4] | 0 | 9 (9.9) | 33 (36.3) | 10 (11.0) | 39 (42.9) | -/0.803 | -/9.7 | -/82 | -/62 | -/63 | -/76 | -/4.97 | -/0.13 |
| Chan et al[11] | 10 (6.2) | 27 (16.8) | 47 (29.2) | 37 (23.0) | 40 (24.8) | -/0.93 | -/9 | -/98 | -/75 | -/57 | -/98 | -/1 | -/0.01 |
| Kim et al[36] | 0 | 10 (7.7) | 37 (28.5) | 16 (12.3) | 67 (51.5) | -/0.84 | -/10.1 | -/76 | -/81 | -/76.1 | -/80.9 | -/- | -/- |
| Marcellin et al[10] | 16 (9.2) | 70 (40.5) | 44 (25.4) | 29 (16.8) | 14 (8.1) | 0.81/0.93 | 7.2/11 | 70/98 | 83/75 | 80/57 | 73/98 | 4/1 | -/0.01 |
| Chang et al[40] | 7 (20.0) | 16 (45.7) | F2-3 (5, 14.3) | 7 (20.0) | -/- | 11.8/- | 90/- | 78/- | -/- | -/- | -/- | -/- | |
| Tanwandee et al[41] | - | - | - | - | - | 0.757/- | 6.9/7.3 | 70/93 | 79/61 | 82/31 | 66/98 | -/- | -/- |
| Choi et al[42] | - | - | - | - | - | 0.88/0.86 | 7.7/10.4 | 88/79 | 88/83 | -/- | -/- | -/- | -/- |
| Chang et al[43] | -14.8 | -30.7 | -14.8 | -21.6 | -17.1 | 0.801/- | 8.8/- | -/- | -/- | -/- | -/- | -/- | -/- |
AUROC: Area under the receive operating characteristic curve; Se: Sensitivity, Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; LR: Likelihood ratio.
The AUROCs for predicting F ≥ 2 fibrosis and F = 4 fibrosis stage in patients with CHC were reported as 0.79-0.83 and 0.97-0.95, respectively in the studies by Ziol et al[2] and Castera et al[44]. However, the AUROCs in Asian studies seems to be lower than those reported in European studies (0.76-0.88 for F ≥ 2 and 0.80-0.93 for F4, Table 4), although TE diagnosed cirrhosis more accurately than significant fibrosis in Asian studies.
Overall, TE is accepted as a promising and accurate tool for the early detection of cirrhosis irrespective of the etiology of CLD, although the optimal cutoff values remain debatable. The range of the optimal cutoff values for diagnosing HBV-related cirrhosis in the Asian population were between 9.0 and 10.1 kPa based on the full-length articles[4,11,39] (Table 4) and the range for cirrhosis was less than 11.0 kPa, which is consistently lower than in patients with CHC[45,46]. These findings can be explained in several ways. The histopathological characteristics of CHC (including portal lymphoid follicles, bile duct damage, lobular activity, and steatosis) may contribute to the variations in cutoff values compared with those in patients with CHB[47]. Another explanation is that the total fibrotic material in CHB may be lower than that in CHC, because CHB tends to make the liver macronodular and heterogeneous[48]. Other researchers have proposed that the different types and extent of liver inflammatory infiltrate within the liver may account for the different cutoff LS values between CHB and CHC[20].
The performance of TE in prediction of cirrhosis is acceptable for the Asian population with CHB, but increased performance in prediction of significant fibrosis and subsequent precise staging, particularly for patients with CHB who are candidates for antiviral treatment, is needed and should be pursued through future studies. This is particularly important, because the decision to start antiviral treatment and the type of antiviral agents to be used may be affected by fibrosis stage.
CONCLUSION
Because of limitations of LB, prohibiting its routine use for the evaluation of liver fibrosis in patients with CHB, interest in the use of noninvasive TE has increased. Ideally, TE can be used to screen the general population to detect high-risk patients with potential liver disease, to identify patients with significant fibrosis who could benefit from the initiation of antiviral treatment, to select patients with cirrhosis who are at a high risk of developing HCC[25], and to identify patients with cirrhosis and portal hypertension. To date, although numerous European studies have demonstrated that this is possible, data on TE for the Asian population are scarce.
We are now fully armed with nuggets of knowledge that the etiologies of CLD, BMI, MS, cardiac function, cholestasis, hepatic steatosis, and ALT levels can influence the performance of TE, and also that several confounders vary between Asian and European populations, for example, the etiology of CLD, major fluctuation in ALT levels, and the prevalence of high BMI and MS. However, because almost all information on TE has thus far originated from European data, for the Asian population with CHB, TE is not popular clinically. Therefore, further studies tailored to the Asian population with CHB will restore the confidence of Asian clinicians to utilize TE.
Footnotes
Supported by A Grant from the Good Health R&D Project from the Ministry of Health, Welfare and Family Affairs, Republic of Korea (A050021)
Peer reviewer: Guglielmo Borgia, MD, Professor, Public Medicine and Social Security, University of Naples “Federico II”, Malattie Infettive (Ed. 18), via S. Pansini 5, I-80131, Naples, Italy
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH
References
- 1.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Ledinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 3.Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouilleres O, de Ledinghen V, Dhumeaux D, Marcellin P, Beaugrand M, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 4.Kim do Y, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, Yoon KT, Paik YH, Lee KS, Chon CY, et al. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci. 2009;54:1758–1763. doi: 10.1007/s10620-008-0541-2. [DOI] [PubMed] [Google Scholar]
- 5.de Ledinghen V, Beaugrand M, Kelleher TB, Foucher J, Castera L, Ziol M, Ganne N, Lai M, Afdhal NH. Prediction of liver fibrosis in non-alcoholic steatohepatitis (NASH): Risk factors and diagnostic potential of liver elasticity using fibroscan. J Hepatol. 2006;44:S39. [Google Scholar]
- 6.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, Gazzard B, Nelson M. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214–219. doi: 10.1097/MCG.0b013e3181b4af1f. [DOI] [PubMed] [Google Scholar]
- 9.Lucidarme D, Foucher J, Le Bail B, Vergniol J, Castera L, Duburque C, Forzy G, Filoche B, Couzigou P, de Ledinghen V. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1083–1089. doi: 10.1002/hep.22748. [DOI] [PubMed] [Google Scholar]
- 10.Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Ledinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 12.Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, de Ledinghen V. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46:628–634. doi: 10.1016/j.jhep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Roulot D, Czernichow S, Le Clesiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606–613. doi: 10.1016/j.jhep.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Kim SU, Choi GH, Han WK, Kim BK, Park JY, Kim do Y, Choi JS, Yang SC, Choi EH, Ahn SH, et al. What are ‘true normal’ liver stiffness values using FibroScan?: a prospective study in healthy living liver and kidney donors in South Korea. Liver Int. 2010;30:268–274. doi: 10.1111/j.1478-3231.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 15.Corpechot C, El Naggar A, Poupon R. Gender and liver: is the liver stiffness weaker in weaker sex? Hepatology. 2006;44:513–514. doi: 10.1002/hep.21306. [DOI] [PubMed] [Google Scholar]
- 16.Sirli R, Sporea I, Tudora A, Deleanu A, Popescu A. Transient elastographic evaluation of subjects without known hepatic pathology: does age change the liver stiffness? J Gastrointestin Liver Dis. 2009;18:57–60. [PubMed] [Google Scholar]
- 17.Colombo S, Belloli L, Buonocore M, Jamoletti C, Zaccanelli M, Badia E, Del Poggio P. Liver Stiffness values in the normal population: a studying voluntary blood donors. Hepatology. 2008;48 Suppl:A995. [Google Scholar]
- 18.Colombo S, Belloli L, Buonocore M, Jamoletti C, Zaccanelli E, Del Poggio P. True normal liver stiffness measurement (LSM) and its determinants. Hepatology. 2009;50 Suppl:A741. [Google Scholar]
- 19.Fung J, Lai CL, Chan SC, But D, Seto WK, Cheng C, Wong DK, Lo CM, Fan ST, Yuen MF. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol. 2010;105:1116–1122. doi: 10.1038/ajg.2009.665. [DOI] [PubMed] [Google Scholar]
- 20.Oliveri F, Coco B, Ciccorossi P, Colombatto P, Romagnoli V, Cherubini B, Bonino F, Brunetto MR. Liver stiffness in the hepatitis B virus carrier: a non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol. 2008;14:6154–6162. doi: 10.3748/wjg.14.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelleher T, MacFarlane C, de Ledinghen V, Beaugrand M, Foucher J, Castera L. Risk factors and hepatic elastography (FibroScan) in the prediction of hepatic fibrosis in nonalcoholic steatohepatitis. Gastroenterology. 2006;130:A736. [Google Scholar]
- 22.Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD) Gut. 2007;56:1330–1331. doi: 10.1136/gut.2007.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foucher J, Castera L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Ledinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411–412. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Chan HL, Wong GL, Tse CH, Chim AM, Yiu KK, Chan HY, Sung JJ, Wong VW. Hepatitis B virus genotype C is associated with more severe liver fibrosis than genotype B. Clin Gastroenterol Hepatol. 2009;7:1361–1366. doi: 10.1016/j.cgh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Imamura J, Goto T, Kanai F, Kato N, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954–1961. doi: 10.1002/hep.22870. [DOI] [PubMed] [Google Scholar]
- 26.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol. 2009;7:227–233. doi: 10.1016/j.cgh.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Maimone S, Calvaruso V, Pleguezuelo M, Squadrito G, Amaddeo G, Jacobs M, Khanna P, Raimondo G, Dusheiko G. An evaluation of transient elastography in the discrimination of HBeAg-negative disease from inactive hepatitis B carriers. J Viral Hepat. 2009;16:769–774. doi: 10.1111/j.1365-2893.2009.01120.x. [DOI] [PubMed] [Google Scholar]
- 28.Lupsor M, Stefanescu H, Sparchez Z, Serban A, Grigorescu M, Iancu S, Suteu T, Badea R. The influence of fatty load on liver stiffness in chronic hepatitis c patients. J Hepatol. 2008;48 Suppl 2:S278. [PubMed] [Google Scholar]
- 29.Gaia S, Carenzi S, Brunello F, Barilli AL, Lagger M, Bugianesi E, Smedile A, Rizzetto M. Is liver stiffness measurement different in patients with NASH or with viral hepatitis? Dig Liver Dis. 2008;40:A128. [Google Scholar]
- 30.Kim SU, Kim DY, Ahn SH, Kim HM, Lee JM, Chon CY, Park YN, Han KH, Park JY. The impact of steatosis on liver stiffness measurement in patients with chronic hepatitis B. Hepatogastroenterology. 2010:In press. [PubMed] [Google Scholar]
- 31.Wong VW, Chan HL, Hui AY, Chan KF, Liew CT, Chan FK, Sung JJ. Clinical and histological features of non-alcoholic fatty liver disease in Hong Kong Chinese. Aliment Pharmacol Ther. 2004;20:45–49. doi: 10.1111/j.1365-2036.2004.02012.x. [DOI] [PubMed] [Google Scholar]
- 32.Nugent C, Younossi ZM. Evaluation and management of obesity-related nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2007;4:432–441. doi: 10.1038/ncpgasthep0879. [DOI] [PubMed] [Google Scholar]
- 33.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Kim SU, Kim do Y, Park JY, Lee JH, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Choi EH, et al. How can we enhance the performance of liver stiffness measurement using FibroScan in diagnosing liver cirrhosis in patients with chronic hepatitis B? J Clin Gastroenterol. 2010;44:66–71. doi: 10.1097/MCG.0b013e3181a95c7f. [DOI] [PubMed] [Google Scholar]
- 35.Calvaruso V, Cammà C, Di Marco V, Maimone S, Bronte F, Enea M, Pleguezuelo M, Xirouchakis E, Misseri M, Manousou P, et al. Error factors for transient elastography in chronic hepatitis C. Hepatology. 2008;48 Suppl:A313. [Google Scholar]
- 36.Yuen MF, Yuan HJ, Hui CK, Wong DK, Wong WM, Chan AO, Wong BC, Lai CL. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416–419. doi: 10.1136/gut.52.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol. 2003;18:246–252. doi: 10.1046/j.1440-1746.2003.02976.x. [DOI] [PubMed] [Google Scholar]
- 38.Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Chan HL. Increased liver stiffness measurement by transient elastography in severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2009;24:1002–1007. doi: 10.1111/j.1440-1746.2009.05779.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim SU, Ahn SH, Park JY, Kang W, Kim do Y, Park YN, Chon CY, Han KH. Liver stiffness measurement in combination with noninvasive markers for the improved diagnosis of B-viral liver cirrhosis. J Clin Gastroenterol. 2009;43:267–271. doi: 10.1097/MCG.0b013e31816f212e. [DOI] [PubMed] [Google Scholar]
- 40.Chang J, Tan HH, Yew BS, Thia K, Ang D, Yap YM, Chau C, Tan CK, Chow WC, Lui HF. Transient elastography (FibroScan®) to assess hepatic fibrosis in Chinese with chronic hepatitis B. Hepatol Int. 2007;1 Suppl:A206. [Google Scholar]
- 41.Tanwandee T, Charatcharoenwitthaya P, Viboolsirikul V, Chotiyaputta W, Chainuvati S, Maneerattanaporn M, Prachayakul V, Pongprasobchai S, Manatsathit S, Leelakucolvong S, et al. Utility of liver stiffness measured by transient elastography for determining significant liver fibrosis in patients with chronic hepatitis B. Hepatology. 2008;48 Suppl:A709. [Google Scholar]
- 42.Choi JW, Kim DY, Park JY, Ahn SH, Yoon KT, Lee JM, Kim JK, Paik YH, Lee KS, Moon BS, et al. Clinical usefulness of liver stiffness measurement in HBeAg-positive chronic hepatitis B patients with ALT level <2 times upper limit of normal. Hepatology. 2008;48 Suppl:A750. [Google Scholar]
- 43.Chang J, Lui HF, Tan CK, Chow WC. Transient elastography (FibroScan®) is reliable for non-invasive diagnosis of significant fibrosis in chronic hepatitis B with mild transaminitis but becomes less reliable at higher ALT levels. Hepatology. 2008;50 Suppl:A501. [Google Scholar]
- 44.Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Ledinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838–845. doi: 10.1002/hep.20814. [DOI] [PubMed] [Google Scholar]
- 46.Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Ledinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheuer PJ, Davies SE, Dhillon AP. Histopathological aspects of viral hepatitis. J Viral Hepat. 1996;3:277–283. doi: 10.1111/j.1365-2893.1996.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 48.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]