Abstract
AIM: To assess the prevalence of advanced liver fibrosis (ALF) in human immunodeficiency virus (HIV), hepatitis C virus (HCV) and HIV/HCV patients using transient elastography, and to identify factors associated with ALF.
METHODS: Between September 2008 and October 2009, 71 HIV mono-infected, 57 HIV/HCV co-infected and 53 HCV mono-infected patients on regular follow-up at our Center were enrolled in this study. Alcohol intake, the main parameters of liver function, presence of HCV-RNA, HIV-RNA, duration of highly active anti-retroviral therapy (HAART) and CD4 cell count were recorded. ALF was defined as liver stiffness (LS) ≥ 9.5 kPa. To estimate liver fibrosis (LF) a further 2 reliable biochemical scores, aspartate aminotransferase platelet ratio index (APRI) and FIB-4, were also used.
RESULTS: LS values of co-infected patients were higher than in either HIV or HCV mono-infected patients (χ2MH = 4, P < 0.04). In fact, LS ≥ 9.5 was significantly higher in co-infected than in HIV and HCV mono-infected patients (χ2 = 5, P < 0.03). Also APRI and the FIB-4 index showed more LF in co-infected than in HIV mono-infected patients (P < 0.0001), but not in HCV mono-infected patients. In HIV⁄HCV co-infected patients, the extent of LS was significantly associated with alcohol intake (P < 0.04) and lower CD4+ cell count (P < 0.02). In HCV patients, LS was correlated with alcohol intake (P < 0.001) and cholesterol levels (P < 0.03). Body mass index, diabetes, HCV- and HIV-viremia were not significantly correlated with LS. In addition, 20% of co-infected patients had virologically unsuccessful HAART; in 50% compliance was low, CD4+ levels were < 400 cells/mm3 and LS was > 9.5 kPa. There was no significant correlation between extent of LF and HAART exposure or duration of HAART exposure, in particular with specific dideoxynucleoside analogues.
CONCLUSION: ALF was more frequent in co-infected than mono-infected patients. This result correlated with lower CD4 levels. Protective immunological effects of HAART on LF progression outweigh its hepatotoxic effects.
Keywords: Liver fibrosis, Transient elastography, Aspartate aminotransferase platelet ratio index, FIB-4 test, Fibrosis evaluation, Human immunodeficiency virus infection, Hepatitis C virus infection
INTRODUCTION
In the last few years, liver disease associated with hepatitis C virus (HCV) has emerged as a significant problem in human immunodeficiency virus (HIV)-infected patients, thanks to improved survival in the highly active anti-retroviral therapy (HAART) era[1]. It has been reported that HIV and HCV co-infection leads to a more rapid progression of liver disease to cirrhosis[2,3]. Other factors such as severe immune suppression and alcohol consumption accelerate the progression of HCV-related fibrosis[4,5]. Virologically successful HAART slows the progression of liver fibrosis (LF) and reduces hepatic necroinflammatory activity in HIV/HCV co-infected patients[2,6]. In contrast, antiretroviral-related liver toxicity could contribute to liver damage in HIV- and HIV/HCV-infected patients[7]. Mitochondrial toxicity of nucleoside analogues[8], and glucose or lipid abnormalities, such as hyperglycemia and lipodystrophy, which are particularly common when using some protease inhibitors[9], may produce or enhance LF progression in HIV mono- and HIV/HCV co-infected patients. Currently, in this respect, a growing number of cases of cryptogenetic liver disease in symptomatic and asymptomatic HIV-infected patients has been reported[10,11].
Percutaneous liver biopsy is the gold standard for assessing LF. However, it may be associated with sampling variability[12], is an invasive technique with rates of morbidity of 3% and mortality of 0.03%[13,14], and as a consequence, is not suitable for repeated assessment, which is required when monitoring LF.
For these reasons, new non-invasive methods for the assessment of LF have been developed. Transient elastography (TE) (Fibro-Scan®; EchoSens, Paris, France) is a rapid, reliable and tolerable imaging technique for the assessment of LF by measuring liver stiffness (LS)[15,16].
On the other hand, many biochemical markers have been implemented to estimate LF, with the aim of reducing the number of liver biopsies[14].
The advent of TE and biochemical markers has been demonstrated to be very helpful in the non-invasive measurement of LF, particularly in asymptomatic HIV-infected patients in whom liver biopsy is not recommended[11]. TE has already been validated for the measurement of LF in HIV and HCV seropositive patients[17,18].
The aim of this study was to assess the prevalence of LF and cirrhosis in a group of HIV mono-infected, HCV mono-infected and HIV/HCV co-infected patients using TE and biochemical markers. In addition, we evaluated which of the factors studied correlated with advanced LF (ALF) and cirrhosis.
MATERIALS AND METHODS
Study population
Between September 2008 and October 2009 all consecutive HIV mono-infected and HIV/HCV co-infected patients on regular follow-up at the AIDS Center of the University of Palermo, as well as HCV mono-infected patients seen consecutively at the Outpatient Clinic of the Department of Clinical Medicine and Emerging Pathologies of the University of Palermo were enrolled in this study.
Information on age, gender, risk factors for HCV and HIV infections, cumulative exposure to non-nucleoside and nucleoside reverse-transcriptase inhibitors, protease inhibitors and specific antiretroviral drugs within each class were all recorded in a database designed for this study.
For all HIV-infected patients the absolute number of CD4+ T-cells and plasma HIV-RNA levels was assessed. In HCV-infected patients, HCV-genotype and plasma HCV-RNA levels were also recorded. In addition, at baseline, complete blood cell counts, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γGT), total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides and glycemia were measured.
Alcohol intake > 20 g/d either at the time of the study or in the past was recorded through patient interviews. Diabetes or impaired fasting glucose (IFG) were defined according to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus criteria[19].
Patients with acute liver decompensation, hepatocellular carcinoma or chronic hepatitis B were excluded.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. Patients were enrolled after written informed consent was obtained.
Assessment of LF
LF was assessed by a single certified operator (trained by the manufacturer) using TE (FibroScan®; EchoSens, Paris, France). TE provides an assessment of LS expressed in kPa units. In brief, an ultrasound transducer probe is mounted on the axis of a vibrator. Vibrations of mild amplitude and low frequency are transmitted by the transducer, inducing an elastic shear wave that propagates through the underlying tissues. The speed of propagation of this vibration across the liver is directly related to tissue stiffness.
The tip of the probe transducer was placed in the intercostal spaces at the right lobe of the liver. Only patients with 10 valid elastometric measures, interquartile ranges > 30% and ≥ 60% success rate (the number of validated measurements divided by the total number of measurements) were considered to be reliable. ALF (severe fibrosis and cirrhosis) was defined as a median LS of 9.5 kPa. As previously published, this cut-off value is strongly correlated with a Metavir score of F3, both in HCV mono-infected and HCV⁄HIV co-infected patients[17,18].
LF was also assessed biologically using 2 different well-validated indices, the aspartate aminotransferase platelet ratio index (APRI) index and the FIB-4 index. The APRI was calculated as follows: AST⁄upper limit of normal × 100⁄platelet count (109⁄L)[20,21]. The FIB-4 index was calculated as follows: age × AST (IU⁄L)⁄{[platelet count (109⁄L)] × [ALT (IU⁄L)]1⁄2}[22]. The prevalence of ALF was estimated using as a reference a FIB-4 index > 3.25 and an APRI index > 1.5[20,22].
Statistical analysis
When data distribution was Gaussian, values were expressed as mean ± SD and their differences were calculated using the Student t-test; otherwise, data were expressed as the median and range and analyzed using the Mann-Whitney U test. Fisher’s exact and χ2 tests, the χ2 test of Mantel Haenszel, Spearman’s rank correlations (ρ) and Pearson’s correlation (r) were used where appropriate. Multiple linear regression analysis was used to study the association between increased values of LS and variables statistically significant at univariate analysis. All analyses were performed using the SPSS software package (version 13.0; Chicago, IL, USA).
RESULTS
Study population
A total of 201 patients on regular follow-up at both our Centers were enrolled in the study.
In 11 patients (4 HIV mono-infected, 6 HCV mono-infected and one co-infected) a valid elastometric assessment could not be obtained because of truncular obesity, therefore 190 patients were finally included in this study. There were 137 HIV-infected patients, including 71 HIV mono-infected and 66 HIV/HCV co-infected, and 53 HCV mono-infected patients. Patient characteristics at the time of LS measurement are summarized in Table 1.
Table 1.
Demographic characteristics of the study populations (mean ± SD) n (%)
| HIV (n = 71) | HCV (n = 53) | HIV/HCV (n = 66) | |
| Age (yr) | 43.3 ± 10.3a | 49.7 ± 12.5 | 45.6 ± 12.5 |
| Male gender | 50 (70) | 36 (67.9) | 49 (74.2) |
| BMI (kg/m2) | 23.6 ± 3.4a | 26.0 ± 3.6 | 22.8 ± 3.0b |
| Risk group | |||
| Transfusion | - | 17 (32.1) | 4 (6.1)b |
| Homosexual men | 21 (29.5) | - | 1 (1.5) |
| Intravenous drug use | 4 (5.6) | 7 (13.2)c | 41 (62.1) |
| Others | - | 12 (22.6) | - |
| Unknown | 9 (12.6) | 16 (30.2) | 8 (12.1) |
| HCV genotype 3:non3 | - | 7:46d | 20:46 |
| HAART | 57 (80.2) | - | 60 (90.9) |
| Alcohol | 6 (8.4) | 4 (7.5) | 5 (7.5) |
| Diabetes + IFG | 2 (2.8) | 4 (7.5)e | 11 (16.6) |
P < 0.002,
P < 0.0001 vs hepatitis C virus (HCV);
P < 0.0001,
P < 0.03,
P < 0.001 vs human immunodeficiency virus (HIV)/HCV. IFG: Impaired fasting glucose; BMI: Body mass index; HAART: Highly active anti-retroviral therapy.
HIV patients were significantly younger than HCV mono-infected individuals (P < 0.002). Body mass index (BMI) was significantly higher in HCV mono-infected patients than in HIV and HIV/HCV co-infected patients (P < 0.002). The most frequent risk factor of HCV contamination was intravenous drug use in co-infected vs mono-infected HCV patients (P < 0.0001), while it was transfusion of blood products in HCV mono-infected vs co-infected patients (P < 0.0001). Most HIV mono-infected (80.2%) and HIV/HCV co-infected patients (90.9%) were under HAART. However, only 62% of HIV mono-infected and 68% of HIV/HCV co-infected patients had an HIV-RNA load of < 47 copies/mL. In addition, 20% of co-infected patients had virologically unsuccessful HAART; in 50% compliance was low, and CD4+ levels were < 400 cells/mm3. HIV⁄HCV co-infected patients were more often infected by HCV genotype 3 compared with HCV mono-infected patients (P < 0.03).
Table 2 shows the main hematological and virological parameters in the 3 study groups. Serum ALT levels were significantly higher in HCV mono-infected patients than in HIV/HCV co-infected and HIV mono-infected patients (P < 0.02). Serum AST, ALT and γGT levels were significantly higher in HIV/HCV co-infected than in HIV mono-infected patients (P < 0.0001). Only γGT levels were more elevated in HIV/HCV co-infected patients than in HCV mono-infected patients (P < 0.01). TG levels were significantly higher in HIV mono-infected and HIV/HCV co-infected patients than in HCV mono-infected patients (P < 0.004).
Table 2.
Main hematological and virological parameters in the three study groups (mean ± SD)
| HIV (n = 71) | HCV (n = 53) | HIV/HCV (n = 66) | |
| AST/ALT | 1 ± 0.4 | 0.72 ± 0.28aj | 0.9 ± 0.4 |
| ALT (U/L), mean (range) | 22 (7-113)fh | 75 (16-551) | 56.5 (9-280)g |
| AST (U/L), mean (range) | 21 (11-56)fh | 52.5 (18-209) | 42.5 (14-281) |
| γGT (IU/L), mean (range) | 33 (9-461)h | 40.5 (10-479)i | 76.5 (11-479) |
| Mean platelet count (109/L) | 208 ± 59 | 184 ± 60 | 173 ± 61b |
| T-Chol (mg/dL) | 201 ± 46 | 175 ± 28d | 171 ± 48c |
| HDL-Chol (mg/dL) | 44 ± 16 | 47 ± 12 | 44 ± 15 |
| LDL Chol (mg/dL) | 123 ± 32 | 108 ± 37.5 | 97 ± 40a |
| TG (mg/dL), mean (range) | 137 (36-615) | 103 (32-354)ej | 118 (49-614) |
| HCV-RNA (IU/mL) > 700.000 | - | 29 (54.7) | 44 (66.6) |
| HIV-RNA (copies/mL), mean (range) | 6050 (50-700 000) | - | 3300 (60-1 100 000) |
| HIV-RNA < 47 copies/mL (%) | 44 (62) | 45 (68.1) | |
| CD4+ count (cells/μL), mean (range) | 466.5 (17-1282) | - | 446 (35-1445) |
| CD4+ < 200 cells/μL | 381 | - | 382 |
P < 0.0001,
P < 0.002,
P < 0.001,
P < 0.004,
P < 0.02 vs human immunodeficiency virus (HIV);
P < 0.0001,
P < 0.02 vs hepatitis C virus (HCV);
P < 0.0001,
P < 0.01,
P < 0.05 vs HIV/HCV.
Data available in 66 patients;
Data available in 56 patients. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; γGT: γ-glutamyl transferase; T-Chol: Total cholesterol; HDL: High density lipoprotein; LDL: Low density lipoprotein; TG: Triglyceride.
Extent of LF
In the overall population LS measured by TE ranged from 3.2 to 48.8 kPa. In 9 HIV/HCV co-infected patients, HCV-RNA was undetectable and for this reason these patients were excluded from the analysis, which was thus carried out only in the remaining 57 co-infected patients. However, their LS was lower than in the remaining co-infected group (data not shown).
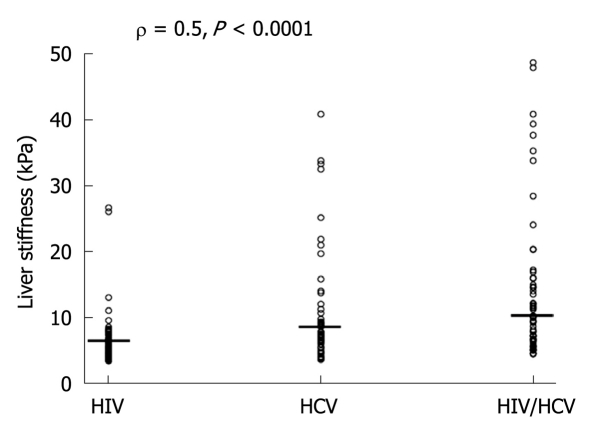
Table 3 shows the distribution of LS values measured in all 3 study groups. Co-infected patients had higher LS values than mono-infected patients (χ2MH = 4, P < 0.04). The HIV⁄HCV-co-infected population had LS ≥ 9.5 kPa (50.9%) more often than HCV and HIV mono-infected patients (28.3%) (χ2 = 5, P < 0.03). In this respect 60% of co-infected patients under virologically unsuccessful HAART showed LS ≥ F3. The individual values of LS increased from HIV to HCV and to HIV/HCV infected patients (ρ = 0.5, P < 0. 0001) (Figure 1).
Table 3.
Estimates of liver fibrosis using transient elastography in the study population n (%)
| LS | Metavir | HIV (n = 71) | HCV (n = 53) | HIV/HCV (n = 57) | |
| < 7.1 | F0-F1 | 56 (78.8) | 23 (43.4) | 19 (33.3) | |
| 7.1-9.4 | F2 | 11 (15.4) | 15 (28.3) | 9 (15.8) | |
| 9.5-12.4 | F3 | 1 (1.4) | 4 (7.5) | 9 (15.8) | |
| ≥ 12.5 | F4 | 3 (4.2) | 11 (20.7) | 20 (35.1) | χ2MH = 4, P < 0.04 |
| ≥ F3 | 4 (5.6) | 15 (28.3) | 29 (50.9) | χ2 = 5, P < 0.03 |
HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; LS: Liver stiffness.
Figure 1.
Median and distribution of liver stiffness in human immunodeficiency virus, hepatitis C virus, human immunodeficiency virus/hepatitis C virus patients. HIV: Human immunodeficiency virus; HCV: Hepatitis C virus.
Overall, by multiple linear regression analysis, the variables significantly associated with ALF were AST values (β = 0.47, P < 0.0001) and HIV/HCV co-infection (β = 0.25, P < 0.002). To better understand which variables were associated with LS in patients with HIV mono- and co-infection, we also performed multiple linear regression analysis of these 2 groups and found that ALF correlated positively with AST serum levels (β = 0.34, P < 0.0001) and presence of HIV/HCV co-infection (β = 0.4, P < 0.0001) and negatively with lower CD4+ cell counts (β = -0.21, P < 0.003).
Median values of APRI and FIB-4 were significantly higher in HCV mono- and co-infected patients than in HIV mono-infected patients. There were no significant differences in APRI and FIB-4 medians in HCV mono- and HIV/HCV co-infected patients (Table 4). Median values of LS were significantly lower in HIV mono-infected than in HCV mono- and HIV/HCV co-infected patients (P < 0.0001). Median values of HIV/HCV co-infected patients were also significantly higher than in HCV mono-infected patients (P < 0.05).
Table 4.
Estimates of liver fibrosis using biochemical markers and transient elastography, mean (range)
| HIV (n = 71) | HCV (n = 53) | HIV/HCV (n = 57) | |
| APRI | 0.31 (0.1-1.6)ab | 0.93 (0.28-6.07) | 0.92 (0.17-19.7) |
| FIB-4 | 0.93 (0.39-4.28)a | 1.62 (0.47-12.6) | 1.63 (0.63-23.8) |
| LS | 5.4 (3.2-26.6)ab | 7.3 (3.4-40.9)c | 9.8 (4.3-48.8) |
P < 0.0001 vs hepatitis C virus (HCV);
P < 0.0001,
P < 0.05 vs human immunodeficiency virus (HIV)/HCV. LS: Liver stiffness; APRI: Aspartate aminotransferase platelet ratio index.
Overall, correlations between LS and APRI values and LS and FIB-4 values were statistically significant (r = 0.60, P < 0.0001 and r = 0.64, P < 0.0001, respectively). However, when correlations were made according to the 3 cut-off values of LS, we found a significant correlation only for values of LS ≥ 9.5 (r = 0.50, P < 0.0001 vs APRI and r =0.53, P < 0.0001 vs FIB-4) (Table 5).
Table 5.
Correlation between liver stiffness and aminotransferase platelet ratio index/FIB-4 values according to the liver stiffness cut-offs
|
LS < 7.1 kPa (n = 98) |
LS (7.1-9.4 kPa) (n = 35) |
LS ≥ 9.5 kPa (n = 48) |
||||
| r | P | r | P | r | P | |
| APRI | 0.01 | NS | 0.23 | NS | 0.50 | < 0.0001 |
| FIB-4 | 0.01 | NS | 0.25 | NS | 0.53 | < 0.0001 |
APRI: Aminotransferase platelet ratio index; LS: Liver stiffness; NS: Not significant.
Correlation between LS and risk factors studied
Table 6 shows the factors associated with LS in the 3 groups. No correlation between the studied parameters and LS was found in the HIV mono-infected group. In HCV mono-infected patients, LS positively correlated with alcohol intake > 20 g/d (P < 0.001) and AST serum level (P < 0.0001), while it negatively correlated with the number of platelets (P < 0.0001) and serum cholesterol levels (P < 0.03). APRI and FIB-4 values were significantly associated with LS both in HCV and HIV/HCV co-infected patients (P < 0.0001).
Table 6.
Correlation (r or ρ) between liver stiffness and studied parameters
|
LS |
|||
| HIV | HCV | HIV/HCV | |
| BMI | 0.11 | -0.1 | 0.02 |
| HCV genotype 3/non 3 | - | -0.1 | -0.26 |
| HCV-RNA | - | - | -0.26 |
| HIV-RNA | 0.21 | - | 0.18 |
| Time HAART | -0.047 | - | 0.09 |
| CD4 cells count | 0.074 | - | -0.32d |
| Alcohol | -0.096 | 0.70a | 0.30e |
| T-Chol | -0.09 | -0.32b | -0.18 |
| TG | 0.06 | 0.18 | 0.1 |
| HDL-Chol | -0.1 | 0.05 | -0.16 |
| Diabetes + IFG | -0.01 | 0.19 | 0.14 |
| Time HCV | - | 0.24 | -0.02 |
| ALT | 0.03 | 0.1 | 0.23 |
| AST | 0.18 | 0.56c | 0.40a |
| Platelets | -0.02 | -0.56c | -0.61c |
| APRI score | 0.04 | 0.70c | 0.50c |
| FIB-4 score | 0.06 | 0.75c | 0.60c |
LS: Liver stiffness; HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; BMI: Body mass index; HAART: Highly active anti-retroviral therapy; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; T-Chol: Total cholesterol; HDL: High density lipoprotein; TG: Triglyceride; IFG: Impaired fasting glucose; APRI: Aminotransferase platelet ratio index.
P < 0.001;
P < 0.03;
P < 0.0001;
P < 0.02;
P < 0.04.
In HIV⁄HCV-co-infected patients the extent of LS was significantly correlated with alcohol intake (P < 0.04) AST level (P < 0.0001) and lower CD4+ cells count (P < 0.02) and was negatively correlated with platelets (P < 0.0001).
BMI, presence of diabetes or IFG, HCV- and HIV-viremia were not significantly correlated with LS in any of the 3 study groups. In addition, there was no significant correlation between the extent of LF under HAART exposure and duration of HAART exposure. There was no significant correlation either between extent of LF and cumulative exposure to each class of antiretroviral drugs (non-nucleoside/nucleoside reverse-transcriptase inhibitors, protease inhibitors) and of specific dideoxynucleoside analogues (didanosine, stavudine, zidovudine).
DISCUSSION
Our results, in agreement with other studies, confirmed that LF is more severe in HIV/HCV co-infected patients than in HCV or HIV mono-infected patients[5,23,24]. In addition, ALF was significantly associated with a lower CD4+cell count in co-infected patients. There is convincing evidence that co-infection with HIV worsens the prognosis of HCV-related liver disease. It has been reported that in patients co-infected with HIV and HCV the risk of progressing to cirrhosis and liver failure is higher than in those infected with only HCV[25,26], especially in individuals with CD4 < 200 cells/μL and alcohol consumption[2].
In our study, 20% of HIV/HCV co-infected patients were under virologically unsuccessful HAART and in 50% CD4+ levels were below 400 cells/mm3 suggesting, in agreement with former findings, that the less successful the response to HAART, the less marked is its clinical benefit. In fact, immune recovery under HAART has been associated with longer overall survival, slower progression of HCV-related liver damage in HIV co-infected patients and with lengthier survival times before death attributable to liver disease[27]. In the same way, Pineda et al[27] demonstrated that liver decompensation emerged earlier in patients who maintained an undetectable HIV viral load for a shorter period during follow-up. Nevertheless, the association between ALF and lower CD4 cell count suggests that the response to HAART, measured using CD4 cell gain and HIV viral load decline, determines the evolution of liver disease and that virologically and immunologically successful HAART may slow progression of LF in HIV/HCV co-infected patients.
On the other hand, antiretroviral-related liver toxicity could have further contributed to liver damage in our HIV population[7]. Mitochondrial toxicity of nucleoside analogues and glucose or lipid abnormalities, particularly common when using some protease inhibitors, may produce or enhance LF progression in HIV-seropositive patients. The correlation between use of antiretroviral drugs and LF in patients with HIV/HCV co-infection has been evaluated in different studies but with contradictory results[21,28-30].
Macías et al[21] reported that HAART regimens, including nevirapine, were associated with an increased degree of LF, while the use of protease inhibitor-based HAART was associated with less severe fibrosis and a slower progression of fibrosis in HIV/HCV co-infected patients. In contrast, Berenguer et al[28] found that exposure to NNRTI was associated with a reduction in LF progression. In addition, Halfon et al[29] showed that exposure to NNRTI was an independent factor in LF while Blanco et al[30] highlighted that exposure to dideoxynucleosides was an independent factor associated with ALF.
In our study, no correlation was found between HAART exposure, duration of HAART exposure or cumulative exposure to any class of antiretroviral drugs and LF. In addition, we analyzed the correlation between duration of exposure to dideoxynucleosides (in particular didanosine, stavudine, zidovudine) and LF but also in this case no correlation was observed, suggesting that these drugs could not play any role in the progression of LF.
ALF was significantly higher in HIV/HCV co-infected patients than in HCV mono-infected patients when the subset of co-infected patients with undetectable HCV-RNA were excluded from the analysis. Overall, HIV/HCV co-infected patients with undetectable HCV-RNA had either no or only mild fibrosis (F0-F1) compared to the remaining co-infected patients, suggesting that the presence of HCV is important in conditioning the progression of LF and that anti-HCV therapy is mandatory in HIV/HCV co-infected patients in order to eradicate the virus. In fact, other authors have reported that achieving HCV clearance may reduce liver-related complications and mortality[31,32] and probably permits at least a partial regression of LF[33]. However, in HIV-positive patients liver cirrhosis may also occur without chronic viral hepatitis, and possible causes of hepatic steatosis in patients with HIV may be due to HIV itself, pathological alcohol use, diabetes mellitus, obesity or antiretroviral medications[34].
Evaluation of LF using the 2 biochemical scores (APRI and FIB-4) was not in full agreement with LS measurement. In fact, these 2 biochemical tests were in agreement with TE values only for high-grade LF, but not in low and moderate LF, suggesting that at least in these cases liver biopsy could be necessary to assess the precise degree of LF. In this respect, we are aware that the lack of liver biopsy, as a reference tool of LF, is a limitation of our study.
More consideration, perhaps, should be given to transaminase levels. In fact, the HCV mono-infected group had the highest levels of transaminases, which may have influenced LS values by increasing them. This result could further support our observation that co-infected patients are at highest risk of LF because of their high AST levels and the immune suppression associated with a low CD4 cell count.
Finally, also in our area, HCV genotype 3 was confirmed to be more associated with HIV-positive patients, because of their habits as drug abusers[35].
In conclusion, in our population, HIV⁄HCV co-infected patients had more ALF than HCV and HIV mono-infected patients. This result was not correlated with long-term exposure to HAART but with a lower CD4 cell count, suggesting that immunologically successful HAART may protect from progression of liver damage in HIV/HCV co-infected patients. In addition, the detection of unsuspected ALF in HIV mono-infected patients confirms that FibroScan® is very useful in this population. HCV infection, with its different pattern of cytolysis, may condition LS values, but viral eradication is mandatory to reduce fibrosis progression. Finally, the use of these non-invasive parameters of LF should be considered with caution. In fact, from our data it emerges that both TE and the biochemical scores may be suitable only for high grades of LF. In contrast, for mild/moderate degrees of fibrosis, they could not replace liver biopsy in the correct evaluation of LF.
COMMENTS
Background
Liver disease associated with hepatitis C virus (HCV) has emerged as a significant problem in human immunodeficiency virus (HIV) patients, thanks to improved survival in the highly active anti-retroviral therapy (HAART) era. Co-infection with HIV is known to lead to a more rapid progression of HCV liver disease to cirrhosis. Other factors such as severe immune suppression and alcohol consumption accelerate the progression of HCV-related fibrosis. In addition, successful HAART slows the progression of liver fibrosis (LF), but antiretroviral-liver toxicity could contributes to hepatic damage in co-infected patients. The advent of transient elastography (TE) has demonstrated to be very helpful for the non-invasive measurement of LF.
Research frontiers
Percutaneous liver biopsy is the gold standard for assessing LF but it is an invasive technique with risk of morbidity and mortality. For these reasons new non-invasive methods for the assessment of LF have recently been developed. TE (FibroScan®) and biochemical markers have demonstrated to be very helpful in the non-invasive measurement of LF. In this study, using these non-invasive tools, i.e. TE plus 2 biochemical tests, aminotransferase platelet ratio index (APRI) and FIB-4, we showed that advanced LF was significantly higher in HIV/HCV co-infected patients than in mono-infected patients and that it was significantly associated with lower CD4+ cells count. The APRI and FIB-4 tests correlated only with the highest values of TE, i.e. ≥ 9.5, suggesting that they are useful tools in diagnosing high grade liver disease, but in the case of a low or moderate degrees of LF liver biopsy remains the best means for correctly diagnosing the degree of fibrosis. Furthermore, the results showed that, overall, a greater number of HIV/HCV co-infected patients with undetectable HCV-RNA had either no or mild fibrosis (F0-F1) compared to the remaining co-infected patients with detectable HCV-RNA.
Innovations and breakthroughs
Several studies have been carried out to identify factors related to an accelerated progression of LF in HIV/HCV co-infected patients. Conflicting results have been reported in the literature about the role of antiretroviral therapy on the progression of LF. In the study, information on alcohol intake, duration of HCV infection and cumulative exposure to non-nucleoside and nucleoside reverse-transcriptase inhibitors, protease inhibitors and specific dideoxynucleoside analogues (didanosine, stavudine, zidovudine) was evaluated. The results showed that on univariate analysis liver stiffness (LS) was significantly associated with alcohol intake > 20 g/d in both HCV mono-infected and co-infected patients, but we did not find any correlations between LF and duration of HCV infection, HAART exposure, duration of HAART exposure or cumulative exposure to any class of antiretroviral drugs.
Applications
A good adherence to antiretroviral therapy, when it is indicated, is important to reduce the risk of progression of LF in co-infected patients. In addition, HCV mono- and co-infected patients should modify negative habits and lifestyles, such as alcohol consumption, which could accelerate the progression of LF. Important fields for further study could include the use and evaluation of the applicability of FibroScan® for repeated assessment in the monitoring of LF.
Terminology
Transient elastometry (Fibro-Scan®; EchoSens, Paris, France) is a rapid, reliable and well-tolerated imaging technique for the assessment of LF by measuring LS.
Peer review
The authors aimed to assess the prevalence of advanced LF (ALF) in HIV, HCV and HIV/HCV patients using TE and to identify factors associated with ALF. They concluded that HIV/HCV co-infected patients had ALF more frequently at TE than HCV and HIV mono-infected patients. The title reflects accurately the contents of the article, and the abstract delineates concisely the research.
Footnotes
Peer reviewer: Dr. Chao-Hung Hung, MD, Associate Professor, Division of Hepatogastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, 123 Ta Pei Road, Niao Sung, Kaohsiung 833, Taiwan, China
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Mocroft A, Soriano V, Rockstroh J, Reiss P, Kirk O, de Wit S, Gatell J, Clotet B, Phillips AN, Lundgren JD. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117–2125. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 3.Pineda JA, Romero-Gómez M, Díaz-García F, Girón-González JA, Montero JL, Torre-Cisneros J, Andrade RJ, González-Serrano M, Aguilar J, Aguilar-Guisado M, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779–789. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 4.Martín-Carbonero L, Benhamou Y, Puoti M, Berenguer J, Mallolas J, Quereda C, Arizcorreta A, Gonzalez A, Rockstroh J, Asensi V, et al. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis. 2004;38:128–133. doi: 10.1086/380130. [DOI] [PubMed] [Google Scholar]
- 5.Mohsen AH, Easterbrook PJ, Taylor C, Portmann B, Kulasegaram R, Murad S, Wiselka M, Norris S. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035–1040. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bräu N, Salvatore M, Ríos-Bedoya CF, Fernández-Carbia A, Paronetto F, Rodríguez-Orengo JF, Rodríguez-Torres M. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Núñez M, Soriano V. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. Drug Saf. 2005;28:53–66. doi: 10.2165/00002018-200528010-00004. [DOI] [PubMed] [Google Scholar]
- 8.John M, Moore CB, James IR, Nolan D, Upton RP, McKinnon EJ, Mallal SA. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS. 2001;15:717–723. doi: 10.1097/00002030-200104130-00007. [DOI] [PubMed] [Google Scholar]
- 9.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Dinh MH, Stosor V, Rao SM, Miller FH, Green RM. Cryptogenic liver disease in HIV-seropositive men. HIV Med. 2009;10:447–453. doi: 10.1111/j.1468-1293.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 11.Castellares C, Barreiro P, Martín-Carbonero L, Labarga P, Vispo ME, Casado R, Galindo L, García-Gascó P, García-Samaniego J, Soriano V. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat. 2008;15:165–172. doi: 10.1111/j.1365-2893.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 12.Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568–571. doi: 10.1016/s0016-5107(96)70192-x. [DOI] [PubMed] [Google Scholar]
- 13.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 14.Montalto G, Soresi M, Carroccio A, Bascone F, Tripi S, Aragona F, Di Gaetano G, Notarbartolo A. Percutaneous liver biopsy: a safe outpatient procedure? Digestion. 2001;63:55–60. doi: 10.1159/000051873. [DOI] [PubMed] [Google Scholar]
- 15.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 17.de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 18.de Lédinghen V, Barreiro P, Foucher J, Labarga P, Castéra L, Vispo ME, Bernard PH, Martin-Carbonero L, Neau D, García-Gascó P, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat. 2008;15:427–433. doi: 10.1111/j.1365-2893.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 20.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 21.Macías J, Girón-González JA, González-Serrano M, Merino D, Cano P, Mira JA, Arizcorreta-Yarza A, Ruíz-Morales J, Lomas-Cabeza JM, García-García JA, et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut. 2006;55:409–414. doi: 10.1136/gut.2005.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Sierra C, Arizcorreta A, Díaz F, Roldán R, Martín-Herrera L, Pérez-Guzmán E, Girón-González JA. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36:491–498. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 24.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 25.Deng LP, Gui XE, Zhang YX, Gao SC, Yang RR. Impact of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. World J Gastroenterol. 2009;15:996–1003. doi: 10.3748/wjg.15.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 27.Pineda JA, García-García JA, Aguilar-Guisado M, Ríos-Villegas MJ, Ruiz-Morales J, Rivero A, del Valle J, Luque R, Rodríguez-Baño J, González-Serrano M, et al. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622–630. doi: 10.1002/hep.21757. [DOI] [PubMed] [Google Scholar]
- 28.Berenguer J, Bellón JM, Miralles P, Alvarez E, Sánchez-Conde M, Cosín J, López JC, Alvarez F, Catalán P, Resino S. Identification of liver fibrosis in HIV/HCV-coinfected patients using a simple predictive model based on routine laboratory data. J Viral Hepat. 2007;14:859–869. doi: 10.1111/j.1365-2893.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 29.Halfon P, Pénaranda G, Carrat F, Bedossa P, Bourlière M, Ouzan D, Renou C, Tran A, Rosenthal E, Wartelle C, et al. Influence of insulin resistance on hepatic fibrosis and steatosis in hepatitis C virus (HCV) mono-infected compared with HIV-HCV co-infected patients. Aliment Pharmacol Ther. 2009;30:61–70. doi: 10.1111/j.1365-2036.2009.03995.x. [DOI] [PubMed] [Google Scholar]
- 30.Blanco F, Barreiro P, Ryan P, Vispo E, Martín-Carbonero L, Tuma P, Labarga P, Medrano J, González-Lahoz J, Soriano V. Risk factors for advanced liver fibrosis in HIV-infected individuals: role of antiretroviral drugs and insulin resistance. J Viral Hepat. 2010:Epub ahead of print. doi: 10.1111/j.1365-2893.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 31.Soriano V, Maida I, Núñez M, García-Samaniego J, Barreiro P, Martín-Carbonero L, González-Lahoz J. Long-term follow-up of HIV-infected patients with chronic hepatitis C virus infection treated with interferon-based therapies. Antivir Ther. 2004;9:987–992. doi: 10.1177/135965350400900616. [DOI] [PubMed] [Google Scholar]
- 32.Berenguer J, Alvarez-Pellicer J, Martín PM, López-Aldeguer J, Von-Wichmann MA, Quereda C, Mallolas J, Sanz J, Tural C, Bellón JM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 33.Soriano V, Labarga P, Ruiz-Sancho A, Garcia-Samaniego J, Barreiro P. Regression of liver fibrosis in hepatitis C virus/HIV-co-infected patients after treatment with pegylated interferon plus ribavirin. AIDS. 2006;20:2225–2227. doi: 10.1097/01.aids.0000247583.38943.95. [DOI] [PubMed] [Google Scholar]
- 34.Sterling RK, Contos MJ, Smith PG, Stravitz RT, Luketic VA, Fuchs M, Shiffman ML, Sanyal AJ. Steatohepatitis: Risk factors and impact on disease severity in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2008;47:1118–1127. doi: 10.1002/hep.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, Chaplinskas S, Peters L, Karlsson A, Katlama C, Toro C, et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337–1344. doi: 10.1086/592171. [DOI] [PubMed] [Google Scholar]