Abstract
AIM: To clarify the clinical significance of high serum IgE in autoimmune pancreatitis (AIP).
METHODS: Forty-two AIP patients, whose IgE was measured before steroid treatment, were analyzed. To evaluate the relationship between IgE levels and the disease activity of AIP, we examined (1) Frequency of high IgE (> 170 IU/mL) and concomitant allergic diseases requiring treatment; (2) Correlations between IgG, IgG4, and IgE; (3) Relationship between the presence of extrapancreatic lesions and IgE; (4) Relationship between clinical relapse and IgE in patients treated with steroids, and (5) Transition of IgE before and after steroid treatment.
RESULTS: IgE was elevated in 36/42 (86%) patients. Concomitant allergic disease was observed in seven patients (allergic rhinitis in three, bronchial asthma in three, and urticaria in one). There were no significant correlations between IgG, IgG4, and IgE (r = -0.168 for IgG, and r = -0.188 for IgG4). There was no significant difference in IgE in the patients with and without extrapancreatic lesions (526 ± 531 IU/mL vs 819 ± 768 IU/mL, P = 0.163), with and without clinical relapse (457 ± 346 IU/mL vs 784 ± 786 IU/mL, P = 0.374). There was no significant difference in IgE between before and after steroid treatment (723 ± 744 IU/mL vs 673 ± 660 IU/mL, P = 0.633).
CONCLUSION: Although IgE does not necessarily reflect the disease activity, IgE might be useful for the diagnosis of AIP in an inactive stage.
Keywords: IgE, IgG4, IgG, Autoimmune pancreatitis
INTRODUCTION
Autoimmune pancreatitis (AIP) is a unique, benign pancreatic disease characterized by irregular narrowing of the pancreatic duct, swelling of the pancreas, lymphoplasmacytic infiltration and fibrosis, and favorable response to steroid therapy[1-8]. Serologically, elevation of IgG and IgG4 is the most remarkable characteristic in this disease[9-12]. A recent study showed that IgM and IgA were decreased in AIP[13]. There has been no detailed clinical analysis of IgE, although some clinicians have noted elevated serum IgE in AIP or IgG4-related diseases[14-17].
In most allergic diseases, total serum IgE levels do not reflect disease activity; however, in allergic bronchopulmonary aspergillosis, it is reported that IgE is a useful marker for therapeutic monitoring[18-21]. The expression of T-helper type 2 (Th2) cytokines [interleukin (IL)-4, IL-5, and IL-13] are upregulated in the affected tissues of AIP[22]. Both IgG4 and IgE production are dependent on help by Th2; therefore, all IgG4-inducing antigens are also efficient IgE inducers[23]. As IgG4 reflects the disease activity of AIP[12], it is reasonable to expect that IgE is also related to the disease activity of AIP and could become a clinically useful marker. Thus, we decided to clarify whether IgE is related to the disease activity of AIP from various viewpoints.
MATERIALS AND METHODS
Patients
Between 1997 and 2009, 67 patients were diagnosed as having AIP at the University of Tokyo hospital and affiliated hospitals. All the patients fulfilled the diagnostic criteria of AIP proposed by the Mayo Clinic[3] or the revised criteria by the Japan Pancreas Society[4]. Serum IgE was measured in 48 patients before steroid treatment. As the method of measurement was different in six patients, these patients were excluded. Thus, 42 patients whose IgE level was measured by the same method before steroid treatment were enrolled in this study. Of the 42 patients, 33 were men and nine were women. The mean age of onset was 65 years old. Thirty-seven patients received steroid treatment. Prednisolone at an initial dose of 30-40 mg/d was administered for 2-4 wk in most cases. It was then tapered by 5 mg every 2-6 wk until 10 mg/d, and 2.5-7.5 mg/d was continued as maintenance therapy in principle.
This retrospective study was approved by the review board of our institute.
Methods
Serum IgE was measured by fluorescence enzyme immunoassay. To evaluate the relationship between IgE levels and disease activity, we examined (1) frequency of high IgE (> 170 IU/mL) and concomitant allergic diseases requiring treatment; (2) correlations among IgG, IgG4, and IgE; (3) relationship between the presence of extrapancreatic lesions and IgE; (4) relationship between clinical relapse and IgE in patients treated with steroids; and (5) transition of IgE before and after steroid treatment.
With regard to allergic diseases, only diseases that required treatment during follow-up were counted. There are many extrapancreatic lesions in AIP; however, in this study, only representative and definite lesions, including sclerosing cholangitis[7,8,24], retroperitoneal fibrosis[8,25], sclerosing sialadenitis[17], interstitial pneumonia[26], and tubulointerstitial nephritis[27] were counted. With regard to the number of extrapancreatic lesions, we used the number of extrapancreatic lesions that were observed when IgE was measured. We do not regard intrapancreatic biliary stricture as an extrapancreatic lesion, because it is influenced by pancreatic edema[24]. We defined “clinical relapse” as AIP-related symptomatic unfavorable events; i.e. obstructive jaundice from distal bile duct stenosis due to exacerbated pancreatitis with pancreatic swelling, increased levels of biliary enzymes caused by sclerosing cholangitis (in which extrapancreatic biliary strictures were confirmed on imaging findings), retroperitoneal fibrosis, interstitial pneumonia, and interstitial nephritis (for which simple observation seemed very inadequate and remission induction therapy was introduced). Concerning clinical relapses and IgE, we analyzed patients whose follow-up after the initiation of steroid treatment was more than 6 mo. With regard to the transition of IgE, IgE measured before steroid treatment and during maintenance steroid treatment (2.5-7.5 mg prednisolone/d) were compared.
Statistical analysis
Categorical variables were compared by the χ2 or Fisher exact test, where appropriate. Continuous variables were reported as mean ± SD and compared by the Student t test, Welch t test, or paired t test, where appropriate. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed by the statistical software JMP 7.0.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Frequency of high IgE and concomitant allergic diseases
The clinical profiles of 42 patients with AIP are summarized in Table 1. Serum IgE was elevated in 36/42 (86%). The average value of IgE was 679 ± 675 IU/mL (range, 67-3000 IU/mL). No patient had concomitant parasitosis. Concomitant allergic diseases were observed in seven patients, comprising allergic rhinitis in three, bronchial asthma in three, and urticaria in one. There was no significant difference between the average IgE values of these seven patients and those of the other 35 patients (970 ± 775 IU/mL vs 621 ± 650 IU/mL, P = 0.216). The frequency of high IgE was 100% (7/7) in these patients, and 63% (29/35) in the others; however, this difference was not statistically significant (P = 0.567).
Table 1.
Clinical profiles of 42 patients with autoimmune pancreatitis
| Patient | Sex | Age (yr) | IgE (< 171 U/mL) | IgG (870-1800 mg/dL) | IgG4 (< 135 mg/dL) | Total bilirubin (0.3-1.3 mg/dL) | Concomitant allergic diseases | Extrapancreatic lesion associated with AIP |
| 1 | F | 58 | 670 | 2542 | 592 | 0.6 | AR | - |
| 2 | M | 63 | 650 | 2055 | 691 | 3.0 | AR | - |
| 3 | F | 53 | 500 | 1527 | 143 | 7.9 | AR | SA |
| 4 | M | 64 | 490 | 2457 | 670 | 0.5 | BA | - |
| 5 | M | 56 | 1800 | 1712 | 436 | 0.5 | BA | - |
| 6 | F | 43 | 340 | 1036 | 223 | 0.9 | BA | - |
| 7 | M | 74 | 2339 | 1481 | 98 | 0.9 | Urticaria | RF |
| 8 | F | 70 | 480 | 2190 | 133 | 5.8 | - | - |
| 9 | M | 57 | 120 | 3793 | 1420 | 10.6 | - | RF |
| 10 | M | 55 | 480 | 1419 | 320 | 1.7 | - | - |
| 11 | M | 61 | 270 | 1878 | 410 | 0.4 | - | SC |
| 12 | M | 66 | 1200 | 1620 | 310 | 0.5 | - | SA |
| 13 | M | 79 | 170 | 1585 | 420 | 3.3 | - | SC |
| 14 | M | 73 | 290 | 1647 | 360 | 5.4 | - | - |
| 15 | M | 79 | 410 | 1404 | 554 | 0.7 | - | - |
| 16 | M | 76 | 1000 | 1728 | 65 | 14.1 | - | SC |
| 17 | F | 72 | 650 | 2384 | 1400 | 1.6 | - | SA |
| 18 | F | 65 | 190 | 1511 | 374 | 0.6 | - | - |
| 19 | M | 68 | 290 | 1656 | 253 | 2.0 | - | SC |
| 20 | M | 61 | 940 | 1448 | 578 | 1.6 | - | - |
| 21 | F | 61 | 640 | 2177 | 354 | 0.4 | - | - |
| 22 | M | 58 | 2289 | 1973 | 481 | 6.9 | - | - |
| 23 | M | 71 | 1915 | 2318 | 470 | 0.6 | - | - |
| 24 | M | 61 | 69 | 2215 | 974 | 0.5 | - | SA |
| 25 | M | 65 | 91 | 3032 | 1260 | 5.4 | - | SC, RF |
| 26 | M | 64 | 330 | 1730 | 361 | 0.4 | - | SC |
| 27 | M | 66 | 1330 | 1849 | 270 | 4.7 | - | - |
| 28 | F | 64 | 328 | 2898 | 456 | 12.8 | - | SC |
| 29 | M | 69 | 440 | 1875 | 270 | 8.7 | - | - |
| 30 | M | 73 | 70.3 | 2395 | 393 | 0.4 | - | RF |
| 31 | M | 67 | 660 | 1683 | 230 | 0.6 | - | - |
| 32 | M | 72 | 3000 | 1579 | 455 | 0.5 | - | - |
| 33 | M | 61 | 467 | 1301 | 331 | 2.9 | - | - |
| 34 | M | 40 | 320 | 1996 | 650 | 1.0 | - | RF |
| 35 | M | 62 | 480 | 1368 | 236 | 0.5 | - | SC, SA |
| 36 | M | 71 | 267 | 1827 | 458 | 1.3 | - | - |
| 37 | M | 59 | 625 | 1876 | 139 | 0.5 | - | RF |
| 38 | M | 73 | 302 | 2834 | 1800 | 0.5 | - | SA |
| 39 | F | 76 | 426 | 1458 | 543 | 0.9 | - | RF |
| 40 | M | 76 | 943 | 1840 | 431 | 0.7 | - | SC |
| 41 | M | 59 | 67 | 1338 | 140 | 0.7 | - | - |
| 42 | M | 64 | 198 | 1709 | 232 | 10.2 | - | - |
AIP: Autoimune pancreatitis; AR: Allergic rhinitis; BA: Bronchial asthma; SA: Sialadenitis; RF: Retroperitoneal fibrosis; SC: Sclerosing cholangitis.
Correlations between IgG, IgG4, and IgE
The values of IgG and IgG4, which were measured at the same time as IgE before steroid treatment, were used in this analysis. Elevation of IgG and IgG4 were observed in 20 (47%) and 39 (93%) patients, respectively. The correlation coefficient of IgG and IgE was -0.168 (not significant, P = 0.290). The correlation coefficient of IgG4 and IgE was -0.188 (not significant, P = 0.235). The correlation coefficient of IgG and IgG4 was 0.698, which was significant (P < 0.0001) (Table 2).
Table 2.
Correlations between IgG, IgG4, and IgE
| Correlation coefficient | P value | |
| IgG and IgE | -0.168 | 0.290 |
| IgG4 and IgE | -0.188 | 0.235 |
| IgG and IgG4 | 0.698 | < 0.0001 |
Relationship between the presence of extrapancreatic lesions and IgE
Extrapancreatic lesions were observed in 20 patients (48%). Two patients had two lesions, and 18 patients had one lesion. Sclerosing cholangitis, retroperitoneal fibrosis, and sclerosing sialadenitis, were observed in nine, seven, and six patients, respectively. The IgE levels of the patients with and without extrapancreatic lesions were compared, and the same analysis was performed for IgG and IgG4. The results are shown in Table 3. IgG tended to be related to the presence of extrapancreatic lesions, although statistical significance was not attained (P = 0.093). No such tendency existed for IgE.
Table 3.
Comparison of IgE, IgG, and IgG4 between patients with and without extrapancreatic lesions
| Patients with extrapancreatic lesions (n = 20) | Patients without extrapancreatic lesions (n = 22) | P value | |
| IgE (IU/mL) | 526 ± 531 | 819 ± 768 | 0.163 |
| IgG (mg/dL) | 2065 ± 644 | 1775 ± 396 | 0.093 |
| IgG4 (mg/dL) | 588 ± 505 | 392 ± 161 | 0.110 |
Relationship between clinical relapse and IgE in the patients treated with steroids
There were 33 patients whose follow-up period was more than 6 mo. The mean follow-up period was 52 mo (range, 8-141 mo). Clinical relapse was observed in five patients. The style of clinical relapse was pancreatitis in two, interstitial pneumonia in two, and sclerosing cholangitis in one. Their relapses occurred 16 mo after the initiation of steroid therapy on average (range, 3-26 mo). The mean follow-up period was the same between the patients with and without clinical relapse (53.0 mo vs 51.8 mo, P = 0.929). IgE levels of the patients with and without clinical relapse were compared, and the same analysis was performed for IgG and IgG4. The results are shown in Table 4. Neither IgE, IgG nor IgG4, were related to later clinical relapses.
Table 4.
Comparison of IgE, IgG, and IgG4 between patients with and without clinical relapses
| Patients with clinical relapses (n = 5) | Patients without clinical relapses (n = 28) | P value | |
| IgE (IU/mL) | 457 ± 346 | 784 ± 786 | 0.374 |
| IgG (mg/dL) | 1898 ± 424 | 1915 ± 579 | 0.953 |
| IgG4 (mg/dL) | 266 ± 157 | 558 ± 429 | 0.148 |
Transition of IgE before and after steroid treatment
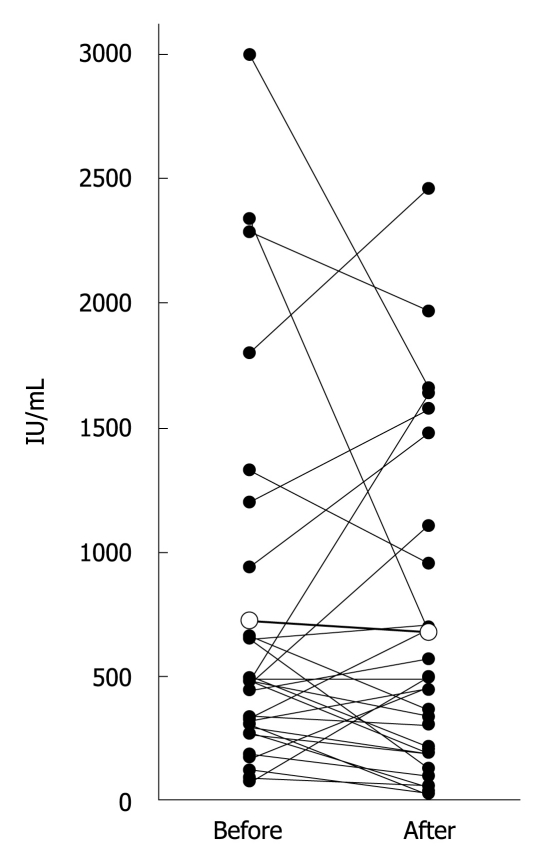
IgE measured before steroid treatment and during maintenance therapy could be compared in 29 patients (Figure 1). IgE increased in 10, and decreased in 18. There was no significant difference in IgE between before and after steroid treatment (723 ± 744 IU/mL vs 673 ± 660 IU/mL, P = 0.633). Abnormally high IgE (> 170 IU/mL) was observed in 90% (26/29) before steroid treatment, and in 79% (23/29) after steroid treatment (P = 0.470). By contrast, IgG and IgG4 did show significant differences before and after steroid treatment (Table 5).
Figure 1.
IgE levels measured before steroid treatment (Before) and during maintenance therapy (After) were compared in 29 patients. White circles show average values.
Table 5.
Transition of IgE, IgG, and IgG4 before and after steroid treatment
| Before | After | P value | |
| IgE (IU/mL) (n = 29) | 723 ± 744 | 673 ± 660 | 0.633 |
| Proportion of high IgE | 26/29 | 23/29 | 0.470 |
| IgG (mg/dL) (n = 30) | 1891 ± 566 | 1155 ± 315 | < 0.0001 |
| Proportion of high IgG | 14/30 | 1/30 | 0.0002 |
| IgG4 (mg/dL) (n = 28) | 557 ± 429 | 229 ± 112 | 0.0002 |
| Proportion of high IgG4 | 27/28 | 20/28 | 0.0248 |
Normal range, IgE: < 171 IU/mL, IgG: 870-1800 mg/dL, IgG: < 135 mg/dL.
DISCUSSION
High IgE in AIP has been frequently documented[14-17], but its frequency and clinical significance were unknown. Kamisawa et al[14] reported that elevation of IgE was observed in 34% (12/35) of patients, and that all the patients with high IgE had present and/or past histories of allergic diseases, although none of the patients with normal IgE had such histories. In the present study, the frequency of high IgE was surprisingly high at 86% (36/42), which might be equal to frequency of high IgG4 (73.3%-94.3%)[12]. On the other hand, unlike the previous report, there seemed no definite relationship between IgE and allergic diseases. It seemed unreasonable to count allergic diseases that occurred decades ago. In addition, it is difficult to accurately judge past histories of mild allergic diseases. Thus, we included only concomitant allergic diseases. For reference, there were at least eight patients who had past histories of allergic diseases, but no concomitant ones. Comparison between patients with (n = 15) and without (n = 27) present and/or past histories of allergic diseases showed no significant difference in mean IgE values (654 ± 605 IU/mL vs 693 ± 721 IU/mL, P = 0.860) and frequency of high IgE (93% vs 81%, P = 0.395); therefore, the presence of past allergic disease did not affect the results.
From the results shown in Table 3, IgE levels appear to be unrelated to disease activity from the viewpoint of extrapancreatic lesions. On the contrary, it is possible that high IgE is associated with lower disease activity, when considering the higher IgE levels in the group without extrapancreatic lesions, and the negative correlation coefficient of IgG (IgG4) and IgE. It is difficult to analyze the results shown in Table 4 because of the small number of patients with clinical relapses. Nevertheless, it is likely that high IgE is not a risk factor for later clinical relapses, especially considering the higher IgE levels in the group without clinical relapses. IgG4 seems a little high in the group without clinical relapses (Table 4), which is similar to previous reports[8,28].
It was of great interest whether IgE could become a useful marker for therapeutic monitoring in AIP, like IgG and IgG4. From the results shown in Table 5, we cannot help but conclude that IgE is not a useful marker. However, this phenomenon is not strange in other allergic diseases. For example, Gunnar et al[18] reported that steroid treatment did not alter IgE levels in patients with atopic dermatitis. Kumar et al[19] showed that changes in serum IgE are not related to severity of asthma or allergic rhinitis. Exceptionally, in allergic bronchopulmonary aspergillosis, it is reported that the response of IgE (35% or more reduction) to steroid treatment is a sensitive marker in the management[20].
Although IgE does not seem to reflect disease activity, we speculate that this feature might be useful for the diagnosis of inactive AIP. Indeed, three patients in the present series showed low IgG4 (65, 98, and 133 mg/dL) at the diagnosis, but all of them showed high IgE (1000, 2339 and 480 IU/mL). When patients with a past history suggestive of AIP, such as voluntarily improved jaundice, do not show high IgG and IgG4, IgE should be measured. If IgE is also low, the possibility that their diagnosis is AIP will be low. If IgE is high, it might indicate AIP in an inactive stage.
In summary, the elevation of serum IgE is very frequent in AIP. It is also observed even in patients without other allergic diseases. IgE might not reflect the disease activity; however, it might be useful for the diagnosis of AIP in an inactive stage.
COMMENTS
Background
It is known that elevation of serum IgE is frequently observed in autoimmune pancreatitis (AIP). However, its clinical significance has not yet been clarified.
Research frontiers
This study demonstrated the frequency of high IgE in AIP, and investigated whether IgE is related to the presence of extrapancreatic lesions and later clinical relapses. In addition, the transition of IgE before and after steroid treatment was investigated to confirm whether IgE can become a marker for therapeutic monitoring.
Innovations and breakthroughs
This study confirmed the high frequency of elevated serum IgE in AIP, although IgE does not seem to be related to the disease activity.
Applications
Measuring IgE might be useful for the diagnosis of AIP especially in an inactive stage.
Peer review
These data about IgE were not positive, which means that IgE is not considered as a useful marker for AIP. However, the author revealed that many (86%) AIP patients have IgE elevation and some AIP patients with low IgG4 have a high level of IgE. IgE should be considered as one of the supportive parameters for diagnosis of AIP and the authors succeeded in clarifying that.
Footnotes
Peer reviewers: Pete Muscarella, MD, Division of Gastrointestinal Surgery, The Ohio State University, N711 Doan Hall, 410 W. 10th Ave., Columbus, OH 43210, United States; Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan; Edward L Bradley III, MD, Professor of Surgery, Department of Clinical Science, Florida State University College of Medicine, 1600 Baywood Way, Sarasota, FL 34231, United States
S- Editor Wang JL L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Shimosegawa T, Kanno A. Autoimmune pancreatitis in Japan: overview and perspective. J Gastroenterol. 2009;44:503–517. doi: 10.1007/s00535-009-0054-6. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki K, Uchida K, Fukui T. Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol. 2008;43:409–418. doi: 10.1007/s00535-008-2190-9. [DOI] [PubMed] [Google Scholar]
- 3.Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016; quiz 934. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- 6.Hirano K, Fukushima N, Tada M, Isayama H, Mizuno S, Yamamoto K, Yashima Y, Yagioka H, Sasaki T, Kogure H, et al. Diagnostic utility of biopsy specimens for autoimmune pancreatitis. J Gastroenterol. 2009;44:765–773. doi: 10.1007/s00535-009-0052-8. [DOI] [PubMed] [Google Scholar]
- 7.Hirano K, Shiratori Y, Komatsu Y, Yamamoto N, Sasahira N, Toda N, Isayama H, Tada M, Tsujino T, Nakata R, et al. Involvement of the biliary system in autoimmune pancreatitis: a follow-up study. Clin Gastroenterol Hepatol. 2003;1:453–464. doi: 10.1016/s1542-3565(03)00221-0. [DOI] [PubMed] [Google Scholar]
- 8.Hirano K, Tada M, Isayama H, Yagioka H, Sasaki T, Kogure H, Nakai Y, Sasahira N, Tsujino T, Yoshida H, et al. Long-term prognosis of autoimmune pancreatitis with and without corticosteroid treatment. Gut. 2007;56:1719–1724. doi: 10.1136/gut.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 10.Hirano K, Komatsu Y, Yamamoto N, Nakai Y, Sasahira N, Toda N, Isayama H, Tada M, Kawabe T, Omata M. Pancreatic mass lesions associated with raised concentration of IgG4. Am J Gastroenterol. 2004;99:2038–2040. doi: 10.1111/j.1572-0241.2004.40215.x. [DOI] [PubMed] [Google Scholar]
- 11.Hirano K, Kawabe T, Yamamoto N, Nakai Y, Sasahira N, Tsujino T, Toda N, Isayama H, Tada M, Omata M. Serum IgG4 concentrations in pancreatic and biliary diseases. Clin Chim Acta. 2006;367:181–184. doi: 10.1016/j.cca.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Morselli-Labate AM, Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: A systematic literature review and meta-analysis. J Gastroenterol Hepatol. 2009;24:15–36. doi: 10.1111/j.1440-1746.2008.05676.x. [DOI] [PubMed] [Google Scholar]
- 13.Taguchi M, Kihara Y, Nagashio Y, Yamamoto M, Otsuki M, Harada M. Decreased production of immunoglobulin M and A in autoimmune pancreatitis. J Gastroenterol. 2009;44:1133–1139. doi: 10.1007/s00535-009-0106-y. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T, Anjiki H, Egawa N, Kubota N. Allergic manifestations in autoimmune pancreatitis. Eur J Gastroenterol Hepatol. 2009;21:1136–1139. doi: 10.1097/meg.0b013e3283297417. [DOI] [PubMed] [Google Scholar]
- 15.Miura H, Miyachi Y. IgG4-related retroperitoneal fibrosis and sclerosing cholangitis independent of autoimmune pancreatitis. A recurrent case after a 5-year history of spontaneous remission. JOP. 2009;10:432–437. [PubMed] [Google Scholar]
- 16.Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, Kasashima F. A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg. 2009;49:1264–1271; discussion 1271. doi: 10.1016/j.jvs.2008.11.072. [DOI] [PubMed] [Google Scholar]
- 17.Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, Takahashi H, Shinomura Y, Imai K, Saeki T, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 18.Gunnar S, Johansson O, Juhlin L. Immunoglobulin E in "healed" atopic dermatitis and after treatment with corticosteroids and azathioprine. Br J Dermatol. 1970;82:10–13. doi: 10.1111/j.1365-2133.1970.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar L, Newcomb RW, Hornbrook M. A year-round study of serum IgE levels in asthmatic children. J Allergy Clin Immunol. 1971;48:305–312. doi: 10.1016/0091-6749(71)90032-7. [DOI] [PubMed] [Google Scholar]
- 20.Ricketti AJ, Greenberger PA, Patterson R. Serum IgE as an important aid in management of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1984;74:68–71. doi: 10.1016/0091-6749(84)90089-7. [DOI] [PubMed] [Google Scholar]
- 21.Pien GC, Orange JS. Evaluation and clinical interpretation of hypergammaglobulinemia E: differentiating atopy from immunodeficiency. Ann Allergy Asthma Immunol. 2008;100:392–395. doi: 10.1016/S1081-1206(10)60605-9. [DOI] [PubMed] [Google Scholar]
- 22.Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–1546. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]
- 23.Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–477. doi: 10.1111/j.1365-2222.2009.03207.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirano K, Tada M, Isayama H, Yamamoto K, Mizuno S, Yagioka H, Yashima Y, Sasaki T, Kogure H, Togawa O, et al. Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc. 2010;71:85–90. doi: 10.1016/j.gie.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, Nakazawa K, Shimojo H, Kiyosawa K. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 26.Hirano K, Kawabe T, Komatsu Y, Matsubara S, Togawa O, Arizumi T, Yamamoto N, Nakai Y, Sasahira N, Tsujino T, et al. High-rate pulmonary involvement in autoimmune pancreatitis. Intern Med J. 2006;36:58–61. doi: 10.1111/j.1445-5994.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 27.Nishi H, Tojo A, Onozato ML, Jimbo R, Nangaku M, Uozaki H, Hirano K, Isayama H, Omata M, Kaname S, et al. Anti-carbonic anhydrase II antibody in autoimmune pancreatitis and tubulointerstitial nephritis. Nephrol Dial Transplant. 2007;22:1273–1275. doi: 10.1093/ndt/gfl672. [DOI] [PubMed] [Google Scholar]
- 28.Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Miyabe K, Yoshida M, Sano H, et al. Clinical significance of extrapancreatic lesions in autoimmune pancreatitis. Pancreas. 2010;39:e1–e5. doi: 10.1097/MPA.0b013e3181bd64a1. [DOI] [PubMed] [Google Scholar]