Abstract
α-Synuclein function is thought to be related to its membrane binding ability. Solution NMR studies have identified several α-synuclein-membrane interaction modes in small unilamellar vesicles (SUVs), but how membrane properties affect binding remains unclear. Here, we use 19F NMR to study α-synuclein-membrane interactions by using 3-fluoro-L-tyrosine (3FY) and trifluoromethyl-L-phenylalanine (tfmF) labeled proteins. Our results indicate that the affinity is affected by both the head group and the acyl chain of the SUV. Negatively charged head groups have higher affinity, but different head groups with the same charge also affect binding. We show that the saturation of the acyl chain has a dramatic effect on the α-synuclein-membrane interactions by studying lipids with the same head group but different chains. Taken together, the data show that α-synuclein's N-terminal region is the most important determinate of SUV binding, but its C-terminal region also modulates the interactions. Our data support the existence of multiple tight phospholipid-binding modes, a result incompatible with the model that α-synuclein lies solely on the membrane surface.
Keywords: binding, 19F NMR, membranes, α-synuclein
Introduction
α-Synuclein (Fig. 1) is a 140 amino-acid, intrinsically-disordered protein associated with Parkinson's disease and other neurodegenerative disorders1–8 whose function is hypothesized to involve its interaction with membranes.8–12 The protein binds lipids and anionic detergents through the seven imperfect, cationic, 11-amino acid repeats located in its N-terminal and hydrophobic regions.6–8,13–20 Electron paramagnetic resonance (EPR) data on its complex with small unilamellar vesicles (SUVs) suggest that the first ∼100 residues of the monomeric protein adopt an α-helical conformation that lies on the membrane surface.21–25 The last ∼40 residues lack defined structure and do not appear to be involved in membrane interactions.13,21 Recent solution NMR data, however, appear incompatible with this model. More specifically, 15N intensity and relaxation data from titration of SUVs sugget there exists several binding modes in which the first 25 residues adopt a helical state that anchor the interaction with SUVs.26,27
Figure 1.

The amino acid sequence of human α-synuclein. The first 11-amino acid repeat (underlined), the negatively charged amino acids (in blue), the positively charged amino acids (in red), and the four tyrosines (bolded) are indicated. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
A variety of other techniques, including circular dichroism spectropolarimetry,12,13,19,28 fluorescence spectroscopy,21,29–31 and EPR21–25 have been used to study how the phospholipid composition of vesicles affects α-synuclein affinity. Although these studies indicate that the properties of phospholipid membranes (i.e., charge, curvature, size, and the identity of the acyl chains) affect binding, there is no consensus, and some studies are contradictory.21,22,24–27 We show that 19F NMR is another useful technique for assessing α-synuclein-membrane interactions.
19F is a good reporter of conformational changes due to its sensitivity to the environment and the fact that few natural biological molecules contain fluorine.32–35 Additionally, adding a few fluorine atoms to a protein has a minimal effect on structure and dynamics.32,33,35,36 Furthermore, the methods listed above provide only the overall affinity of α-synuclein for membranes. 19F NMR detects α-synuclein-membrane interactions at the level of individual residues.
Here, we use 19F NMR as a probe to monitor systematically the binding of α-synuclein to SUVs with different head groups and acyl chains. There are four tyrosines in α-synuclein (Fig. 1). One is at position 39. The other three are near the C-terminus, at positions 125, 133, and 136. We substituted these tyrosines with either 3-fluoro-L-tyrosine (3FY) or trifluoromethyl-L-phenylalanine (tfmF). The 19F signal is detectable in the free state. Once the protein binds and exchanges slowly with the free state, the 19F signal is undetectable because the slow tumbling of the large SUV (∼20 nm diameter)37 broadens the resonance into the baseline. The decrease in signal corresponds to binding. We also combine site-directed mutagenesis with 19F NMR to determine which segment of α-synuclein binds to SUVs and to estimate binding affinities.
Results and Discussion
Binding of 3FY labeled α-synuclein to spherical micelles, rod-like micelles, and SUVs
SDS micelles are generally used as membrane mimics. At low salt concentrations, they form spherical micelles with diameter of ∼5 nm with highly curved surfaces.38 Rod-like micelles form at high salt concentrations.24,38
Three 19F peaks are observed from 3FY-labeled α-synuclein in buffer [Fig. 2(A)]. The middle peak is twice as large because the resonance from residues 39 and 125 overlap.32 In 200 mM SDS, all four 19F resonances are observed [Fig. 2(B)]. Upon binding micelles, the resonance from 3FY39 decreases and shifts from −59.9 ppm to −60.2 ppm. As shown in Figure 2(C), increasing the salt concentration to 250 mM24 broadens the position 39 resonance beyond detection, but the resonances from the three C-terminal residues remain unchanged. Increasing the temperature to 50°C, causes the 3FY39 resonance to reappear, although it is broad [Fig. 2(D)]. The resonances from the C-terminal residues shift to lower field due to strong temperature-sensitivity of 19F chemical shifts.
Figure 2.
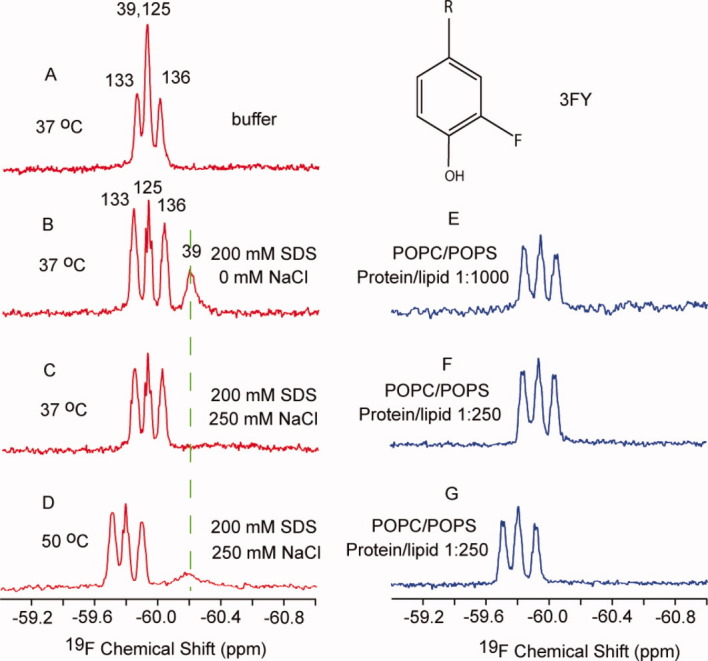
Spectra of 3FY-labeled α-synuclein in buffer (A), in the presence of spherical micelles (B), rod-like micelles (C and D), and small unilamillar vesicles (E–G). The assignments32 are indicated above the spectra. The protein concentration was 250 μM. The structure of the 3FY side chain is shown above panel E. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In contrast to SDS micelles, the 3FY39 resonance was barely observed in palmitoyl-oleoyl-phosphatidylcholine (POPC)/palmitoyl-oleoyl-phosphatidylserine (POPS) SUVs, even at 50°C [Fig. 2(E–G)]. The resonances from the C-terminal residues do not change at molar protein/lipid ratios of 1/250 and 1/1000 [Fig. 2(E–G)].
SDS binding induces a conformational change in α-synuclein.28 Our data [Fig. 2(B)] are consistent both with this conclusion and with conclusions based on 15N NMR data, which show that the N-terminal region of the protein binds SDS while the C-terminal region remains disordered.13,20,24,28,31,32
Solution NMR is a powerful tool for accessing processes that occur over a range of timescales.26 In 250 mM NaCl, SDS forms larger rod-like micelles, decreasing the tumbling rate of the α-synuclein-micelle complex. The slow tumbling results in the absence of a detectable resonance for residue 39 [Fig. 2(C)]. Increasing the exchange and tumbling rates by increasing the temperature facilitates detection of the resonance [Fig. 2(D)]. The 3FY39 resonance was not observed even at 50°C in SUVs [Fig. 2(E–G)] because tight binding leads to slower exchange and because the large SUVs tumble more slowly than micelles. The chemical shift of free 3FY (−59.6 ppm) is close to that observed for the C-terminal 3FY resonance region of the protein under all conditions, confirming that C-terminal region of α-synuclein is disordered. The data in Figure 2 show that the 3FY labeled protein provides important qualitative information, but quantification is difficult because of the overlap of the resonance from 3FY39 and 3FY125.
Influence of lipid head groups probed with tfmF labeled α-synuclein
To overcome the incomplete resolution of the 3FY resonances, we used an orthogonal t-RNA synthase system39 to label the protein specifically with tfmF at position 39 and then analyzed the influence of membrane charge on binding. As described previously,33 we used mass spectrometry to confirm the identity of the labeled protein. We prepared SUVs containing neutral (PC, PE) or negatively charged (PS, PG, and PA) head groups. Two samples with the same amount of 19F labeled α-synuclein were prepared, one with the desired SUV and one without it. After acquiring a 19F spectrum of 150-μM α-synuclein in buffer, the same parameters were used to acquire the spectrum with SUVs at a protein-to-lipid ratio of 1/100. The results are shown in Figure 3.
Figure 3.
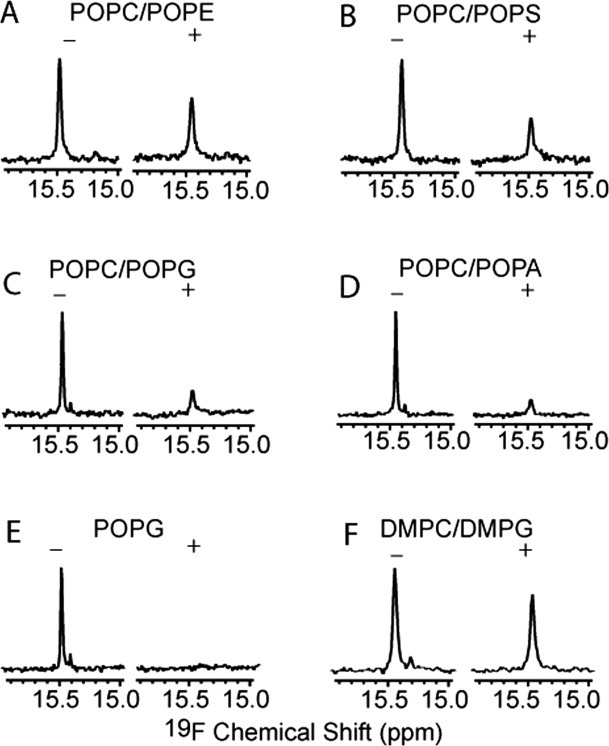
Spectra of α-synuclein with tfmF labeled at position 39 in the absence (−) and in the presence (+) of SUVs. The protein concentration was 150 μM. The molar ratio of protein to lipid was 0.01. The protein concentration is the same in all experiments. Day-to-day differences in shimming account for the small difference in width at half-height for the spectra acquired in the absence of lipids.
SUVs attenuate the tfmF resonance, but its chemical shift remains unchanged, indicating slow exchange between the free and bound states. The resonance from the bound state is broadened beyond detection because of the slow tumbling of the SUVs. The decrease in the area under the resonance corresponds to the bound population. Thus, comparing the decreases for different SUVs provides information about the affinity of α-synuclein for the vesicles.
In SUVs made from a 7:3 mixture of the zwitterionic lipids, POPC and palmitoyl-oleoyl-phosphatidylethanolamine (POPE), 62% of the original α-synuclein signal is observed, indicating that 38% is vesicle-associated [Figs. 3(A) and 4]. Changing POPE to a negatively charged lipid, increases the bound fraction to 50%, 70%, 75%, and 100% in 7:3 mixtures of POPC/POPS, POPC/palmitoyl-oleoyl-phosphatidylglycerol (POPG), POPC/palmitoyl-oleoyl-phosphatidic acid (POPA), and POPG alone [Fig. 3(B–E)]. The results are summarized in Figure 4.
Figure 4.
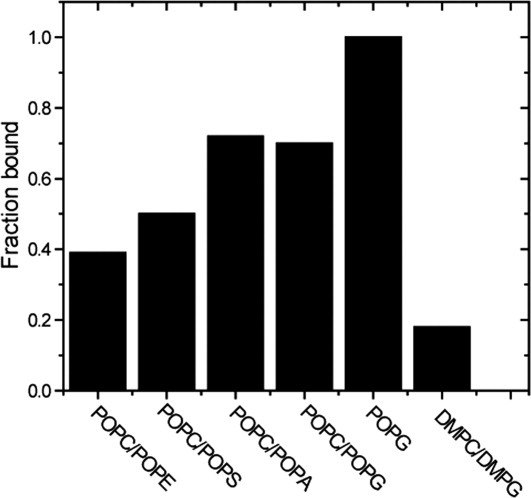
Histogram of the fraction of bound α-synuclein versus lipid composition. The typical uncertainty is ±0.05.
Conflicting results have been reported for the effect of head group charge on α-synuclein binding. Negatively charged head groups were reported to have a higher affinity in some studies,15,19,30,40 but not in others.41 Others report that α-synuclein binds weakly to phospholipids with neutral head groups, such as PC and PE.30,40 Our data show that α-synuclein prefers negatively charged phospholipids over neutral phospholipids. Nevertheless, the strength of α-synuclein-membrane interaction varies, even for acid phospholipids with the same charge, indicating that head group charge is not the only factor.30 We also find that α-synuclein prefers POPA-containing SUVs to POPS-containing SUVs, which agrees with earlier reports.15,19 This observation reinforces the idea that although electrostatic interaction plays a significant role, other types of interactions are also involved. The preference of α-synuclein for negatively charged lipids can be explained by the fact that N-terminal region of α-synuclein contains many positively charged residues (Fig. 1). The C-terminal region of the protein remains unstructured upon membrane binding because this region contains many negatively charged residues (Fig. 1).
Influence of the acyl chain on binding probed with tfmF labeled protein
We compared 19F data from SUVs made with PC and PG head groups containing unsaturated (POPC/POPG) or saturated dimyristoyl-phosphatidylcholine (DMPC)/dimyristoyl-phosphatidylglycerol (DMPG) acyl chains. The data [Fig. 3(C,F)] show that ∼70% of the α-synuclein binds POPC/POPG SUVs, but only ∼20% binds DMPC/DMPG SUVs.
The dramatic effect of acyl chain saturation indicates that hydrophobic interactions modulate α-synuclein binding. The experiments were performed at 37°C, where DMPC/DMPG and POPC/POPG SUVs are in the liquid crystalline phase. Neutron diffraction data show that in this phase DMPC/DMPG and POPC/POPG bilayer hydrophobic thicknesses are ∼26Å and ∼39Å, respectively.42 The presence of the double band in the POPC/POPG also makes the membrane more dynamic.43,44 In summary, increasing the hydrophobic thickness and dynamics of the acyl chain lead to higher affinity.
In the α-synuclein-membrane interaction model,21–25,31 ∼100 N-terminal residues lie on the membrane surface as an extended helix, and the remaining residues are disordered. Accordingly, the buried acyl chain should have little effect on α-synuclein interactions. This supposition, however, is inconsistent with our data, which agree with the conclusion of Bodner et al. that the first 25 residues adopt a helical structure state which anchors the interaction to SUVs.26,27 In that model, α-synuclein prefers SUV defects, which are affected by head group size, charge, and the hydrophobic thickness. The reduced binding of α-synuclein to DMPC/DMPG SUVs is due to the increase in the curvature that arises as a consequence of the acyl chain, which agree with the conclusion of Nuscher et al.45 As we observe, these properties affect α-synuclein-membrane interactions.
Identifying the region that binds SUVs by using tfmF as a probe
19F spectra of proteins labeled at positions 39 and 133 are shown in Figure 5. The tfmF 39 signal completely disappears in the presence of SUVs made from POPG, while the signal from tfmF 133 decreases only ∼30%. These data show that the N-terminal region interacts strongly with POPG SUVs while the C-terminal region is involved in weaker membrane interactions, a conclusion consistent with other 15N NMR studies.26,27
Figure 5.

Spectra of labeled α-synuclein in the absence (black) and presence of (red) POPG SUV. The protein concentration was 150 μM. The molar ratio of protein to lipid was 0.01. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Conclusions
Binding mainly involves the N-terminal region of α-synuclein, and both the head group and the acyl chain of phospholipids are important. Although the protein prefers negatively charged lipids, other properties, including the saturation of the lipid also affect α-synuclein membrane interactions. Hydrophobic thickness and acyl chain saturation dramatically affect protein-membrane interactions. Our data support the idea of multiple tight binding modes.26,27
19F labeling is well suited for monitoring protein-membrane interactions, and the method described here should be applicable to other membrane associated proteins. Using this approach, it is easy to quantify how much protein is membrane bound by comparing the signal intensity in the presence or in the absence of SUVs. In addition, site-specific labeling with tfmF provides a simple way to determine which protein segments bind the membrane.
Materials and Methods
3FY and tfmF labeled α-synuclein
The labeled proteins were prepared as described.32,33
SDS micelle-bound protein samples
Spherical micelles and rod-like micelles were prepared as described.24 3FY α-synuclein was added to solutions of SDS in 10 mM Na2HPO4 (pH 7.4) with or without 250 mM NaCl to a final protein concentration of 250 μM.
SUV-bound protein samples
POPC, POPE, POPS, POPG, POPA, DMPC, and DMPG were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. SUVs were prepared as described.25 Briefly, the lipids were weighed to produce the desired ratios and dissolved in CHCl3. The solvent was evaporated by using a gentle stream of N2 (g). The resulting film was dried overnight under vacuum. Dulbecco's phosphate buffered saline (DPBS, 1x free of calcium and magnesium ions 14190-144, GIBCO) was added to the container of lipid film. The sample was vortexed, incubated for 15 min, and then tip sonicated (2W) for 30 min. The sonicated sample was centrifuged for 20 min at 16 000g at room temperature. The supernatant was recovered and mixed with labeled α-synuclein. The final protein concentration was 150 μM.
NMR spectroscopy
19F spectra were acquired at 37°C on a Varian Inova 600-MHz spectrometer equipped with a 5 mm 19F z-gradient probe. The spectra comprised 512 transients, a 30 kHz sweep width, with a 2 s delay between transients. 19F chemical shifts are referenced to trifluoroethanol at 0 ppm.
Acknowledgments
The authors thank Ryan A. Mehl and Peter Lansbury for supplying the expression systems, Marc ter Horst for spectrometer maintenance, and Elizabeth Pielak for helpful comments on the manuscript.
Glossary
Abbreviations:
- DMPC
dimyristoyl-phosphatidylcholine
- DMPG
dimyristoyl-phosphatidylglycerol
- EPR
electron paramagnetic resonance
- 3FY
3-fluoro-L-tyrosine
- NMR
nuclear magnetic resonance
- POPA
palmitoyl-oleoyl-phosphatidic acid
- POPC
palmitoyl-oleoyl-phosphatidylcholine
- POPE
palmitoyl-oleoyl-phosphatidylethanolamine
- POPG
palmitoyl-oleoyl-phosphatidylglycerol
- POPS
palmitoyl-oleoyl-phosphatidylserine
- SDS
sodium dodecyl sulfate
- SUV
small unilamellar vesicle
- tfmF
trifluoromethyl-L-phenylalanine
References
- 1.Maroteaux L, Campanelli JT, Scheller RH. Synuclea neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DAC, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwai A, Masliah E, Yoshimoto M, Ge NF, Flanagan L, Desilva HAR, Kittel A, Saitoh T. The precursor protein of non-Aβ component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb PH, Zhen WG, Poon AW, Conway KA, Lansbury PT. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Bussell R, Eliezer D. A structural and functional role for 11-mer repeats in α-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 7.Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of α-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 8.Bussell R, Ramlall TF, Eliezer D. Helix periodicity, topology, and dynamics of membrane-associated α-synuclein. Protein Sci. 2005;14:862–872. doi: 10.1110/ps.041255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293:321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 10.Uversky VN, Gillespie JR, Fink AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins. 2000;41:415–427. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Jensen PH, Nielsen MS, Jakes R, Dotti G, Goedert M. Binding of α-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 12.Bussell R, Eliezer D. Effects of Parkinson's disease-linked mutations on the structure of lipid-associated α-synuclein. Biochemistry. 2004;43:4810–4818. doi: 10.1021/bi036135+. [DOI] [PubMed] [Google Scholar]
- 13.Chandra S, Chen XC, Rizo J, Jahn R, Südhof TC. A broken α-helix in folded α-synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 14.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 15.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 16.Schluter OM, Fornai F, Alessandri MG, Takamori S, Geppert M, Jahn R, Südhof TC. Role of α-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine-induced parkinsonism in mice. Neuroscience. 2003;118:985–1002. doi: 10.1016/s0306-4522(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 17.Jo EJ, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. α-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 18.McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of α-synuclein in intact neurons—effect of Parkinson's disease-linked mutations. J Biol Chem. 2000;275:8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 19.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human α-synuclein and Parkinson's disease variants with phospholipids—structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 20.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human α-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 21.Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound α-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci USA. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bortolus M, Tombolato F, Tessari I, Bisaglia M, Mammi S, Bubacco L, Ferrarini A, Maniero AL. Broken helix in vesicle and micelle-bound α-synucleinsights from site-directed spin labeling-EPR experiments and MD simulations. J Am Chem Soc. 2008;130:6690–6691. doi: 10.1021/ja8010429. [DOI] [PubMed] [Google Scholar]
- 23.Drescher M, Godschalk F, Veldhuis G, van Rooijen BD, Subramaniam V, Huber M. Spin-label EPR on α-synuclein reveals differences in the membrane binding affinity of the two antiparallel helices. ChemBioChem. 2008;9:2411–2416. doi: 10.1002/cbic.200800238. [DOI] [PubMed] [Google Scholar]
- 24.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound α-synuclein forms an extended helix: long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound α-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci USA. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodner CR, Dobson CM, Bax A. Multiple tight phospholipid-binding modes of α-synuclein revealed by solution NMR spectroscopy. J Mol Biol. 2009;390:775–790. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodner CR, Maltsev AS, Dobson CM, Bax A. Differential phospholipid binding of α-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy. Biochemistry. 2010;49:862–871. doi: 10.1021/bi901723p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreon ACM, Deniz AA. α-Synuclein multistate folding thermodynamics: implications for protein misfolding and aggregation. Biochemistry. 2007;46:4499–4509. doi: 10.1021/bi602461y. [DOI] [PubMed] [Google Scholar]
- 29.Lee JC, Langen R, Hummel PA, Gray HB, Winkler JR. α-Synuclein structures from fluorescence energy-transfer kinetics: implications for the role of the protein in Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:16466–16471. doi: 10.1073/pnas.0407307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhoades E, Ramlall TF, Webb WW, Eliezer D. Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreon ACM, Gambin Y, Lemke EA, Deniz AA. Interplay of α-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci USA. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CG, Lutz EA, Slade KM, Ruf RAS, Wang GF, Pielak GJ. 19F NMR studies of α-synuclein conformation and fibrillation. Biochemistry. 2009;48:8578–8584. doi: 10.1021/bi900872p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li CG, Wang GF, Wang YQ, Creager-Allen R, Lutz EA, Scronce H, Slade KM, Ruf RAS, Mehl RA, Pielak GJ. Protein 19F NMR in Escherichia coli. J Am Chem Soc. 2010;132:321–327. doi: 10.1021/ja907966n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eccleston JF, Molloy DP, Hinds MG, King RW, Feeney J. Conformational differences between complexes of elongation factor Tu studied by 19F-NMR spectroscopy. Eur J Biochem. 1993;218:1041–1047. doi: 10.1111/j.1432-1033.1993.tb18463.x. [DOI] [PubMed] [Google Scholar]
- 35.Danielson MA, Falke JJ. Use of 19F NMR to probe protein structure and conformational changes. Annu Rev Biophys Biomol Struct. 1996;25:163–195. doi: 10.1146/annurev.bb.25.060196.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frieden C, Hoeltzli SD, Ropson IJ. NMR and protein folding: equilibrium and stopped-flow studies. Protein Sci. 1993;2:2007–2014. doi: 10.1002/pro.5560021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lentz BR, Carpenter TJ, Alford DR. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987;26:5389–5397. doi: 10.1021/bi00391a026. [DOI] [PubMed] [Google Scholar]
- 38.Mazer NA, Benedek GB, Carey MC. An investigation of micellar phase of sodium dodecyl sulfate in aqueous sodium chloride solutions using quasielastic light scattering spectroscopy. J Phys Chem. 1976;80:1075–1085. [Google Scholar]
- 39.Hammill JT, Miyake-Stoner S, Hazen JL, Jackson JC, Mehl RA. Preparation of site-specifically labeled fluorinated proteins for 19F-NMR structural characterization. Nat Protoc. 2007;2:2601–2607. doi: 10.1038/nprot.2007.379. [DOI] [PubMed] [Google Scholar]
- 40.Zhu M, Li J, Fink AL. The association of α-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishnan M, Jensen PH, Marsh D. α-Synuclein association with phosphatidylglycerol probed by lipid spin labels. Biochemistry. 2003;42:12919–12926. doi: 10.1021/bi035048e. [DOI] [PubMed] [Google Scholar]
- 42.Cheng JTJ, Hale JD, Elliot M, Hancock REW, Straus SK. Effect of membrane composition on antimicrobial peptides aurein 2.2 and 2.3 from Australian Southern Bell frogs. Biophys J. 2009;96:552–565. doi: 10.1016/j.bpj.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ipsen JH, Mouritsen OG, Bloom M. Relationships between lipid membrane area, hydrophobic thickness, and acyl-chain orientational order. The effects of cholesterol. Biophys J. 1990;57:405–412. doi: 10.1016/S0006-3495(90)82557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooijen BD, Claessens MMAE, Subramaniam V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim Biophys Acta. 2009;1788:1271–1278. doi: 10.1016/j.bbamem.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Nuscher B, Kamp F, Mehnert T, Odoy S, Haass C, Kahle PJ, Beyer K. α-Synuclein has a high affinity for packing defects in a bilayer membrane - a thermodynamics study. J Biol Chem. 2004;279:21966–21975. doi: 10.1074/jbc.M401076200. [DOI] [PubMed] [Google Scholar]


