Abstract
Hepatic glucose production (HGP) plays a vital role in maintaining the supply of glucose to the body, and transcription factor FoxO1 is known to confer hormone responsiveness onto HGP. Mice with a liver-specific FoxO1 deletion (L-FoxO1) show reduced HGP and reduced expression of glucose production genes. To determine the contribution of additional transcription factors to HGP, we created double and triple liver-specific knock-outs lacking FoxO1, FoxO3, and FoxO4 or the related protein FoxA2. We show that, when compared with single knock-out of FoxO1, triple ablation of FoxO genes causes more pronounced fasting hypoglycemia, increased glucose tolerance, and enhanced insulin sensitivity, with decreased plasma insulin levels. In contrast, combined ablation of FoxO1 and FoxA2 phenocopied the single knock-out of FoxO1. These data indicate that FoxOs work in concert to regulate multiple aspects of hepatic glucose metabolism.
Keywords: Diabetes, Gluconeogenesis, Glucose Metabolism, Insulin, Liver
Introduction
Blood glucose levels are maintained within a narrow range under physiological circumstances, partly by the action of hormones to stimulate or inhibit glucose production, which occurs mainly in liver (1). Hepatic glucose production (HGP)2 is a combination of gluconeogenesis and glycogenolysis, and although it is generally accepted that HGP is high in patients with diabetes, the physiology of this abnormality remains disputed (2–4).
FoxO1 is a transcription factor that confers hormone sensitivity onto HGP (5). In hepatocytes, FoxO1 interacts with coactivator Pgc1α to promote the expression of gluconeogenic and glycogenolytic genes including glucose-6-phosphatase (6). This activity is blocked by insulin through the PI 3-kinase-Akt pathway, wherein phosphorylation of FoxO1 by Akt causes its nuclear exclusion and inactivation (7). Fittingly, FoxO1 haplo-insufficiency or hepatic expression of dominant negative FoxO1 in mice reduces glucogenetic gene expression and rescues the diabetic phenotype of insulin resistance (8, 9). We have shown that mice with a liver-specific knock-out of FoxO1 (L-FoxO1) display reduced glucogenetic gene expression, improved glucose tolerance, and reduced HGP in hyperinsulinemic clamps (10). Moreover, hepatic FoxO1 ablation in mice lacking either the insulin receptor or the insulin receptor substrates IRS1 and IRS2 rescues the diabetic phenotypes of these mice (10, 11).
L-FoxO1 mice demonstrate that FoxO1 plays a physiological role in the regulation of HGP by hormones. It remains unclear whether residual HGP in L-FoxO1 mice reflects non-hormonal (basal) glucose turnover or whether there is another hormone-regulated component that is dependent on additional transcription factors. Candidates include Crtc2 (Torc2), FoxA2, and other FoxOs (12, 13). Although ablations of transcription factors such as C/Ebpα or Fxr cause low HGP, these proteins are not regulated by cAMP or insulin signaling and appear to control basal, as opposed to hormone-dependent, rates of HGP (14, 15).
There are three additional FoxO proteins in mammals: FoxO3, FoxO4, and FoxO6. FoxO3 and FoxO4 both contain the same conserved Akt phosphorylation sites as FoxO1, and all three of these are found in liver (16–19). FoxO6 is regulated differently and is expressed primarily in brain (20). FoxOs share a target consensus sequence and some overlapping functions (21), although some functions appear to be unique (22). Notably, FoxO3- and FoxO4-null mice are viable, but FoxO1-null mice die in embryogenesis (23).
Given the recent demonstration that hepatic Crtc2 ablation does not affect HGP (24), in this study, we focused on the contribution of FoxO3, FoxO4, and FoxA2 to HGP. We crossed L-FoxO1 mice with those bearing FoxO3lox/lox and 4lox/lox alleles (21) to create double and triple liver-specific FoxO knock-outs. We monitored glucose metabolic parameters, including glucose and insulin levels, glucose tolerance, and gene expression. We found that ablation of all three FoxOs caused considerably greater defects in glucose metabolism, beyond those in L-FoxO1 mice, whereas FoxA2 had no apparent additional effect.
EXPERIMENTAL PROCEDURES
Mice
L-FoxO1 mice and FoxO3lox/lox, FoxO4lox/lox, and FoxA2lox/lox mice have been described (10, 21, 25). Mice were fed a standard chow diet, and only males were studied. The Columbia University Institutional Animal Care and Use Committee approved all experiments.
Metabolic Tests
Glucose monitor and strips were OneTouch products (LifeScan, Inc., Milpitas, CA). Intraperitoneal glucose tolerance tests (GTTs) were performed in adults as described, using glucose at 2 g/kg (8). Insulin was measured by ELISA (Millipore, Billerica, MA).
Gene Expression
We isolated RNA using TRIzol (Invitrogen), synthesized cDNA using qScript (Quanta BioSciences, Gaithersburg, MD), and performed qPCR using SYBR Green (New England Biolabs). Genes were normalized to 18 S. Primer sequences are available by request.
Statistical Analysis
Data are presented as mean ± S.E. and were analyzed by two-tailed Student's t tests or one-way analysis of variance followed by post hoc Tukey tests, where appropriate.
RESULTS
Absence of Compensatory Changes in FoxO Isoform Expression in Single Hepatic FoxO Knock-outs
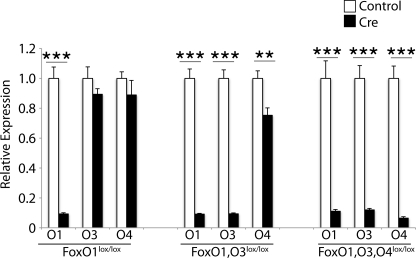
We crossed L-FoxO1 mice with mice bearing FoxO3lox/lox and FoxO4lox/lox (21) to create triple (L-FoxO1,O3,O4), as well as double (L-FoxO1,O3), liver-specific knock-outs. L-FoxO1,O3,O4 mice were born at lower than expected Mendelian ratios. Mating mice homozygous for all three “floxed” FoxO alleles, where one parent contained a single copy of α1 antitrypsin-Cre, yielded 40% L-FoxO1,O3,O4 pups (54 out of 136) instead of the expected 50% (p < 0.01 by χ2 test). Surviving L-FoxO1,O3,O4 mice, as well as L-FoxO1,O3 mice, which were born at expected ratios, had no gross abnormalities, nor differences in body weight, when compared with littermate controls.
To identify any compensatory changes in FoxO expression due to deletion of other family members, we examined FoxO mRNAs. Knock-outs showed >90% reduced expression of the expected mRNAs (Fig. 1), without changes in FoxO3 or FoxO4 expression in L-FoxO1 mice when compared with controls. In L-FoxO1,O3 mice, FoxO4 was slightly decreased.
FIGURE 1.
FoxO expression in single and compound knock-out mice. Mice were fasted for 5 h prior to sacrifice, and livers were analyzed by qPCR (n = 6–7). Values shown are relative to same strain littermate controls. **, p < 0.01, ***, p < 0.001.
Regulation of Fasting Glycemia in Combined FoxO Knock-outs
L-FoxO1 mice have a defect in HGP initiation at birth, when pups are first required to maintain their own glycemia, by inducing gluconeogenesis and glycogenolysis (26); this manifests as fasting hypoglycemia (10). We found that L-FoxO1 mice, as reported (10), have lower glucose at postnatal day 2 when compared with littermate controls (Fig. 2A). L-FoxO1,O3 mice showed a similar phenotype. L-FoxO1,O3,O4 mice had a more profound defect in postnatal HGP, with 58% lower glucose when compared with littermate controls (p < 0.001). We noted differences in glucose and insulin levels between control groups from different strains; these are likely due to the mixed genetic backgrounds, which are known to affect glucose metabolism and insulin levels (27, 28). However, even considering the genetic contribution from background strain, it is clear that triple FoxO ablation causes hypoglycemia to an extent not seen in any of the single knock-outs.
FIGURE 2.
Glucose metabolism. A, glucose at postnatal day 2 (n = 4–16). B, serum insulin during ad libitum feeding or after a 5-h fast (n = 6–21). C, glucose tolerance tests (n = 9–18/group). The mean values of all control mice are shown here. D, area under the curve, relative to same strain littermate controls. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
Single and compound FoxO knock-outs also showed relative hypoglycemia as adults (p < 0.05). However, this phenotype was extremely variable because of potential extrahepatic contributions to this process and genetic background effects.
FoxO Ablation Improves Insulin Sensitivity
We measured ad libitum fed and 5-h fasted insulin levels (Fig. 2B). L-FoxO1 mice showed a trend toward reduced fed insulin, and L-FoxO1,O3 mice showed a magnification of this phenotype, with fed insulin around half that of littermate controls. L-FoxO1,O3,O4 mice had even more dramatically reduced insulin, 66% lower than controls (p < 0.01), and also had lower insulin after a 5-h fast (p < 0.05). Notably, although all other control and knock-out strains showed higher insulin during ad libitum feeding than after a 5-h fast, L-FoxO1,O3,O4 mice appear to have minimal differences in insulin between fed and fasted states. This suggests exquisite insulin sensitivity.
The Three FoxOs Regulate Glucose Excursions during GTT
We found that, as reported (10), L-FoxO1 mice have improved glucose tolerance, with an 18% decrease in area under the curve (AUC) (p < 0.01) (Fig. 2, C and D). The magnitude of this phenotype was doubled in L-FoxO1,O3,O4 mice, with a 37% decrease in AUC when compared with controls (p < 0.0001). L-FoxO1,O3 mice showed an intermediate phenotype. To directly compare knock-out strains, we calculated the AUC for each mouse, relative to the mean AUC of same strain littermates. Analysis by one-way analysis of variance revealed that L-FoxO1,O3,O4 mice have improved glucose tolerance when compared with both L-FoxO1 and L-FoxO1,O3 (Fig. 2D).
FoxA2 Ablation Fails to Exacerbate the Phenotype of L-FoxO1
It has been suggested that transcription factor FoxA2 is regulated similarly to FoxO1 (29) and that it has a role in HGP (12, 30), although mice lacking hepatic FoxA2 have no defects in glucose metabolism (25). To determine whether FoxA2 contributed to HGP similarly to FoxOs, we crossed L-FoxO1 mice with those bearing FoxA2lox/lox alleles (25). Unlike L-FoxO1,O3,O4 mice, however, L-FoxO1,A2 mice showed no difference in GTT when compared with L-FoxO1 (Fig. 2D). These mice also showed no difference in insulin levels (Fig. 2B), indicating no enhancement of insulin sensitivity. These data confirm that FoxA2 does not contribute significantly to HGP.
Gene Expression Studies
Next, we measured the expression of genes involved in glucose homeostasis (Fig. 3). Glucose-6-phosphatase (encoded by G6pc), phosphoenolpyruvate carboxykinase (encoded by Pck1), and insulin-like growth factor-binding protein-1 (encoded by Igfbp1) are reported FoxO1 targets, and we found them down-regulated in L-FoxO1 mice, as reported (10). However, these genes were not further down-regulated in double and triple FoxO knock-outs, and in some cases, these genes were expressed at normal levels. Liver pyruvate kinase (encoded by Pklr) was also normal in all three strains, as was pyruvate dehydrogenase kinase-4 (encoded by Pdk4), although it showed a trend toward up-regulation. In contrast, glucokinase (encoded by Gck) was slightly elevated in L-FoxO1 and L-FoxO1,O3 mice and was induced 3-fold in L-FoxO1,O3,O4 mice, suggesting that the further reduction in glucose output in these mice is due to a combination of reduced gluconeogenesis and rapid metabolism of glucose into glucose-6-phosphate.
FIGURE 3.
Gene expression. mRNA was extracted from livers of mice fasted for 5 h. qPCR analyses were performed with n = 6–7/group, and values shown are relative to same strain controls. **, p < 0.01 versus same strain controls.
DISCUSSION
The ability of FoxO1 to promote HGP through increased expression of glucogenetic genes has been confirmed extensively (5, 6, 8–11, 31). FoxO3 and FoxO4, however, have not previously been reported to be involved in this pathway, and no glucose metabolism defects have been described in either FoxO3−/− or FoxO4−/− mice (23). Thus the discovery of their role in HGP was somewhat unexpected. Furthermore, FoxO4 is X-linked, and our studies were limited to males, indicating that even a single FoxO allele is sufficient to sustain FoxO activity. This intimates a multipartite system of redundancy to ensure normal HGP.
Our observations reveal that the most profound effects occur with knock-out of all three FoxOs, similar to other FoxO effects (21). However, we have not studied FoxO1,O4 double knockouts; thus we cannot rule out that the products of these two genes predominate over glucose homeostasis.
It is also interesting that compound FoxO knock-outs do not necessarily display further decreases in canonical FoxO1 targets. Instead, these mice demonstrate that some targets, such as G6pc and Igfbp1, may be preferentially regulated by FoxO1, whereas other targets that contribute to glucose homeostasis are regulated redundantly by multiple FoxOs. Gck is regulated by insulin through an unclear mechanism (32), although FoxO1 has been suggested as a repressor (33, 34). Our observation that Gck is up-regulated in L-FoxO1,O3,O4 mice supports the possibility that FoxOs act redundantly to maintain its levels or that Gck may be a target of FoxO4 specifically.
Acknowledgments
We thank Dr. R. A. DePinho for providing mice and Ana Flete for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants P01HL87123, R01DK57539, and P30DK63608 (to D. A.), and T32DK07328 (to R. A. H.).
- HGP
- hepatic glucose production
- GTT
- glucose tolerance test
- qPCR
- quantitative PCR
- AUC
- area under the curve.
REFERENCES
- 1.Cherrington A. D. (1999) Diabetes 48, 1198–1214 [DOI] [PubMed] [Google Scholar]
- 2.Nuttall F. Q., Ngo A., Gannon M. C. (2008) Diabetes Metab. Res. Rev. 24, 438–458 [DOI] [PubMed] [Google Scholar]
- 3.Barrett E. J. (2003) J. Clin. Invest. 111, 434–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherrington A. D. (2005) J. Clin. Invest. 115, 1136–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakae J., Kitamura T., Silver D. L., Accili D. (2001) J. Clin. Invest. 108, 1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. (2003) Nature 423, 550–555 [DOI] [PubMed] [Google Scholar]
- 7.Nakae J., Park B. C., Accili D. (1999) J. Biol. Chem. 274, 15982–15985 [DOI] [PubMed] [Google Scholar]
- 8.Nakae J., Biggs W. H., 3rd, Kitamura T., Cavenee W. K., Wright C. V., Arden K. C., Accili D. (2002) Nat. Genet. 32, 245–253 [DOI] [PubMed] [Google Scholar]
- 9.Altomonte J., Richter A., Harbaran S., Suriawinata J., Nakae J., Thung S. N., Meseck M., Accili D., Dong H. (2003) Am. J. Physiol. Endocrinol. Metab. 285, E718–E728 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M., Pocai A., Rossetti L., Depinho R. A., Accili D. (2007) Cell Metab. 6, 208–216 [DOI] [PubMed] [Google Scholar]
- 11.Dong X. C., Copps K. D., Guo S., Li Y., Kollipara R., DePinho R. A., White M. F. (2008) Cell Metab. 8, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfrum C., Asilmaz E., Luca E., Friedman J. M., Stoffel M. (2004) Nature 432, 1027–1032 [DOI] [PubMed] [Google Scholar]
- 13.Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 14.Ma K., Saha P. K., Chan L., Moore D. D. (2006) J. Clin. Invest. 116, 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N. D., Finegold M. J., Bradley A., Ou C. N., Abdelsayed S. V., Wilde M. D., Taylor L. R., Wilson D. R., Darlington G. J. (1995) Science 269, 1108–1112 [DOI] [PubMed] [Google Scholar]
- 16.Furuyama T., Nakazawa T., Nakano I., Mori N. (2000) Biochem. J. 349, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W. H., 3rd, Wright C. V., White M. F., Arden K. C., Accili D. (2002) J. Clin. Invest. 110, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 19.Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. (1999) Nature 398, 630–634 [DOI] [PubMed] [Google Scholar]
- 20.Jacobs F. M., van der Heide L. P., Wijchers P. J., Burbach J. P., Hoekman M. F., Smidt M. P. (2003) J. Biol. Chem. 278, 35959–35967 [DOI] [PubMed] [Google Scholar]
- 21.Paik J. H., Kollipara R., Chu G., Ji H., Xiao Y., Ding Z., Miao L., Tothova Z., Horner J. W., Carrasco D. R., Jiang S., Gilliland D. G., Chin L., Wong W. H., Castrillon D. H., DePinho R. A. (2007) Cell 128, 309–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arden K. C. (2008) Oncogene 27, 2345–2350 [DOI] [PubMed] [Google Scholar]
- 23.Hosaka T., Biggs W. H., 3rd, Tieu D., Boyer A. D., Varki N. M., Cavenee W. K., Arden K. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Lay J., Tuteja G., White P., Dhir R., Ahima R., Kaestner K. H. (2009) Cell Metab. 10, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sund N. J., Ang S. L., Sackett S. D., Shen W., Daigle N., Magnuson M. A., Kaestner K. H. (2000) Mol. Cell. Biol. 20, 5175–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hume R., Burchell A., Williams F. L., Koh D. K. (2005) Early Hum. Dev. 81, 95–101 [DOI] [PubMed] [Google Scholar]
- 27.Toye A. A., Lippiat J. D., Proks P., Shimomura K., Bentley L., Hugill A., Mijat V., Goldsworthy M., Moir L., Haynes A., Quarterman J., Freeman H. C., Ashcroft F. M., Cox R. D. (2005) Diabetologia 48, 675–686 [DOI] [PubMed] [Google Scholar]
- 28.Goren H. J., Kulkarni R. N., Kahn C. R. (2004) Endocrinology 145, 3307–3323 [DOI] [PubMed] [Google Scholar]
- 29.Wolfrum C., Besser D., Luca E., Stoffel M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11624–11629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J. C., Stafford J. M., Scott D. K., Sutherland C., Granner D. K. (2000) J. Biol. Chem. 275, 14717–14721 [DOI] [PubMed] [Google Scholar]
- 31.Zhang W., Patil S., Chauhan B., Guo S., Powell D. R., Le J., Klotsas A., Matika R., Xiao X., Franks R., Heidenreich K. A., Sajan M. P., Farese R. V., Stolz D. B., Tso P., Koo S. H., Montminy M., Unterman T. G. (2006) J. Biol. Chem. 281, 10105–10117 [DOI] [PubMed] [Google Scholar]
- 32.Iynedjian P. B. (2009) Cell. Mol. Life Sci. 66, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganjam G. K., Dimova E. Y., Unterman T. G., Kietzmann T. (2009) J. Biol. Chem. 284, 30783–30797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirota K., Sakamaki J., Ishida J., Shimamoto Y., Nishihara S., Kodama N., Ohta K., Yamamoto M., Tanimoto K., Fukamizu A. (2008) J. Biol. Chem. 283, 32432–32441 [DOI] [PubMed] [Google Scholar]