Abstract
Endoplasmic reticulum (ER) stress is a causative factor of inflammatory bowel diseases. ER stress mediators, including CCAAT enhancer-binding protein (C/EBP) homologous protein (CHOP), are elevated in intestinal epithelia from patients with inflammatory bowel diseases. The present study arose from the question of how chemical ER stress and CHOP protein were associated with nuclear factor-κB (NF-κB)-mediated epithelial inflammatory response. In a human intestinal epithelial cell culture model, chemical ER stresses induced proinflammatory cytokine interleukin-8 (IL-8) expression and the nuclear translocation of CHOP protein. CHOP was positively involved in ER-activated IL-8 production and was negatively associated with expression of peroxisome proliferator-activated receptor γ (PPARγ). ER stress-induced IL-8 production was enhanced by NF-κB activation that was negatively regulated by PPARγ. Mechanistically, ER stress-induced CHOP suppressed PPARγ transcription by sequestering C/EBPβ and limiting availability of C/EBPβ binding to the PPARγ promoter. Due to the CHOP-mediated regulation of PPARγ action, ER stress can enhance proinflammatory NF-κB activation and maintain an increased level of IL-8 production in human intestinal epithelial cells. In contrast, PPARγ was a counteracting regulator of gut inflammatory response through attenuation of NF-κB activation. The collective results support the view that balances between CHOP and PPARγ are crucial for epithelial homeostasis, and disruption of these balances in mucosal ER stress can etiologically affect the progress of human inflammatory bowel diseases.
Keywords: Epithelium, ER Stress, Inflammation, Intestine, NF-kappaB, PPAR
Introduction
Endoplasmic reticulum (ER)2 is a protein biosynthesis organelle in which newly synthesized proteins are accurately folded into their proper conformation. However, under diverse pathological stress, folding may occur improperly, or proteins may unfold; the aberrant proteins trigger a severe stress response called the ER stress response (1). Phosphorylation of eukaryotic translation initiation factor 2-α (eIF2α) is a highly conserved point of convergence for the distinct signaling pathways that adapt eukaryotic cells to diverse stressful conditions, including ER stress (2, 3). It provides stress resistance by global protein translational arrest and induction of numerous stress-triggered genes. CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) is a representative stress-responsive factor induced by eIF2α phosphorylation-dependent cellular insults, such as ER stress and nutritional deprivation (4–6). CHOP is a transcription factor that primarily mediates stress-linked apoptosis. Among various pathogenic conditions, ER stress is closely associated with inflammatory diseases in many organs, including intestine, lung, liver, kidney, and central nervous system, and is mediated by proinflammatory triggers, such as microbes, cytokines, and reactive radicals (7–9). CHOP is also involved in various inflammatory responses (10–12). Endotoxemia enhances CHOP activation, leading to caspase-processed activation of interleukin-1β (8), and the proinflammatory cytokine tumor necrosis factor-α (TNF-α) induces ER stress and CHOP expression (13). In an experimental ulcerative colitis model, mucosal inflammatory response is critically modulated by CHOP protein that mediates production of proinflammatory cytokines and caspase-dependent cytotoxicity (11). ER stress can be a causative factor of inflammatory bowel diseases (IBD), including Crohn's disease and ulcerative colitis. ER stress indicators, including CHOP, are elevated in intestinal epithelia from IBD patients (14, 15).
Gut epithelial tissues are directly confronted with a variety of xenobiotic factors, including intestinal microbiota and dietary components that can trigger host immune responses (16, 17). In response to these triggers, epithelial tissues become tolerant by suppressing excessive mucosal sensitivity to avoid harmful effects of the inflammatory response. Particularly, tolerant mucosal epithelia can be hyporesponsive to commensal bacteria and their components via pattern recognition receptors (18). Mechanistically, pre-exposure to commensals or their components can desensitize human cells to the pattern recognition receptor-linked proinflammatory signals, such as nuclear factor κB (NF-κB) and MAPK signal transduction (19). Epithelial recognition of microbial fingerprints attenuates subsequent triggering of proinflammatory cytokine production. Many gastrointestinal disorders, including IBD, are associated with mucosal intolerance derived from disruption of the epithelial barrier (20–22). One critical factor mediating mucosal tolerance is peroxisome proliferator-activated receptor γ (PPARγ). PPARγ is a member of the nuclear receptor superfamily of transcription factors and is a ligand-dependent nuclear receptor. PPARγ is abundantly expressed in adipocytes and colonic epithelium (23). PPARγ has been investigated as a critical regulator of gut homeostasis because epithelial PPARγ activation generally reduces gene expression of proinflammatory mediators by suppressing NF-κB-linked signals (24–26). Down-regulation of PPARγ expression may exist within intestinal epithelial cells of IBD patients, which are susceptible to uncontrolled inflammation, and ligands of PPARγ can be efficient in the treatment of IBD (27, 28). PPARγ expressed in gut epithelium has a protective effect against colonic inflammatory responses to both commensal and pathogenic insults.
The present study investigated the nature of chemical ER stress and CHOP protein association with epithelial inflammatory response, including NF-κB-mediated cytokine production in human intestinal epithelial cells. As well, how the mucosal regulatory factor PPARγ is involved in ER stress-mediated cytokine production was assessed.
MATERIALS AND METHODS
Cell Culture Conditions and Reagents
The human epithelial cell lines HCT-8, HT-29, and HCT-116 were sourced from human embryonic jejunum and ileum and were purchased from the American Type Culture Collection (Rockville, MD). They were maintained in RPMI medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich), 50 units/ml penicillin (Sigma-Aldrich), and 50 μg/ml streptomycin (Sigma-Aldrich) in a 5% CO2 humidified incubator at 37 °C. Cell number was assessed by trypan blue (Sigma-Aldrich) dye exclusion using a hemacytometer. Thapsigargin (TG) was purchased from Assay design (Ann Arbor, MI), and Bay 11-7082 was purchased from Calbiochem. All other chemicals were purchased from Sigma-Aldrich.
Construction of Plasmid
Dominant negative CHOP (dnCHOP) and wild type CHOP constructs were provided by Dr. Tomomi Gotoh (Kumamoto University), and FLAG-tagged WT PPARγ plasmid was provided by Dr. Krishna Chatterjee (University of Cambridge). Interleukin-8 (IL-8) transcriptional activity was measured using IL-8 promoter-luciferase reporter constructs (a part of the human IL-8 promoter ranging from nucleotides −416 to +44), which was kindly provided by Dr. Hsing-Jien Kung (University of California, Davis, CA). The human CHOP promoter, ranging from nucleotides −649 to +91, was kindly provided by Dr. Pierre Fafournoux (The National Institute for Agricultural Research (INRA), France). PPARγ transcriptional activity was measured using PPARγ3 promoter-luciferase reporter constructs (a part of the human PPARγ promoter ranging from nucleotides −851 to −1 in generated pGL3 basic vectors. After PCR of the promoter region with Pfu turbo DNA polymerase (Stratagene, La Jolla, CA), the fragment was cloned into the TA vector (Invitrogen), sequenced, and subcloned into the pGLBasic3 vector. Cytomegalovirus (CMV)-driven small hairpin interference RNA (shRNA) was constructed by inserting an shRNA template into pSilencer 4.1-CMV-neo vector (Ambion, Austin, TX). The C/EBPβ shRNA-containing vector was designated ShC/EBPβ; it targeted the sequence GAAGAAACGTCTATGTGTA.
Western Immunoblot Analysis
Levels of protein expression were compared by Western immunoblot analysis using anti-human actin, anti-C/EBP, anti-I-κBα rabbit polyclonal antibody, and anti-CHOP mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Cells were washed with ice-cold phosphate buffer, lysed in boiling lysis buffer (1% (w/v) SDS, 1.0 mm sodium orthovanadate, and 10 mm Tris, pH 7.4), and sonicated for 5 s. Lysates containing proteins were quantified using the BCA protein assay kit (Pierce). Fifty micrograms of protein was separated by minigel electrophoresis (Bio-Rad). Proteins were transferred onto a polyvinylidene fluoride membrane (Amersham Biosciences), and the blots were blocked for 1 h with 5% skim milk in Tris-buffered saline plus Tween 0.1% (TBS-T) and probed with each antibody for a further 2 h at room temperature or overnight at 4 °C. After washing three times with TBS-T, blots were incubated with horseradish-conjugated secondary antibody for 1 h and washed with TBS-T a further three times. Protein was detected by ECL chemiluminescent substrate (Amersham Biosciences).
IL-8 Enzyme-linked Immunosorbent Assay (ELISA)
IL-8 was quantified from each cell supernatants using ELISA. HCT8 cells were dispensed at 3 × 104 cells in each well of a 24-well plate and allowed to adhere. After treatment with deoxynivalenol or vehicle, cell culture medium was collected and centrifuged to remove cell debris. Levels of IL-8 were determined by ELISA using an OptEIA human IL-8 ELISA kit (BD Biosciences) according to the manufacturer's instructions. Briefly, capture antibody was coated onto ELISA plates overnight at 4 °C. After washing with Tween 20-containing PBS (PBST) and blocking with PBS supplemented with 10% (v/v) FBS overnight at 4 °C, plates were incubated with serial dilutions of IL-8 samples and standards. After treatment with detection antibody and tetramethyl benzidine substrate, absorbance was measured at 405 nm using an ELISA reader. The assay detection limit was 3.1 pg/ml of IL-8.
Reverse Transcription (RT) Conventional PCR and Real-time PCR
RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's instructions. RNA (100 ng) from each sample was transcribed to cDNA by Prime RT premix (Genetbio, Nonsan, South Korea). The amplification was performed with Takara HS ExTaq DNA polymerase (Takara Bio, Shiga, Japan) in a Mycycler Thermal Cycler (Bio-Rad) using the following parameters: denaturation at 94 °C for 2 min and 25 cycles of reactions of denaturation at 98 °C for 10 s, annealing at 59 °C for 30 s, and elongation at 72 °C for 45 s. An aliquot of each PCR product was subjected to 1.2% (w/v) agarose gel electrophoresis and visualized by staining with ethidium bromide. The 5′ forward and 3′ reverse complement PCR primers for amplification of each gene were as follows: human PPAR-γ (5′-TTC AGA AAT GCC TTG CAG TG-3′ and 5′-CAC CTC TTT GCT CTG CTC CT-3′), human CHOP (5′-CTT GGC TGA CTG AGG AGG AG-3′ and 5′-TCA CCA TTC GGT CAA TCA GA-3′), human IL-8 (5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′ and 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′), and human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (5′-TCA ACG GAT TTG GTC GTA TT-3′ and 5′-CTG TGG TCA TGA GTC CTT CC-3′). In real-time PCR, 6-carboxyl-fluorescein was used as fluorescent reporter dye and conjugated to 5′-ends of probes to detect amplified cDNA in iCycler Thermal Cycler (Bio-Rad) using the following parameters: denaturation at 94 °C for 2 min and 40 cycles of reactions of denaturation at 98 °C for 10 s, annealing at 59 °C for 30 s, and elongation at 72 °C for 45 s. Each sample was tested in triplicate to ensure statistical significance. The relative quantification of gene expression was performed using the comparative Ct method. The Ct value is defined as the point where a statistically significant increase in the fluorescence has occurred. The number of PCR cycles (Ct) required for the 6-carboxyl-fluorescein intensities to exceed a threshold just above background was calculated for the test and reference reactions. In all experiments, GAPDH was used as the endogenous control. Results were analyzed in a relative quantitation study with the vehicle treated.
Transient and Stable Transfection
Cells were transfected with a mixture of plasmids using Lipofectamine 2000 (Invitrogen) or Carrigene reagent (Kinovate, Oceanside, CA) according to the manufacturer's protocol. For transfection of the luciferase reporter gene, a mixture of 2 μg of firefly luciferase reporter and 0.2 μg of Renilla luciferase, pRL-null vector (Promega, Madison, WI) per 2 μl of Carrigene was applied to wells of a 6-well culture plate. For the luciferase assay, 12 h after transfection, cells were exposed to chemicals for a further 12 h for IL-8 promoter-luciferase reporter constructs and lysed for the dual luciferase reporter assay system (Promega). All transfection efficiency was maintained at around 50–60%, which was confirmed with pMX-enhanced green fluorescent protein vector. To create stable cell lines, cells were transfected using Lipofectamine 2000 reagent. After 48 h, cells were subjected to selection for stable integrants by exposure to 700 μg/ml G418 (Invitrogen) in complete medium containing 10% FBS. Selection was continued until monolayers were formed. The transfectants were then maintained in medium supplemented with 10% FBS and 350 μg/ml G418.
Luciferase Assay
Cells were washed with cold PBS, lysed with passive lysis buffer (Promega), and centrifuged at 12,000 × g for 4 min. The supernatant was collected, isolated, and stored at −80 °C until assessed for luciferase activity. Luciferase activity was measured with a model TD-20/20 dual mode luminometer (Turner Designs, Sunnyvale, CA) after briefly mixing the supernatant (10 μl) with 50 μl of firefly luciferase assay substrate solution, followed by 50 μl of stopping Renilla luciferase assay solution (Promega). The firefly luciferase activity was normalized against Renilla luciferase activity by dividing the former activity by the latter.
Chromatin Immunoprecipitation (ChIP) Assay
Cells were cross-linked for 10 min in 1% formaldehyde. The reaction was stopped by the addition of glycine to 125 mm, and cells were washed twice with 1× PBS. Chromatin was fragmented by sonication for 10 s to a size of 1000–2000 bp in lysis buffer (1% (w/v) SDS, 10 mm EDTA, pH 8.0, 50 mm Tris-HCl, pH 8.0), protease inhibitor mixture) using Vibra-Cell (Sonics & Materials, Inc., Newtown, CT). The soluble chromatin was immunoprecipitated with 2 μg of mouse monoclonal anti-GADD153 antibody and rabbit polyclonal anti-C/EBPβ antibody in a mixture of nine parts dilution buffer (1% Triton X-100, 150 mm NaCl, 2 mm EDTA, pH 8.0, 20 mm Tris, pH 8.0, and protease inhibitor mixture) and one part lysis buffer. After rotating overnight at 4 °C, protein G-Sepharose 4 fast flow (GE Healthcare) was added in 100 μl of a 9:1 mixture of dilution buffer and lysis buffer containing 100 μg/ml BSA) (Promega) and 500 μg/ml salmon sperm DNA (Invitrogen) per sample. After centrifugation of the protein G-Sepharose mixture, each sample was washed twice in dilution buffer, and finally the chromatin was resuspended in the 9:1 dilution buffer/lysis buffer solution and incubated at 37 °C with proteinase K and RNase A (500 μg/ml for each sample). Chromatin was purified using a MEGAquick-spinTM kit (Intron, SungNam, South Korea).
Co-immunoprecipitation Assay
Cellular lysate was prepared in immunoprecipitation lysis buffer (50 mm Tris, pH 7.2, 150 mm NaCl, 1 mm EDTA, 400 mm Na3VO4, and 2.5 mm phenylmethanesulfonylfluoride) containing 0.1% Triton X-100 and incubated on ice for 40 min. Mouse monoclonal anti-CHOP was added and rotated overnight at 4 °C. Protein G-Sepharose in antibody-cell lysate were incubated by rotating at 4 °C for 3 h. Immunoprecipitates were collected by centrifugation and subjected to SDS-PAGE.
Cellular Viability Assay
Colorimetric analysis of cell growth was performed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (5 × 104/well) were cultured in a 96-well plate for each time, and MTT (20 μl from 5 mg/ml stock solution) was added to cells for 2 h. Supernatant was removed and dissolved with 200 μl of DMSO. Optical density was read at 560 nm, which was subtracted by background optical density at 670 nm. Optical density was directly correlated with cell quantity.
Statistical Analyses
Data were analyzed using SigmaStat for Windows (Jandel Scientific, San Rafael, CA). For comparative analysis of two groups of data, Student's t test was performed. For comparative analysis of multiple groups, data were subjected to analysis of variance, and pairwise comparisons were made by the Student-Newman-Keuls method. Data not meeting normality assumptions were subjected to a Kruskal-Wallace analysis of variance on ranks, and then pairwise comparisons were made by the Student-Newman-Keuls method.
RESULTS
Chemical ER Stresses Induce Proinflammatory Cytokine Interleukin-8 Expression
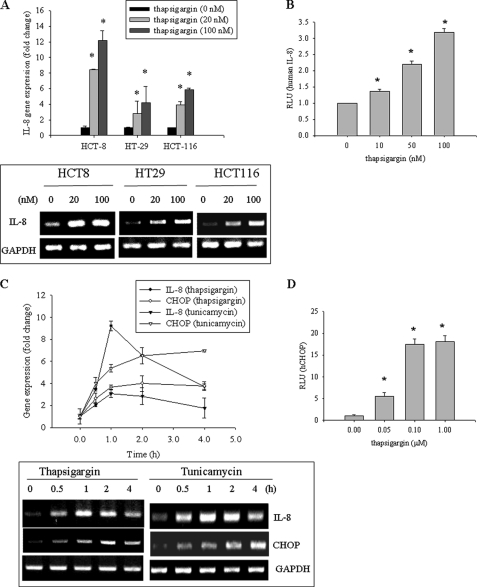
ER stress response has been implicated as an etiological factor of human intestinal inflammatory diseases in various models (9, 11, 14, 29). TG as a representative ER stress inducer was used to assess effects on proinflammatory gene IL-8 in different human intestinal epithelial cells, including HCT-8, HT-29, and HCT-116. In all tested cells, IL-8 mRNA expression was induced by TG in a dose-dependent manner, and among these cell lines, HCT-8 cells were the most sensitive IL-8 producer in response to chemical ER stress (Fig. 1A). HCT-8 cells are a frequently used human epithelial cell culture model for microbial infection and inflammatory diseases (30–32) and were presently used hereafter in the assessment of ER stress. In addition to IL-8 mRNA, transcriptional activity of IL-8 gene expression was also enhanced by TG in a dose-dependent way (Fig. 1B). Moreover, tunicamycin, another ER stress inducer, was shown to promote gene expression of IL-8 and CHOP, one representative indicator of ER stress response (Fig. 1C). In agreement with experiments in diverse tissues and cell types, ER stress also triggered CHOP transcriptional activation in human intestinal epithelial cells (Fig. 1D). Because the CHOP transcription factor is also involved in various inflammatory responses (10–12), the next experiments were performed to assess the effect of CHOP on ER stress-induced IL-8 production.
FIGURE 1.
Effects of ER stress on IL-8 and CHOP production in human intestinal epithelial cells. A, HCT-8, HT29, or HCT-116 cells were treated with TG for 1 h, and each mRNA was measured using RT-real-time PCR. The boxed panel below the graph indicates representative results of three independent experiments using RT-conventional PCR. B, HCT-8 cells transfected with IL-8 reporter plasmid were treated with 0.1 μm TG for 12 h. C, HCT-8 cells were treated with 0.1 μm TG or 1 μg/ml tunicamycin, and each mRNA was measured using RT-real-time PCR. The boxed panel below the graph indicates representative results of three independent experiments using RT-conventional PCR. D, HCT-8 cells transfected with CHOP reporter plasmid were treated with TG for 12 h. Groups with an asterisk are significantly different (p < 0.05) from the vehicle control group. Error bars, S.D.
CHOP Is Positively Involved in ER-activated IL-8 Production and Negatively Associated with Expression of PPARγ
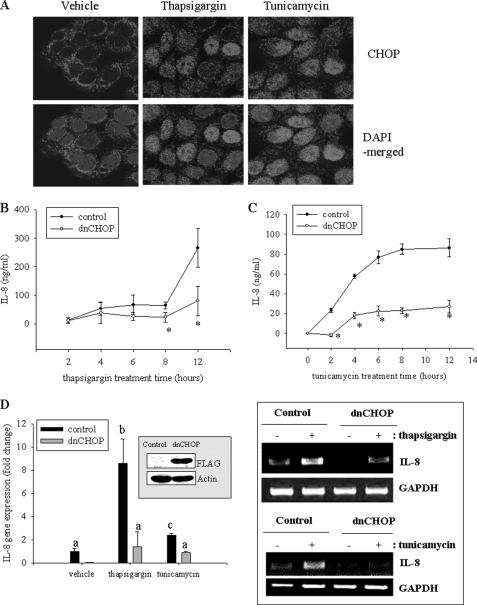
CHOP is a C/EBP homologous protein that plays crucial roles in cellular translational stress conditions, making epithelial cells sensitive to xenobiotic-induced injuries (33). In the present study, chemical ER stresses were assessed for their effects on stress-inducible CHOP expression and its association with inflammatory cytokine production. First, effects of chemical ER stress on CHOP expression and cellular localization were observed. Both TG and tunicamycin promoted nuclear translocation of CHOP protein as well as its gene induction in human intestinal epithelial cells (Fig. 2A). To check effects of CHOP on IL-8 induction, cells expressing dnCHOP were compared with the empty vector-expressing control cells for its inducible effects on IL-8 production. Suppression of CHOP reduced IL-8 production (Fig. 2, B and C), which implied a positive relationship between CHOP and IL-8 induction in human intestinal epithelial cells. This association was confirmed by measuring IL-8 mRNA, where it was observed that CHOP suppression reduced ER stress-induced IL-8 mRNA expression (Fig. 2D).
FIGURE 2.
Involvement of CHOP in TG-induced IL-8 production. A, HCT-8 cells were treated with 0.1 μm TG for 6 h, fixed, and stained for confocal microscopy analysis. B and C, stable cell lines (empty vector- or dnCHOP-expressing HCT-8) were treated with 0.1 μm TG or 1 μg/ml tunicamycin. Culture supernatant was analyzed for IL-8 secretion using ELISA. Groups with an asterisk are significantly different (p < 0.05) from the treatment control group. D, stable cell lines (empty vector- or dnCHOP-expressing HCT-8) were treated with 0.1 μm TG or 1 μg/ml tunicamycin for 1 h, and each mRNA was measured using RT-real-time PCR. The inset in the graph indicates Western blot of cellular lysates from each stable cell lines (empty vector- or dnCHOP-expressing HCT-8). Different letters above each bar represent significant differences between two groups (p < 0.05). The boxed panel to the right of the graph indicates representative results of three independent experiments using RT-conventional PCR. Error bars, S.D.
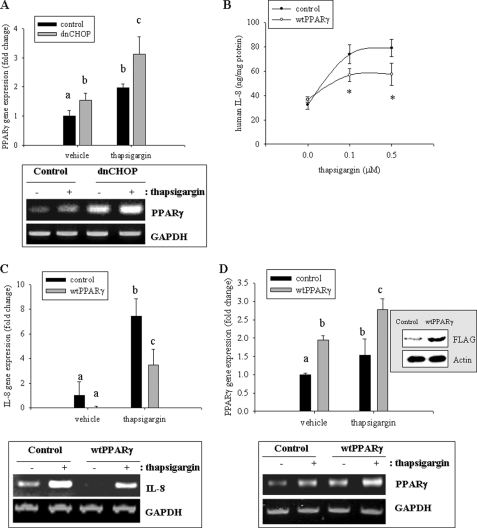
Because CHOP is a well known dominant negative factor of C/EBPβ action (34), C/EBPβ- downstream targets, including PPARγ, can be suppressed (35, 36). Moreover, PPARγ is an anti-inflammatory modulator of the NF-κB signaling cascade. Appropriately, the influence of the suppression of CHOP on levels of PPARγ in response to proinflammatory stimulation was assessed. Whereas CHOP mediated up-regulation of proinflammatory IL-8 (Fig. 2), CHOP suppression enhanced mRNA levels of PPARγ (Fig. 3A), indicating negative association between CHOP and PPARγ expression. The next experiment tested whether PPARγ could suppress CHOP-enhanced IL-8 production and its gene expression. Cells with exogenously introduced PPARγ (Fig. 3D) showed reduced gene expression and release of IL-8 compared with the control human intestinal epithelial HCT-8 cells (Fig. 3, B and C).
FIGURE 3.
Involvement of PPARγ in ER stress-induced IL-8 production. A, stable cell lines (empty vector- or dnCHOP-expressing HCT-8) were treated with 0.1 μm TG, and each mRNA was measured using RT-real-time PCR. Different letters above each bar represent significant difference between two groups (p < 0.05). The boxed panel below the graph indicates representative data of three independent experiments using RT-conventional PCR. B, stable cell lines (empty vector- or WT PPARγ-expressing HCT-8) were treated with 0.1 μm TG. Culture supernatant was analyzed for IL-8 secretion using the ELISA method. Groups with an asterisk are significantly different (p < 0.05) from the treatment control group. C and D, stable cell lines (empty vector- or WT PPARγ-expressing HCT-8) were treated with 0.1 μm TG, and each mRNA was measured using RT-real-time PCR. Different letters above each bar represent significant difference between two groups (p < 0.05). The inset in the graph indicates Western blot of cellular lysates from each stable cell line (empty vector- or WT PPARγ-expressing HCT-8). Boxed panels below each graph indicate representative results of three independent experiments using RT-conventional PCR. Error bars, S.D.
ER Stress-induced CHOP Suppresses PPARγ Expression by Limiting C/EBPβ
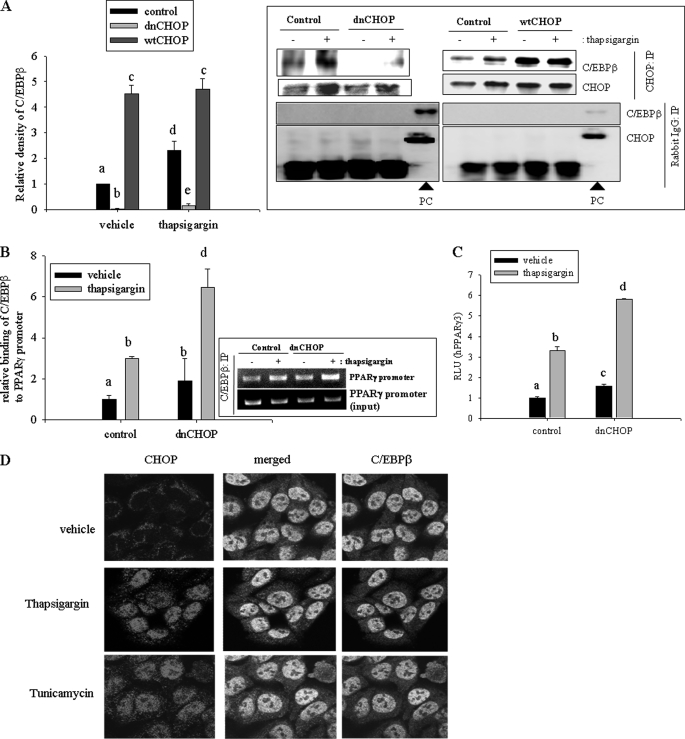
CHOP is a dominant negative form of C/EBPβ. C/EBP members can form homodimers or heterodimers (37), but CHOP cannot compose its homodimer. Moreover, CHOP has no ability to bind functional DNA binding domains, including C/EBP binding sites (38), but CHOP interacts with the other C/EBP members to form a heterodimer, which competitively inhibits the action of the other C/EBPs. In the present study, the cross-talk between CHOP and C/EBPβ protein was assessed in response to chemical ER stress. To observe protein interaction between C/EBPβ and CHOP, a ChIP assay was performed, demonstrating that CHOP formed a complex with C/EBPβ protein in response to ER stress (Fig. 4A). Exogenous expression of dnCHOP interfered with the interaction between CHOP and C/EBPβ in response to ER stress. In terms of regulation of CHOP and C/EBPβ for IL-8 transcription, binding action of CHOP and C/EBPβ to the IL-8 promoter was not altered (data not shown). Based on an assumption that CHOP can interfere with binding of C/EBPβ to the PPARγ promoter by forming a heterodimer with C/EBPβ, it can be speculated that binding of C/EBPβ to the PPARγ promoter would be increased if the action of CHOP was inhibited. Binding of C/EBPβ to the PPARγ promoter was more enhanced in CHOP-suppressed cells than the control cells in response to ER stress (Fig. 4B). Therefore, CHOP-suppressed cells using dominant negative CHOP showed a greater increase in PPARγ promoter activity than control cells (Fig. 4C). In response to ER stress, CHOP expression was induced at an early time (3 h) and gradually decreased. C/EBPβ was also induced with ER stress but showed another late induction (12 h) with decreasing CHOP expression (supplemental Fig. S1A). PPARγ also had a biphasic induction pattern with delayed time because C/EBPβ is a critical inducer of PPARγ expression. Based on the assumption that CHOP is a negative regulator of C/EBPβ-mediated PPARγ expression, binding of C/EBPβ to the PPARγ promoter was measured using a ChIP assay (supplemental Fig. S1B). Compared with the early binding of C/EBPβ to the promoter, it was more enhanced when CHOP drop and C/EBPβ went up again (12 h), indicating suppression of C/EBPβ-mediated PPARγ by CHOP protein in response to ER stress. In addition, to assess the direct interaction between CHOP and C/EBPβ protein in cells in the presence of ER stress, cellular localization of both proteins was analyzed using confocal microscopy. In response to ER stress agent, both proteins were observed to interact mostly in the nuclear area of human intestinal epithelial cells (Fig. 4D).
FIGURE 4.
Regulation of PPARγ expression by ER stress-triggered CHOP with C/EBPβ. A, stable cell lines (empty vector-, dnCHOP-, or WT CHOP-expressing HCT-8) were treated with 0.1 μm TG, and total lysates were immunoprecipitated with anti-CHOP or IgG antibody. Precipitated samples were subjected to Western blot analysis. The graph indicates statistical analysis of three independent experiments. Different letters above each bar represent significant difference between two groups (p < 0.05). The boxed panel below the graph indicates representative results of three independent experiments. The arrows indicate lysate samples from cells transfected with expression plasmid of each recombinant gene (CHOP and C/EBPβ) as the positive control (PC). B, stable cell lines (empty vector- or dnCHOP-expressing HCT-8) were treated with 0.1 μm TG, and cellular chromatin was immunoprecipitated with anti-C/EBPβ antibody. Collected chromatin was subjected to real-time PCR. Different letters above each bar represent significant difference between two groups (p < 0.05). The boxed panel below the graph indicates representative results of three independent experiments using conventional PCR. C, stable cell lines (empty vector- or dnCHOP-expressing HCT-8) were transfected with PPARγ reporter plasmid and were treated with 0.1 μm TG for 24 h. Different letters above each bar represent significant difference between two groups (p < 0.05). D, HCT-8 cells were treated with 0.1 μm TG or 1 μg/ml tunicamycin for 6 h, fixed, and stained for confocal microscopy analysis. Error bars, S.D.
ER Stress-induced IL-8 Production Is Enhanced by NF-κB Activation That Is Negatively Regulated by PPARγ
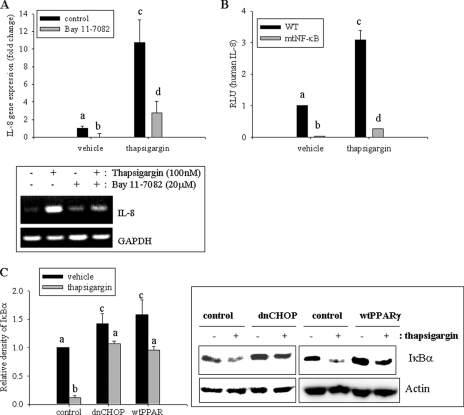
The NF-κB pathway is activated by diverse cellular stresses, including ER stress (39). Chemical-induced ER stress was also assessed for the induction of the proinflammatory cytokine IL-8 via the NF-κB signaling pathway using the inhibitor Bay 11-7082, which specifically blocks I-κB kinase (IKK) phosphorylation. Induction of IL-8 mRNA by ER stress was mediated by NF-κB signals (Fig. 5A), and cis-activation of the NF-κB site was crucial in ER stress-mediated IL-8 gene expression in human intestinal epithelial cells (Fig. 5B). Cells transfected with the mutant IL-8 reporter in the NF-κB binding site displayed repressed IL-8 activation in response to ER stress. ER stress-induced CHOP had negative regulatory effects on PPARγ expression, which could be expected to suppress NF-κB-mediated proinflammatory responses. Chemical ER stress promoted degradation of IκBα in human intestinal epithelial cells, but cells stably transfected with dnCHOP or overexpression of PPARγ had less NF-κB activation in response to ER stress (Fig. 5C). The observations are consistent with the view that ER stress-activated CHOP repressed PPARγ expression, which attenuated repression of NF-κB-linked production of proinflammatory cytokine in human intestinal epithelial cells (Fig. 6).
FIGURE 5.
NF-κB-mediated IL-8 production via CHOP and PPARγ. A, HCT-8 cells were pretreated with 20 μm (E)-3-[(4-methylphenyl)sulfonyl]-2-propenenitrile (BAY 11-7082) for 2 h and treated with 0.1 μm TG for 1 h. Each mRNA was measured using RT-real-time PCR. Different letters above each bar represent significant difference between two groups (p < 0.05). The boxed panel below the graph indicates representative results of three independent experiments using RT-conventional PCR. B, HCT-8 cells were transfected with wild type IL-8 reporter plasmid or mutant IL-8 reporter in NF-κB binding site and were treated with 0.1 μm TG for 12 h. Different letters above each bar represent significant difference between two groups (p < 0.05). C, stable cell lines (empty vector-, dnCHOP-, or WT PPARγ-expressing HCT-8) were treated with 0.1 μm TG for 30 min, and total cell lysate was subjected to Western blot analysis. Different letters above each bar represent significant difference between two groups (p < 0.05). Right panels indicate representative results of three independent experiments. Error bars, S.D.
FIGURE 6.
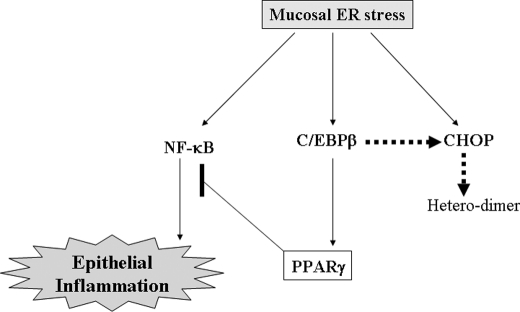
A putative involvement of CHOP and PPARγ in ER stress-induced IL-8 production in human intestinal epithelial cells. In the schematic signaling pattern, ER stress induces epithelial proinflammatory cytokine production, which is mediated by NF-κB signals. Epithelial NF-κB-mediated proinflammatory responses are suppressed by PPARγ expression that is regulated by CHOP protein. CHOP limits the transcriptional activation of PPARγ by C/EBPβ protein via a heterodimer formation in human intestinal epithelial cells.
DISCUSSION
The present study provides molecular insights into ER stress-linked NF-κB activation via regulation of PPARγ by CHOP protein in human enterocytes, which can explain up-regulation of diverse proinflammatory mediators, including cytokines, eicosanoids, and adhesion molecules in response to ER stress. Another recent study also suggested that CHOP can affect interleukin-1β activation, which is only limited to caspase-processed cytokines (8, 12). Other than CHOP-linked modulation, ER stress sensor molecules, such as inositol-requiring ER-to-nucleus signal kinase 1 (IRE1), RNA-dependent protein kinase-like ER kinase (PERK), and activating transcription factor 6 (ATF6), can trigger the NF-κB signaling pathway to induce proinflammatory mediators (40). IRE1 interacts with the C terminus of TNF receptor-associated factor 2, which involves ER stress-induced NF-κB activation (41). PERK activates NF-κB via phosphorylation of eIF2α, inhibiting translation. Phosphorylation of eIF2α also triggers activation of NF-κB by eukaryotic initiation factor 2 α kinase 4, another kinase of eIF2α (39). ATF6 also activates NF-κB signals via the protein kinase B signaling pathway. ER stress has been positively associated with chronic proinflammatory diseases (7, 42, 43). Particularly in intestinal epithelia, ER stress-induced X-box binding protein 1 confers genetic susceptibility to human IBD, including ulcerative colitis and Crohn's disease (9).
Because NF-κB activation occurs earlier than CHOP induction by ER stress, the CHOP-mediated proinflammatory response may be crucial in maintaining the whole inflammatory process. Early activation of NF-κB signals may be associated with other ER stress sensor molecules, such as IRE1, PERK, and ATF6. Instead, CHOP was presently associated with maintenance of low levels of PPARγ in human epithelia. Without CHOP action in the epithelial cells, PPARγ was strongly up-regulated, which abolished proinflammatory cytokine production by ER stress. Therefore, CHOP is expected to be a crucial mediator to support prolonged inflammatory responses to mucosal insults by ER stress. In addition, CHOP was also partly involved in ER stress-induced cell death (supplemental Fig. S2, A and B). Chemical ER stress inducers, including TG and tunicamycin, at higher doses than those in proinflammatory responses caused epithelial cell death, which was also associated with CHOP protein. Particularly, ER stress has been associated with diverse chronic inflammatory disease rather than acute inflammatory responses (11, 44, 45). Additional proinflammatory signals can exist to support persistent NF-κB signals in response to ER stress. One example is boosting kinases, including small GTPase p21-activated kinase, for prolonged phosphorylation of NF-κB (46). In contrast, the results of the present study support the suggestion that CHOP controls cellular levels of PPAR-suppressing NF-κB signals, finally achieving a high level of proinflammatory cytokine production.
Intestinal epithelial NF-κB can contribute to up-regulation of proinflammatory cytokines related to chronic intestinal inflammatory diseases, such as IBD. In response to the proinflammatory stress, PPARγ is a critical regulator of gut homeostasis by suppressing NF-κB-linked signals as shown both previously (24–26) and presently. Impaired expression of PPARγ without any mutation of PPARγ sequences in patients with ulcerative colitis indicates that PPARγ is a key mediator of anti-inflammatory responses in human gut epithelia and can be a crucial target of therapeutic agents of IBD (47). The human gut is continuously confronted by commensal bacteria, and human intestinal epithelial cells are generally the first contact targets of the bacteria. Gut epithelial cells show hyporesponsiveness to bacterial pattern moiety, particularly attenuation of proinflammatory NF-κB signals by PPARγ. Mechanistically, commensal-mediated PPARγ attenuates epithelial inflammatory responses by triggering nuclear export of p65 protein in complex with PPARγ (48). Moreover, PPARγ also can affect proinflammatory cytokine production via an indirect activation of NF-κB. PPARγ interferes with the cytosol-to-membrane translocation of protein kinase Cα, which induces cellular desensitization to proinflammatory stimulation (49). Whereas C/EBPβ can suppress NF-κB activation by enhancing PPARγ production in the present study, another explanation can be that C/EBPβ interferes with NF-κB signals by directly snatching p65 protein (50). Moreover, bacterial lipopolysaccharide delays the activation of PPARγ, which attenuates proinflammatory responses by oxidative stress or mucosal ribotoxic stresses in human epithelial cells (51, 52). However, although activation of intestinal epithelial NF-κB increases harmful proinflammatory cytokines related to chronic intestinal inflammatory diseases or colon cancers, it is not always bad news in terms of the defense against microbial infection. In diverse mechanical injury models, NF-κB is involved in protective action, including wound healing responses, in particular by promoting cellular proliferation (53, 54). Moreover, NF-κB promotes the reconstitution of injured epithelial monolayer via NF-κB target genes, such as inducible nitric-oxide synthase and cyclooxygenase-2, which are strong mediators of epithelial migration to the injury site (55, 56). Thus, it can be speculated that ER stress-activated NF-κB can protect intestinal epithelial cells from the toxic insults of proinflammatory response and even facilitates the wound healing process after epithelial injury to lessen microbial translocation (57). Therefore, more careful observations are needed for homeostatic counteraction between activated NF-κB and PPARγ-mediated regulation in the intestinal epithelia. In particular, the present study provides a promising insight into the molecular mechanism of epithelial decision in the progress of ER stress-linked IBD.
Supplementary Material
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2009-0087028).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- ER
- endoplasmic reticulum
- C/EBP
- CCAAT enhancer-binding protein
- CHOP
- C/EBP homologous protein
- dnCHOP
- dominant negative CHOP
- IBD
- inflammatory bowel disease(s)
- PPARγ
- peroxisome proliferator-activated receptor γ
- TG
- thapsigargin
- NF-κB
- nuclear factor-κB
- IRE1
- inositol-requiring ER-to-nucleus signal kinase 1
- PERK
- RNA-dependent protein kinase-like ER kinase
- ATF6
- activating transcription factor 6.
REFERENCES
- 1.Kopito R. R. (2000) Trends Cell Biol. 10, 524–530 [DOI] [PubMed] [Google Scholar]
- 2.Wek R. C., Jiang H. Y., Anthony T. G. (2006) Biochem. Soc. Trans. 34, 7–11 [DOI] [PubMed] [Google Scholar]
- 3.Moenner M., Pluquet O., Bouchecareilh M., Chevet E. (2007) Cancer Res. 67, 10631–10634 [DOI] [PubMed] [Google Scholar]
- 4.Bruhat A., Jousse C., Wang X. Z., Ron D., Ferrara M., Fafournoux P. (1997) J. Biol. Chem. 272, 17588–17593 [DOI] [PubMed] [Google Scholar]
- 5.Fujii J., Wood K., Matsuda F., Carneiro-Filho B. A., Schlegel K. H., Yutsudo T., Binnington-Boyd B., Lingwood C. A., Obata F., Kim K. S., Yoshida S., Obrig T. (2008) Infect. Immun. 76, 3679–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. (1998) Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin W., Harding H. P., Ron D., Popko B. (2005) J. Cell Biol. 169, 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo M., Mori M., Akira S., Gotoh T. (2006) J. Immunol. 176, 6245–6253 [DOI] [PubMed] [Google Scholar]
- 9.Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo M., Oyadomari S., Suga M., Mori M., Gotoh T. (2005) J. Biochem. 138, 501–507 [DOI] [PubMed] [Google Scholar]
- 11.Namba T., Tanaka K., Ito Y., Ishihara T., Hoshino T., Gotoh T., Endo M., Sato K., Mizushima T. (2009) Am. J. Pathol. 174, 1786–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suyama K., Ohmuraya M., Hirota M., Ozaki N., Ida S., Endo M., Araki K., Gotoh T., Baba H., Yamamura K. (2008) Biochem. Biophys. Res. Commun. 367, 176–182 [DOI] [PubMed] [Google Scholar]
- 13.Xue X., Piao J. H., Nakajima A., Sakon-Komazawa S., Kojima Y., Mori K., Yagita H., Okumura K., Harding H., Nakano H. (2005) J. Biol. Chem. 280, 33917–33925 [DOI] [PubMed] [Google Scholar]
- 14.Shkoda A., Ruiz P. A., Daniel H., Kim S. C., Rogler G., Sartor R. B., Haller D. (2007) Gastroenterology 132, 190–207 [DOI] [PubMed] [Google Scholar]
- 15.Burczynski M. E., Peterson R. L., Twine N. C., Zuberek K. A., Brodeur B. J., Casciotti L., Maganti V., Reddy P. S., Strahs A., Immermann F., Spinelli W., Schwertschlag U., Slager A. M., Cotreau M. M., Dorner A. J. (2006) J. Mol. Diagn. 8, 51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sansonetti P. J. (2008) Curr. Opin. Gastroenterol. 24, 435–439 [DOI] [PubMed] [Google Scholar]
- 17.Cario E. (2008) Curr. Opin. Gastroenterol. 24, 725–732 [DOI] [PubMed] [Google Scholar]
- 18.McCole D. F., Barrett K. E. (2007) Curr. Opin. Gastroenterol. 23, 647–654 [DOI] [PubMed] [Google Scholar]
- 19.Medvedev A. E., Kopydlowski K. M., Vogel S. N. (2000) J. Immunol. 164, 5564–5574 [DOI] [PubMed] [Google Scholar]
- 20.Tsianos E. V., Katsanos K. (2009) World J. Gastroenterol. 15, 521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan M., Summerlin D. J. (2009) Clin. Immunol. 133, 411–421 [DOI] [PubMed] [Google Scholar]
- 22.Schölmerich J. (2006) Ann. N.Y. Acad. Sci. 1072, 365–378 [DOI] [PubMed] [Google Scholar]
- 23.Mansén A., Guardiola-Diaz H., Rafter J., Branting C., Gustafsson J. A. (1996) Biochem. Biophys. Res. Commun. 222, 844–851 [DOI] [PubMed] [Google Scholar]
- 24.Jiang C., Ting A. T., Seed B. (1998) Nature 391, 82–86 [DOI] [PubMed] [Google Scholar]
- 25.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. (1998) Nature 391, 79–82 [DOI] [PubMed] [Google Scholar]
- 26.Su C. G., Wen X., Bailey S. T., Jiang W., Rangwala S. M., Keilbaugh S. A., Flanigan A., Murthy S., Lazar M. A., Wu G. D. (1999) J. Clin. Invest. 104, 383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely G. B., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., Lehmann J. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belluzzi A., Brignola C., Campieri M., Pera A., Boschi S., Miglioli M. (1996) N. Engl. J. Med. 334, 1557–1560 [DOI] [PubMed] [Google Scholar]
- 29.Bertolotti A., Wang X., Novoa I., Jungreis R., Schlessinger K., Cho J. H., West A. B., Ron D. (2001) J. Clin. Invest. 107, 585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcantara Warren C., Destura R. V., Sevilleja J. E., Barroso L. F., Carvalho H., Barrett L. J., O'Brien A. D., Guerrant R. L. (2008) J. Infect. Dis. 198, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sifuentes L. Y., Di Giovanni G. D. (2007) Appl. Environ. Microbiol. 73, 7548–7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thébault S., Deniel N., Marion R., Charlionet R., Tron F., Cosquer D., Leprince J., Vaudry H., Ducrotté P., Déchelotte P. (2006) Proteomics 6, 3926–3937 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y. J., Tan B. C., Cheng Y. Y., Chen J. S., Lee S. C. (2010) Nucleic Acids Res. 38, 764–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira R. C., Delany A. M., Canalis E. (2004) Endocrinology 145, 1952–1960 [DOI] [PubMed] [Google Scholar]
- 35.Hamm J. K., Park B. H., Farmer S. R. (2001) J. Biol. Chem. 276, 18464–18471 [DOI] [PubMed] [Google Scholar]
- 36.Wu Z., Bucher N. L., Farmer S. R. (1996) Mol. Cell Biol. 16, 4128–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Z., Umek R. M., McKnight S. L. (1991) Genes Dev. 5, 1538–1552 [DOI] [PubMed] [Google Scholar]
- 38.Ron D., Habener J. F. (1992) Genes Dev. 6, 439–453 [DOI] [PubMed] [Google Scholar]
- 39.Jiang H. Y., Wek S. A., McGrath B. C., Scheuner D., Kaufman R. J., Cavener D. R., Wek R. C. (2003) Mol. Cell Biol. 23, 5651–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung J. H., Su I. J., Lei H. Y., Wang H. C., Lin W. C., Chang W. T., Huang W., Chang W. C., Chang Y. S., Chen C. C., Lai M. D. (2004) J. Biol. Chem. 279, 46384–46392 [DOI] [PubMed] [Google Scholar]
- 41.Kaneko M., Niinuma Y., Nomura Y. (2003) Biol. Pharm. Bull. 26, 931–935 [DOI] [PubMed] [Google Scholar]
- 42.Kahle P. J., Haass C. (2004) EMBO Rep. 5, 681–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araki E., Oyadomari S., Mori M. (2003) Intern. Med. 42, 7–14 [DOI] [PubMed] [Google Scholar]
- 44.Jorgensen E., Stinson A., Shan L., Yang J., Gietl D., Albino A. P. (2008) BMC Cancer 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koumenis C. (2006) Curr. Mol. Med. 6, 55–69 [DOI] [PubMed] [Google Scholar]
- 46.Orr A. W., Hahn C., Blackman B. R., Schwartz M. A. (2008) Circ. Res. 103, 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubuquoy L., Jansson E. A., Deeb S., Rakotobe S., Karoui M., Colombel J. F., Auwerx J., Pettersson S., Desreumaux P. (2003) Gastroenterology 124, 1265–1276 [DOI] [PubMed] [Google Scholar]
- 48.Kelly D., Campbell J. I., King T. P., Grant G., Jansson E. A., Coutts A. G., Pettersson S., Conway S. (2004) Nat. Immunol. 5, 104–112 [DOI] [PubMed] [Google Scholar]
- 49.von Knethen A., Soller M., Tzieply N., Weigert A., Johann A. M., Jennewein C., Köhl R., Brüne B. (2007) J. Cell Biol. 176, 681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwergal A., Quirling M., Saugel B., Huth K. C., Sydlik C., Poli V., Neumeier D., Ziegler-Heitbrock H. W., Brand K. (2006) J. Immunol. 177, 665–672 [DOI] [PubMed] [Google Scholar]
- 51.Moon Y., Yang H., Park S. H. (2008) Toxicol. Appl. Pharmacol. 231, 94–102 [DOI] [PubMed] [Google Scholar]
- 52.Von Knethen A. A., Brüne B. (2001) FASEB J. 15, 535–544 [DOI] [PubMed] [Google Scholar]
- 53.Ishida Y., Kondo T., Kimura A., Matsushima K., Mukaida N. (2006) J. Immunol. 176, 5598–5606 [DOI] [PubMed] [Google Scholar]
- 54.Egan L. J., de Lecea A., Lehrman E. D., Myhre G. M., Eckmann L., Kagnoff M. F. (2003) Am. J. Physiol. Cell Physiol. 285, C1028–C1035 [DOI] [PubMed] [Google Scholar]
- 55.Noiri E., Peresleni T., Srivastava N., Weber P., Bahou W. F., Peunova N., Goligorsky M. S. (1996) Am. J. Physiol. 270, C794–C802 [DOI] [PubMed] [Google Scholar]
- 56.Cowan M. J., Coll T., Shelhamer J. H. (2006) J. Appl. Physiol. 101, 1127–1135 [DOI] [PubMed] [Google Scholar]
- 57.Fukata M., Michelsen K. S., Eri R., Thomas L. S., Hu B., Lukasek K., Nast C. C., Lechago J., Xu R., Naiki Y., Soliman A., Arditi M., Abreu M. T. (2005) Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1055–G1065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.