Abstract
The evolutionarily conserved lethal giant larvae (Lgl) tumor suppressor gene has an essential role in establishing apical-basal cell polarity, cell proliferation, differentiation, and tissue organization. However, the precise molecular mechanism by which the Lgl carries out its function remains obscure. In the current study, we have identified Ran-binding protein M (RanBPM) as a novel binding partner of Mgl-1, a mammalian homolog of Drosophila tumor suppressor protein lethal (2) giant larvae (L(2)gl) by yeast two-hybrid screening. RanBPM seems to act as a scaffolding protein with a modulatory function with respect to Mgl-1. The Mgl-1 and RanBPM association was confirmed by co-immunoprecipitation and GST pull-down experiments. Additionally, expression of RanBPM resulted in inhibition of Mgl-1 degradation, and thereby extended the half-life of Mgl-1. Furthermore, the ability of Mgl-1 activity in cell migration and colony formation assay was enhanced by RanBPM. Taken together, our findings reveal that RanBPM plays a novel role in regulating Mgl-1 stability and contributes to its biological function as a tumor suppressor.
Keywords: Cell Migration, Protein Stability, Tumor, Tumor Suppressor, Ubiquitination
Introduction
The lethal giant larvae (Lgl)2 gene was identified as the first recessive oncogene in Drosophila (1–3). Lgl function is reported to be essential for the development of polarized epithelia (4, 5), localization of cell fate determinant Numb in Drosophila neuroblasts (6, 7) and association with cytoskeletal complex (8). It also has been reported that Lgl prevents tumor formation by antagonizing Decapentaplegic (Dpp) signaling by semaphonrin 5c in the brain (9). Lgl functions in concert with two other tumor suppressor genes: discs large (dlg) and scribble (scrib), primarily involved in maintenance of basolateral membrane domain and basal protein targeting (4, 5, 7). In addition, Lgl functions competitively with Par3 in order to make a complex with Par6-aPKC protein complexes that are crucial for the apical membrane domain (10, 11).
Homologs of Lgl have been identified in many species including human, mouse, rat, bovine, insect, worm, slime mold, and yeast (12–15). The two Lgl homologs, Lgl1 and Lgl2 have conserved function in the maintenance of cell polarity and tissue homeostasis. In mouse, changes in Lgl1 activity leads to the loss of apical junctional complex in neuroblasts and hyperplasia (16). Hugl-l, a human homolog of Lgl is strongly down-regulated in malignant melanoma (17). A significant reduction in the expression of Hugl-1 was reported in tumor tissues from colorectal cancer patients. Thus, down-regulation of Hugl-1 correlates with occurrence of colorectal cancers whereas its expression leads to increase in cell adhesion and decreased cell migration (18). Hugl-1 plays a key role in the regulation of proteins, which are involved in epithelial-mesenchymal transition (EMT), a process that enables an epithelial cell to gain mesenchymal and migratory properties (17). In mouse embryonic fibroblasts, a mutant of Mgl-1 lacking five serine residues reduced cell polarization in an in vitro wounding assay (11). Recently, it has been shown that Lgl2 acts as a tumor suppressor in zebrafish epidermis (19). These results suggest that the Lgl protein primarily acts as a tumor suppressor.
Previously, we reported the spatial expression of Mgl-1 (NM_008502.1), the mammalian homologue of Lgl tumor suppressor gene family in early embryonic development (20). Here, we report the regulation of tumor suppressor activity of Mgl-1 protein. Using yeast two-hybrid system, we identified RanBPM as a novel cellular protein involved in the regulation of Mgl-1 protein stability and function. RanBPM was originally identified as a Ran-GTPase binding protein (21, 22). RanBPM is distributed in the nucleus, cytoplasm, plasma membrane and cell junctions (22–25). RanBPM primarily acts as a scaffolding protein involved in localization of several proteins (24, 26). Recent studies on RanBPM have evidenced its function as a critical regulator contributing to protein stabilization and promoting the function of several proteins upon interaction. For example, RanBPM is involved in the stabilization of p73α and increases its proapoptotic activity (27). RanBPM interacts with the Plexin-A receptor and strongly inhibits axonal outgrowth in vitro and in vivo (28). RanBPM promotes BACE1 processing of amyloid precursor protein and amyloid β peptide generation (26). Recently, RanBPM was reported as an activator of proapoptotic pathway in response to DNA damage (29).
In this study, we showed exogenous and endogenous interaction between Mgl-1 and RanBPM by co-immunoprecipitation studies. We also demonstrated that the N-terminal region of Mgl-1 was responsible for binding with RanBPM, and the Mgl-1-interacting region in RanBPM was mapped to the N-terminal region containing SPRY domain. We showed that RanBPM contributes to the stability of Mgl-1 protein and functionally extends the half-life of Mgl-1 by preventing its protein turnover through the ubiquitin-proteasomal pathway. In summary, we propose that RanBPM is an active binding partner that robustly promotes Mgl-1 tumor suppressor activity.
EXPERIMENTAL PROCEDURES
Cell Culture
MDCK (Madin Darby Canine Kidney cell line), HEK293T (Human Embryonic Kidney cell line), and HeLa (human cervical cancer cell line) were grown in Dulbecco's modified Eagle's medium (DMEM, GIBCO BRL, Rockville, MD) supplemented with 10% fetal bovine serum (FBS, GIBCO BRL) and 1% penicillin and streptomycin (GIBCO BRL).
Yeast Two-hybrid Assay
The yeast two-hybrid Matchmaker GAL4 Two-Hybrid System 3 (Clontech, Palo Alto, CA) was employed to identify the specific binding partner for Mgl-1. Full-length Mgl-1 was cloned into pGBKT7 vector containing DNA binding domain. Mouse brain cDNA library in the vector pACT2 (Clontech) was used to screen the binding proteins. pGBKT7-Mgl-1 and mouse brain cDNA library were co-transformed into AH109 strain and cultured in yeast drop-out minimal mediums lacking leucine, tryptophan, histidine, adenine, and containing X-gal. Positive clones were picked for nucleotide sequences. From sequence result and BLAST search, we identified a list of putative binding proteins, which are encoded in cDNA plasmids. To confirm interaction between a putative binding protein and Mgl-1, we performed co-transformation of two constructs along with positive and negative controls into AH109 yeast strain and plated them on yeast drop-out minimal mediums lacking leucine, tryptophan, histidine, adenine, and confirmed by assaying for β galactosidase activity. Positive clones were sequenced and compared with reference sequences available in GenBankTM.
Construction of Expression Vectors
Isolation of full-length cDNA for Mgl-1 has been described previously (20). Mgl-1 was subcloned into pcDNA3–6myc expression vector. A mammalian expression vector encoding for FLAG-tagged RanBPM was kindly provided by Dr. Yoshiaki Ishigatsubo (Yokohama City University School of Medicine). Expression vectors were constructed in pcDNA3–6myc and pCS4–3FLAG. Expression constructs of Mgl-1 and RanBPM are as follows: pcDNA3–6myc-Mgl-1-Full, aa 1–1034; pcDNA3–6myc-Mgl-1-N1, aa 1–680; pcDNA3–6myc-Mgl-1-C, aa 638–1034; pCMV-FLAG-RanBPM-Full, aa 1–729; pCS4–3FLAG-RanBPM-N, aa 1–340; pCS4–3FLAG-RanBPM-C, aa 335–729, and pCS4–3FLAG-RanBPMΔSPRY, Δaa 212–333. The mammalian expression vector, pSilencer 1.0-U6 (Ambion, Austin, TX), was used for the expression of two RanBPM shRNAs. The insert sequences used for designing shRNA are: RanBPM gene-specific insert a 19-nucleotide sequence corresponding to nucleotides 516–534 (GGA CAA GTT CAG CTA CAT C) and 866–884 (CAA TAC CTG CTT TTA CAC C) of human RanBPM cDNA; and a scrambled shRNA (Silencer Control 3 shRNA) was used as a negative control.
Antibodies
To generate a polyclonal antibody against Mgl-1, C-terminal (788–1034 aa) fragment was subcloned in-frame into the pGEX4T-1 vector and expressed in BL21 cells. Recombinant GST-tagged protein was purified by a glutathione-Sepharose column. After the final booster injection, the whole blood was drawn and the serum was separated. A mouse anti-β-actin mAb, a mouse anti-HA mAb, anti-9E10 mAb, and a goat anti-RanBPM polyclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). In some experiments an anti-Mgl-1 antibody from Dr. Patrick Brennwald (Department of Cell and Developmental Biology, University of North Carolina at Chapel Hill, NC) and an anti-RanBPM antibody from Dr. Elisabetta Bianchi (Department of Immunology, Institute Pasteur) were used (24). A mouse anti-Flag mAb was purchased from Sigma.
Pull-down Assay
HEK293T cells were transfected with Flag-RanBPM. After 48 h of incubation, the cells were harvested and suspended in Buffer A (20 mm Tris-HCl at pH 8.0, 1 mm EDTA, 1 mm dithiothreitol, 150 mm NaCl, 1% Triton X-100) containing 1X protease inhibitor mixture (CompleteTM: Roche Applied Science, Mannheim, Germany). The cell extract was applied onto full-length of GST-Mgl-1 immobilized on 100 μl of glutathione-Sepharose beads (Pharmacia Biotech, Uppsala, Sweden). Beads were extensively washed with Buffer A. The bound proteins were eluted by boiling in the SDS sample buffer for 10 min. The samples were analyzed by 8% SDS-PAGE and immunoblotted with an anti-RanBPM antibody and an anti-GST antibody.
Immunoprecipitation and Western Blot Analysis
Myc-tagged Mgl-1 and Flag-tagged RanBPM were transfected individually and co-transfected in HEK293T and MDCK cells. After 48 h of transfection, the cells were lysed in a lysis buffer (1% Triton X, 150 mm NaCl, 50 mm Tris-HCl, pH 8, 1 mm PMSF) for 20 min. Cell lysates were centrifuged at 13,000 rpm at 4 °C, supernatant was collected into a fresh Eppendorf tube. The cell lysates were incubated with anti-Myc (9E10, Santa Cruz Biotechnology) and anti-Flag (Sigma) antibodies overnight at 4 °C. 20 μl of protein A/G Sepharose (Santa Cruz Biotechnology) was added to the lysate and incubated at 4 °C for 1 h. Beads were washed with lysis buffer for four times, elution was done with 2× SDS loading dye and boiled for 5 min. The eluted samples were loaded on to 8% SDS-PAGE gel followed by Western blotting.
Colony Formation Assay
HeLa cells were transfected with pCDNA3–6myc vector, Mgl-1, RanBPM, Mgl-1/RanBPM, Mgl-1/RanBPM shRNA1, and Mgl/RanBPM shRNA2. MDCK cells were transfected with empty vector, RanBPM, RanBPMΔSPRY, RanBPM shRNA1, and shRNA2 were equally seeded and maintained in G418 (400 μg/ml) containing culture medium. 2 weeks after selection, the colonies were fixed in ice cold methanol for 10 min, and stained with 1% crystal violet in methanol for 15 min. The colonies were finally rinsed with water, and then counted.
Wounding Assay
Migration and proliferation rates were assayed by wounding assays as previously described (30). Stably transfected MDCK cells were cultured to near 90% confluence in 100Φ dish. A line was drawn with a marker on the bottom of the dish and cell culture medium was aspirated before wounding. A scratch was made on MDCK monolayer with a sterile 200 μl pipette tip in a definite array. The wounded cell layer was washed twice with PBS and incubated in complete medium and wound closure was documented at 0 h and 16 h. Each analysis was performed in triplicate.
RESULTS
Identification of RanBPM as a Novel Mgl-1-interacting Protein
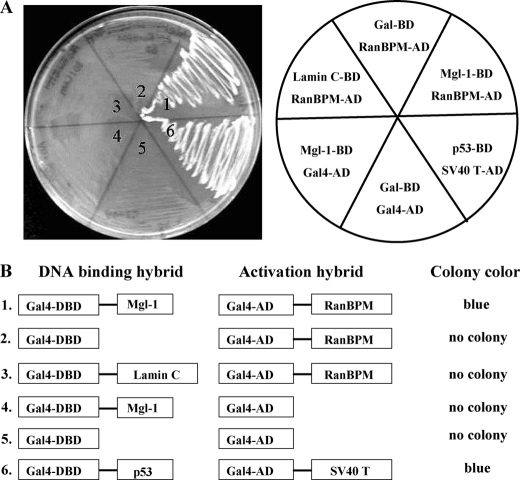
To identify novel-interacting proteins with Mgl-1, we performed the yeast two-hybrid screening using a mouse brain library with full-length Mgl-1 cDNA as bait and found RanBPM as a putative binding protein for Mgl-1 (Fig. 1A). To analyze the specificity of interaction between Mgl-1 and RanBPM, several control yeast mating experiments were performed. The co-expression of the DBD-Mgl-1 and AD-RanBPM in yeast strain AH109 was able to form blue colonies on SD/-Leu/-Trp/-His/-Ade minimal plates (Fig. 1B, lane 1). In contrast, Gal4 DNA binding domain (Gal4-DBD) did not show any affinity to bind with RanBPM (Fig. 1B, lane 2), and Gal4 activation domain (Gal4-AD) failed to bind to the Mgl-1 (Fig. 1B, lane 4). Interaction between Gal4-DBD and Gal4-AD was not observed, suggesting that there is no leaky expression of reporter genes. As a positive control, we conducted fusion between murine p53 and SV-40 large T-antigen and obtained blue colonies (Fig. 1B, lane 6).
FIGURE 1.
Identification of RanBPM as a novel interacting protein of Mgl-1. A, yeast strain AH109 strain containing the indicated BD plasmids (Mgl-1, Lamin C, p53, and Gal-BD) was co-transformed with AD plasmids (RanBPM, SV40 LT, and Gal-AD). Growth of yeast cells containing different constructs was compared by streaking onto triple drop-out plates. The Gal4 BD and the Gal4 AD were incorporated as a negative control, p53 and simian virus 40 large T-antigen were used as positive controls. B, schematic summary of the result of yeast two-hybrid assay between Mgl-1 and RanBPM along with positive and negative controls. Yeast color was observed after 3 days of incubation at 30 °C.
In Vitro and in Vivo Association between Mgl-1 and RanBPM
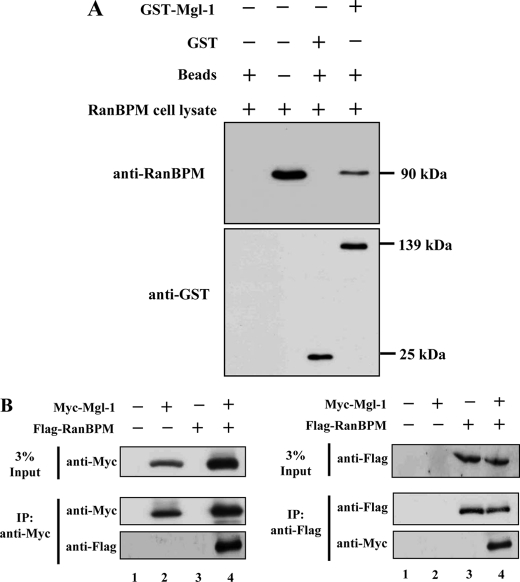
To verify the potential interaction obtained with the yeast two-hybrid screening, we performed GST pull-down assay. Flag-tagged full-length RanBPM expressed in 293T cells bound to the immobilized GST-Mgl-1 but not with GST alone (Fig. 2A). Further, we conducted binding assays by co-immunoprecipitation and Western blot analyses in mammalian cells. Myc-tagged full-length Mgl-1 and Flag-tagged full-length RanBPM were co-expressed in HEK293T cells and immunoprecipitated with either an anti-Myc or an anti-Flag antibody. Reciprocal co-immunoprecipitation of Mgl-1 and RanBPM was conducted to support the interaction between these two proteins (Fig. 2B). Our data showed both in vitro and in vivo interaction between Mgl-1 and RanBPM.
FIGURE 2.
Mgl-1 associates with RanBPM both in vitro and in vivo. A, pull-down assay. Recombinant GST or GST-Mgl-1 fusion proteins purified by glutathione-SepharoseTM 4B were incubated with RanBPM cell lysates, and the bound proteins were analyzed by immunoblotting with an anti-RanBPM antibody. B, Myc-tagged full-length Mgl-1 and Flag-tagged full-length RanBPM were co-transfected into 293T cells, and immunoprecipitation was performed by using either an anti-Myc or an anti-Flag antibody, followed by Western blotting with anti-Myc and anti-Flag antibodies.
Determination of the Binding Site of RanBPM and Mgl-1
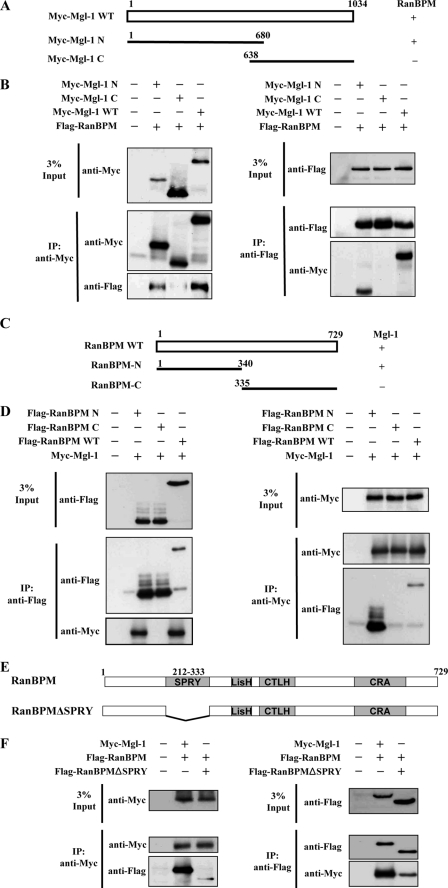
To determine the RanBPM binding region on Mgl-1, we generated the N-terminal and C-terminal constructs of Mgl-1 (Fig. 3A). RanBPM bound to both full-length and the N-terminal, but not to the C-terminal region of Mgl-1. This suggests that the site of interaction exists within amino acids 1–680 of Mgl-1 (Fig. 3B). We next determined the Mgl-1 binding site on RanBPM by generating the N-terminal and C-terminal constructs of RanBPM (Fig. 3C). Immunoprecipitation assay revealed that Mgl-1 bound to RanBPM full-length and the N terminus, but not to the C terminus (Fig. 3D). RanBPM has a modular domain structure comprising of SPRY, LISH, CTLH, and CRA domains (Fig. 3E). SPRY is the only domain located toward N terminus region of RanBPM. Therefore, we generated SPRY domain-deleted mutant RanBPM construct to verify its potential for interaction with Mgl-1 (Fig. 3E). Interestingly, SPRY domain-deleted RanBPM showed significantly less interaction with Mgl-1 when compared with RanBPM full-length (Fig. 3F). These results suggest that the SPRY domain of RanBPM contributes for the interaction between Mgl-1 and RanBPM.
FIGURE 3.
Determination of binding region between Mgl-1 and RanBPM. A, RanBPM interacts with the N terminus of Mgl-1. Schematic structure of Mgl-1 deletion constructs. Wild-type Mgl-1 (WT), Mgl-1 deletion constructs Mgl-1-N1-(1–680), Mgl-1-C-(638–1034) were used to analyze the region of Mgl-1 that interacts with RanBPM. B, 293T cells were co-transfected with FLAG-RanBPM WT, Myc-Mgl-1 WT, and Mgl-1 deletion constructs Myc-Mgl-1-N1-(1–680), and Myc-Mgl-1-C-(638–1034). Immunoprecipitation was performed with either an anti-Myc or an anti-Flag antibody, followed by Western blotting with anti-Myc and anti-Flag antibodies. C, Mgl-1 interacts with the N terminus of RanBPM. Schematic structure of RanBPM deletion constructs. Wild-type RanBPM, RanBPM deletion mutants RanBPM-N-(1–340), RanBPM-C-(335–729) were used to examine the region of RanBPM that interacts with Mgl-1. D, 293T cells were co-transfected with Myc-Mgl-1 WT and FLAG-RanBPM WT and RanBPM deletion constructs FLAG-RanBPM-N-(1–340), and FLAG-RanBPM-C-(335–729). Immunoprecipitation was performed by either an anti-Myc or an anti-Flag antibody, followed by Western blotting with anti-Myc and anti-Flag antibodies. E, Mgl-1 interacts both with RanBPM and RanBPMΔSPRY. Schematic structure of SPRY domain deletion construct of RanBPM (Δaa 212–333). F, 293T cells were transfected with Myc-Mgl-1 WT and Flag-RanBPM WT or Flag-RanBPMΔSPRY. Immunoprecipitation was performed by either an anti-Myc or an anti-Flag antibody and immunoblotted with anti-Flag and anti-Myc antibodies.
Endogenous Association of Mgl-1 with RanBPM
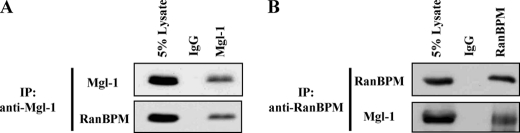
To investigate endogenous interaction between Mgl-1 and RanBPM, we checked for endogenous expression of Mgl-1 and RanBPM in several cell lines. Endogenous Mgl-1 protein expression was detected only in MDCK cells, while endogenous RanBPM protein expression was detected in most of the cell lines used for analysis (data not shown). Thus, we further assessed our endogenous studies in MDCK cells. To demonstrate endogenous interaction between Mgl-1 and RanBPM, we performed co-immunoprecipitation assay from MDCK cell lysate. We found that anti-Mgl-1 antibodies could co-precipitate endogenous RanBPM (Fig. 4A). In reciprocal co-immunoprecipitation, anti-RanBPM antibodies could co-precipitate endogenous Mgl-1 (Fig. 4B).
FIGURE 4.
Endogenous association of Mgl-1 and RanBPM. A, in vivo interaction of endogenous Mgl-1 with RanBPM in MDCK cells. Cell lysates were immunoprecipitated with an anti-Mgl-1 antibody and blotted with an anti-RanBPM antibody. B, cell lysates were immunoprecipitated with an anti-RanBPM antibody and blotted with an anti-Mgl-1 antibody.
RanBPM Stabilizes Mgl-1
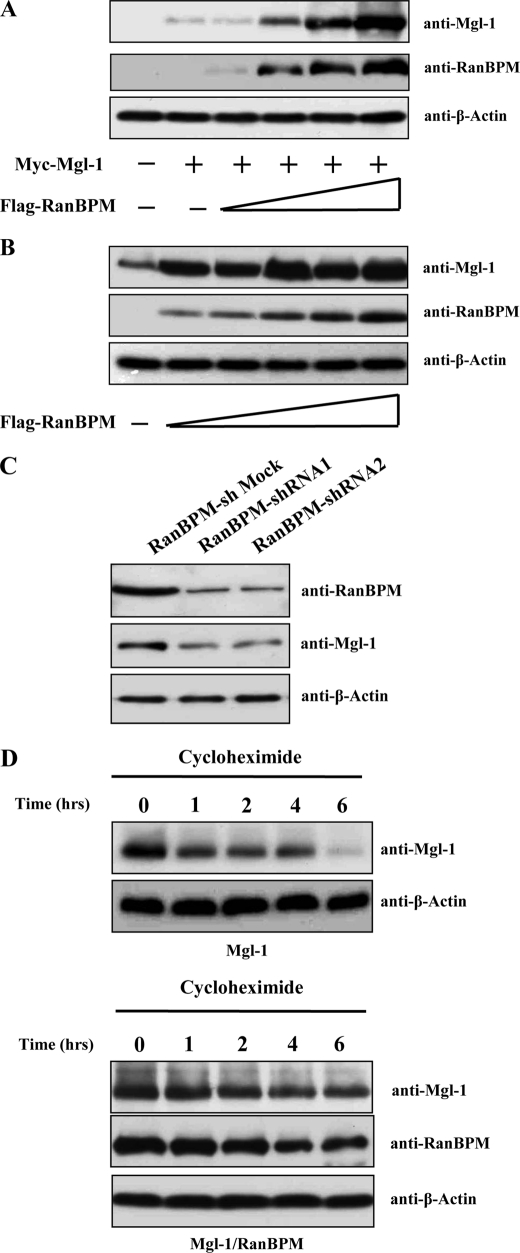
To assess the functional consequence of the interaction between Mgl-1 and RanBPM, we examined whether RanBPM affects the stabilization of Mgl-1. 293T cells were transfected with constant amount of Mgl-1 alone or together with the increasing amount of RanBPM. The level of Mgl-1 protein was dramatically increased in presence of RanBPM in a dose-dependent manner (Fig. 5A), though the Mgl-1 messenger RNA (mRNA) expression levels remained unchanged (data not shown). We further demonstrated stabilization of endogenous Mgl-1 in MDCK cells by transfecting with RanBPM in a dose-dependent manner and checked for the levels of endogenous expression of Mgl-1. The endogenous expression level of Mgl-1 was dramatically increased when the expression of RanBPM increased in a dose-dependent manner (Fig. 5B). To test the specificity of Mgl-1 stabilization conferred by interaction with RanBPM, we employed an antisense strategy to reduce the expression level of endogenous RanBPM and analyzed the status of endogenous Mgl-1 protein. We designed two sets of shRNA corresponding to nucleotides 516–534 (shRNA1) or to nucleotides 866–884 (shRNA2) of the RanBPM cDNA. MDCK cells were transfected with mock shRNA, RanBPM shRNA1 and RanBPM shRNA2. The expression of RanBPM shRNA1 and 2 resulted in significant reduction of endogenous RanBPM protein, thus leading to a corresponding decline in the expression level of endogenous Mgl-1 (Fig. 5C). These results led us to conclude that RanBPM specifically stabilizes the protein level of Mgl-1.
FIGURE 5.
RanBPM increases the stability of Mgl-1. A, RanBPM increases the expression level of Mgl-1. 293T cells were co-transfected with the constant amount of Myc-Mgl-1 (0.5 μg) together with or without the increasing amounts of Flag-RanBPM (1, 2, 3, and 4 μg). At 48 h after transfection, cell lysates were prepared, and subjected to immunoblotting with the indicated antibodies. B, RanBPM significantly increases the expression level of endogenous Mgl-1. MDCK cells were transfected with or without increasing the amounts of Flag-RanBPM, and were processed for immunoblotting as described above. C, knockdown efficiency of RanBPM-shRNA1 and RanBPM-shRNA2 was checked by Western blot analysis in MDCK cells (upper panel) and its effect on endogenous expression of Mgl-1 (middle panel). D, RanBPM increases the half-life of Mgl-1. 293T cells were transfected with Myc-Mgl-1 alone (0.5 μg) (upper panels) or together with Flag-RanBPM (2.0 μg) (bottom panels). After 48 h of transfection, cells were treated with cycloheximide (100 μg/ml) and harvested at the indicated time periods. Cell lysates were used for immunoblotting with the indicated antibodies.
RanBPM Extends the Half-life of Mgl-1
We determined the half-life of Mgl-1 and wished to investigate the half-life of Mgl-1 in the presence of RanBPM. For this purpose, 293T cells were transfected with Myc-Mgl-1 together with or without Flag-RanBPM. At 48 h post-transfection, cells were treated with cycloheximide (100 μg/ml). Cells were harvested at different time intervals and analyzed for Mgl-1 by Western blotting. Ectopically expressed Mgl-1 was found to have a half-life of less than 4 h, whereas the degradation level of Mgl-1 was greatly reduced in the presence of RanBPM (Fig. 5D). This further signifies RanBPM-mediated stabilization of Mgl-1 toward enhancement of its half-life.
RanBPM Prevents Mgl-1 Ubiquitination
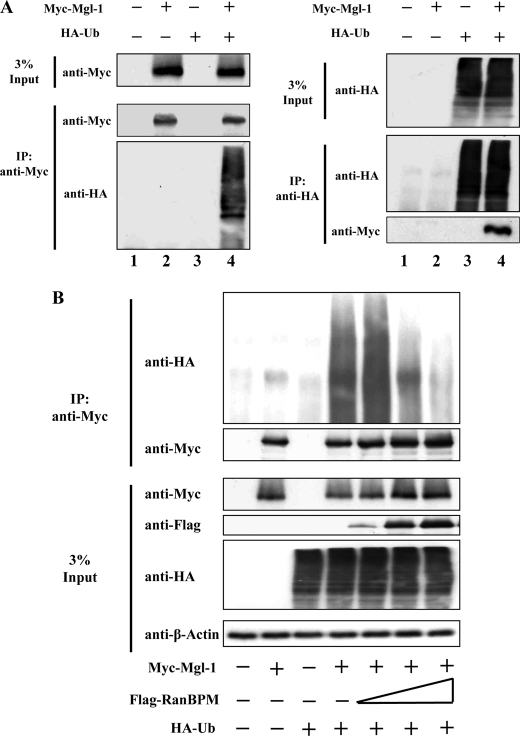
We further investigated whether Mgl-1 protein levels are turned over by the ubiquitin-proteasomal pathway. Firstly, to determine whether Mgl-1 gets ubiquitinated, 293T cells were transfected with Myc-Mgl-1 and HA-ubiquitin, and co-immunoprecipitated. A smear of ubiquitinated proteins was observed when immunoprecipitated with an anti-Myc antibody and blotted with an anti-HA antibody to detect ubiquitinated proteins (Fig. 6A). This observation is indicative of the fact that Mgl-1 undergoes polyubiquitination and protein turnover is regulated through the ubiquitin-proteasomal pathway.
FIGURE 6.
RanBPM inhibits ubiquitination of Mgl-1. A, in vivo ubiquitination of Mgl-1. 293T cells were transfected with Myc-Mgl-1 and HA-ubiquitin individually or together. Ubiquitination of Mgl-1 was confirmed by co-immunoprecipitation with an anti-Myc antibody and the counter blot was detected by an anti-HA antibody. B, RanBPM decreases Mgl-1 ubiquitination level. 293T cells were transfected with constant amount of Myc-Mgl-1 and HA-ubiquitin, together with or without the increasing amount of FLAG-RanBPM. Ubiquitination level was checked by co-immunoprecipitation with an anti-Myc antibody and immunoblotted with an anti-HA antibody.
A previous report (27), suggests that RanBPM contributes to the stability of p73 protein by preventing its degradation through the ubiquitin-proteasomal pathway. Thus, we further hypothesized that the increase in half-life of Mgl-1 may be mediated by a similar mechanism involving prevention of ubiquitination and subsequent turnover via proteasomal pathway. To investigate whether ubiquitination of Mgl-1 was affected by RanBPM, we co-transfected constant amount of Mgl-1 and HA-ubiquitin in combination with increasing amounts of RanBPM. Interestingly, the ubiquitinated proteins were dramatically reduced in cells expressing RanBPM in a dose-dependent manner (Fig. 6B).
RanBPM Inhibits Mgl-1-mediated Cell Proliferation
The RNA interference method was used to examine the impact of reduced RanBPM level on Mgl-1 function. It is known that Lgl-1 family proteins show strong reduction in colony formation and migration in various cell lines (17). To demonstrate the role of RanBPM on stabilizing Mgl-1, we investigated its effect on cell growth by colony formation assay. HeLa cells were transfected with a mock control, Mgl-1, Mgl-1, and RanBPM, Mgl-1 and RanBPM shRNA1 or RanBPM shRNA2. Cells were maintained in the presence of G418 (400 μg/ml) for 2 weeks. The results showed significantly reduced number of colony formation on plates transfected with Mgl-1 and RanBPM in comparison with Mgl-1 alone. In contrast, Mgl-1 and shRNA of RanBPM-transfected plates showed similar number of colonies as Mgl-1 alone (Fig. 7, A and B). Thus, our result suggests an enhancement of the tumor suppression function of Mgl-1 by inhibition of colony formation ability through its interaction with RanBPM. Furthermore, we demonstrated the negative regulatory activity of RanBPM on cell proliferation ability of endogenous Mgl-1 in MDCK cells (Fig. 7C). MDCK cells exhibiting endogenous Mgl-1 were transfected with various constructs of RanBPM along with a mock control. RanBPM significantly reduced the number of colonies formed on the plates in comparison to the mock control. Additionally, the number of colonies formed on RanBPMΔSPRY transfected plates was higher than the RanBPM transfected plates (Fig. 7, C and D). Our results are suggestive of RanBPM ability to interact and stabilize Mgl-1 expression to effect inhibition of cell proliferation. RanBPMΔSPRY having lesser interaction with Mgl-1 showed a correspondingly lower inhibitory effect of Mgl-1 on cell proliferation.
FIGURE 7.
RanBPM inhibits Mgl-1 cell proliferation. A, Colony formation assay. HeLa cells transfected with respective constructs and kept in the medium containing G418 (400 μg/ml), and surviving colonies were counted after 2 weeks. B, results represent the average number of colonies formed from three individual experiments. n = 3. *, p < 0.05. C, MDCK cells transfected with empty vector, RanBPM, RanBPMΔSPRY, RanBPM shRNA1, and RanBPM shRNA2 were selected in the DMEM medium containing G418 for 2 weeks. D, results represent the average number of colonies formed from three individual experiments. n = 3. *, p < 0.05.
RanBPM Inhibits Mgl-1-mediated Cell Migration
To further evaluate the interaction between RanBPM and Mgl-1 to affect cell migration and proliferation, wound healing assay was performed (Fig. 8A). Mgl-1-transfected cells showed significant reduction in migration when compared with the controls. The co-transfection of Mgl-1 and RanBPM displayed significantly reduced migration in comparison with cells transfected with Mgl-1 alone (Fig. 8B). In addition, we demonstrated the inhibitory effect of RanBPM on migration ability of MDCK cells that express endogenous Mgl-1. For this purpose, we transfected only RanBPM constructs such as RanBPM, RanBPMΔSPRY and RanBPM shRNA1 or RanBPM shRNA2 and observed their effects on endogenous Mgl-1 cell migration (Fig. 8C). Analysis of the migratory behavior of the transfected MDCK cells indicates that RanBPM reduced endogenous Mgl-1 cell migration activity, but RanBPMΔSPRY did not show any significant effect on endogenous Mgl-1 cell migration. RanBPM shRNA1 and RanBPM shRNA2 showed similar migratory behavior as that of mock control (Fig. 8D). Taken together, it is suggested that RanBPM has a stabilizing effect on Mgl-1 and it enhances the tumor suppressor activity of Mgl-1 by reducing cell migratory ability in mammalian cell lines.
FIGURE 8.
RanBPM inhibits Mgl-1 cell migration. A, stably transfected MDCK cells with respective constructs were checked for Mgl-1-mediated migratory and invasive potential by wound healing assays. Images were captured at a time interval between 0 and 16 h. Assays were performed in triplicate. B, percentage of migration was statistically analyzed from three separate experiments. n = 3. *, p < 0.05. C, stably transfected MDCK cells with empty vector, RanBPM, RanBPMΔSPRY, RanBPM shRNA1, and RanBPM shRNA2 were analyzed for endogenous Mgl-1 cell migration by wound healing assay. D, percentage of migration was statistically analyzed from three separate experiments. n = 3. *, p < 0.05.
DISCUSSION
In this study, we investigated the interaction and regulatory effect of RanBPM on the function of Mgl-1, the mammalian homologue of Drosophila tumor suppressor Lgl. We initiated this study to determine if the tumor suppressor function of Mgl-1 can be regulated by any cellular protein through screening for potential Mgl-1-interacting candidate proteins using yeast two-hybrid systems. Here, we describe novel structural and functional intracellular interactions between Mgl-1 and RanBPM, a membrane scaffolding protein primarily characterized as a binding protein for Ran, a small GTPase. We validated the exogenous and endogenous interaction between Mgl-1 and RanBPM by GST pull-down assay and co-immunoprecipitation experiments. Further, we determined by means of co-immunoprecipitation assays that the N-terminal region of Mgl-1 binds to the N-terminal region of RanBPM. To determine the functional significance of their interaction, we examined the possible effect of RanBPM on the Mgl-1 tumor suppressor function. RanBPM was shown to stabilize Mgl-1 protein in a dose-dependent manner and extend its half-life. Our experiments suggest that this function is modulated by RanBPM preventing the ubiquitination and subsequent cellular turnover of Mgl-1 protein via proteasomal degradation pathway. Finally, we showed that RanBPM enhances tumor suppressor activity of Mgl-1 by performing migration and colony formation assays.
Disruption of cell polarity plays an important role as proposed in the models that explain EMT contributing to cancer development or progression. A number of evidences suggest that Lgl family proteins are responsible for asymmetric cell division. Lgl-1 is necessary for the regulation of asymmetric cell division and cell fate determination in developing mammalian brain through proper Numb localization (16). Loss of Lgl-1 causes an expansion of neural progenitor cells and an overall increase in cell proliferation (16). Mutated Lgl in Drosophila showed a metastatic behavior leading to uncontrolled cell proliferation (31). Apart from Lgl-1, many other proteins including Scribble and Dlg are involved in the regulation of cell adhesion, maintenance of tissue architecture, suppression of tumor growth and invasion (4, 32). Loss of Lgl-2 function leads to over-proliferation of epidermal cells in zebrafish (33). Recent report indicate that the pen/lgl2 mutants activate ErbB signaling resulting in the promotion of EMT through delocalization of E-cadherin as well as proliferation (19). There is also a significant correlation between loss of Hugl-1 and disease progression (17, 18, 34). Several reports indicate that loss of cell-cell and cell-matrix contacts and aberration in the cell cytoskeletal organization results in early development of neoplastic diseases (35), during which proteins involved in EMT process are grossly deregulated (36, 37). The expression of Hugl-1 was reported to regulate the expression of matrix-metalloproteinases (MMPs) and cell-cell adhesion molecules. The Hugl-1 expressing cell lines reduced their migratory potential with transcriptional up-regulation of the cell adhesion molecule E-cadherin, while strongly down-regulate the expression level of MMP-9, MMP-14, notch4, fibronectin and β-catenin which are known to be involved in the EMT process (17). Aberrant splicing of Hugl-1 mRNA results in hepatocellular carcinoma progression (38). Previously, we characterized that expression of Mgl-1, the mammalian homologue of Drosophila tumor suppressor Lgl, can partially restore salt tolerance ability in yeast (20), further we showed that the conserved amino acids at WD-40 repeats motifs are associated with salt tolerance and temperature sensitivity in yeast (40).
To identify the possible cellular proteins involved in the regulation of Mgl-1 protein stability, we screened a cDNA library derived from mouse brain using the full-length Mgl-1 as bait in a yeast two-hybrid screening system. RanBPM was found to have high β-galactosidase activity and was repeatedly isolated (Fig. 1). RanBPM, a Ran-binding protein is expressed widely in most of the tissues and cell lines (41). The expression of RanBPM was found to be elevated in many tumor cell lines (42). RanBPM has about 96% of high homology of amino acid sequence between human RanBPM (NM_005493) and mouse RanBPM (NM_019930). RanBPM is a multifunctional protein with a variety of intracellular interaction with proteins including MET (41), androgen receptor (43), HIPK2 (44), USP11 (45), Twa1 (25), calbindin D28K (47), p75NTR (48), TrkA (49), muskelin (50), LRP (26), and MOP (51), suggesting that RanBPM is involved in diverse biological processes. In particular, RanBPM was shown to interact with p73 and Ache to promote cell apoptosis (27, 52).
In the present study, we confirmed the interaction between Mgl-1 and RanBPM by GST pull-down and co-immunoprecipitation assays. In support of yeast two-hybrid results, these observations suggest that Mgl-1 strongly binds to RanBPM (Fig. 2). Mgl-1 has a conserved WD-40 repeat domain at its N-terminal region which is responsible for protein-protein interactions (53). RanBPM has four putative domains: SPRY, LISH, CTLH, and CRA (54). SPRY domain at the N-terminal region in RanBPM was characterized for protein-protein interactions (41, 55, 56). In agreement to the previous findings, we have shown that the N-terminal region of Mgl-1 containing WD-40 repeat domains interacts with the N-terminal region of RanBPM containing SPRY domain. Furthermore, SPRY domain deleted RanBPM showed lesser interaction with Mgl-1 in comparison with full-length RanBPM (Fig. 3). We also performed co-immunoprecipitation experiment to confirm the endogenous interaction between Mgl-1 and RanBPM (Fig. 4).
The functional interaction of RanBPM with Mgl-1 could affect the stability of Mgl-1 protein contributes to an increase in the half-life of Mgl-1 (Fig. 5). The stability of such tumor suppressor proteins is very essential for the normal cell cycle process. It is also apparent that most of cellular proteins have to undergo ubiquitin-mediated protein degradation to maintain cellular homeostasis. Ubiquitin is attached to the target proteins in order to regulate half-life, localization and activity of targeted proteins (57). The process of ubiquitination is a well-established event involving a complex yet organized milieu of ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligase (E3) enzymes that mediate ligation of ubiquitin to the lysine residues of proteins that are targeted to the 26 S proteasome for degradation (39, 46, 58). The stability of Mgl-1 by RanBPM due to its interaction led us to investigate whether Mgl-1 protein is regulated at least in part through the ubiquitin-proteasomal pathway. In our study, we showed that Mgl-1 interacts with ubiquitin to undergo polyubiquitination. We further showed that RanBPM increased the stability of Mgl-1 protein by decreasing its ubiquitination level (Fig. 6). RanBPM was reported as a crucial regulator of cell proliferation, differentiation, and apoptosis as well as migration (27, 41). The tumor suppressor function of Mgl-1 was examined in the presence of its interacting partner RanBPM. Our data suggest that RanBPM interacts with Mgl-1, thus acting in concert to suppress cell proliferation and migration in mammalian cell lines (Figs. 7 and 8).
In conclusion, our studies demonstrate exogenous as well as endogenous interaction between Mgl-1 and RanBPM and the functional significance thereof. We showed that RanBPM prevents Mgl-1 ubiquitination through 26 S proteasomal degradation pathway and thus contributes to its stability by increasing its half-life. Additionally, RanBPM enhances the tumor suppressor activity of Mgl-1 by suppressing cell proliferation and cell migration ability. Our findings implicate a novel role of RanBPM in the regulation of Mgl-1 stability and function.
Acknowledgments
We thank Professor Yoshiaki Ishigatsubo at Yokohama City University, Japan for the pCMV-FLAG-RanBPM construct, Professor Elisabetta Bianchi at Pasteur Institute, France for the RanBPM monoclonal antibody, and Professor Patrick Brennwald at University of North Carolina at Chapel Hill for providing us the anti-Mgl-1 antibody. We also thank Sai Kiran Sharma at University of Alberta, Canada for his critical comments on our manuscript. We thank the members of Baek's laboratory at CHA University for helpful discussion.
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (00001602).
- Lgl
- lethal giant larvae
- Gal4-DBD
- Gal4 DNA binding domain
- Gal4-AD
- Gal4 activation domain
- MDCK
- Madin Darby Canine Kidney cell line
- Mgl-1
- mammalian homolog of Drosophila tumor suppressor protein lethal giant larvae
- RanBPM
- Ran-binding protein M
- aa
- amino acid
- MMP
- matrix-metalloproteinases.
REFERENCES
- 1.Baek K. H. (1999) Mutat Res. 436, 131–136 [DOI] [PubMed] [Google Scholar]
- 2.Baek K. H. (2004) Int. J. Oncol. 24, 1257–1261 [PubMed] [Google Scholar]
- 3.Gateff E. (1978) Science 200, 1448–1459 [DOI] [PubMed] [Google Scholar]
- 4.Bilder D., Li M., Perrimon N. (2000) Science 289, 113–116 [DOI] [PubMed] [Google Scholar]
- 5.Müsch A., Cohen D., Yeaman C., Nelson W. J., Rodriguez-Boulan E., Brennwald P. J. (2002) Mol. Biol. Cell. 13, 158–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohshiro T., Yagami T., Zhang C., Matsuzaki F. (2000) Nature 408, 593–596 [DOI] [PubMed] [Google Scholar]
- 7.Peng C. Y., Manning L., Albertson R., Doe C. Q. (2000) Nature 408, 596–600 [DOI] [PubMed] [Google Scholar]
- 8.Strand D., Jakobs R., Merdes G., Neumann B., Kalmes A., Heid H. W., Husmann I., Mechler B. M. (1994) J. Cell. Biol. 127, 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodhouse E. C., Fisher A., Bandle R. W., Bryant-Greenwood B., Charboneau L., Petricoin E. F., 3rd., Liotta L. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11463–11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betschinger J., Mechtler K., Knoblich J. A. (2003) Nature 422, 326–330 [DOI] [PubMed] [Google Scholar]
- 11.Plant P. J., Fawcett J. P., Lin D. C., Holdorf A. D., Binns K., Kulkarni S., Pawson T. (2003) Nat. Cell. Biol. 5, 301–308 [DOI] [PubMed] [Google Scholar]
- 12.Baek K. H., Kim Y. S., Jung S., Lee K. Y., Choi H. K., Kim K. S. (2002) Int. J. Oncol. 20, 739–744 [PubMed] [Google Scholar]
- 13.Kim Y. S., Baek K. H., Lee K. Y., Chung H. M., Lee K. A., Ko J. J., Cha K. Y. (2002) Int. J. Oncol. 20, 1219–1225 [PubMed] [Google Scholar]
- 14.Kuwabara K., Takahashi Y., Tomotsune D., Takahashi N., Kominami R. (1994) Genomics 20, 337–338 [DOI] [PubMed] [Google Scholar]
- 15.Strand D., Unger S., Corvi R., Hartenstein K., Schenkel H., Kalmes A., Merdes G., Neumann B., Krieg-Schneider F., Coy J. F. (1995) Oncogene 11, 291–301 [PubMed] [Google Scholar]
- 16.Klezovitch O., Fernandez T. E., Tapscott S. J., Vasioukhin V. (2004) Genes Dev. 18, 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuphal S., Wallner S., Schimanski C. C., Bataille F., Hofer P., Strand S., Strand D., Bosserhoff A. K. (2006) Oncogene 25, 103–110 [DOI] [PubMed] [Google Scholar]
- 18.Schimanski C. C., Schmitz G., Kashyap A., Bosserhoff A. K., Bataille F., Schäfer S. C., Lehr H. A., Berger M. R., Galle P. R., Strand S., Strand D. (2005) Oncogene 24, 3100–3109 [DOI] [PubMed] [Google Scholar]
- 19.Reischauer S., Levesque M. P., Nüsslein-Volhard C., Sonawane M. (2009) PLoS Genet. 5, e1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y. S., Song J., Kim Y., Kim I. O., Kang I., Baek K. H. (2003) Int. J. Oncol. 23, 1515–1519 [PubMed] [Google Scholar]
- 21.Nakamura M., Masuda H., Horii J., Kuma K., Yokoyama N., Ohba T., Nishitani H., Miyata T., Tanaka M., Nishimoto T. (1998) J. Cell. Biol. 143, 1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishitani H., Hirose E., Uchimura Y., Nakamura M., Umeda M., Nishii K., Mori N., Nishimoto T. (2001) Genes Dev. 272, 25–33 [DOI] [PubMed] [Google Scholar]
- 23.Chang Y., Paramasivam M., Girgenti M. J., Walikonis R. S., Bianchi E., LoTurco J. J. (2010) Dev. Neurobiol. 70, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denti S., Sirri A., Cheli A., Rogge L., Innamorati G., Putignano S., Fabbri M., Pardi R., Bianchi E. (2004) J. Biol. Chem. 279, 13027–13034 [DOI] [PubMed] [Google Scholar]
- 25.Umeda M., Nishitani H., Nishimoto T. (2003) Genes Dev. 303, 47–54 [DOI] [PubMed] [Google Scholar]
- 26.Lakshmana M. K., Yoon I. S., Chen E., Bianchi E., Koo E. H., Kang D. E. (2009) J. Biol. Chem. 284, 11863–11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer S., Ozaki T., Miyazaki K., Kato C., Hanamoto T., Nakagawara A. (2005) Oncogene 24, 938–944 [DOI] [PubMed] [Google Scholar]
- 28.Togashi H., Schmidt E. F., Strittmatter S. M. (2006) J. Neurosci. 26, 4961–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atabakhsh E., Bryce D. M., Lefebvre K. J., Schild-Poulter C. (2009) Mol. Cancer Res. 7, 1962–1972 [DOI] [PubMed] [Google Scholar]
- 30.Qiao M., Shapiro P., Fosbrink M., Rus H., Kumar R., Passaniti A. (2006) J. Biol. Chem. 281, 7118–7128 [DOI] [PubMed] [Google Scholar]
- 31.Bilder D. (2001) Curr. Biol. 11, R132–R135 [DOI] [PubMed] [Google Scholar]
- 32.Pagliarini R. A., Xu T. (2003) Science 302, 1227–1231 [DOI] [PubMed] [Google Scholar]
- 33.Sonawane M., Carpio Y., Geisler R., Schwarz H., Maischein H. M., Nuesslein-Volhard C. (2005) Development 132, 3255–3265 [DOI] [PubMed] [Google Scholar]
- 34.Tsuruga T., Nakagawa S., Watanabe M., Takizawa S., Matsumoto Y., Nagasaka K., Sone K., Hiraike H., Miyamoto Y., Hiraike O., Minaguchi T., Oda K., Yasugi T., Yano T., Taketani Y. (2007) Oncol. Res. 16, 431–435 [DOI] [PubMed] [Google Scholar]
- 35.Hendrix M. J., Seftor E. A., Chu Y. W., Trevor K. T., Seftor R. E. (1996) Cancer Metastasis Rev. 15, 507–525 [DOI] [PubMed] [Google Scholar]
- 36.Pla P., Moore R., Morali O. G., Grille S., Martinozzi S., Delmas V., Larue L. (2001) J. Cell. Physiol. 189, 121–132 [DOI] [PubMed] [Google Scholar]
- 37.Tester A. M., Ruangpanit N., Anderson R. L., Thompson E. W. (2000) Clin. Exp. Metastasis. 18, 553–560 [DOI] [PubMed] [Google Scholar]
- 38.Lu X., Feng X., Man X., Yang G., Tang L., Du D., Zhang F., Yuan H., Huang Q., Zhang Z., Liu Y., Strand D., Chen Z. (2009) Clin. Cancer. Res. 15, 3287–3296 [DOI] [PubMed] [Google Scholar]
- 39.Koegl M., Hoppe T., Schlenker S., Ulrich H. D., Mayer T. U., Jentsch S. (1999) Cell 96, 635–644 [DOI] [PubMed] [Google Scholar]
- 40.Kim Y. K., Kim Y. S., Baek K. H. (2005) Biochem. Biophys. Res. Commun. 331, 922–928 [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Li Z., Messing E. M., Wu G. (2002) J. Biol. Chem. 277, 36216–36222 [DOI] [PubMed] [Google Scholar]
- 42.Emberley E. D., Gietz R. D., Campbell J. D., HayGlass K. T., Murphy L. C., Watson P. H. (2002) BMC Cancer 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao M. A., Cheng H., Quayle A. N., Nishitani H., Nelson C. C., Rennie P. S. (2002) J. Biol. Chem. 277, 48020–48027 [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Marion Schneider E., Li X., Duttenhöfer I., Debatin K., Hug H. (2002) Biochem. Biophys. Res. Commun. 297, 148–153 [DOI] [PubMed] [Google Scholar]
- 45.Ideguchi H., Ueda A., Tanaka M., Yang J., Tsuji T., Ohno S., Hagiwara E., Aoki A., Ishigatsubo Y. (2002) Biochem. J. 367, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amerik A. Y., Hochstrasser M. (2004) Biochim Biophys. Acta. 1695, 189–207 [DOI] [PubMed] [Google Scholar]
- 47.Lutz W., Frank E. M., Craig T. A., Thompson R., Venters R. A., Kojetin D., Cavanagh J., Kumar R. (2003) Biochem. Biophys. Res. Commun. 303, 1186–1192 [DOI] [PubMed] [Google Scholar]
- 48.Bai D., Chen H., Huang B. R. (2003) Biochem. Biophys. Res. Commun. 309, 552–557 [DOI] [PubMed] [Google Scholar]
- 49.Yuan Y., Fu C., Chen H., Wang X., Deng W., Huang B. R. (2006) Neurosci Lett. 407, 26–31 [DOI] [PubMed] [Google Scholar]
- 50.Valiyaveettil M., Bentley A. A., Gursahaney P., Hussien R., Chakravarti R., Kureishy N., Prag S., Adams J. C. (2008) J. Cell. Biol. 182, 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talbot J. N., Skifter D. A., Bianchi E., Monaghan D. T., Toews M. L., Murrin L. C. (2009) Neurosci Lett. 466, 154–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong X., Ye W., Zhou H., Ren X., Li Z., Zhou W., Wu J., Gong Y., Ouyang Q., Zhao X., Zhang X. (2009) Acta. Biochim Biophys Sin. 41, 883–891 [DOI] [PubMed] [Google Scholar]
- 53.Li D., Roberts R. (2001) Cell. Mol. Life. Sci. 58, 2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menon R. P., Gibson T. J., Pastore A. (2004) J. Mol. Biol. 343, 43–53 [DOI] [PubMed] [Google Scholar]
- 55.Ponting C., Schultz J., Bork P. (1997) Trends Biochem. Sci. 22, 193–194 [DOI] [PubMed] [Google Scholar]
- 56.Wu Y., Sun X., Kaczmarek E., Dwyer K. M., Bianchi E., Usheva A., Robson S. C. (2006) Biochem. J. 396, 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glickman M. H., Ciechanover A. (2002) Physiol. Rev. 82, 373–428 [DOI] [PubMed] [Google Scholar]
- 58.Ciechanover A. (1998) EMBO J. 17, 7151–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]