Abstract
LAT (linker for activation of T cells) is a transmembrane adaptor protein that plays an essential role in TCR-mediated signaling and thymocyte development. Because LAT-deficient mice have an early block in thymocyte development, we utilized an inducible system to delete LAT in primary T cells to study LAT function in T cell activation, homeostasis, and survival. Deletion of LAT caused primary T cells to become unresponsive to stimulation from the TCR and impaired T cell homeostatic proliferation and long term survival. Furthermore, deletion of LAT led to reduced expression of Foxp3, CTLA-4, and CD25 in Treg cells and impaired their function. Consequently, mice with LAT deleted developed a lymphoproliferative syndrome similar to that in LATY136F mice, although less severe. Our data implicate that LAT has positive and negative roles in the regulation of mature T cells.
Keywords: Adaptor Proteins, Immunology, Signal Transduction, T-cell Receptor, Tyrosine-protein Kinase (Tyrosine Kinase)
Introduction
Upon engagement of the TCR (T cell receptor),3 LAT (linker for activation of T cells) is phosphorylated at multiple tyrosine residues by ZAP-70 kinase (1) and functions as a protein scaffold to assemble a large membrane-tethered signalosome (1–3). LAT binding of Gads leads to the membrane recruitment of SLP-76, which in turn interacts with Itk, as well as Vav1 and PLC-γ1. Through its interaction with LAT and SLP-76, PLC-γ1 is also brought to the plasma membrane. Upon activation by ZAP-70 and Itk, PLC-γ1 hydrolyzes phosphatidylinositol 4,5-bisphosphate into the secondary messengers inositol trisphosphate and diacylglycerol (4, 5). Inositol trisphosphate induces Ca2+ mobilization, whereas diacylglycerol binds to RasGRP1 to activate the Ras-MAPK pathway (6, 7). LAT also contributes to Ras-MAPK activation through recruitment of the Grb2-Sos complex to the plasma membrane to activate Ras (1, 8). Initiation of these signaling cascades eventually leads to activation of transcription factors that regulate the genes critical for T cell proliferation and effector functions.
The essential role of LAT in T cell activation was initially demonstrated in LAT-deficient Jurkat T cells. Although the proximal signals upstream of LAT phosphorylation remain intact in these cells, TCR-mediated calcium mobilization, Ras-MAPK activation, and NFAT activation are all abolished (9, 10). LAT function during T cell development has also been well characterized. Thymocyte development in LAT−/− mice is completely blocked at the CD25+CD44− DN3 stage, indicating an absolute requirement for LAT in pre-TCR signaling (11). Our recent studies using LAT conditional knock-out mice show that LAT is also required during thymocyte development at the DP to SP transition, as positive selection in LAT-deficient DP thymocytes is markedly compromised in these mice. Consequently, the maturation of SP thymocytes is severely blocked (12). In addition, a point mutation at LAT tyrosine 136 (Y136F), which specifically abolishes the LAT-PLC-γ1 interaction, renders both positive and negative thymic selection processes defective in the LATY136F knock-in mice (13), highlighting the importance of LAT-mediated signaling in the regulation of thymocyte development.
Although early studies using LAT-deficient Jurkat cells clearly indicate the importance of LAT in TCR-mediated signaling, not many studies have focused on its function in primary T cells. This issue is complicated by the fact that mature αβ T cells are absent in LAT−/− mice (11). Additionally, LAT4YF knock-in mice harboring mutations of the corresponding tyrosines (Tyr-136, -175, -195, and -235) exhibit the same phenotype as LAT−/− mice (14, 15). In LAT3YF knock-in mice with mutations at the Tyr-175, -195, and -235 residues, αβ T cell development is also totally blocked at the DN3 thymocyte stage (15). Studies using CD4+ T cells from LATY136F mice show that the loss of the LAT-PLC-γ1 interaction leads to diminished PLC-γ1 activation and calcium mobilization (16, 17). Surprisingly, ERK activation is normal in these T cells despite the low level of surface TCR expression (17). Because these mutant T cells are hyperproliferative and are present in an environment with high levels of Th2 cytokines, those results may not truly reflect the function of LAT in mature T cells under normal physiological conditions. Moreover, whether LAT regulates resting T cell homeostasis and cell survival has yet to be fully understood. Previous studies have shown that both homeostatic proliferation and long term survival of T cells require signaling after the engagement of the TCR with self-peptide·MHC complexes. One such study showed that CD8+ and CD4+ T cells fail to undergo proliferation upon transfer into lymphopenic hosts that lack MHC class I and class II molecules, respectively (18, 19). Furthermore, T cells lacking expression of the TCR exhibit defective long term survival (20, 21). Similarly, the disruption of TCR signaling by deleting Src family PTKs, Lck and Fyn, also leads to a shortened T cell lifespan (22). These data underscore the importance of TCR signaling in regulating T cell homeostasis and cell survival. However, the specific components of such basal TCR signaling in resting T cells have not been clearly identified.
To investigate LAT function in primary T cells, we used the ERCre transgenic system to induce deletion of LAT in T cells. Our results showed that LAT deficiency severely diminished TCR-mediated calcium mobilization as well as Ras-ERK, Akt, and NFκB activation, rendering T cells unresponsive to TCR stimulation. Deletion of LAT also impaired long term cell survival and lymphopenia-driven homeostatic proliferation of naive T cells. In addition, deletion of LAT led to reduced expression of Foxp3, CTLA-4, and CD25 in Treg cells and impaired their suppressive function. Furthermore, over time, the ablation of LAT in peripheral T cells induced a LATY136F-like lymphoproliferative syndrome.
EXPERIMENTAL PROCEDURES
Mice
The LATf/f knock-in mouse model used in this study was described previously (12). The ERCre transgenic line was kindly provided by Dr. Thomas Ludwig (Columbia University, New York). All mice were used in accordance with the National Institutes of Health guidelines. The procedures performed for this study were reviewed and approved by the Duke University Institutional Animal Care and Use Committee. Mice were housed in specific pathogen-free conditions at the Duke University animal facility.
In Vivo Deletion of LAT and Reconstitution of LAT−/− Mice
Tamoxifen (Sigma) was dissolved in autoclaved corn oil to the final concentration of 10 mg/ml. Eight to 10-week-old ERCre+LATf/−, ERCre+LATf/+, and LATf/− mice were injected intraperitoneally with 100 μl of tamoxifen on 2 consecutive days. For short term deletion, tamoxifen-treated mice were sacrificed 4–5 days after the first injection. For long term deletion, mice were initially treated twice as described above and then treated once a week for 4 additional weeks.
For reconstitution of LAT−/− mice, single-cell suspensions were prepared from the spleens of ERCre+LATf/− or LATf/− mice. 2 × 107 splenocytes were injected into 6-week-old LAT−/− recipients via tail veins. For reconstitution of LAT−/− mice with normal Treg cells and ERCre+LATf/− T cells, CD4+CD25+ T cells from Thy1.1+ B6 mice were purified using a Treg separation kit (StemCell Technology), and CD4+ T cells from Thy1.2+ ERCre+LATf/− or LATf/− mice were purified using a CD4+ T cell separation kit (StemCell Technology). 1.1 × 106 purified Thy1.1+ CD4+CD25+ T cells were mixed with 2.2 × 106 total CD4+ T cells from Thy1.2+ ERCre+LATf/− mice and intravenously injected into LAT−/− recipients. For control groups, LAT−/− recipients received 3.3 × 106 CD4+ cells from either Thy1.2+ERCre+LATf/− mice or Thy1.2+ LATf/− mice. Five weeks after reconstitution, these mice were treated with tamoxifen for 4 weeks as described above before FACS analysis.
Flow Cytometry Analysis
Texas red-conjugated anti-IgM was purchased from Southern Biotech. 7-Aminoactinomycin D was purchased from Invitrogen. All other fluorochrome-conjugated antibodies were purchased from eBioscience. For staining of cell surface markers, single cell suspensions were first incubated with anti-CD32/16 (2.4G2) and then stained with different antibodies. For intracellular staining of Foxp3 and CTLA-4, cells were fixed, permeabilized, and stained with antibodies using the eBioscience Foxp3 staining buffer set per the manufacturer's guidelines. For intracellular staining of IL-4 and IFN-γ, splenocytes were either left untreated or stimulated with PMA (20 ng/ml) and ionomycin (0.5 μg/ml) for 1 h. GolgiStopTM (BD Biosciences) was then added to the culture. Four hours later, cells were intracellularly stained with the appropriate antibodies using the eBioscience Foxp3 staining buffer set. FACS data were collected on FACSCanto or FACSVantage (BD Biosciences) and subsequently analyzed using Flowjo software.
IL-2 Production and T Cell Proliferation
Splenocytes (5 × 105 cells/150 μl) were seeded in U-bottom 96-well plates in triplicate and stimulated with 5 μg/ml of anti-CD3ϵ or PMA (20 ng/ml) plus ionomycin (0.5 μg/ml). IL-2 concentration in the overnight culture from each well was determined by ELISA (eBioscience). For T cell proliferation, cells stimulated for 36 h were pulsed with 1 μCi/well of [3H]thymidine (GE Healthcare) for an additional 6 h and then harvested for scintillation counting.
Calcium Flux
Splenocytes were first loaded with Indo-1 acetoxymethyl ester (Molecular Probes) and then stained with phycoerythrin-conjugated anti-CD4 antibody. Calcium mobilization was initiated by the addition of biotin-anti-CD3ϵ (5 μg/ml) with biotin-anti-CD4 (1 μg/ml), followed by cross-linking with streptavidin (25 μg/ml, Sigma), and was monitored using FACStar (BD Biosciences). Free intracellular Ca2+ was measured by the fluorescence emission ratio at 405/510 nm and analyzed using Flowjo software.
Immunoprecipitation and Western Blotting
T cells activated in vitro were used for biochemical analysis of TCR-mediated signaling. Briefly, splenocytes from ERCre+LATf/− and LATf/− littermates were activated by plate-bound anti-CD3ϵ (5 μg/ml) for 2 days and then transferred to a new flask and expanded in the presence of mouse IL-2 (10 ng/ml) and 50 nm 4-OHT (4-hydroxytamoxifen, Sigma) for 4 days. These activated T cells were rested in complete medium without IL-2 for 4 h before being stimulated by anti-CD3ϵ cross-linking. These cells were lysed at different time points and post-nuclear protein lysates were used in immunoprecipitation and Western blotting. Immunoprecipitated proteins or total lysates were resolved on SDS-PAGE and transferred onto nitrocellulose membranes (Bio-Rad). Western blot analysis was done as previously described (23).
EMSA
Splenocytes from LATf/− and ERCre+LATf/− littermates were activated and treated with 4-OHT as described above. 5 × 106 activated T cells were either unstimulated or stimulated with plate-bound anti-CD3ϵ (5 μg/ml) and soluble anti-CD28 (1 μg/ml) for 16 h. Nuclear extracts were prepared and used in EMSA with NFκB binding oligonucleotide (5′-ACCAAGAGGGATTTCACCTAAATC-3′) as described (24).
T Cell Homeostatic Proliferation
Splenocytes from tamoxifen-treated ERCre+LATf/− and LATf/− mice were labeled with 5 μm Cell Proliferation Dye eFluor® 670 (eBioscience) for 10 min and washed. 2 × 107 of these splenocytes were then transferred to LAT−/− mice via tail vein injection. Seven days later, the recipients were sacrificed and the dilution of fluorescent dye in the donor T cells was analyzed by FACS.
Long Term Survival of T Cells
Splenocytes from age and sex-matched tamoxifen-treated CD45.1+CD45.2+ ERCre+LATf/+ and CD45.2+ ERCre+LATf/− mice were harvested and mixed at a ratio of 1:1. 4 × 107 of these mixed cells were adoptively transferred into CD45.1+ syngeneic B6 recipients via tail vein injection. At the indicated time points, splenocytes from the recipients were analyzed by FACS.
Regulatory T Cell in Vitro Suppression Assay
CD4+CD25+ cells (Treg cells) and Thy1.1+CD4+CD25− T cells (responders) were purified using a regulatory T cell isolation kit (StemCell Technologies). Responders were labeled with 5 μm Carboxy fluorescein succinimidyl ester (CFSE) for 10 min and washed three times with 5% FBS/PBS. 2 × 104 responders were cultured for 66 to 72 h with 2 × 104 Treg cells, 1 μg/ml of αCD3 (2C11), and 4 × 104 APCs (splenocytes from LAT−/− mice). Cells were cultured in duplicate; harvest of cells in culture was followed by FACS analysis.
RESULTS
Effective Deletion of LAT in Tamoxifen-treated ERCre+LATf/− T Cells
To induce LAT deletion in mature T cells, we used a well characterized ERCre system in which Cre is fused with the ligand-binding domain of the mouse estrogen receptor (ER) (25–27). Upon binding tamoxifen or 4-OHT, this ERCre fusion protein translocates to the nucleus and deletes floxed genes. The generation of LAT knock-in mice (LATf/f) has been described previously (12). Cre-mediated deletion of lat allows for the expression of a LAT-GFP fusion protein while simultaneously rendering LAT non-functional (12). We crossed ERCre transgenic mice with LATf/f and LAT−/− mice to generate ERCre+LATf/− mice, in which only one floxed allele needs to be deleted to cause LAT deficiency. LATf/− littermates were used as controls.
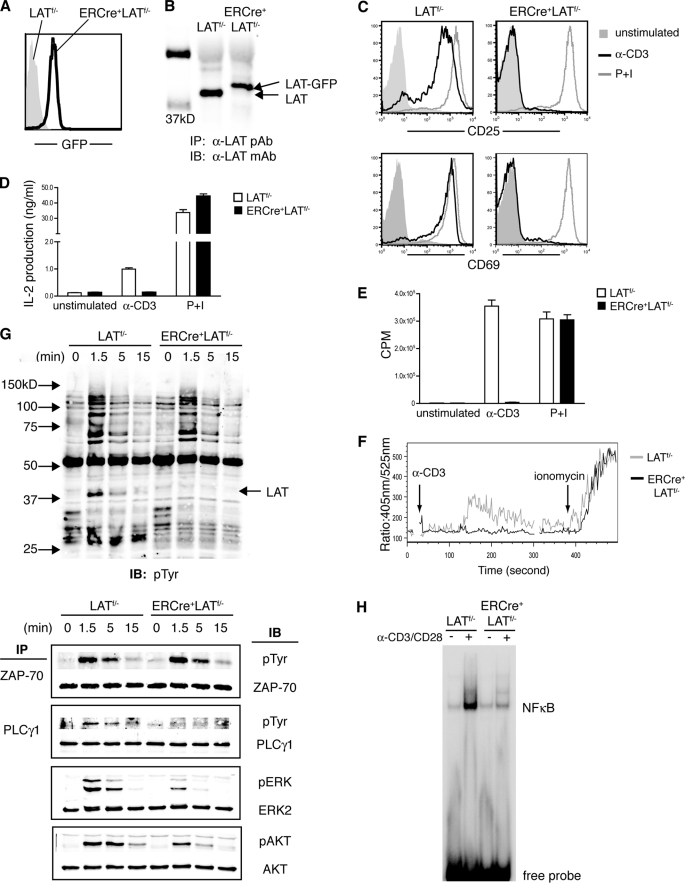
As expected, ERCre+LATf/− mice displayed normal thymocyte development as compared with LATf/− mice (data not shown). To induce LAT deletion, 8–10-week-old ERCre+LATf/− and LATf/− littermates were injected intraperitoneally with tamoxifen on 2 consecutive days. As shown in Fig. 1A, 48 h after the first treatment, more than 95% of ERCre+LATf/− peripheral T cells expressed GFP, indicating that deletion of lat was efficient. At day 4 post-tamoxifen injection, LAT protein was absent in ERCre+LATf/− splenocytes; a non-functional LAT-GFP fusion protein could be detected (Fig. 1B). Together, these data indicated that our tamoxifen-inducible deletion system worked efficiently.
FIGURE 1.
LAT deletion in mature T cells impairs TCR-mediated signaling and cell proliferation. A and B, deletion of LAT in T cells. Eight to 10-week-old LATf/− and ERCre+LATf/− littermates were treated with tamoxifen. A, the efficiency of LAT deletion, as measured by GFP expression, in TCRβ+ splenocytes was examined by FACS. B, lysates of splenocytes were subjected to anti-LAT immunoprecipitation, followed by anti-LAT Western blotting. C-E, the effect of LAT deletion on T cell activation. Splenocytes were stimulated with either plate-bound anti-CD3 or P + I. Data shown are representative of at least three independent experiments. C, after overnight culture, expression of CD25 and CD69 on CD4+ T cells was examined by FACS. D, IL-2 concentrations were determined by ELISA. E, 36 h after stimulation, cells were pulsed with [3H]thymidine for an additional 6 h before scintillation counting. Triplicates were performed for each sample. F, calcium mobilization. Splenocytes were loaded with Indo-1 and then stimulated by cross-linking CD3ϵ; LATf/− CD4+ T cells (gray line); ERCre+LATf/− GFP+CD4+ T cells (black line). Data shown are representative of three independent experiments. G, biochemical analysis of TCR signaling pathways. Splenocytes were cultured with plate-bound anti-CD3ϵ plus IL-2 for 2 days and then treated with 4-OHT for 4 more days. Cells were then rested, stimulated by anti-CD3ϵ, and subsequently lysed. ZAP-70 and PLC-γ1 were immunoprecipitated and analyzed by Western blotting with anti-Tyr(P) antibody. Total protein lysates were also subjected to Western blotting with anti-Tyr(P), anti-AKT, and anti-pERK1/2 antibodies. Anti-ZAP-70, PLC-γ1, AKT, and ERK2 blots are shown as protein loading controls. Data shown are representative of four independent experiments. H, 4-OHT-treated T cells in G were rested and either left untreated (−) or stimulated with anti-CD3/CD28 (+) for 16 h. Nuclear extracts were then subjected to EMSA assay with NFκB-binding oligonucleotide. Data shown are one representative of three independent experiments.
TCR-mediated Signaling in LAT-deficient Mature T Cells
Next, we examined how T cells deficient in LAT respond to stimulation from the TCR. ERCre+LATf/− and LATf/− littermates were treated with tamoxifen as described above. At day 4 post-treatment, splenocytes from both mice were harvested and stimulated with plate-bound anti-CD3ϵ overnight. This short term deletion of LAT had no effect on TCR surface expression on GFP+ERCre+LATf/− T cells (data not shown). As shown in Fig. 1C, there was an increase in the expression of CD25 and CD69 on LATf/− CD4+ T cells after stimulation with anti-CD3ϵ; however, up-regulation of these two activation markers was completely abolished in ERCre+LATf/− T cells. Similarly, tamoxifen-treated ERCre+LATf/− T cells failed to produce IL-2 (Fig. 1D) or to proliferate upon anti-CD3ϵ stimulation (Fig. 1E). In contrast, these ERCre+LATf/− T cells were able to respond normally upon stimulation with PMA and ionomycin.
We next investigated the signaling defects in LAT-deficient T cells. Calcium mobilization and Ras-MAPK activation are two major signaling events downstream of TCR ligation. To examine calcium flux, tamoxifen-treated LATf/− and ERCre+LATf/− splenocytes were loaded with Indo-1; the changes in intracellular calcium after anti-CD3ϵ cross-linking were monitored by flow cytometry. Although ionomycin-induced calcium response remained intact in GFP+ ERCre+LATf/− T cells, these cells failed to mobilize calcium after stimulation via the TCR (Fig. 1F).
We further performed biochemical analysis of TCR-mediated signaling events. To obtain sufficient numbers of T cells, splenocytes from ERCre+LATf/− and LATf/− littermates were activated with plate-bound anti-CD3ϵ for 2 days before 4-OHT treatment. Deletion of LAT was complete 4 days after the treatment (data not shown). 4-OHT-treated T cells were then rested for 4 h before being stimulated by anti-CD3ϵ. As shown in Fig. 1G, the overall tyrosine phosphorylation of proteins in ERCre+LATf/− and LATf/− T cells was comparable. Phosphorylated LAT was clearly missing in ERCre+LATf/− cells as expected. ZAP-70 phosphorylation appeared to be normal, suggesting that LAT deficiency did not affect signaling upstream of LAT. Consistent with the defect in TCR-mediated calcium flux, PLC-γ1 phosphorylation was abolished in the LAT-deficient T cells. Although ERK phosphorylation was severely diminished, it was not completely absent. We further examined the effect of LAT deficiency on the activation of the PI3K pathway. Phosphorylated LAT recruits the regulatory subunit of PI3K (1). As shown in Fig. 1G, phosphorylation of AKT was markedly reduced in LAT-deficient T cells, suggesting that PI3K activation was also impaired. In addition, TCR-mediated NFκB activation was also drastically abolished in LAT-deficient T cells as assayed by EMSA (Fig. 1H). Altogether, our data showed that deletion of LAT in mature T cells severely impaired TCR-mediated calcium mobilization as well as Ras-ERK, PI3K, and NFκB activation, rendering T cells unresponsive to TCR engagement in vitro.
LAT Deficiency Impairs T Cell Homeostatic Proliferation
T cell homeostasis consists of two components: homeostatic proliferation and cell survival. Lymphopenia-driven homeostatic proliferation of T cells can be further divided into two distinct types: slow and rapid proliferation (28, 29). Upon transfer into a partially lymphopenic environment, such as sublethally irradiated mice, T cells undergo slow homeostatic proliferation that requires both IL-7 and self-peptide·MHC complexes. However, a fraction of T cells can also undergo rapid homeostatic proliferation upon transfer into a chronically, completely lymphopenic environment, e.g. RAG−/− mice. This “rapid” proliferation is independent of IL-7, yet seemingly dependent upon agonistic antigen-induced TCR signaling (28, 29). The specific TCR signaling components that are involved in either scenario are not yet clearly identified.
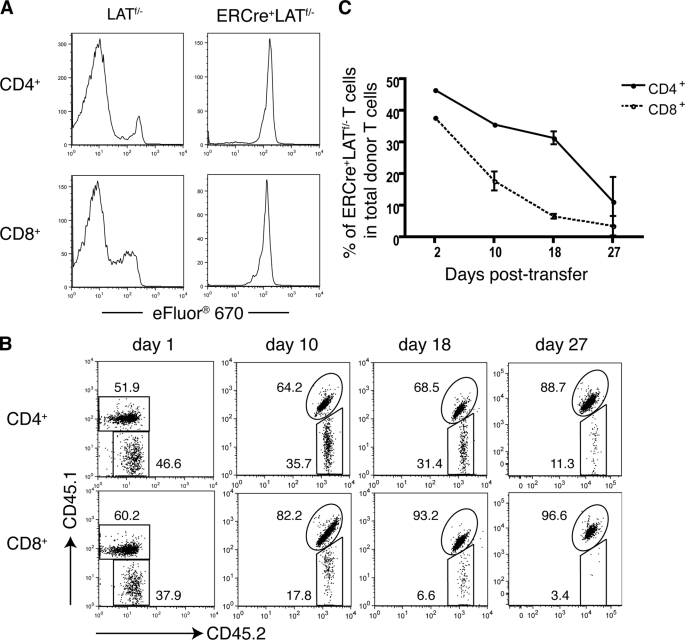
To investigate LAT function in homeostatic proliferation in a lymphopenic environment, we used LAT−/− mice as recipients, which are completely devoid of mature T cells (11). Splenocytes from tamoxifen-treated ERCre+LATf/− or LATf/− littermates were labeled with the cell proliferation dye eFluor 670 and subsequently transferred into syngeneic LAT−/− hosts. Seven days later, the dilution of eFluor 670 fluorescence in donor T cells was analyzed by FACS. As shown in Fig. 2A, in LAT−/− mice that received LATf/− splenocytes, the majority of donor CD4+ and CD8+ T cells had completely lost the eFluor 670 fluorescence, suggesting that they were the progeny of donor T cells that had undergone rapid homeostatic proliferation. Meanwhile, a small percentage of T cells remained eFluor 670 positive. Dilution of the dye could be detected, particularly in CD8+ T cells. These were likely the progeny of donor T cells that had undergone multiple rounds of slow homeostatic proliferation.
FIGURE 2.
LAT deletion impairs T cell homeostatic proliferation and long term survival. A, LAT in homeostatic proliferation. Splenocytes from tamoxifen-treated LATf/− and ERCre+LATf/− littermates were labeled with Cell Proliferation Dye eFluor 670 and subsequently transferred into LAT−/− mice via tail vein injection. Seven days later, the eFluor 670 signal in CD4+ (top panel) and CD8+ (bottom panel) T cells was analyzed by flow cytometry. ERCre+LATf/− cells were gated on GFP+ cells. Data are one representative of three independent experiments. B and C, LAT in T cell survival. Splenocytes from tamoxifen-treated CD45.1+CD45.2+ ERCre+LATf/+ and CD45.2+ ERCre+LATf/− mice were mixed. The initial ratio of CD45.1+CD45.2+ to CD45.2+ CD4+GFP+ T cells was 1:1. The cell mixtures were transferred to CD45.1+ syngeneic B6 hosts. Data shown are representative of three independent experiments with three mice per experiment. B, at the indicated days following transfer, the CD45.1 versus CD45.2 expression on CD4+GFP+ (top panel) or CD8+GFP+ (bottom panel) donor T cells was analyzed by flow cytometry. The data shown are from one representative of nine mice analyzed. The numbers represent the average percentages of the gated populations. C, percentages of CD45.2+ LAT-deficient CD4+ (solid line) or CD8+ (dotted line) cells among total donor CD4+ or CD8+ T cells on the indicated days post-transfer.
In sharp contrast, very few ERCre+LATf/− donor T cells lost their eFluor 670 fluorescence, indicating that LAT-deficient T cells failed to undergo rapid homeostatic proliferation. Moreover, even among the eFluor 670-positive T cells, dilution of the dye was minimal in ERCre+LATf/− donor cells when compared with that in the LATf/− controls. This finding suggests that the slow homeostatic proliferation of T cells was also impaired. Altogether, our data clearly demonstrated that LAT plays a critical role in lymphopenia-driven homeostatic proliferation.
LAT Deficiency Impairs Long Term Survival of T Cells
Next, we examined if LAT is required for the long term survival of T cells. CD45.1+CD45.2+ ERCre+LATf/+ and CD45.2+ ERCre+LATf/− syngeneic mice were treated with tamoxifen. T cells from both strains of mice were marked by GFP expression after deletion; however, ERCre+LATf/+ T cells still had one wild type lat allele, which served as a control for ERCre+LATf/− T cells. After tamoxifen treatment for 4 days, splenocytes from CD45.1+CD45.2+ ERCre+LATf/+ and CD45.2+ ERCre+LATf/− mice were mixed at a ratio of 1:1. 2 × 107 mixed splenocytes were then transferred into CD45.1+ syngeneic B6 mice. These mice were sacrificed at days 1, 10, 18, and 27 after the adoptive transfer. The relative percentage of both donor T cells at each time point was determined by FACS.
As shown in Fig. 2B, 1 day after the adoptive transfer, the ratio of CD45.1+CD45.2+ and CD45.2+ cells (gated on CD4+GFP+ T cells) remained ∼1:1, indicating that engraftment of both LAT-sufficient and LAT-deficient T cells was equally successful. However, the percentage of ERCre+LATf/− cells among total donor CD4+ T cells decreased significantly at day 10 post-transfer, and continued to decline at later time points. At day 27, only ∼11% of donor CD4+GFP+ T cells were CD45.2+, suggesting that the survival of LAT-deficient CD4+ T cells was severely impaired.
A similar trend was also observed with CD8+ T cells. We consistently noticed that the CD4:CD8 ratio in tamoxifen-treated ERCre+LATf/− mice was slightly skewed toward CD4+ (data not shown). Although the ratio of ERCre+LATf/+ and ERCre+LATf/− CD4+ T cells was at 1:1 after mixing, the initial percentage of ERCre+LATf/− CD8+ T cells (CD45.2+) was only ∼40% of total donor CD8+ T cells. This observation implied that LAT-deficient CD8+ T cells might have a more severe survival defect than CD4+ T cells. Indeed, after the transfer, LAT-deficient CD8+ T cells disappeared much faster than their CD4+ counterparts (Fig. 2C). At day 10 post-transfer, the percentage of LAT-deficient CD8+ T cells among total donor CD8+ T cells was ∼17.8%. At day 27, only 2.7% of donor CD8+ T cells were LAT deficient. Altogether, our data demonstrated that, whereas LAT is required for the survival of both CD4+ and CD8+ T cells, LAT deficiency has a more severe impact on the survival of CD8+ T cells.
LAT Deficiency Causes a LATY136F-like Lymphoproliferative Syndrome
Despite being widely regarded as a positive regulator of TCR-mediated signaling, LAT may have a negative role in T cell homeostasis, as suggested by studies on LATY136F knock-in mice. CD4+ T cells from these mice undergo an uncontrolled expansion and produce large amounts of Th2 cytokines. It has been suggested that the Y136F mutation may disrupt a delicate balance between LAT-mediated positive and negative signaling pathways (16, 17). To test this hypothesis, we examined whether such a lymphoproliferative disease would arise in the absence of LAT.
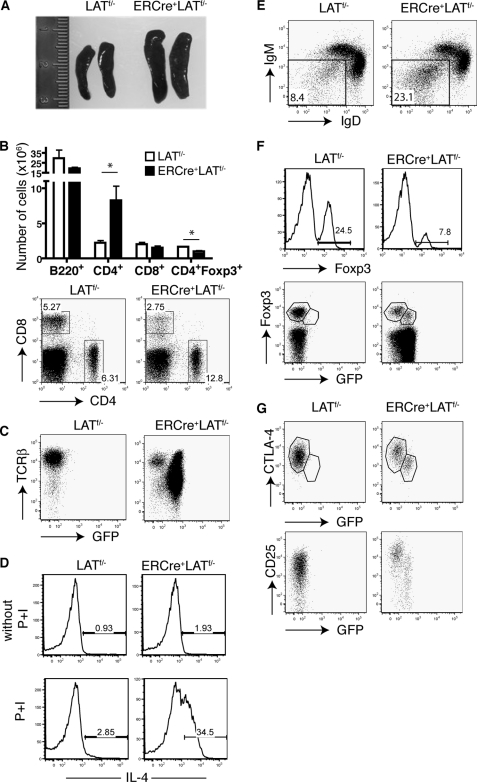
To avoid possible complications arising from T cells newly emigrated from the thymus, we reconstituted LAT−/− mice with 2 × 107 splenocytes from untreated ERCre+LATf/− or LATf/− littermates. We waited 5 weeks to allow for homeostatic expansion of T cells in these mice. At 5 weeks after the transfer, similar percentages of CD4+ and CD8+ T cells were found in the peripheral blood of these mice (data not shown). To delete LAT, these mice were first treated with tamoxifen on 2 consecutive days and were then treated once every week for 5 weeks. After 5 weeks of tamoxifen treatment, LAT−/− mice reconstituted with ERCre+LATf/− splenocytes exhibited splenomegaly, whereas mice that received LATf/− splenocytes had normal sized spleens (Fig. 3A). Similar to the disease observed in LATY136F mice, the splenomegaly in mice reconstituted with ERCre+LATf/− cells was caused by the expansion of CD4+ T cells. As shown in Fig. 3B (upper panel), the total number of CD4+ cells was increased in the spleens of LAT-deficient mice receiving ERCre+LATf/− cells as compared with the controls; this was accompanied by an increase in the CD4:CD8 ratio (Fig. 3B, lower panel). Most of these CD4+ T cells were GFP+, suggesting that LAT deletion was efficient. Interestingly, the majority of ERCre+LATf/− GFP+ CD4+ T cells had down-regulated TCR surface expression, as compared with the GFP− T cells (Fig. 3C). This phenomenon did not represent a global defect in protein expression as CD4 expression was normal on LAT-deficient cells (data not shown).
FIGURE 3.
LAT ablation in peripheral T cells results in a LATY136F-like lymphoproliferative syndrome. 2 × 107 splenocytes from untreated ERCre+LATf/− littermates were adoptively transferred to syngeneic LAT−/− recipients; LATf/− splenocytes were used as controls. Five weeks later, the recipients were treated with tamoxifen on 2 consecutive days. Tamoxifen treatment was then repeated once a week for 4–5 weeks. Data are representative of two independent experiments with three mice per genotype in each experiment. A, enlarged spleens from mice that received ERCre+LATf/− cells. B, total numbers of CD4+, CD8+, and CD4+Foxp3+ cells in spleens (top panel) and expression of CD4 versus CD8 in spleens (bottom panel). A two-tailed Student's t test analysis was performed; * represents p < 0.05. C, expression of GFP versus TCRβ in spleens, gated on CD4+ cells. D, cytokine production. Splenocytes from both groups of recipients were either left untreated or stimulated with PMA plus ionomycin for 5 h. Intracellular staining of IL-4 in CD4+-gated splenocytes is shown. The numbers on the FACS plots represent the percentages of the gated populations. E, the IgM versus IgD profile of B220+-gated splenocytes. F, percentage of Foxp3+ cells in CD4+ splenocytes (top panel). Foxp3 versus GFP expression in CD4+ splenocytes (bottom panel). G, CTLA-4 (top panel) and CD25 (bottom panel) versus GFP in CD4+Foxp3+ splenocytes.
To examine whether these LAT-deficient CD4+ T cells also produced large amounts of cytokines, we stimulated splenocytes from mice reconstituted with ERCre+LATf/− or LATf/− T cells with PMA plus ionomycin for 5 h and performed intracellular staining of cytokines. Strikingly, ∼35% of LAT-deficient CD4+ T cells produced IL-4 as compared with ∼3% of control T cells (Fig. 3D). These data indicated that, similar to LATY136F T cells, LAT-deficient CD4+ T cells were hyperproliferative and also displayed a distinct Th2 phenotype. In addition, the percentage of IgD−IgM− B cells in mice with LAT-deficient T cells was increased (Fig. 3E), suggesting that these B cells had undergone maturation and isotype switching. Consequently, high levels of serum IgE and IgG1 were detected in these mice (data not shown). Altogether, our data showed that deletion of LAT in peripheral T cells could induce a similar autoimmune-like lymphoproliferative disorder to that observed in LATY136F mice.
The Effect of LAT Deficiency on Treg Cells
Our data in Fig. 2 showed that LAT-deficient T cells were defective in both long term survival and lymphopenia-driven homeostatic proliferation. These data seemed to contradict the findings that these cells could also drive the development of a LATY136F-like lymphoproliferative disease. It is possible that LAT deletion caused a breakdown in peripheral tolerance by affecting Treg cell maintenance or survival. Indeed, there was a significant decrease in the total number of CD4+Foxp3+ cells (Fig. 3B). Among CD4+ T cells from the LAT−/− mice reconstituted with ERCre+LATf/− splenocytes, only ∼8% of them expressed Foxp3 as compared with ∼25% by control cells (Fig. 3F). Moreover, a notable percentage of the Foxp3+ population of CD4+ ERCre+LATf/− T cells appeared to be GFP−, indicating that those Foxp3-expressing cells had escaped deletion (Fig. 3F). Therefore, LAT-deficient Treg cells did not undergo the same drastic proliferation as conventional CD4+ T cells. Intriguingly, compared with the GFP− CD4+Foxp3+ T cells, LAT-deficient Treg cells (GFP+) had down-regulated the expression of Foxp3 (Fig. 3F), CTLA-4, and CD25 (Fig. 3G).
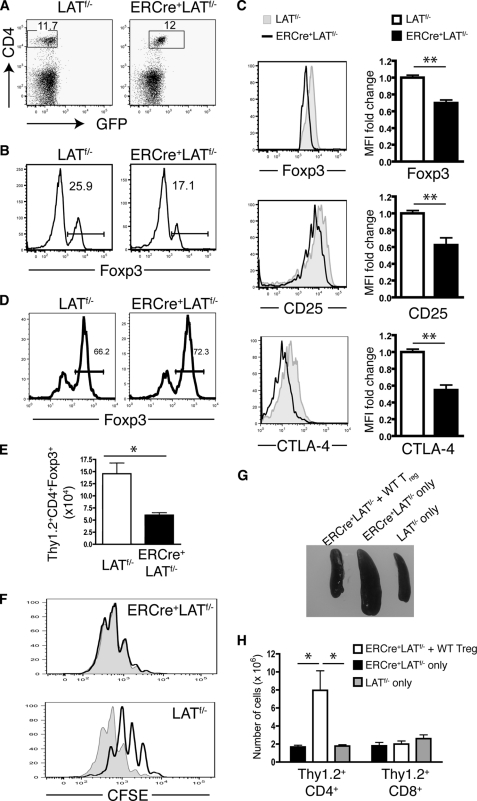
To confirm that such observed phenomena were directly caused by the loss of LAT and not by the autoimmune-like environment, we examined the effects of LAT deletion on Treg cells before the onset of the disease. ERCre+LATf/− and LATf/− littermates were treated with tamoxifen on 2 consecutive days. Ten days after the first treatment, spleens from both groups of mice remained a normal size (data not shown), and the percentages of CD4+ T cells were comparable (Fig. 4A). However, the percentage of CD4+Foxp3+ cells was decreased in ERCre+LATf/− spleens (Fig. 4B); this decrease was even more drastic in the lymph nodes (not shown). Similar to the data in Fig. 3, despite being Foxp3+, LAT-deficient Treg cells had reduced expression of Foxp3 (Fig. 4C, top panel). Moreover, expression of CTLA-4 and CD25 was decreased in GFP+ CD4+Foxp3+ Treg cells (Fig. 4C, middle and bottom panels).
FIGURE 4.
LAT deficiency leads to loss of number and function of Treg cells and reduced expression of Foxp3. A-C, ERCre+LATf/− and LATf/− littermates were treated with tamoxifen on 2 consecutive days. Ten days after the initial treatment, splenocytes were harvested and analyzed by FACS. Data shown are representative of three independent experiments using two mice per genotype in each experiment. A, the CD4 versus GFP profile of splenocytes. The numbers on the FACS plots represent the percentages of the gated populations. B, the percentages of Foxp3+ cells in CD4+ T cells. C, comparison of expression levels of Foxp3 (top), CD25 (middle), and CTLA-4 (bottom) in CD4+GFP−Foxp3+ cells from LATf/− mice and CD4+GFP+Foxp3+ cells from ERCre+LATf/− mice. Bar graphs in the right column represent fold-change in mean fluorescence intensity (MFI) in ERCre+LATf/− compared with LATf/− controls. Data shown are representative of three independent experiments using two mice per genotype for each experiment. D and E, LAT−/− mice were reconstituted with Thy1.1+ wild type CD4+CD25− T cells mixed with Thy1.2+ CD4+CD25+ T cells from either ERCre+LATf/− mice or LATf/− mice followed by tamoxifen treatment. Mice were analyzed 10 days after initial treatment. D, the percentages of Foxp3+ cells in Thy1.2+CD4+ splenocytes. E, the number of Thy1.2+CD4+Foxp3+ cells in spleens. Two-tailed Student's t test; * represents p < 0.05. F, in vitro suppression assay using a 1:1 ratio of responder cells to Treg cells from either ERCre+LATf/− or LATf/− mice. Proliferation of the responder cells is shown by CFSE dilution (the shaded region represents responder cells cultured without Treg cells). The figure shown is one representative of three experiments performed. G and H, LAT−/− mice were reconstituted with ERCre+LATf/− CD4+ T cells mixed with wild type CD4+CD25+ T cells followed by 4 weeks of tamoxifen treatment. Mice reconstituted with only ERCre+LATf/− or LATf/− CD4+ T cells were used as controls. G, spleens from one representative of three mice of each genotype analyzed. H, the total number of Thy1.2+CD4+ and Thy1.2+CD8+ splenocytes in above mice.
The decrease in Treg cells (CD4+Foxp3+) observed in Figs. 3B and 4B suggested that LAT is crucial for Treg cell survival. However, it could also be argued that this loss of Treg cells may result from their conversion to cytokine-producing effector cells, termed exFoxp3 cells (30). To investigate this possibility, CD4+CD25+ cells from either ERCre+LATf/− or LATf/− mice (Thy1.2+) were mixed with Thy1.1+ WT CD4+CD25− cells and transferred into LAT−/− mice. After reconstitution, the recipients were treated with tamoxifen on 2 consecutive days. Ten days after the initial tamoxifen treatment, similar percentages of the Thy1.2+ donor T cells in both groups remained Foxp3+ (Fig. 4D), suggesting that there was no obvious conversion of Treg cells into exFoxp3 cells. In addition, the ability of donor Thy1.2+Foxp3− cells to produce IFN-γ in response to P + I stimulation was similar between the two groups neither of which produced detectable IL-4 (data not shown). Consistent with our findings in Figs. 3B and 4B, the absolute number of Thy1.2+ ERCre+LATf/− CD4+Foxp3+ cells was significantly decreased compared with their LATf/− counterparts (Fig. 4E). Together, these data demonstrated that the loss of peripheral Treg cells after LAT deletion is not caused by their conversion to cytokine-producing exFoxp3 cells.
We further assayed the suppressive function of Treg cells from ERCre+LATf/− mice after tamoxifen treatment. These Treg cells were not able to suppress the expansion of conventional T cells in vitro (Fig. 4F). Together, our data above demonstrated that deletion of LAT caused a reduction of Foxp3 expression in Treg cells, as well as a decrease in the number of Treg cells in the periphery; this suggests that LAT plays a critical role in Treg cell maintenance or survival. As Treg cells play an essential role in maintaining peripheral tolerance, the autoimmune-like disorder observed in mice with LAT-deficient T cells may be caused by a lack of suppression by Treg cells.
To confirm that this autoimmune-like disorder can be corrected by Treg cells with normal function, we reconstituted LAT−/− mice with CD4+ T cells from ERCre+LATf/− mice (Thy1.2+) mixed with CD4+CD25+ cells from wild type mice (Thy1.1+). For control groups, LAT−/− mice were transferred with the same number of CD4+ T cells from either ERCre+LATf/− mice or LATf/− mice. The recipient mice were treated with tamoxifen for 4 weeks. As shown in Fig. 4, G and H, co-transfer of wild type Treg cells prevented splenomegaly as well as the expansion of CD4+ T cells that were otherwise observed in LAT−/− hosts receiving ERCre+LATf/− T cells alone. In addition, ERCre+LATf/− T cells did not produce large amounts of IL-4 in the presence of wild type Treg cells (data not shown). These findings indicate that normal Treg cells can indeed prevent the development of the LATY136F-like lymphoproliferative disease.
Comparison of the Autoimmune-like Syndromes Caused by LAT Deficiency and the LATY136F Mutation
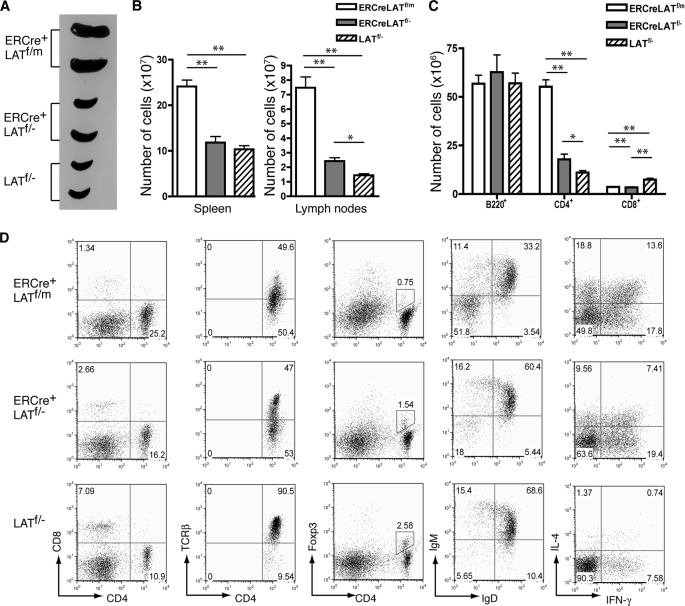
Our data in Fig. 3 showed that deletion of LAT in peripheral T cells caused the development of an autoimmune-like syndrome similar to the one observed in LATY136F mice. To directly compare the differences between the syndromes caused by LAT deficiency or the Y136F mutation, we treated ERCre+LATf/− and ERCre+LATf/m mice with tamoxifen to induce deletion of LAT (where the “m” in ERCre+LATf/m refers to a LATY136F mutant allele of lat). LATf/− mice were used as controls. These mice were injected with tamoxifen once a week for 5 weeks before analysis to ensure efficient and continuous deletion of LAT. As shown in Fig. 5A, ERCre+LATf/m mice had severe splenomegaly. The weight of their spleens (407 ± 39 mg) was approximately four times of that of LATf/− mice (86 ± 7 mg). In contrast, the spleens of ERCre+LATf/− mice were modestly enlarged (137 ± 30 mg). The numbers of splenocytes were increased in these mice, as expected (Fig. 5B). A similar increase of cells was also seen in the lymph nodes. Although the numbers of B220+ cells in the spleens of ERCre+LATf/−, ERCre+LATf/m, and LATf/− mice were similar, the numbers of CD4+ T cells were drastically different (Fig. 5, C and D). ERCre+LATf/m mice had ∼5 times more CD4+ T cells than LATf/− mice (55 ± 7 × 106 versus 11 ± 2 × 106), whereas ERCre+LATf/− mice had ∼60% more (18 ± 5 × 106). In contrast, the number of ERCre+LATf/− CD8+ T cells was reduced to 50% of that in LATf/− mice.
FIGURE 5.
Comparison of the autoimmune syndromes in ERCre+LATf/m and ERCre+LATf/− mice. ERCre+LATf/m, ERCre+LATf/−, and LATf/− mice were treated with tamoxifen for 5 weeks before analysis. A, spleens from two mice of each genotype. B, total numbers of cells in spleens and lymph nodes (n = 4). Two-tailed t test; *, p ≤ 0.01; **, p ≤ 0.001. C, total numbers of B220+, CD4+, and CD8+ splenocytes (n = 4). Two-tailed t test; *, p ≤ 0.01; **, p ≤ 0.001. D, FACS analysis of splenocytes. From left to right: CD8 versus CD4 expression on total cells; TCRβ expression on CD4+ cells; Foxp3 versus CD4 expression on total cells; IgM versus IgD expression on B220+ cells; IL-4 versus IFNγ expression in CD4+ T cells after a 4-h P + I stimulation. The numbers on the FACS plots represent the percentages of the gated populations. FACS plots shown are representative of four mice per genotype.
Further analysis of T cells from these mice showed a down-regulation of TCR expression in ERCre+LATf/m mice similar to that observed in the LATY136F mice (16, 17). This down-regulation of the TCR was also seen in ERCre+LATf/− T cells, although a large percentage of CD4+ T cells still retained a normal level of TCR expression (Fig. 5D). Analysis of Foxp3 expression in CD4+ T cells showed that ERCre+LATf/m mice had the lowest percentage of Treg cells. The percentages of Treg cells in ERCre+LATf/m, ERCre+LATf/−, and LATf/− mice were 0.71, 1.55, and 2.64%, respectively. Although the percentage of Treg cells was low in ERCre+LATf/m mice, the number of Foxp3+ Treg cells in these mice was similar to that in LATf/− mice (2.5 × 106 versus 2.1 × 106 per spleen). On the other hand, the number of Foxp3+ Treg cells in the spleens of ERCre+LATf/− mice was only ∼1.2 × 106, which is in agreement with our data showing that LAT is required for Treg cell maintenance or survival (Fig. 4). We also analyzed cytokine production by CD4+ T cells from these mice. As shown in Fig. 5D, T cells from both ERCre+LATf/m and ERCre+LATf/− mice produced large amounts of cytokines, such as IL-4 and IFN-γ, compared with those from LATf/− mice. However, more ERCre+LATf/m T cells produced IL-4 compared with ERCre+LATf/− T cells. In addition, the fluorescence intensity of anti-IL-4 staining in T cells from ERCre+LATf/m mice appeared higher, indicating that they produced more IL-4. In LATY136F mice, B cells are activated and undergo maturation and isotype switching (16). Similar activation was also seen in ERCre+LATf/m and ERCre+LATf/− mice treated with tamoxifen. Among splenocytes from ERCre+LATf/− and ERCre+LATf/m mice, the percentage of IgM−IgD− B cells were 18.0 and 51.8%, respectively, indicating that more B cells in ERCre+LATf/m mice were activated and had undergone isotype switching. Together, these results indicated that, although mice with LAT-deficient T cells developed a similar autoimmune-like syndrome, this disease is less severe than the disease observed in LATY136F mice.
DISCUSSION
Our biochemical analysis of TCR-mediated signaling in LAT-deficient T cells yielded observations that differed from those reported in a recent study (31). First, whereas the earlier study showed that TCR-induced Ras-ERK activation was completely abolished in LAT-deficient T cells, our data consistently showed a reduced level of ERK phosphorylation (Fig. 1G). LAT-independent Ras-MAPK activation has been documented previously. It was shown that the Grb2-Sos complex directly interacts with the phosphorylated TCR-ζ chain, bypassing the need for ZAP-70 activation and LAT phosphorylation to activate Ras (32). Similarly, ERK activation was observed in ZAP-70-deficient Jurkat cells upon TCR stimulation (33). Our published data also showed this residual ERK activation in CD4Cre+LATf/− DP thymocytes (12). Our data, together with previously published data, indicate that, whereas TCR-mediated ERK activation is mainly dependent upon LAT, it can also be activated independently of LAT. Second, we consistently detected reduced AKT phosphorylation in both 4-OHT-treated ERCre+LATf/− activated T cells (Fig. 1G) and tamoxifen-treated primary T cells (data not shown). This result is different from the recent report that asserts that AKT activation is intact in primary T cells in the absence of LAT (31). What causes the observed differences is not clear.
Both T cell survival and homeostatic proliferation depend upon signaling induced by weak interactions between the TCR and self-peptide·MHC ligands (18–21). Unlike the signaling pathways that are induced by agonists, which have been very well characterized, the signaling pathways activated by the engagement of the TCR by self-peptide·MHC complexes are poorly understood. Similarly, whereas LAT function in T cell activation has been extensively studied, its function in survival and homeostatic proliferation remains unclear. Our finding that LAT is required for both long term survival and homeostatic proliferation of T cells strongly suggests that LAT is a critical component of the signaling pathway downstream of the interaction between the TCR and self-peptide·MHC complexes.
The mechanism by which LAT transduces a basal level of TCR signaling in resting T cells to regulate these cellular processes is not yet known. Although the function of LAT in TCR signaling is relatively clear, questions remain as to whether LAT plays a similar role in self-ligand-induced signaling. First, is LAT constitutively phosphorylated at a low level? It has been suggested that TCR signaling upon interaction with self-ligands leads to a basal phosphorylation of TCRζ in naive T cells (34, 35). Such TCRζ phosphorylation is abolished in the absence of both Lck and Fyn (22), resulting in defective T cell homeostatic proliferation and long term survival (22, 36, 37). In addition, ZAP-70 is constitutively associated with phosphorylated TCRζ (35). It is possible that TCRζ-associated ZAP-70 phosphorylates LAT at a low level in naive T cells. Second, do T cell survival and homeostatic proliferation require different LAT-mediated signaling complexes? Accumulating data show that whereas these cellular processes are driven by the same environmental factors, namely IL-7 and self-peptide·MHC ligands, they are probably regulated differently. One convincing line of evidence comes from recent studies on IL-7Rα Y449F knock-in mice. The point mutation at tyrosine 449, which is essential for STAT5 activation, specifically abolishes IL-7-mediated homeostatic proliferation, but not survival (38). Furthermore, both Lck and Fyn are capable of maintaining TCRζ phosphorylation; thus, either kinase can provide survival signals in the absence of the other. However, Lck-deficient T cells fail to proliferate in a mildly lymphopenic environment, suggesting that homeostatic proliferation requires unique Lck-mediated signals that cannot be provided by Fyn (22, 36, 37). These data suggest that T cell survival and homeostatic proliferation are differentially regulated; how LAT signaling may be involved in these two processes requires further investigation.
Interestingly, despite a clear deficiency in cell survival and homeostatic proliferation, LAT-deficient CD4+ T cells were capable of causing a lymphoproliferative syndrome and producing large amounts of cytokines, such as IL-4 (Fig. 3). After tamoxifen treatment, LAT−/− mice reconstituted with ERCre+LATf/− T cells developed a Th2-type autoimmune-like syndrome characterized by splenomegaly and IgG1/IgE hypergammaglobulinemia. This phenotype is very similar to the one seen in LATY136F knock-in mice, in which the LAT-PLC-γ1 interaction is abolished (16, 17). Data from our laboratory (39) and others (40) have demonstrated that this disease arises independently of abnormal thymic selection in the knock-in mice. Instead, the LATY136F mutation in peripheral T cells is sufficient to confer pathogenicity and induce a similar disease. Moreover, LAT3YF knock-in mice, which have mutations at Tyr-175, -195, and -235, also exhibit a similar syndrome mediated by mutant γδ T cells (15). Despite being a critical positive regulator in TCR activation, LAT may also play a negative role.
Although both ERCre+LATf/m and ERCre+LATf/− CD4+ T cells produce abnormally high levels of cytokines and undergo seemingly uncontrolled expansion, our data showed that ERCre+LATf/m T cells were more hyperactive and triggered a more drastic disease. What causes the difference in the severity of the autoimmune syndromes is not clear. Such a negative role of LAT, if it exists, should be missing in T cells from ERCre+LATf/m and ERCre+LATf/− mice after tamoxifen treatment. It is possible that in the absence of this negative regulation by LAT, the LATY136F mutant is able to drive more expansion of CD4+ T cells. The signals that drive this expansion remain to be determined. Previous studies have shown that CD4+ T cells expressing the LATY136F mutant can expand in the absence of MHC class II (41), indicating that the engagement of the TCR with self-peptide·MHC complexes is likely not involved in the development of this syndrome. This speculation is supported by the fact that TCR-mediated LAT and PLC-γ1 phosphorylation is severely impaired in LATY136F T cells (17). However, it is still possible that LAT-mediated tonic signals from the TCR, which are absent in ERCre+LATf/− T cells, are able to drive faster expansion of LATY136F T cells. Even though the LATY136F mutant fails to bind PLC-γ1, it still binds to Grb2 and Gads, thus partially functioning in TCR-mediated signaling (42). This partial or unbalanced signaling is supported by data showing that ERK activation in the mutant T cells is enhanced compared with that in normal T cells (17). Not only did the LATY136F T cells, as those in ERCre+LATf/m mice, expand faster, they also produced more cytokines, such as IL-4, compared with ERCre+LATf/− T cells (Fig. 5), likely driving the expansion of these mutant T cells as well as the maturation and isotype switching of B cells.
Furthermore, our data showed that, after tamoxifen treatment, the percentage and number of CD4+Foxp3+ Treg cells in ERCre+LATf/− mice were decreased. Additionally, Foxp3 expression in Treg cells, as well as CTLA-4 and CD25 expression, was reduced. Moreover, the effect of LAT deficiency on the expression of these proteins occurred within 10 days after tamoxifen treatment, well before the onset of the lymphoproliferative disorder. These data suggested that the mechanisms behind the T cell hyperproliferation are complex and may involve a breakdown in Treg-mediated peripheral tolerance due to the loss of LAT.
We have previously shown that the LAT-PLC-γ1 interaction plays a critical role in the development of CD4+CD25+ Treg cells, as LATY136F mice lack this population (43). However, the data from the studies presented here suggested that LAT is not only important in the development of Treg cells, but also in the maintenance or survival as well as the function of mature Treg cells. How TCR signaling regulates the expression of Foxp3 during Treg development has been answered with some satisfaction; however, the role of TCR signaling in regulating mature Treg homeostasis is not yet clear. Interestingly, Kim et al. (44) recently reported that specific abrogation of TCR signaling in Treg cells by inactivation of p56Lck resulted in defective Treg turnover, loss of suppressive function, as well as an altered gene expression profile, including reduced CTLA-4 expression. These data, together with our results, suggest that mature Treg homeostasis in the periphery is dependent on sustained TCR signaling. Intriguingly, a minimal decrease of Foxp3 expression was observed in p56Lck-inactivated Treg cells (44), whereas a noticeable reduction of Foxp3 expression was consistently observed in our LAT-depleted Treg cells. As a transcription factor, Foxp3 has long been established as the master regulator of Treg development and function (45–47). Interestingly, recent data demonstrated that Foxp3 expression in mature Treg cells is not stable (30). Treg cells with transient Foxp3 expression can convert into inflammatory effector T cells. This finding highlighted the importance of understanding the precise mechanisms controlling Foxp3 expression. Our results implicate a potential role of LAT-mediated signaling in maintaining Foxp3 expression in Treg cells. Recent studies have demonstrated that c-Rel, a member of NFκB family, is required for thymic Foxp3+ Treg differentiation and regulates Foxp3 transcription by directly binding to the cis-regulatory elements at the Foxp3-locus (48, 49). Although the role of c-Rel in Foxp3 maintenance is yet unclear, LAT may regulate Foxp3 expression as well as Treg cell maintenance and function through activation of the NFκB pathway.
Acknowledgments
We thank the Duke University Cancer Center Flow Cytometry and Transgenic Mouse facilities for their excellent services.
This work was supported, in whole or in part, by National Institutes of Health Grants AI048674 and AI056156.
- TCR
- T cell receptor
- LAT
- linker for activation of T cells
- PLC
- phospholipase C
- 4-OHT
- 4-hydroxytamoxifen
- ER
- estrogen receptor
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1.Zhang W., Sloan-Lancaster J., Kitchen J., Trible R. P., Samelson L. E. (1998) Cell 92, 83–92 [DOI] [PubMed] [Google Scholar]
- 2.Samelson L. E. (2002) Annu. Rev. Immunol. 20, 371–394 [DOI] [PubMed] [Google Scholar]
- 3.Horejsi V., Zhang W., Schraven B. (2004) Nat. Rev. Immunol. 4, 603–616 [DOI] [PubMed] [Google Scholar]
- 4.Wu J. N., Koretzky G. A. (2004) Semin. Immunol. 16, 379–393 [DOI] [PubMed] [Google Scholar]
- 5.Jordan M. S., Singer A. L., Koretzky G. A. (2003) Nat. Immunol. 4, 110–116 [DOI] [PubMed] [Google Scholar]
- 6.Ebinu J. O., Stang S. L., Teixeira C., Bottorff D. A., Hooton J., Blumberg P. M., Barry M., Bleakley R. C., Ostergaard H. L., Stone J. C. (2000) Blood 95, 3199–3203 [PubMed] [Google Scholar]
- 7.Dower N. A., Stang S. L., Bottorff D. A., Ebinu J. O., Dickie P., Ostergaard H. L., Stone J. C. (2000) Nat. Immunol. 1, 317–321 [DOI] [PubMed] [Google Scholar]
- 8.Roose J. P., Mollenauer M., Ho M., Kurosaki T., Weiss A. (2007) Mol. Cell. Biol. 27, 2732–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W., Irvin B. J., Trible R. P., Abraham R. T., Samelson L. E. (1999) Int. Immunol. 11, 943–950 [DOI] [PubMed] [Google Scholar]
- 10.Finco T. S., Kadlecek T., Zhang W., Samelson L. E., Weiss A. (1998) Immunity 9, 617–626 [DOI] [PubMed] [Google Scholar]
- 11.Zhang W., Sommers C. L., Burshtyn D. N., Stebbins C. C., DeJarnette J. B., Trible R. P., Grinberg A., Tsay H. C., Jacobs H. M., Kessler C. M., Long E. O., Love P. E., Samelson L. E. (1999) Immunity 10, 323–332 [DOI] [PubMed] [Google Scholar]
- 12.Shen S., Zhu M., Lau J., Chuck M., Zhang W. (2009) J. Immunol. 182, 5596–5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommers C. L., Lee J., Steiner K. L., Gurson J. M., Depersis C. L., El-Khoury D., Fuller C. L., Shores E. W., Love P. E., Samelson L. E. (2005) J. Exp. Med. 201, 1125–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommers C. L., Menon R. K., Grinberg A., Zhang W., Samelson L. E., Love P. E. (2001) J. Exp. Med. 194, 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuñez-Cruz S., Aguado E., Richelme S., Chetaille B., Mura A. M., Richelme M., Pouyet L., Jouvin-Marche E., Xerri L., Malissen B., Malissen M. (2003) Nat. Immunol. 4, 999–1008 [DOI] [PubMed] [Google Scholar]
- 16.Aguado E., Richelme S., Nuñez-Cruz S., Miazek A., Mura A. M., Richelme M., Guo X. J., Sainty D., He H. T., Malissen B., Malissen M. (2002) Science 296, 2036–2040 [DOI] [PubMed] [Google Scholar]
- 17.Sommers C. L., Park C. S., Lee J., Feng C., Fuller C. L., Grinberg A., Hildebrand J. A., Lacaná E., Menon R. K., Shores E. W., Samelson L. E., Love P. E. (2002) Science 296, 2040–2043 [DOI] [PubMed] [Google Scholar]
- 18.Ernst B., Lee D. S., Chang J. M., Sprent J., Surh C. D. (1999) Immunity 11, 173–181 [DOI] [PubMed] [Google Scholar]
- 19.Goldrath A. W., Bevan M. J. (1999) Immunity 11, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrecque N., Whitfield L. S., Obst R., Waltzinger C., Benoist C., Mathis D. (2001) Immunity 15, 71–82 [DOI] [PubMed] [Google Scholar]
- 21.Polic B., Kunkel D., Scheffold A., Rajewsky K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon B., Zamoyska R. (2002) J. Immunol. 169, 2997–3005 [DOI] [PubMed] [Google Scholar]
- 23.Zhu M., Koonpaew S., Liu Y., Shen S., Denning T., Dzhagalov I., Rhee I., Zhang W. (2006) Immunity 25, 757–768 [DOI] [PubMed] [Google Scholar]
- 24.Sun Z., Arendt C. W., Ellmeier W., Schaeffer E. M., Sunshine M. J., Gandhi L., Annes J., Petrzilka D., Kupfer A., Schwartzberg P. L., Littman D. R. (2000) Nature 404, 402–407 [DOI] [PubMed] [Google Scholar]
- 25.Feil R., Brocard J., Mascrez B., LeMeur M., Metzger D., Chambon P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 10887–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Riesterer C., Ayrall A. M., Sablitzky F., Littlewood T. D., Reth M. (1996) Nucleic Acids Res. 24, 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998) Curr. Biol. 8, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 28.Kieper W. C., Troy A., Burghardt J. T., Ramsey C., Lee J. Y., Jiang H. Q., Dummer W., Shen H., Cebra J. J., Surh C. D. (2005) J. Immunol. 174, 3158–3163 [DOI] [PubMed] [Google Scholar]
- 29.Min B., Yamane H., Hu-Li J., Paul W. E. (2005) J. Immunol. 174, 6039–6044 [DOI] [PubMed] [Google Scholar]
- 30.Zhou X., Bailey-Bucktrout S. L., Jeker L. T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J. A. (2009) Nat. Immunol. 10, 1000–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mingueneau M., Roncagalli R., Grégoire C., Kissenpfennig A., Miazek A., Archambaud C., Wang Y., Perrin P., Bertosio E., Sansoni A., Richelme S., Locksley R. M., Aguado E., Malissen M., Malissen B. (2009) Immunity 31, 197–208 [DOI] [PubMed] [Google Scholar]
- 32.Chau L. A., Madrenas J. (1999) J. Immunol. 163, 1853–1858 [PubMed] [Google Scholar]
- 33.Shan X., Balakir R., Criado G., Wood J. S., Seminario M. C., Madrenas J., Wange R. L. (2001) Mol. Cell. Biol. 21, 7137–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Oers N. S., Tao W., Watts J. D., Johnson P., Aebersold R., Teh H. S. (1993) Mol. Cell. Biol. 13, 5771–5780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Oers N. S., Killeen N., Weiss A. (1994) Immunity 1, 675–685 [DOI] [PubMed] [Google Scholar]
- 36.Seddon B., Legname G., Tomlinson P., Zamoyska R. (2000) Science 290, 127–131 [DOI] [PubMed] [Google Scholar]
- 37.Seddon B., Zamoyska R. (2002) J. Immunol. 169, 3752–3759 [DOI] [PubMed] [Google Scholar]
- 38.Osborne L. C., Dhanji S., Snow J. W., Priatel J. J., Ma M. C., Miners M. J., Teh H. S., Goldsmith M. A., Abraham N. (2007) J. Exp. Med. 204, 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chuck M. I., Zhu M., Shen S., Zhang W. (2010) J. Immunol. 184, 2476–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Archambaud C., Sansoni A., Mingueneau M., Devilard E., Delsol G., Malissen B., Malissen M. (2009) J. Immunol. 182, 2680–2689 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Kissenpfennig A., Mingueneau M., Richelme S., Perrin P., Chevrier S., Genton C., Lucas B., DiSanto J. P., Acha-Orbea H., Malissen B., Malissen M. (2008) J. Immunol. 180, 1565–1575 [DOI] [PubMed] [Google Scholar]
- 42.Zhang W., Trible R. P., Zhu M., Liu S. K., McGlade C. J., Samelson L. E. (2000) J. Biol. Chem. 275, 23355–23361 [DOI] [PubMed] [Google Scholar]
- 43.Koonpaew S., Shen S., Flowers L., Zhang W. (2006) J. Exp. Med. 203, 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J. K., Klinger M., Benjamin J., Xiao Y., Erle D. J., Littman D. R., Killeen N. (2009) PLoS One 4, e6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hori S., Nomura T., Sakaguchi S. (2003) Science 299, 1057–106112522256 [Google Scholar]
- 46.Khattri R., Cox T., Yasayko S. A., Ramsdell F. (2003) Nat. Immunol. 4, 337–342 [DOI] [PubMed] [Google Scholar]
- 47.Fontenot J. D., Gavin M. A., Rudensky A. Y. (2003) Nat. Immunol. 4, 330–336 [DOI] [PubMed] [Google Scholar]
- 48.Ruan Q., Kameswaran V., Tone Y., Li L., Liou H. C., Greene M. I., Tone M., Chen Y. H. (2009) Immunity 31, 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long M., Park S. G., Strickland I., Hayden M. S., Ghosh S. (2009) Immunity 31, 921–931 [DOI] [PubMed] [Google Scholar]