Abstract
Reverse pharmacology, also called the “bedside to bench” approach, that deals with new uses for a well known molecular entity has been used extensively in cancer drug development to identify novel compounds and delineate their mechanisms of action. Here, we show that nimbolide, a triterpenoid isolated from Azadirachta indica, enhanced the apoptosis induced by inflammatory cytokines and chemotherapeutic agents in tumor cells. This limonoid abrogated the expression of proteins associated with cell survival (Bcl-2, Bcl-xL, IAP-1, and IAP-2), proliferation (cyclin D1), invasion (MMP-9), and angiogenesis (VEGF), all regulated by nuclear factor (NF)-κB. Nimbolide inhibited the activation of NF-κB induced by carcinogens and inflammatory stimuli. Constitutively active NF-κB found in most tumor cells was also inhibited. We found that suppression of NF-κB activation by nimbolide was caused by inhibition of IκB kinase (IKK), which led to suppression of IκBα phosphorylation and degradation, nuclear translocation, DNA binding, and gene transcription. Reducing agent reversed the action of the limonoid, suggesting the involvement of a cysteine residue. Replacement of Cys179 of IKK-β with alanine abolished the effect of nimbolide, suggesting that Cys179 plays a critical role in inhibiting the NF-κB activation. Overall, our results indicate that nimbolide can sensitize tumor cells to chemotherapeutic agents through interaction with IKK, leading to inhibition of NF-κB-regulated proteins.
Keywords: Apoptosis, Cancer Therapy, Inflammation, NF-kappa B, Tumor Necrosis Factor (TNF), Chemotherapeutic Agents, Limonoid
Introduction
Extensive research during the past 2 decades has made it clear that cancer is a disease in which multiple cell signaling pathways are dysregulated and thus mono-targeted therapies are unlikely to provide a significant benefit (1). Natural products derived from fruits, vegetables, spices, legumes, cereals, and traditional medicines are preferred as potential therapeutics for cancer and other chronic diseases because of their safety, affordability, long term use, and ability to target multiple cell signaling pathways (2). Promiscuous drugs that interact with multiple targets as compared with a single target are becoming a virtue in drug development as in case of imatinib, sunitinib, or sorafenib (3). Neem (Azadirachta indica, a tree in the Mahogany family; Azad Dirakht in Persian means “free tree”; called Muarubaini in Swahili, which means “treats 40 different diseases”) is one such traditional medicinal plant, the extract of which has been used for thousands of years for most acute and chronic diseases in India and Africa and thus has been named “Heal All,” “Nature's Drugstore,” “Village Pharmacy,” and “Panacea for all Diseases.” Over 300 bioactive components from this plant have been isolated, one-third of which are limonoids (4–6). One of the components of this plant is nimbolide, which was first isolated from the leaves and flowers of neem (4, 7). Nimbolide, a tetranortriterpenoid, consists of a classical limonoid skeleton with an α,β-unsaturated ketone system and a δ-lactonic ring (Fig. 1A) (8). This limonoid has been shown to exhibit numerous biologic activities, including anti-feedant (inhibits normal feeding behavior) (9), anti-malarial (10), anti-microbial (11), and anti-cancer (7, 12–16) activities.
FIGURE 1.
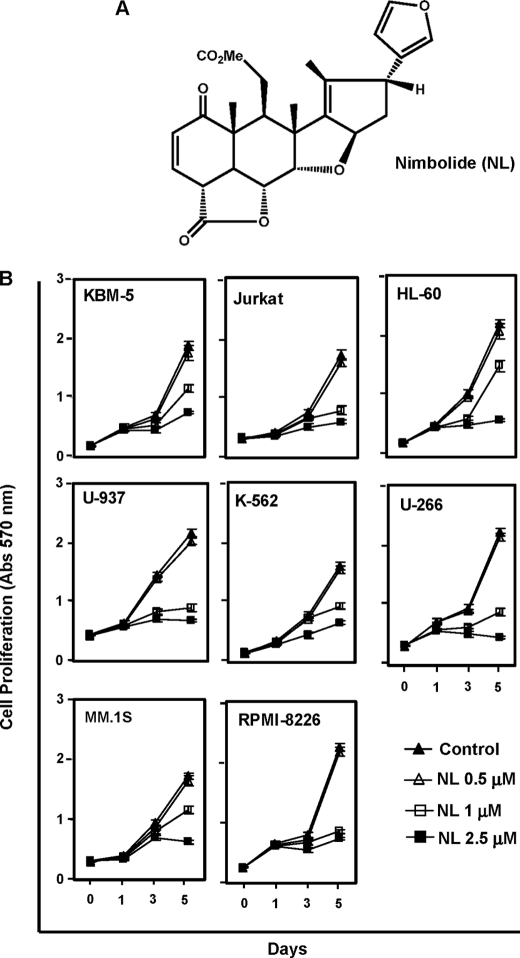
Nimbolide suppresses cell proliferation of tumor cells. A, chemical structure of nimbolide. B, cells (3000 cells/100 μl) were plated in four replicates, treated with the indicated concentrations of nimbolide, and subjected to an MTT assay on days 0, 1, 3, and 5. Cell proliferation was analyzed by measuring the absorbance at 570 nm.
This triterpenoid exhibits anti-proliferative activity in a wide variety of tumor cells, including neuroblastoma cells (12), osteosarcoma cells, choriocarcinoma cells (17), macrophages, leukemia cells, and melanoma cells (16). How nimbolide exerts its antiproliferative effects is not fully understood. Nimbolide has been shown to induce G0/G1 cell cycle arrest (16), increase reactive oxygen species generation, activate caspases, and modulate the activity of cyclins, cyclin-dependent kinase inhibitors, proliferating cell nuclear antigen, and p53 (18). In animal tumor models, nimbolide (10–100 mg/kg) has been shown to exhibit chemopreventive activity against 7,12-dimethylbenz[α]anthracene (DMBA)3-induced oral carcinogenesis (18, 19). Furthermore, the α,β-unsaturated ketone structural element of nimbolide has been linked to its anticancer activity (20). Amide derivatives modified on the lactone ring were also found to enhance the cytotoxic activity of nimbolide (15). Because of the critical role of NF-κB in apoptosis, tumor cell survival, proliferation, invasion, and angiogenesis and its activation by various carcinogens, including DMBA, we hypothesized that nimbolide may modulate this cell signaling pathway.
NF-κB is a ubiquitous and evolutionarily conserved transcription factor that is activated in response to a number of carcinogens and inflammatory stimuli, including cytokines (e.g. tumor necrosis factor (TNF)), tumor promoters, cigarette smoke, environmental pollutants, ionizing radiation, and stress. In resting cells, NF-κB is kept in an inactive state in the cytoplasm as a heterotrimer consisting of p50, p65, and IκB proteins, including IκBα, IκBβ, and IκBϵ (21). In response to activation signals, the IκBα subunit is phosphorylated at serine residues 32 and 36 and ubiquitinated at lysine residues 21 and 22, which target them for proteasome-mediated degradation. The p65 subunit is then phosphorylated and translocated to the nucleus where it binds to a specific DNA sequence and activates the transcription of over 500 genes involved in immunoregulation, growth regulation, inflammation, carcinogenesis, and apoptosis. The phosphorylation of IκBα is catalyzed by IκBα kinase (IKK), which consists of three subunits, IKK-α, IKK-β, and IKK-γ (also called NEMO). Some natural compounds have been reported to inhibit NF-κB activation through modification of a specific cysteine residue (Cys179) in the activation loop of IKK-β (22–26).
In this study, we investigated whether nimbolide modulates the NF-κB signaling pathway in tumor cells. Our results demonstrate that this limonoid inhibits the NF-κB activation pathway induced by carcinogens, tumor promoters, inflammatory stimuli, and growth factors through direct interaction with Cys179 of IKK-β, leading to suppression of IκBα phosphorylation and degradation, inhibition of p65 nuclear translocation, down-regulation of NF-κB-regulated gene products, inhibition of cell proliferation, and potentiation of apoptosis induced by TNF-α and chemotherapeutics in tumor cells.
EXPERIMENTAL PROCEDURES
Reagents
Nimbolide (Fig. 1A) was isolated from Azadirachta indica leaves as reported previously (27). A 50 mm solution of this limonoid was prepared in dimethyl sulfoxide and stored in small aliquots at −20 °C. Nimbolide was diluted in the culture medium just before use. Human recombinant TNF-α purified from bacterial cells to homogeneity with a specific activity of 5 × 107 units/mg was provided by Genentech (South San Francisco, CA). Cigarette smoke condensate (CSC) was provided by Dr. C. Gary Gairola (University of Kentucky, Lexington). Penicillin, streptomycin, RPMI 1640 medium, Iscove's modified Dulbecco's medium, Dulbecco's modified Eagle's medium, and fetal bovine serum (FBS) were obtained from Invitrogen. Phorbol myristate acetate (PMA), lipopolysaccharide (LPS), okadaic acid (OA), and antibodies against FLAG and β-actin were obtained from Sigma. Antibodies against p65, p50, cyclin D1, cyclooxygenase-2 (COX-2), matrix metalloproteinase-9 (MMP-9), poly(ADP-ribose) polymerase (PARP), inhibitor of apoptosis protein-1 (IAP-1), IAP-2, Bcl-2, Bcl-xL, intercellular adhesion molecule-1 (ICAM-1), c-Myc, caspase-3, -8, and -9, and the annexin V staining kit were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-specific anti-IκBα (Ser32/36) and anti-p65 (Ser536) were purchased from Cell Signaling (Danvers, MA). An antibody against p65, which was used for immunocytochemical analyses, was obtained from Abcam (Cambridge, MA). The vascular endothelial growth factor (VEGF) antibody was purchased from NeoMarkers (Fremont, CA). Anti-IκBα, -IKK-α, and -IKK-β antibodies were obtained from Imgenex (San Diego, CA).
Cell Lines
The cell lines KBM-5 (human chronic myeloid leukemia), U937 (human leukemic monocyte lymphoma), HL-60 (human promyelocytic leukemia), Jurkat (T-cell leukemia), A293 (human embryonic kidney), H1299 (human lung adenocarcinoma), U266 (human multiple myeloma), MCF-7 (breast cancer), SCC-4 (human squamous cell carcinoma), and RPMI-8226 and MM.1S (human multiple myeloma) were obtained from the American Type Culture Collection (Manassas, VA). K-562 (human chronic myeloid leukemia) was a gift from Dr. Hesham Amin (University of Texas M. D. Anderson Cancer Center, Houston). KBM-5 cells were cultured in Iscove's modified Dulbecco's medium with 15% FBS; K-562, HL-60, Jurkat, H1299, MCF-7, U937, RPMI-8226, MM.1S, and U266 cells were cultured in RPMI 1640 medium with 10% FBS; and A293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS. SCC-4 cells were cultured in Dulbecco's modified Eagle's medium containing 10% FBS, nonessential amino acids, pyruvate, glutamine, and vitamins. All culture media were supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin.
Electrophoretic Mobility Shift Assay
To determine NF-κB activation, we prepared nuclear extracts and performed an electrophoretic mobility shift assay (EMSA) as described previously (28).
Western Blot Analysis
The cytoplasmic, nuclear, and whole-cell extracts were prepared, and Western blot analysis was performed as described previously (29).
Enzyme-linked Immunosorbent Assay
Human ELISA systems kits (eBioscience, San Diego) were used for the detection of interleukin-6 (IL-6) and TNF-α. The U-266 cells were treated with different concentrations of nimbolide, and cell-free supernatants were collected after 24 h. The cytokine level was determined by following the manufacturer's protocol.
Invasion Assay
A membrane invasion culture system was used to assess cell invasion. We used the BD BioCoat tumor invasion system (BD Biosciences), which contains an 8-μm polyethylene terephthalate membrane with a thin layer of reconstituted Matrigel basement membrane matrix. The H1299 cells (2.5 × 104) were suspended in serum-free medium and seeded into the upper wells. After incubation overnight, cells were treated with 2.5 μm nimbolide for 4 h and then stimulated with 1 nm TNF in the presence of 1% FBS. After 24 h of incubation, the invaded cells were fixed, stained with Diff-Quik stain, and counted in five random microscopic fields.
IKK Assay
We performed IKK assay to determine the effect of nimbolide on TNF-α-induced IKK activation using a method described previously (30).
Immunocytochemical Analysis for NF-κB p65 Localization
We performed immunocytochemical analysis to determine the effect of nimbolide on TNF-α-induced p65 nuclear translocation, as described previously (24). Stained slides were analyzed using a Labophot-2 fluorescence microscope (Nikon, Tokyo, Japan). Images were captured with a Photometrics Coolsnap CF color camera (Nikon, Lewisville, TX) and analyzed with MetaMorph version 4.6.5 software (Universal Imaging, Sunnyvale, CA).
NF-κB-dependent Reporter Gene Expression Assay
The effect of nimbolide on the induction of NF-κB-dependent reporter gene transcription by TNF-α, TNF receptor-1 (TNFR1), TNF receptor-associated death domain (TRADD), TNF receptor-associated factor 2 (TRAF2), NF-κB-inducing kinase (NIK), TAK1/TAB1-β, and IKK-β was analyzed using a secretory alkaline phosphatase (SEAP) assay, as described previously (31).
Apoptosis by Live/Dead Assay
To determine plasma membrane integrity and intracellular esterase activity, we performed a live/dead assay. It is a two-color fluorescence assay that simultaneously determines live cells and dead cells. Intracellular esterases from live cells convert nonfluorescent cell-permeable calcein acetoxymethyl (calcein AM) to the intensely fluorescent calcein. Cleaved calcein is retained within cells. It also examines dead cells that have damaged membranes; the ethidium homodimer-1 (EthD-1) enters damaged cells and is fluorescent when bound to nucleic acids. EthD-1 produces a bright red fluorescence in damaged or dead cells. This assay was performed as described previously (32).
Apoptosis by Phosphatidylserine Externalization Assay
The annexin V assay provides a simple and effective method to detect apoptosis at a very early stage. This assay takes advantage of the fact that phosphatidylserine (PS) is translocated from the inner (cytoplasmic) leaflet of the plasma membrane to the outer (cell surface) leaflet soon after the induction of apoptosis and that the annexin V protein has a strong, specific affinity for PS. PS on the outer leaflet is available to bind labeled annexin V, providing the basis for a simple staining assay. We measured the loss of membrane asymmetry that occurs when PS moves to the extracellular surface of the membrane using an annexin V staining kit (Santa Cruz Biotechnology). The assay was performed by following the manufacturer's instructions.
Measurement of Apoptosis and Cell Proliferation by the 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl Tetrazolium Bromide Method
The effects of nimbolide on the proliferation of leukemic cells and on the cytotoxic potential of TNF-α and other chemotherapeutic agents were determined by measuring mitochondrial dehydrogenase activity, using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) as the substrate (33).
Statistical Analysis
Different parameters were monitored in normal and treated cells. Experiments were repeated a minimum of three times. Data are given as the mean ± S.D. The statistical analysis was carried out using a two-tailed unpaired Student's t test. A value of p < 0.05 was considered statistically significant.
RESULTS
Our goal in this study was to determine whether nimbolide affects the NF-κB activation pathway, NF-κB-regulated gene products, and NF-κB-mediated cellular responses in tumor cells. We focused primarily on TNF-α-induced cellular effects because the role of TNF-α in the NF-κB activation pathway is relatively well defined. When nimbolide or NF-κB activators were added alone, at the concentrations and the exposure times employed in our study, cell viability was not affected.
Nimbolide Suppresses Cell Proliferation in Tumor Cells
Because NF-κB has been implicated in the survival and proliferation of various tumor cells, we examined the effect of nimbolide on cell proliferation. The limonoid suppressed the proliferation of various myeloid leukemic (KBM-5, HL-60, U-937, and K-562), T-cell lymphoma (Jurkat), and myeloma (U266, MM.1S, and RPMI-8226) cells in a dose- and time-dependent manner. The suppression of cell proliferation could be observed at nimbolide concentrations as low as 1 μm (Fig. 1B). No significant inhibition of cell growth was noted after 1 day. These results indicate that nimbolide exhibits potent anti-proliferative effects on both leukemic and myeloid cells.
Nimbolide Potentiates the Apoptosis Induced by TNF-α in Tumor Cells
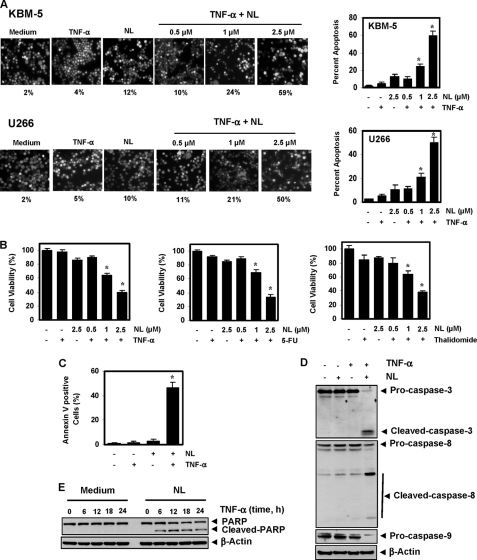
Among the cytokines, TNF-α is a well known apoptosis-inducing agent (34). We investigated whether nimbolide could potentiate TNF-α-induced apoptosis. When apoptosis was examined by measuring intracellular esterase activity, we found that nimbolide treatment increased TNF-α-induced apoptosis in KBM-5 and U266 cells in a dose-dependent manner. The TNF-α-induced apoptosis increased from 4 to 59% in KBM-5 cells and from 5 to 50% in U-266 cells (Fig. 2A). The results of the MTT assay also indicated that nimbolide enhanced the cytotoxic effects of TNF-α in a dose-dependent manner (Fig. 2B).
FIGURE 2.
Nimbolide potentiates the apoptotic effects of TNF-α and chemotherapeutic agents in chronic myeloid leukemic and multiple myeloma cells. A, nimbolide enhances TNF-α-induced apoptosis. KBM-5 and U266 cells were pretreated with the indicated concentrations of nimbolide for 4 h and then incubated with 1 nm TNF-α for 24 h. The cells were stained with a live/dead assay reagent for 30 min and analyzed under a fluorescence microscope. Values below each photomicrograph represent percent apoptotic cells. B, nimbolide enhances TNF-α-, 5-fluorouracil-, and thalidomide-induced cytotoxicity. We seeded 5000 cells in four replicates, pretreated them with the indicated concentrations of nimbolide, and then incubated them with chemotherapeutic agents (1 nm TNF-α, 0.1 μm 5-fluorouracil, and 10 μg/ml thalidomide) for 24 h. Cell viability was analyzed by the MTT assay. C, nimbolide potentiates TNF-α-induced early apoptosis in KBM-5 cells, as determined by the annexin V assay. Cells were pretreated with 2.5 μm nimbolide for 4 h and then incubated with 1 nm TNF-α for 24 h. The cells were incubated with an fluorescein isothiocyanate-conjugated annexin V antibody and then analyzed using flow cytometry. D, nimbolide induces TNF-α-induced caspase activation. Cells were incubated with 2.5 μm nimbolide for 4 h and then treated with 1 nm TNF-α for 24 h. Whole-cell extracts were prepared and analyzed by Western blotting, using the indicated antibodies. E, nimbolide induces PARP cleavage. Cells were pretreated with 2.5 μm nimbolide for 4 h and then incubated with 1 nm TNF-α for the indicated times. Whole-cell extracts were prepared and analyzed by Western blotting using an anti-PARP antibody. Figures are representative of one of three independent experiments. The values in the histograms represent the means ± S.D. of three independent replicates. * indicates the significance of difference compared with TNF-α group; p < 0.05. NL, nimbolide; 5-FU, 5-fluorouracil.
When apoptosis was examined by a PS externalization assay, significantly more annexin V-positive cells were found after treatment with nimbolide and TNF-α than after treatment with the cytokine alone (Fig. 2C). TNF-α binds to TNFR1 and sequentially recruits TRADD, FADD, and the FADD-like interleukin-1β-converting enzyme (caspase-8), leading to activation of caspase-9 and -3. Activated caspase-3 then induces PARP cleavage, a sign of apoptosis. Thus, we examined whether nimbolide could enhance the TNF-α-induced activation of caspase-3, -8, and -9. We found that TNF-α alone had little effect on caspase activation, but nimbolide sensitized the cells to caspase activation (Fig. 2D) and subsequent PARP cleavage (Fig. 2E). Overall, these results indicate that nimbolide significantly increases the apoptotic potential of TNF-α in tumor cells.
Nimbolide Potentiates the Apoptosis Induced by Chemotherapeutic Agents in Tumor Cells
Although most tumor cells are initially sensitive to chemotherapeutic agents, gradually they develop chemoresistance in part because of induction of NF-κB-mediated multidrug resistance. Whether nimbolide can potentiate the apoptotic effects of chemotherapeutic agents was examined. We found that this limonoid potentiated the apoptotic effects of both 5-fluorouracil and thalidomide (Fig. 2B) in KBM-5 cells. These results indicate that nimbolide significantly enhances the apoptotic potential of chemotherapeutic agents.
Nimbolide Suppresses the Expression of NF-κB-regulated Anti-apoptotic Gene Products
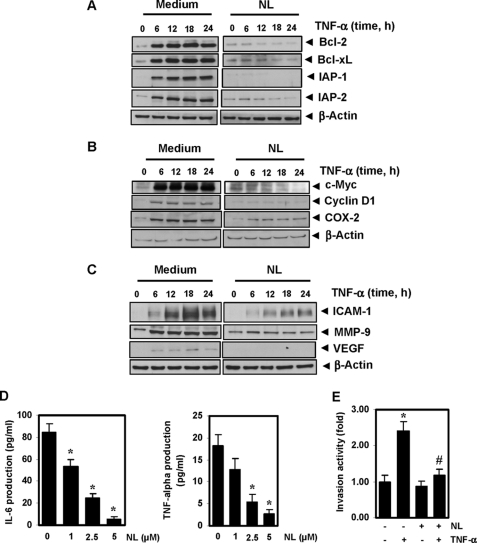
We investigated in detail how nimbolide potentiates the effect of cytokines or chemotherapeutic agents. The anti-apoptotic proteins Bcl-2, Bcl-xL, IAP-1, and IAP-2 are all known to be induced by TNF-α and to play a major role in cell survival. We found that nimbolide down-regulated the expression of all these TNF-α-induced proteins (Fig. 3A).
FIGURE 3.
Nimbolide inhibits TNF-α-induced expression of NF-κB-dependent anti-apoptotic, proliferative, and metastatic proteins. KBM-5 cells were incubated with 2.5 μm nimbolide for 4 h and then treated with 1 nm TNF-α for the indicated times. Whole-cell extracts were prepared and analyzed by Western blotting using antibodies against anti-apoptotic (A), proliferative (B), and metastatic proteins (C). One of the three independent experiments is shown. D, nimbolide down-regulates IL-6 and TNF-α production in U266 cells. The cells (2 × 106) were treated with the indicated concentrations of nimbolide, and cell-free supernatants were harvested after 24 h. The levels of IL-6 and TNF-α were detected by ELISA. E, nimbolide suppresses TNF-α induced cell invasion. H1299 cells (2.5 × 104 cells) were seeded into the top chamber of a Matrigel invasion chamber system overnight in the absence of serum and then treated with 2.5 μm nimbolide. After incubation for 4 h, the cells were treated with TNF-α in the presence of 1% serum and then assayed for invasion as described under “Experimental Procedures.” The value for control was set to 1.0. The values given in histograms represent the means ± S.D. of three independent replicates. * and # indicates the significance of difference compared with control and TNF-α group, respectively; p < 0.05. NL, nimbolide.
Nimbolide Suppresses the Expression of Proliferation Proteins in Tumor Cells
We next examined whether suppression of cell proliferation by nimbolide is due to down-regulation of proteins such as cyclin D1, c-Myc, and COX-2. We found that the limonoid inhibited the expression of all three of these TNF-α-induced proteins (Fig. 3B).
Nimbolide Suppresses the Expression of Proteins Linked to Invasion and Metastasis
MMP-9, by virtue of its ability to degrade the extracellular matrix, has been implicated in cellular invasion (35). ICAM-1, an adhesion molecule, has also been shown to be required for tumor metastasis (36), and VEGF plays a critical role in angiogenesis by promoting vascular endothelial cell growth and enhancing vascular permeability (37). Thus, we examined whether nimbolide can modulate the expression of these gene products. Our results revealed that nimbolide down-regulated the expression of all these proteins (Fig. 3C).
Nimbolide Down-regulates Pro-inflammatory Cytokine Production in Multiple Myeloma Cells
Both IL-6 and TNF-α have been linked with the proliferation of leukemic cells. We investigated whether nimbolide has an effect on the production of IL-6 and TNF-α in U266 cells. The production of IL-6 and TNF-α in U266 cells was suppressed by nimbolide in a concentration-dependent manner (Fig. 3D).
Nimbolide Suppresses TNF-α-induced Tumor Cell Invasion
We next examined whether nimbolide can affect tumor cell invasion. As shown in Fig. 3E, TNF-α increased tumor cell invasion by almost 2.4-fold, and pretreating cells with nimbolide suppressed the TNF-α-induced cell invasion.
Nimbolide Suppresses TNF-α-induced NF-κB Activation
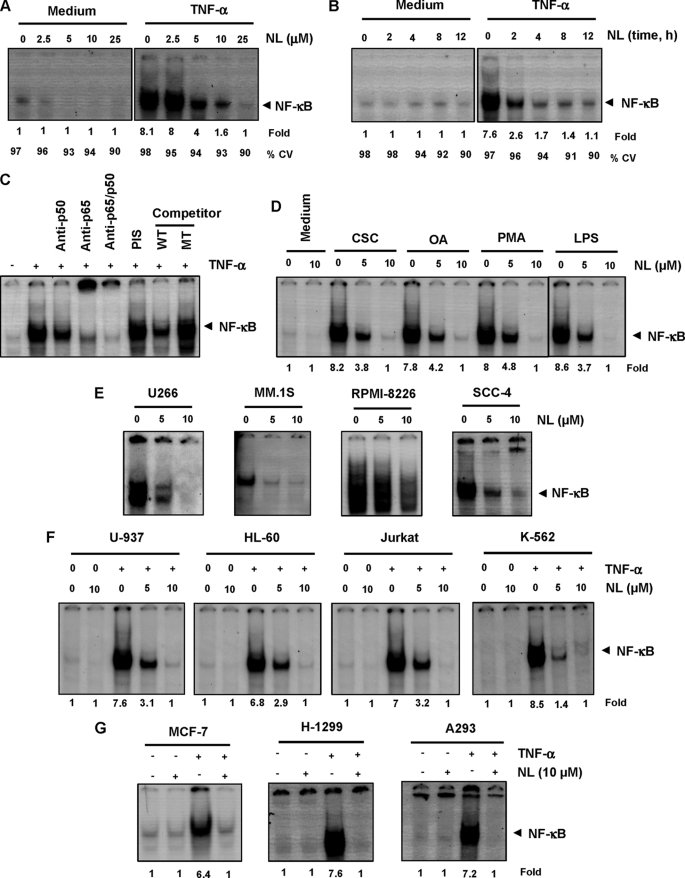
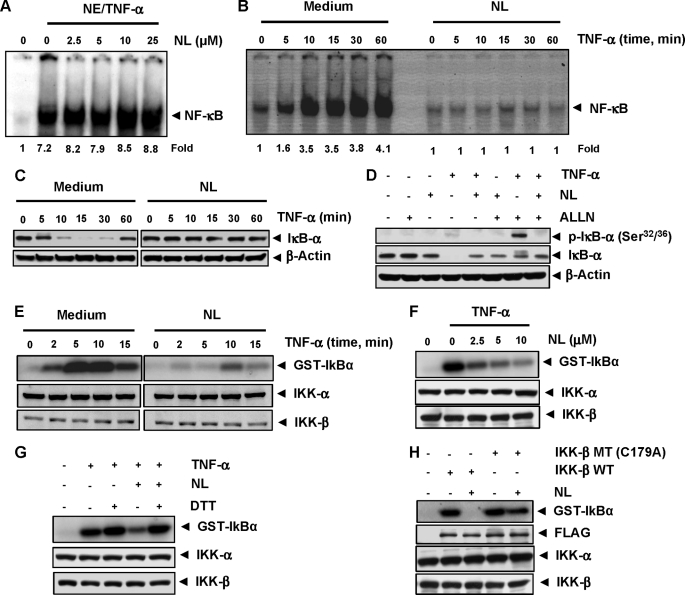
Whether enhancement of apoptosis or down-regulation of anti-apoptotic, proliferative, and metastatic gene products, all require the activation of NF-κB. Therefore, we investigated whether the TNF-α-induced activation of NF-κB is modulated by nimbolide. We found that TNF-α induces NF-κB activation, whereas nimbolide alone did not. However, pretreatment of cells with nimbolide inhibited the TNF-α-induced NF-κB activation in a dose- (Fig. 4A) and time-dependent (Fig. 4B) manner. Treatment of cells with 10 μm nimbolide for 4 h was optimal for inhibiting the TNF-α-induced NF-κB activation without affecting cell viability.
FIGURE 4.
Nimbolide down-regulates constitutive and TNF-α-induced NF-κB activation in different cell lines. Dose-dependent (A) and time-dependent (B) effects of nimbolide on TNF-α-induced NF-κB activation. KBM-5 cells were incubated with the indicated concentrations of nimbolide for 4 h (A) or with 10 μm nimbolide for the indicated times (B) and then treated with 0.1 nm TNF-α for 30 min. Nuclear extracts were prepared and assayed for NF-κB activation using EMSA. The fold activation of NF-κB as compared with control and percentage of viable cells (CV) are shown at the bottom. C, TNF-α-induced NF-κB is composed of p65 and p50 subunits. Nuclear extracts from untreated cells or cells treated with 0.1 nm TNF-α were incubated with the indicated antibodies, an unlabeled NF-κB oligoprobe, or a mutant oligoprobe. They were then assayed for NF-κB activation by EMSA. D, nimbolide inhibits NF-κB activation induced by CSC, OA, PMA, and LPS. KBM-5 cells were incubated with 5 and 10 μm nimbolide for 4 h and then treated with 10 μg/ml CSC for 1 h, 500 nm OA for 4 h, 100 ng/ml PMA for 2 h, or 10 μg/ml LPS for 30 min. Nuclear extracts were analyzed for NF-κB activation using EMSA. The fold activation of NF-κB as compared with control is shown at the bottom. E, effect of nimbolide on constitutive NF-κB activation. U266, MM.1S, RPMI-8226, and SCC-4 cells were incubated with nimbolide at the indicated concentrations for 4 h. Nuclear extracts were prepared and analyzed for NF-κB activation by EMSA. F and G, effect of nimbolide on TNF-α-induced NF-κB activation in other types of cells. U-937, HL-60, Jurkat, K-562, MCF-7, H-1299, and A293 cells were incubated with 10 μm nimbolide for 4 h and then treated with 0.1 nm TNF-α for 30 min. Nuclear extracts were assayed for NF-κB activation using EMSA. The fold activation of NF-κB as compared with control is shown at the bottom. One of three independent experiments is shown. NL, nimbolide.
NF-κB is a complex of proteins in which various combinations of Rel or NF-κB proteins constitute active NF-κB heterodimers that bind to a specific DNA sequence. In our study, when nuclear extracts from TNF-α-activated cells were incubated with p50 (NF-κB) or p65 (RelA) antibodies, the resulting bands were shifted to higher molecular masses, suggesting that the TNF-α-activated complex consisted of p50 and p65. Preimmune serum had no effect on DNA binding, and the addition of excess unlabeled NF-κB (cold oligonucleotide; 100-fold excess) caused a decrease in the intensity of the band, whereas the addition of a mutated oligonucleotide had no effect on DNA binding (Fig. 4C). These results indicated that the band visualized in TNF-α-treated cells was indeed NF-κB.
Nimbolide Inhibits NF-κB Activation Induced by Carcinogens and Inflammatory Stimuli
Numerous agents, including cigarette smoke, OA, PMA, and LPS, are potent activators of NF-κB, but they work by different mechanisms. We determined whether nimbolide affects the NF-κB activation induced by these agents using EMSA. All of these agents activated NF-κB in KBM-5 cells, and nimbolide suppressed this activation in a dose-dependent manner (Fig. 4D). These results suggest that nimbolide acts at a step in the NF-κB activation pathway that is common to all four agents.
Nimbolide Inhibits Constitutive NF-κB Activation in Myeloid Cells
Human multiple myeloma cells (U266, MM.1S, and RPMI-8226) are known to constitutively express NF-κB. We found that nimbolide inhibited constitutive NF-κB activation in these cells (Fig. 4E), indicating that it can suppress not only inducible but also constitutively active NF-κB in tumor cells. Nimbolide also inhibited constitutive NF-κB in SCC-4 cells (Fig. 4E).
Inhibition of NF-κB Activation by Nimbolide Is Not Cell-specific
We next determined whether the nimbolide-mediated inhibition in TNF-α-induced NF-κB activation is cell type-specific. The inhibitory effect of nimbolide on TNF-α-induced NF-κB activation was observed not only in KBM-5 cells but also in other leukemic cells (U-937, HL-60, Jurkat, K-562; Fig. 4F) and in MCF-7, H-1299, and A293 cells (Fig. 4G).
Nimbolide Does Not Directly Affect the Binding of NF-κB to DNA
Some NF-κB inhibitors, such as plumbagin (38), l-phenylalanine chloromethyl ketone (a serine protease inhibitor) (25), and caffeic acid phenethyl ester (39), directly modify NF-κB to suppress its DNA binding. We determined whether nimbolide mediates suppression of NF-κB activation through a similar mechanism using EMSA. Nimbolide did not modify the DNA-binding ability of NF-κB proteins prepared from TNF-α-treated cells (Fig. 5A), suggesting that it inhibits NF-κB activation using a mechanism different from that of plumbagin, l-phenylalanine chloromethyl ketone, and caffeic acid phenethyl ester.
FIGURE 5.
Down-regulation of TNF-α-induced NF-κB activation by nimbolide involves cysteine 179 of IKK. A, direct effect of nimbolide on NF-κB-DNA binding. Nuclear extracts prepared from untreated cells or cells treated with 0.1 nm TNF-α were incubated for 30 min with the indicated concentrations of nimbolide in vitro. The effect of nimbolide on NF-κB-DNA binding was then analyzed by EMSA. B, nimbolide inhibits the TNF-α-induced activation of NF-κB. KBM-5 cells were preincubated with 10 μm nimbolide for 4 h, treated with 0.1 nm TNF-α for the indicated times, and then analyzed for NF-κB activation by EMSA. The fold activation of NF-κB as compared with control is indicated at the bottom. C, nimbolide inhibits TNF-α-induced degradation of IκBα. KBM-5 cells were incubated with 10 μm nimbolide for 4 h and treated with 0.1 nm TNF-α for the indicated times. Cytoplasmic extracts were prepared and analyzed for IκBα by Western blotting. D, nimbolide inhibits TNF-α-induced phosphorylation of IκBα. KBM-5 cells were treated with 10 μm nimbolide for 4 h, incubated with 50 μg/ml N-acetyl-leucyl-leucyl-norleucinal (ALLN) for 30 min, and then treated with 0.1 nm TNF-α for 10 min. Cytoplasmic extracts were analyzed by Western blotting using a phospho-specific IκBα antibody (Ser32/36). The same membrane was reprobed with IκBα and β-actin antibody. E, effect of nimbolide on TNF-α-induced IKK activation. KBM-5 cells were preincubated with 10 μm nimbolide for 4 h and then treated with 1 nm TNF-α for the indicated times. Whole-cell extracts were immunoprecipitated with an antibody against IKK-β and analyzed using an immune complex kinase assay. The effect of nimbolide on IKK protein expression was determined by Western blot analysis using anti-IKK-α and anti-IKK-β antibodies. F, direct effect of nimbolide on IKK activation induced by TNF-α. Whole-cell extracts were prepared from KBM-5 cells treated with 1 nm TNF-α and immunoprecipitated with an anti-IKK-β antibody. The immunoprecipitated complex was incubated with the indicated concentrations of nimbolide, and an immunocomplex kinase assay was performed. G, nimbolide-induced suppression of TNF-α-induced IKK activation was reversed by the reducing agent DTT. Assays were performed as indicated in Fig. 4F, except that IKK activity was also determined in the presence of DTT (100 μm). H, kinase activity of mutated IKK (C179A) is unaffected by nimbolide. A293 cells were transfected with wild-type FLAG-IKK-β (IKK-β WT) or mutated FLAG-IKK-β (IKK-β MT (C179A)). Whole-cell extracts were prepared, immunoprecipitated, incubated with nimbolide, and subjected to an IKK assay. One of three independent experiments is shown. NL, nimbolide.
Nimbolide Inhibits TNF-α-dependent IκBα Phosphorylation and Degradation
IκBα is the inhibitory subunit present in the NF-κB complex. The translocation of NF-κB to the nucleus is preceded by the phosphorylation, ubiquitination, and proteolytic degradation of IκBα. To determine whether the inhibition of TNF-α-induced NF-κB activation was due to suppression of IκBα degradation, we pretreated KBM-5 cells with nimbolide and exposed them to TNF-α for 0–60 min. We then analyzed the DNA binding of NF-κB in the nuclear fraction by EMSA and IκBα degradation in the cytoplasmic fraction by Western blotting. TNF-α induced NF-κB in control cells within 5 min, and the level plateaued after 30 min. However, in the cells pretreated with nimbolide, TNF-α was unable to induce NF-κB activation (Fig. 5B). TNF-α induced IκBα degradation in KBM-5 cells after 10 min, and the maximum level of degradation was seen after 30 min; IκBα was resynthesized after 60 min. In nimbolide-pretreated cells, the TNF-α-induced IκBα degradation was completely suppressed (Fig. 5C). These results indicate that nimbolide inhibits both TNF-α-induced NF-κB activation and IκBα degradation.
To determine whether the inhibition of TNF-α-induced IκBα degradation was due to inhibition of IκBα phosphorylation, we used the proteasome inhibitor N-acetyl-leucyl-leucyl-norleucinal to block IκBα degradation. Western blotting with an antibody that recognized the phosphorylated form of IκBα (Ser32 and Ser36) revealed that TNF-α induced the phosphorylation of IκBα, and nimbolide completely suppressed this phosphorylation (Fig. 5D). These results indicate that nimbolide inhibits TNF-α-induced IκBα degradation by inhibiting IκBα phosphorylation.
Nimbolide Directly Inhibits TNF-α-induced IKK Activation
The TNF-α-induced phosphorylation of IκBα is mediated through IKK. The inhibition in TNF-α-induced IκBα phosphorylation by nimbolide prompted us to determine the effect of nimbolide on IKK activation. A kinase assay revealed that TNF-α activated IKK in a time-dependent manner and that nimbolide completely suppressed this activation (Fig. 5E). Neither TNF-α nor nimbolide had any effect on the expression of IKK-α or IKK-β proteins.
To determine whether nimbolide suppresses IKK activity directly or indirectly by suppressing its activation, we incubated an immune complex prepared from TNF-α-stimulated cells with various concentrations of nimbolide and then assayed for IKK activity. Nimbolide inhibited IKK activity (Fig. 5F), suggesting that it directly modulates TNF-α-induced IKK activation.
Nimbolide Binds to and Inhibits IKK Activation
Because the IKK-β subunit of the IKK complex contains various cysteine residues, we hypothesized that nimbolide may inhibit IKK through direct modification of one or more of these cysteine residues. We used a reducing agent, dithiothreitol (DTT), to investigate whether the modulation of IKK activity by nimbolide was caused by the oxidation of critical cysteine residues. The addition of DTT to the kinase reaction mixture reversed the nimbolide-mediated inhibition of TNF-α-induced IKK activity (Fig. 5G), suggesting that a cysteine residue is involved in the pathway. IKK-β contains a cysteine at position 179 in its activation loop that is critical for its activity. To determine whether this residue is involved in nimbolide-mediated IKK inhibition, we transfected A293 cells with wild-type FLAG-IKK-β or FLAG-IKK-β with a C179A mutation. Nimbolide inhibited wild-type IKK-β. In contrast, nimbolide had no apparent effect on the activity of the mutated IKK-β (Fig. 5H). These findings suggest that nimbolide inhibits IKK-β activity by modifying Cys179.
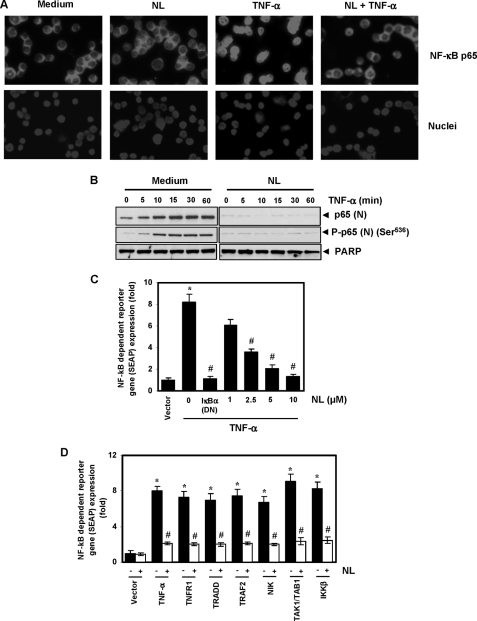
Nimbolide Inhibits TNF-α-induced p65 Nuclear Translocation and Phosphorylation
IκBα degradation is required for the nuclear translocation of p65. Thus, we determined whether nimbolide can affect TNF-α-induced p65 nuclear translocation. An immunocytochemical analysis revealed that TNF-α induced the translocation of p65 to the nucleus in KBM-5 cells, whereas pretreatment of cells with nimbolide suppressed this translocation. In untreated cells and cells treated with nimbolide alone, p65 was localized to the cytoplasm. These results confirmed that nimbolide inhibited the TNF-α-induced p65 nuclear translocation (Fig. 6A).
FIGURE 6.
Nimbolide inhibits TNF-α-induced phosphorylation and nuclear translocation of p65 and NF-κB-dependent reporter gene expression induced by TNF-α and different molecules in the NF-κB-signaling pathway. A, immunocytochemical analysis of p65 localization. KBM-5 cells were treated with 10 μm nimbolide for 4 h, exposed to 0.1 nm TNF-α for 15 min, and analyzed for p65 localization. B, KBM-5 cells were untreated or pretreated with 10 μm nimbolide for 4 h and then treated with 0.1 nm TNF-α for the indicated times. Nuclear extracts were prepared and analyzed by Western blotting using antibodies against p65 and phospho-p65 (Ser536). PARP was used as an internal control. Figures are representative of one of three independent experiments. C, nimbolide inhibits TNF-α-induced NF-κB-dependent SEAP expression. A293 cells were transiently transfected with an NF-κB-containing plasmid linked with the SEAP gene. Cells were treated with nimbolide for 4 h at the indicated concentrations, followed by 1 nm TNF-α for 24 h. Cell supernatants were collected and assayed for SEAP activity. The results are expressed as the change in activity relative to the vector control. DN, dominant negative. D, nimbolide inhibits NF-κB-dependent reporter gene expression induced by TNF-α, TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, and IKK-β. A293 cells were transfected with pNF-κB-SEAP plasmid, expression plasmid, and control plasmid for 24 h and treated with nimbolide. The cell supernatants were then assayed for SEAP activity. Where indicated, the cells were exposed to 1 nm TNF-α for 12 h. The results are expressed as the change in activity relative to the vector control. Values given are the mean ± S.D. of three independent replicates. * and # indicate the significance of difference compared with control and TNF-α/plasmid alone group respectively; p < 0.05. NL, nimbolide.
The effect of nimbolide on TNF-α-induced p65 nuclear translocation was further confirmed by Western blot analysis. TNF-α-induced p65 translocation in a time-dependent manner, and nimbolide suppressed this translocation (Fig. 6B). In addition, phosphorylation of p65 is required for its transcriptional activity. Thus, we measured levels of phosphorylated p65 in cells treated with and without nimbolide. We found that p65 phosphorylation at Ser536 occurred in a time-dependent manner in TNF-α-treated cells but not in cells that had also been treated with the limonoid (Fig. 6B).
Nimbolide Represses TNF-α-induced NF-κB-dependent Reporter Gene Expression
Using DNA binding assays, we demonstrated that nimbolide inhibits NF-κB activation; however, DNA binding alone is not always associated with NF-κB-dependent gene transcription, suggesting that additional regulatory steps are involved. Therefore, we determined whether nimbolide affects TNF-α-induced reporter gene transcription. We transiently transfected A293 cells with an NF-κB-regulated SEAP reporter construct, pretreated the cells with nimbolide, and stimulated them with TNF-α. We observed 8.2 times higher SEAP activity than in cells expressing the vector control; this induction was completely abolished by the dominant negative IκBα, indicating the specificity of the assay. When cells were pretreated with nimbolide, the TNF-α-induced NF-κB-dependent SEAP expression was inhibited in a dose-dependent manner (Fig. 6C). These results indicate that nimbolide inhibits the NF-κB-dependent reporter gene expression induced by TNF-α.
Because TNF-α-induced NF-κB activation is mediated through the sequential interaction of TNFR1 with TRADD, TRAF2, TAK1, and IKK-β, we determined the site of action for nimbolide in the TNF-α signaling pathway. A SEAP assay revealed that TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, and IKK-β plasmids significantly induced reporter gene expression, and nimbolide treatment substantially suppressed NF-κB-dependent gene expression (Fig. 6D).
DISCUSSION
The objective of this study was to determine whether nimbolide, a triterpenoid isolated from a traditionally used plant called neem, exhibits its activities through modulation of the NF-κB activation pathway, which has been associated with inflammation, tumor cell survival, proliferation, invasion, and angiogenesis (40). We found that nimbolide inhibited the proliferation of various tumor cells; potentiated the apoptosis induced by the inflammatory cytokine TNF-α and chemotherapeutic agents; suppressed the expression of NF-κB-dependent gene products involved in cell survival, cell proliferation, metastasis, and angiogenesis; and suppressed the production of cytokines. These effects were mediated through the suppression of constitutive and inducible NF-κB activation by inhibition of IKK activation, leading to the suppression of IκBα phosphorylation and degradation, and p65 nuclear translocation and phosphorylation.
This is the first report to suggest that nimbolide can inhibit the NF-κB pathway. Among all the cytokines, TNF-α is perhaps the most potent activator of NF-κB, a cell survival factor. TNF-α, however, is known to activate both the NF-κB and apoptosis pathways simultaneously. Thus, suppression of the TNF-α-induced NF-κB activation potentiated TNF-α-induced apoptosis in tumor cells. Similarly, apoptosis induced by chemotherapeutic agents, such as 5-fluorouracil and thalidomide, was also enhanced by the limonoid. Although the pro-apoptotic effects of the limonoid have been reported previously, to our knowledge, this is the first report of its effects in combination with cytokines or chemotherapeutic agents in tumor cells.
We also found that nimbolide suppressed the NF-κB-regulated expression of anti-apoptotic genes, including Bcl-2, Bcl-xL, IAP-1, and IAP-2. Overexpression of these genes has been associated with tumor cell survival, chemoresistance, and radioresistance in numerous tumor cell types. Suppression of NF-κB activation by nimbolide was concomitant with the suppression of these gene products, which may account for the potentiation of apoptosis induced by TNF-α and chemotherapeutic agents. This is in agreement with the findings of a previous report that demonstrated that nimbolide exhibits pro-apoptotic effects through modulation of the cell survival protein Bcl-2 (18). Furthermore, an increase in caspase-3, -8, and -9 cleavage products suggests that the flavonone potentiates apoptosis through both extrinsic and intrinsic pathways.
Our results also show for the first time that nimbolide suppresses the expression of NF-κB-regulated genes involved in cell proliferation and metastasis. Down-regulation of the expression of the gene products involved in cell proliferation (c-Myc, cyclin D1, and COX-2) by nimbolide was concomitant with an inhibition of cell proliferation in various tumor cells. This result is further supported by a previous report that showed the ability of nimbolide to suppress the proliferation of a wide variety of cancer cells (12, 18, 19). In addition, the ability of nimbolide to suppress the TNF-α-induced expression of ICAM-1 and MMP-9 suggests that it has a role in preventing metastasis and tumor invasion. This result was further confirmed by an earlier study showing that the nimbolide suppresses the TNF-α-induced invasion activity of cancer cells. In addition, we showed that nimbolide inhibits TNF-α-induced VEGF expression. VEGF is a specific mitogen for vascular endothelial cells and is involved in carcinogenesis as an angiogenic factor. These results are in agreement with those of a recent report that demonstrated that this limonoid has anti-invasive and anti-angiogenic activities in cancer cells, although the mechanism by which this occurs has not been described (19).
Nimbolide has been shown to suppress DMBA-induced carcinogenesis in an animal model of oral oncogenesis (18). However, the authors of that study did not report its mechanism of suppression. Because DMBA has been shown to activate NF-κB (41), the inhibitory effects of nimbolide on the NF-κB activation pathway could also account for its anticarcinogenic activity.
We found that nimbolide targets IKK to suppress the TNF-α-induced phosphorylation and degradation of IκBα that is concomitant with the inhibition of nuclear translocation and phosphorylation of p65. We showed that nimbolide directly binds to and inhibits TNF-α-activated IKK. Furthermore, the addition of a reducing agent (DTT) reversed the effects of nimbolide on IKK activation, suggesting the involvement of cysteine residues. Indeed, the mutation of residue Cys179 of IKK-β to alanine abolished the inhibitory effect of nimbolide on IKK activation. These results indicate that despite the involvement of more than 50 different proteins in the NF-κB activation pathway (42), nimbolide exerts its inhibitory effect by modifying Cys179 in the IKK-β activation loop.
Previous reports have shown that Cys179 of IKK-β is prone to redox recycling (43), which results in the generation of reactive oxygen species. Indeed, nimbolide has been known to induce the production of reactive oxygen species (17). However, because nimbolide inhibited IKK both in vitro and in vivo, it is unlikely that its effects are mediated through the generation of reactive oxygen species. Our results suggest that nimbolide modifies Cys179. An α- and β-unsaturated ketone group of nimbolide has been linked to its anticancer activity (20). Thus, it is possible that this ketone group interacts with the cysteine residue of IKK. In addition, nimbolide appears to specifically target Cys179 of IKK-β, as it did not interfere with the DNA binding ability of NF-κB through direct interaction with the cysteine residue present in the p65 subunit (39).
The IKK complex is an important site for the integration of signals that regulate the NF-κB pathway. Several agents, including aspirin, sulindac, cyclopentenone prostaglandins, cytochrome P450 epoxygenase-derived eicosanoids, and viral proteins, have been shown to inhibit the NF-κB pathway through IKK (44–46), but their mechanisms of inhibition remain elusive. Our results reveal the critical role of a cysteine residue in the activation loop of IKK-β. This same residue is targeted by nitric oxide (22), butein (24), cobrotoxin (23), and xanthohumol (26). Given the relevance of NF-κB in cancer, our data suggest that IKK is a highly attractive target for therapeutic development. Some of the selective small molecule inhibitors of the IKK complex that have been recently developed by the pharmaceutical industry include PS-1145, BMS-345541, and AS602828 (47, 48).
We also found that nimbolide inhibited constitutive and inducible NF-κB activation in myeloid and leukemic cells. Constitutively activated NF-κB has been found to be critical for the survival and proliferation of various tumor cell types (49). Some of the potential mechanisms of constitutive NF-κB activation are IκBα overexpression, IκBα gene mutations, enhanced IκBα degradation, constitutive IKK activation, and constitutive expression of inflammatory cytokines (33, 49, 50). We found that nimbolide inhibits the constitutive secretion of IL-6 and TNF-α by U-266 cells, which could account for its inhibition of constitutive NF-κB activation and suppression of cell proliferation.
The suppression of NF-κB activation induced by CSC, TNF-α, OA, PMA, and endotoxin suggests that nimbolide may act at a step common to all these activators. TNF-α activates NF-κB through the sequential recruitment of TNFR1, TRADD, TRAF2, NIK, TAK1/TAB1, and IKK. Nimbolide suppressed the activation of NF-κB induced by overexpression of these intermediates.
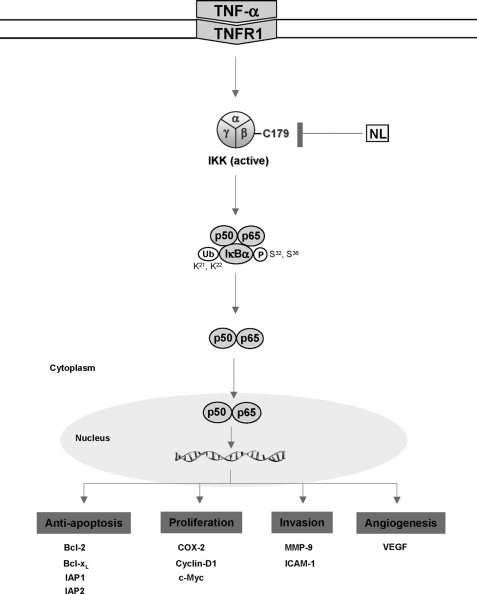
In summary, our results suggest that nimbolide exhibits anti-carcinogenic, anti-proliferative, anti-inflammatory, and apoptotic effects through the suppression of the IKK-induced NF-κB pathway, which is activated by a wide variety of carcinogens and inflammatory agents. On the basis of our findings, we propose that nimbolide inhibits IKK by modifying Cys179, which blocks NF-κB activation and the transcription of NF-κB-regulated gene products (Fig. 7). This limonoid may exhibit activities against human myeloid, leukemia, lymphoma, and other types of cancer. However, further studies in animals and humans will be required to determine fully the potential of this fascinating molecule for cancer prevention or treatment.
FIGURE 7.
A schematic diagram showing nimbolide (NL) activity in the TNF-α-induced NF-κB signaling pathway.
Acknowledgments
We thank Kate Newberry for carefully proofreading the manuscript and Drs. Veera Baladandayuthapani and Wei Qiao for statistical advice.
This work was supported, in whole or in part, by National Institutes of Health Core Grant CA-16672 and Program Project Grant CA-124787-01A2. This work was also supported by a grant from the Clayton Foundation for Research (to B. B. A.) and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center.
- DMBA
- 7,12-dimethylbenz[α]anthracene
- IKK
- IκB kinase
- PMA
- phorbol myristate acetate
- OA
- okadaic acid
- PARP
- poly(ADP-ribose) polymerase
- NIK
- NF-κB-inducing kinase
- TRADD
- TNF receptor-associated death domain
- SEAP
- secretory alkaline phosphatase
- PS
- phosphatidylserine
- CSC
- cigarette smoke condensate
- MTT
- 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide.
REFERENCES
- 1.Sartore-Bianchi A., Ricotta R., Cerea G., Maugeri M. R., Siena S. (2007) Int. J. Biol. Markers 22, S77–S87 [PubMed] [Google Scholar]
- 2.Singh S. (2007) Cell 130, 765–768 [DOI] [PubMed] [Google Scholar]
- 3.Mencher S. K., Wang L. G. (2005) BMC Clin. Pharmacol. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui S. (1942) Curr. Sci. 11, 278–279 [Google Scholar]
- 5.Biswas K., Chattopadhyay I., Banerjee R. K., Bandyopadhyay U. (2002) Curr. Sci. 82, 1336–1345 [Google Scholar]
- 6.Subapriya R., Nagini S. (2005) Curr. Med. Chem. Anticancer Agents 5, 149–156 [DOI] [PubMed] [Google Scholar]
- 7.Ekong D. E. U. (1967) Chem. Commun. 808 [Google Scholar]
- 8.Anitha G., Josepha Lourdu Raj J., Narasimhan S., Anand Solomon K., Rajan S. S. (2006) J. Asian Nat. Prod. Res. 8, 445–449 [DOI] [PubMed] [Google Scholar]
- 9.Suresh G., Gopalakrishnan G., Wesley S. D., Pradeep Singh N. D., Malathi R., Rajan S. S. (2002) J. Agric. Food Chem. 50, 4484–4490 [DOI] [PubMed] [Google Scholar]
- 10.Rochanakij S., Thebtaranonth Y., Yenjai C., Yuthavong Y. (1985) Southeast Asian J. Trop. Med. Public Health 16, 66–72 [PubMed] [Google Scholar]
- 11.Rojanpo W., Suwanno S., Somjaree R., Glinsukon T., Thebtaranont Y. J. (1985) Sci. Soc. Thailand 11, 177–188 [Google Scholar]
- 12.Cohen E., Quistad G. B., Casida J. E. (1996) Life Sci. 58, 1075–1081 [DOI] [PubMed] [Google Scholar]
- 13.Kigodi P. G., Blaskó G., Thebtaranonth Y., Pezzuto J. M., Cordell G. A. (1989) J. Nat. Prod. 52, 1246–1251 [DOI] [PubMed] [Google Scholar]
- 14.Khalid S. A., Duddeck H., Gonzalez-Sierra M. (1989) J. Nat. Prod. 52, 922–926 [DOI] [PubMed] [Google Scholar]
- 15.Sastry B. S., Suresh Babu K., Hari Babu T., Chandrasekhar S., Srinivas P. V., Saxena A. K., Madhusudana Rao J. (2006) Bioorg. Med. Chem. Lett. 16, 4391–4394 [DOI] [PubMed] [Google Scholar]
- 16.Roy M. K., Kobori M., Takenaka M., Nakahara K., Shinmoto H., Isobe S., Tsushida T. (2007) Phytother. Res. 21, 245–250 [DOI] [PubMed] [Google Scholar]
- 17.Harish Kumar G., Chandra Mohan K. V., Jagannadha Rao A., Nagini S. (2009) Invest. New Drugs 27, 246–252 [DOI] [PubMed] [Google Scholar]
- 18.Harish Kumar G., Vidya Priyadarsini R., Vinothini G., Vidjaya Letchoumy P., Nagini S. (2010) Invest. New Drugs 28, 392–401 [DOI] [PubMed] [Google Scholar]
- 19.Priyadarsini R. V., Manikandan P., Kumar G. H., Nagini S. (2009) Free Radic. Res. 43, 492–504 [DOI] [PubMed] [Google Scholar]
- 20.Pezzuto J. M. (1986) in New Trends in Natural Products Chemistry (Atta-ur-Rahaman, LeQuence P. W. eds) Vol. 26, pp. 371–386, Elsevier Science, Amsterdam, The Netherlands [Google Scholar]
- 21.Ting J. P., Duncan J. A., Lei Y. (2010) Science 327, 286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynaert N. L., Ckless K., Korn S. H., Vos N., Guala A. S., Wouters E. F., van der Vliet A., Janssen-Heininger Y. M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8945–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park M. H., Song H. S., Kim K. H., Son D. J., Lee S. H., Yoon D. Y., Kim Y., Park I. Y., Song S., Hwang B. Y., Jung J. K., Hong J. T. (2005) Biochemistry 44, 8326–8336 [DOI] [PubMed] [Google Scholar]
- 24.Pandey M. K., Sandur S. K., Sung B., Sethi G., Kunnumakkara A. B., Aggarwal B. B. (2007) J. Biol. Chem. 282, 17340–17350 [DOI] [PubMed] [Google Scholar]
- 25.Ha K. H., Byun M. S., Choi J., Jeong J., Lee K. J., Jue D. M. (2009) Biochemistry 48, 7271–7278 [DOI] [PubMed] [Google Scholar]
- 26.Harikumar K. B., Kunnumakkara A. B., Ahn K. S., Anand P., Krishnan S., Guha S., Aggarwal B. B. (2009) Blood 113, 2003–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair M. S., Gopal S., Issac D. (1997) Phytochemistry 46, 1177–1178 [Google Scholar]
- 28.Chaturvedi M. M., Mukhopadhyay A., Aggarwal B. B. (2000) Methods Enzymol. 319, 585–602 [DOI] [PubMed] [Google Scholar]
- 29.Sethi G., Ahn K. S., Pandey M. K., Aggarwal B. B. (2007) Blood 109, 2727–2735 [DOI] [PubMed] [Google Scholar]
- 30.Takada Y., Aggarwal B. B. (2003) J. Immunol. 171, 3278–3286 [DOI] [PubMed] [Google Scholar]
- 31.Darnay B. G., Ni J., Moore P. A., Aggarwal B. B. (1999) J. Biol. Chem. 274, 7724–7731 [DOI] [PubMed] [Google Scholar]
- 32.Takada Y., Khuri F. R., Aggarwal B. B. (2004) J. Biol. Chem. 279, 26287–26299 [DOI] [PubMed] [Google Scholar]
- 33.Bharti A. C., Donato N., Singh S., Aggarwal B. B. (2003) Blood 101, 1053–1062 [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal B. B. (2003) Nat. Rev. Immunol. 3, 745–756 [DOI] [PubMed] [Google Scholar]
- 35.John A., Tuszynski G. (2001) Pathol. Oncol. Res. 7, 14–23 [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi H., Boelte K. C., Lin P. C. (2007) Curr. Med. Chem. 14, 377–386 [DOI] [PubMed] [Google Scholar]
- 37.Bose D., Meric-Bernstam F., Hofstetter W., Reardon D. A., Flaherty K. T., Ellis L. M. (2010) Lancet Oncol. 11, 373–382 [DOI] [PubMed] [Google Scholar]
- 38.Sandur S. K., Ichikawa H., Sethi G., Ahn K. S., Aggarwal B. B. (2006) J. Biol. Chem. 281, 17023–17033 [DOI] [PubMed] [Google Scholar]
- 39.Natarajan K., Singh S., Burke T. R., Jr., Grunberger D., Aggarwal B. B. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 9090–9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn K. S., Sethi G., Chao T. H., Neuteboom S. T., Chaturvedi M. M., Palladino M. A., Younes A., Aggarwal B. B. (2007) Blood 110, 2286–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D. W., Sovak M. A., Zanieski G., Nonet G., Romieu-Mourez R., Lau A. W., Hafer L. J., Yaswen P., Stampfer M., Rogers A. E., Russo J., Sonenshein G. E. (2000) Carcinogenesis 21, 871–879 [DOI] [PubMed] [Google Scholar]
- 42.Chen L. F., Greene W. C. (2004) Nat. Rev. Mol. Cell Biol. 5, 392–401 [DOI] [PubMed] [Google Scholar]
- 43.Ahmad R., Raina D., Meyer C., Kharbanda S., Kufe D. (2006) J. Biol. Chem. 281, 35764–35769 [DOI] [PubMed] [Google Scholar]
- 44.Yin M. J., Yamamoto Y., Gaynor R. B. (1998) Nature 396, 77–80 [DOI] [PubMed] [Google Scholar]
- 45.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. (1999) Science 285, 1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M. G. (2000) Nature 403, 103–108 [DOI] [PubMed] [Google Scholar]
- 47.Gupta S. C., Sundaram C., Reuter S., Aggarwal B. B. (2010) Biochim. Biophys. Acta, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro A. C., Dang L. C., Soucy F., Grenier L., Mazdiyasni H., Hottelet M., Parent L., Pien C., Palombella V., Adams J. (2003) Bioorg. Med. Chem. Lett. 13, 2419–2422 [DOI] [PubMed] [Google Scholar]
- 49.Jackson-Bernitsas D. G., Ichikawa H., Takada Y., Myers J. N., Lin X. L., Darnay B. G., Chaturvedi M. M., Aggarwal B. B. (2007) Oncogene 26, 1385–1397 [DOI] [PubMed] [Google Scholar]
- 50.Kato T., Jr., Gotoh Y., Hoffmann A., Ono Y. (2008) Genes Cells 13, 509–520 [DOI] [PubMed] [Google Scholar]