Abstract
Semen was recently shown to contain amyloid fibrils formed from a self-assembling peptide fragment of the protein prostatic acid phosphatase. These amyloid fibrils, termed semen-derived enhancer of virus infection, or SEVI, have been shown to strongly enhance HIV infectivity and may play an important role in sexual transmission of HIV, making them a potential microbicide target. One novel approach to target these fibrils is the use of small molecules known to intercalate into the structure of amyloid fibrils, such as derivatives of thioflavin-T. Here, we show that the amyloid-binding small molecule BTA-EG6 (the hexa(ethylene glycol) derivative of benzothiazole aniline) is able to bind SEVI fibrils and effectively inhibit both SEVI-mediated and semen-mediated enhancement of HIV infection. BTA-EG6 also blocks the interactions of SEVI with HIV-1 virions and HIV-1 target cells but does not cause any inflammation or toxicity to cervical epithelial cells. These results suggest that an amyloid-binding small molecule may have utility as a microbicide, or microbicidal supplement, for HIV-1.
Keywords: Amyloid, Antiviral Agents, HIV, Human Immunodeficiency Virus, Virus, SEVI, Microbicide, Semen, Virus Infection
Introduction
The global HIV-1 epidemic remains a pressing public health threat nearly 30 years after its identification. The vast majority of cases worldwide are acquired by heterosexual transmission, especially male to female (1). Therefore, a microbicide that could limit the sexual transmission of HIV might provide an affordable and feasible way to decrease the spread of the virus, especially in developing countries.
Recently, Münch et al. (2) showed that human semen can enhance HIV infection as a result of the presence of amyloid fibrils formed from a self-assembling peptide consisting of amino acid residues 248–286 of prostatic acid phosphatase (PAP),2 an abundant protein secreted into semen from the prostate. These fibrils, which Münch et al. termed the “semen-derived enhancer of virus infection” (SEVI), are highly cationic and enhance infectivity by at least two mechanisms. First, they decrease the electrostatic repulsion between the negatively charged surface of the virion and the target cell. Second, they bind virions and increase their rate of sedimentation onto the target cell surface, enhancing the likelihood of receptor-mediated viral entry (3). The effect of SEVI fibrils is greatest with low levels of infectious virus (2), similar to the conditions seen in a mucosal transmission of HIV, where relatively few virions must cross the mucosal barrier (4).
Other amyloid fibrils, especially amyloid-β (Aβ), which is associated with Alzheimer disease (5), have been well studied as potential therapeutic or diagnostic targets. One approach to target Aβ fibrils is the use of small molecules that bind the fibrils at a high density and thereby sterically inhibit their interactions with proteins (6). The amyloid imaging agent, thioflavin-T (ThT) is one such molecule. Substituted 2-(4-aminophenyl)benzothiazoles (commonly referred to as “benzothiazole aniline” or BTA) are biocompatible analogues of ThT that bind to an increased number of sites along the Aβ fibril axis (7). Specifically, the hexa(ethylene glycol) derivative of BTA, BTA-EG6, has been shown to bind to Aβ fibrils and to inhibit the interaction of Aβ fibrils with other proteins (6, 8). ThT and its derivatives are believed to bind to Aβ based on the β-sheet structure that is common to all amyloid fibrils (9). Therefore, we investigated the ability of these small molecules to bind to SEVI and interfere with SEVI-mediated enhancement of HIV-1 infectivity.
We show here that BTA-EG6 is able to inhibit both SEVI-mediated and semen-mediated enhancement of HIV-1 infectivity in a dose-dependent fashion while remaining nontoxic to cervical cells. Therefore, BTA-EG6 might be a good candidate for addition to potential microbicide formulations.
EXPERIMENTAL PROCEDURES
Synthesis of BTA-EG6
BTA-EG6 was synthesized as described previously (6).
Cell Culture
CEM-M7 (a gift from N. Landau, New York University, New York, NY) and Jurkat cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (50 units/ml), and streptomycin (50 μg/ml). SiHa cells were cultured in DMEM medium supplemented with 10% fetal bovine serum, penicillin (50 units/ml), and streptomycin (50 μg/ml). A2En cells (a gift from S. Greene, Louisiana State University Health Sciences Center, New Orleans, LA), and 3EC1 cells (a gift from R. Pyles, University of Texas Medical Branch, Galveston, TX) were cultured in keratinocyte serum-free medium (Invitrogen) supplemented with bovine pituitary extract (50 mg/liter), recombinant epidermal growth factor (5 μg/liter), CaCl2 (44.1 mg/liter), and Primocin (0.1 mg/ml). PBMCs were isolated from whole blood by Lymphoprep density gradient centrifugation. PBMCs were stimulated for 48 h in RPMI 1640 medium supplemented with 5% human IL-2 (ZeptoMetrix), 5 μg/ml PHA (Sigma), 20% fetal bovine serum, penicillin (50 units/ml), and streptomycin (50 μg/ml).
SEVI and Semen
PAP248–286 and biotinylated PAP248–286, in which a biotin was added to the amino terminus of the peptide, was synthesized by New England Peptide and dissolved in PBS at a concentration of 10 mg/ml. Fibrils were formed by agitation in an Eppendorf Thermomixer at 1400 rpm and 37 °C for 72 h. Semen samples were obtained from the Strong Fertility Center (Rochester, NY) and Fairfax CryoBank (Fairfax, VA). Samples were pooled, aliquoted, and stored at −80 °C.
Fluorescence Polarization
100 μg/ml SEVI was mixed with 16 μg/ml FITC-heparin and concentrations of BTA-EG6 ranging from 0 to 200 μg/ml. Samples were incubated 1 h at room temperature and read on a PerkinElmer Life Sciences Envision 2012 multilabel reader at an excitation λ = 480 nm and emission λ = 535 nm. The horizontal and vertical polarized fluorescence intensities were recorded, and the calculated polarization was determined in millipolarization units.
Determination of the Binding Affinity of BTA-EG6 and SEVI Fibrils
Binding of BTA-EG6 to SEVI fibrils was measured according to the centrifugation assay described by Levine (10) for BTA-1 to Aβ fibrils. Briefly, 200 μl of various concentrations of BTA-EG6 in PBS were incubated in the presence or absence of 10 μg of SEVI fibrils to give a final volume of 220 μl of solution. These incubations were performed in duplicate runs and allowed to equilibrate overnight at room temperature. After equilibration, each solution was centrifuged at 16,000 × g for 30 min. The supernatants were separated from the pelleted fibrils, and 220 μl of fresh PBS was added to resuspend the pellets. A-100 μl aliquot of each resuspended pellet was pipetted into a cuvette (ultra-microcuvette, 10-mm light path, Hellma®, Müllheim, Germany), and the fluorescence of BTA-EG6 was determined at 355 nm excitation and 430 nm emission using a spectrofluorometer (Photon Technology International, Inc., Birmingham, NJ). Error bars represent standard deviations from the mean. The graph was plotted and fitted using the following one-site specific binding algorithm to determine Kd: Y = Bmax ×X/(Kd+X), where X is the concentration of BTA-EG6, Y is the specific binding fluorescence intensity, and Bmax corresponds to the apparent maximal observable fluorescence upon binding of BTA-EG6 to SEVI fibrils. The data were processed using Origin 7.0 (MicroCal Software, Inc., Northampton, MA).
Flow Cytometry
105 Jurkat cells were incubated with biotinylated SEVI fibrils (40 μg/ml) with and without BTA-EG6 at a concentration of 10 (low) or 30 μg/ml (high) or heparin (100 μg/ml) as a positive control for interfering with SEVI binding to the cell surface. Cells were incubated for 1 h at 37 °C, washed, and stained for 1 h with a covalent conjugate of streptavidin and fluorescein isothiocyanate (SA-FITC). Cells were washed and run on an Accuri C6 Flow Cytometer. Data were analyzed using FlowJo (TreeStar Inc, Ashland, OR).
Infectivity Assays
For infection of CEM-M7 cells, X4 tropic HIV-1IIIB (21 ng/ml p24) or R5 tropic HIV-1ADA (60 ng/ml p24) was pretreated for 10 min at room temperature with 15 μg/ml SEVI in the presence or absence of BTA-EG6. Treated virions were then added to 5 × 104 CEM-M7 cells/well in 96-well flat-bottomed tissue culture plates. After 2 h, the medium axis were replaced. Infection was assayed after 48 h by quantifying luciferase expression using the Promega Dual-Luciferase assay and a Beckman Coulter DTX880 plate reader.
For infections using semen, pooled human semen samples were added to virions at a 1:1 dilution and incubated for 15 min at room temperature in the presence or absence of BTA-EG6. After 15 min, the semen and virus mixture was diluted 1:15 into 5 × 104 CEM-M7 cells/well in a 96-well plate. Cells were washed after 1 h, and infection was assayed at 48 h as above.
For infections of PBMCs, R5 tropic HIV-1BaL preincubated with 15 μg/ml SEVI in the presence or absence of BTA-EG6 was added to 2 × 105 PHA/IL-2-stimulated PBMCs/well in 96-well flat-bottomed tissue culture plates. Cells were washed at 3 h, and infection was analyzed at day 4 using the HIV-1 p24 antigen capture assay (Advanced Bioscience Laboratory).
Virus Binding Assay
HIV-1 IIIB or ADA virions were pretreated with 15 μg/ml SEVI and added to 5 × 104 Jurkat cells, or A2En cells, in the presence or absence of BTA-EG6. After 90 min, cells were washed to remove any unbound virus, and bound virions were detected using an HIV-1 p24 antigen capture assay (Advanced Bioscience Laboratory).
Cytokine and Chemokine Studies
HIV-1 BaL virions were pretreated with 15 μg/ml SEVI and added to 5 × 104 A2En cells in the presence or absence of BTA-EG6. Supernatant was collected at 6 and 24 h, and the production of the chemokines IL-8 and Mip-3α was measured by ELISA (R&D Systems).
To assess semen-mediated chemokine production, SiHa cells were treated with semen, as described above, in the presence or absence of BTA-EG6. After 6 h, supernatants were collected, and the production of the chemokines IL-8 and Mip-3α was measured by ELISA (R&D systems).
Toxicity Studies
The cervical epithelial cell lines SiHa, A2En (endocervical, data not shown), and 3EC1 (ectocervical, data not shown) were treated for 12 h with BTA-EG6 at concentrations up to 66 μg/ml, 10 times the IC50. After 12 h, cell viability was analyzed by measuring cellular metabolic activity using the resazurin cytotoxicity assay, alamarBlue® (Invitrogen), in accordance with the manufacturer's protocol. Cytokine and chemokine production was assessed at 12 h by ELISA (R&D systems). Cells were also treated with 0.1% nonoxynol-9 as a positive control for cytotoxicity and with 0.1 μg/ml FSL1, a synthetic diacylated lipoprotein derived from Mycoplasma salivarium (InvivoGen), as a positive control for chemokine production.
RESULTS
The Thioflavin-T Analog BTA-EG6 Binds SEVI Fibrils
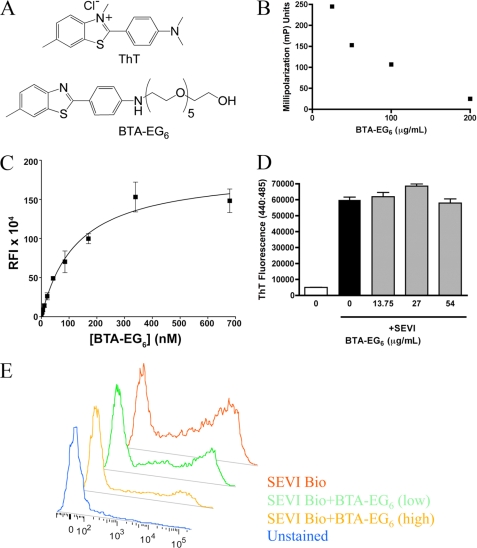
ThT is able to intercalate into the generic β-sheet structure of amyloid fibrils. The benzothiazole aniline derivative, BTA-EG6, is a ThT analog carrying a hexa(ethylene glycol) moiety (Fig. 1A). This molecule has been previously shown to bind Aβ fibrils and interfere with the ability of Aβ-binding proteins to interact with the fibrils (6). Based on these interactions with Aβ, we hypothesized that BTA-EG6 might also bind to SEVI fibrils. To test this hypothesis, we used fluorescence polarization to measure the ability of BTA-EG6 to bind SEVI. Increasing concentrations of BTA-EG6 were added to 50 μg/ml SEVI that had been preincubated with 16 μg/ml FITC-heparin, a known SEVI binder (3). BTA-EG6 was able to displace fluorescent heparin from the SEVI fibrils in a dose-dependent fashion (Fig. 1B), suggesting an interaction between these molecules and the fibrils.
FIGURE 1.
The thioflavin-T analog BTA-EG6 binds SEVI fibrils. A, chemical structure of ThT and BTA-EG6. B, BTA-EG6 binds SEVI fibrils as measured by fluorescence polarization. 100 μg/ml SEVI was mixed with 16 μg/ml FITC-heparin in varying concentrations of BTA-EG6 ranging from 0 to 200 μg/ml. Samples were incubated 1 h at room temperature, and polarized fluorescence intensities were measured. Decreased millipolarization units (mP) indicate a displacement of FITC-heparin from SEVI fibrils due to BTA binding. C, binding of BTA-EG6 to SEVI fibrils as determined by a previously reported centrifugation assay (10). Briefly, various concentrations of BTA-EG6 in PBS were incubated overnight at room temperature in the presence or absence of SEVI fibrils. After equilibration, each solution was centrifuged, and the supernatants were separated from the pelleted fibrils. The fluorescence of BTA-EG6 was determined from the resuspended pellets in PBS solution. Error bars represent ± S.D. of duplicate measurements. The Kd was determined by fitting the data to a one-site specific binding algorithm: Y = Bmax × X/(Kd+X), where X is the concentration of BTA-EG6, Y is the specific binding fluorescence intensity, and Bmax corresponds to the apparent maximal observable fluorescence upon binding of BTA-EG6 to SEVI fibrils. RFI, relative fluorescence intensity. D, BTA-EG6 does not affect the stability of SEVI fibrils. Preformed SEVI fibrils were incubated with increasing concentrations of BTA-EG6 for 3 h. Fibril stability was measured by ThT fluorescence. Results shown are average values ± S.D. of triplicate measurements from one of three independent experiments that yielded equivalent results. E, BTA-EG6 binding to SEVI inhibits the interaction of SEVI fibrils with the cell surface. Jurkat T cells were incubated with SEVI-biotin for 1 h in the presence or absence of 5.5 μg/ml (low) or 27 μg/ml (high) BTA-EG6. Surface-bound fibrils were detected with SA-FITC and measured by flow cytometry. Results are summarized in Table 1 and are representative of three experiments that were performed with similar results.
Having observed an interaction between these molecules, we wanted to more closely assess the binding of BTA-EG6 to SEVI fibrils by quantifying its binding affinity. We therefore used a previously reported fluorescence-based assay (10) to determine the Kd between BTA-EG6 and the SEVI fibrils. Fig. 1C shows the relative fluorescence intensity (RFI) of BTA-EG6 bound to SEVI fibrils as a function of exposure of the SEVI peptides to increasing concentrations of BTA-EG6. Fitting the data in Fig. 1C with a one-site specific binding algorithm revealed a value of Kd = 127 ± 22 nm (R2 = 0.98). For comparison, we used this same fluorescence binding assay (10) to measure the affinity of BTA-EG6 for binding to aggregated Alzheimer disease-related Aβ (1–42) peptides, which gave a value of Kd = 111 ± 32 nm (R2 = 0.95, data not shown); this value was similar to the Kd value for binding of BTA-EG6 to SEVI fibrils.
To examine whether the interaction of BTA-EG6 with SEVI impacted the stability of the fibrils, preformed SEVI fibrils were incubated with BTA-EG6 for 3 h. After that time, fibrillar structures were examined by ThT fluorescence. ThT changes in fluorescence intensity when intercalated into the β-sheet structure common to amyloid fibrils; therefore, ThT fluorescence serves as a surrogate measure for fibrillar structure of SEVI and for the stability of SEVI fibrils. As seen in Fig. 1D, the addition of BTA-EG6 had no effect on fibrillar stability as measured by ThT. Unlike in the case with ThT, the fluorescence intensity of BTA-EG6 does not change upon binding to amyloid fibrils (10). The intrinsic fluorescence of BTA-EG6, therefore, does not interfere with the analysis of fibril stability using this assay.
To further explore the interactions between SEVI fibrils and BTA-EG6, we tested whether binding of this compound to SEVI could inhibit the ability of the fibrils to interact with the negatively charged surface of mammalian cells (2). Jurkat T-cells were incubated with 35 μg/ml SEVI-biotin fibrils, which were formed by fibrillization of a biotinylated PAP248–286 peptide, in the presence of 5.5 or 13 μg/ml BTA-EG6. Heparin was used as a positive control as this polyanionic compound has been shown to inhibit the binding of SEVI fibrils to the cell surface (3). Binding of the fibrils to the cell surface was detected using SA-FITC. As seen in Fig. 1E and Table 1, increasing concentrations of BTA-EG6 inhibited the ability of SEVI fibrils to interact with and bind the cell surface, as measured by both the percentage of cells with bound fibrils and the mean fluorescence intensity of the cells.
TABLE 1.
BTA-EG6 binding to SEVI inhibits interaction of SEVI fibrils with the cell surface
Jurkat cells were incubated with SEVI-biotin (SEVI-Bio) for 1 h in the presence or absence of 5.5 (low) or 27 μg/ml (high) BTA-EG6. Surface-bound fibrils were detected with SA-FITC and measured by flow cytometry. Results are shown as percentage of cells with bound fibrils (SA-FITC+) as well as mean fluorescent intensity (MFI). Results are shown from one of three experiments in which similar results were obtained.
| Sample | SA-FITC+ | MFI |
|---|---|---|
| % | ||
| Unstained | 1.65 | 28 |
| SEVI-Bio | 48.1 | 2525 |
| SEVI-Bio + BTA-EG6 (low) | 36.6 | 95.3 |
| SEVI-Bio + BTA-EG6 (high) | 21.2 | 38.7 |
| SEVI-Bio + heparin | 31.7 | 376 |
BTA-EG6 Inhibits SEVI-mediated Enhancement of HIV-1 Infection
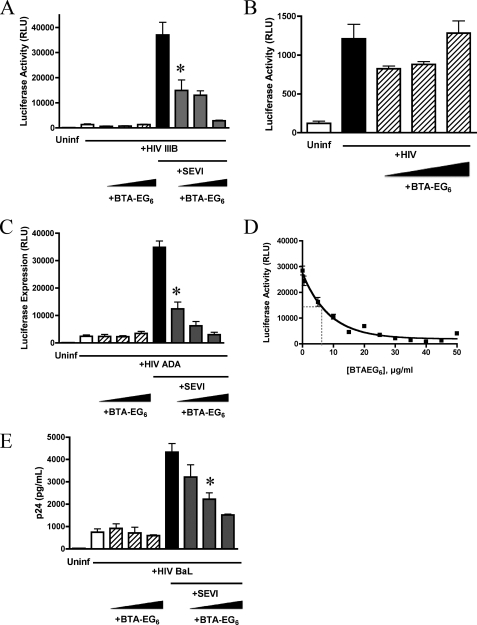
Having observed that BTA-EG6 was able to inhibit the interaction of SEVI with the cell surface, we investigated whether it could effectively inhibit SEVI-mediated enhancement of HIV-1 infection. CEM-M7 cells were infected with HIV-1 strain IIIB plus 15 μg/ml SEVI fibrils in the presence of increasing concentrations of BTA-EG6. CEM-M7 cells are a CD4+ CCR5+ CXCR4+ T/B cell hybrid cell line and contain HIV LTR-driven luciferase and GFP reporter gene cassettes. The HIV LTR is a weak transcriptional regulator in the absence of its cognate, virally encoded trans-activator, Tat. As a result, luciferase and GFP expression levels in these cells are directly responsive to HIV-1 infection; this property therefore provides a convenient method to determine the extent of viral infection.
BTA-EG6 was able to effectively inhibit SEVI-mediated enhancement of HIV infection in a dose-dependent fashion, reducing infectivity nearly back to baseline levels (i.e. levels detected in the absence of SEVI) at the highest concentrations tested (Fig. 2A). Importantly, BTA-EG6 had no effect on the infectivity of HIV virus alone, even at the highest concentrations (Fig. 2B), suggesting that this effect was not due to direct inhibition of intrinsic virus infectivity.
FIGURE 2.
BTA-EG6 inhibits SEVI-mediated enhancement of HIV-1 infection. A, HIV-1 IIIB virions were preincubated with increasing concentrations of BTA-EG6 (0, 5.5, 11, and 22.5 μg/ml) and with or without SEVI (15 μg/ml) as indicated. The samples were then added to CEM-M7 cells. Cells were washed at 2 h, and infection was assayed at 48 h by measuring Tat-driven luciferase expression. Results shown are average values ± S.D. of triplicate measurements from one of four independent experiments that yielded equivalent results. * indicates p < 0.05 when compared with control cells exposed to HIV-1IIIB + SEVI alone by ANOVA with Tukey's post test. RLU, relative luciferase units; Uninf, uninfected. B, zoom in of panel A to show data for cells treated with HIV-IIIB virions with and without increasing concentrations of BTA-EG6, in the absence of SEVI. BTA-EG6 had no effect on the infectivity of HIV alone; concentrations of BTA-EG6 are noted above for panel A. C, CEM-M7 cells were infected with HIV-1ADA, as in panel A. D, CEM-M7 cells were infected with HIV-1ADA + SEVI with concentrations of BTA-EG6 ranging from 0.4 to 50 μg/ml. An exponential decay curve was then fit to the data and used to calculate the IC50 of the inhibitory effect of BTA-EG6 on SEVI-mediated enhancement of HIV-1 infection. E, human PBMCs were stimulated with IL-2/PHA and infected with HIV-1BAL and increasing concentrations of BTA-EG6 (0, 5.5, 11, and 22.5 μg/ml) with and without SEVI (15 μg/ml). Cells were washed at 3 h, and infection was assayed at 4 days by measuring p24. Results shown are average values ± S.D. of triplicate measurements. * indicates p < 0.01 when compared with control PBMCs exposed to HIV-1ADA + SEVI alone (ANOVA with Tukey's post test).
Because most sexually transmitted HIV-1 infections are the result of R5 viruses (11), we also examined whether the effect of BTA-EG6 extended to a well characterized R5 strain. CEM-M7 cells were infected with HIV-1ADA and 15 μg/ml SEVI, with and without increasing concentrations of BTA-EG6. Once again, BTA-EG6 showed a significant dose-dependent inhibition of SEVI-mediated enhancement of HIV-1 infection (Fig. 2C). No effect on the infectivity of HIV-1ADA was observed in the absence of SEVI (Fig. 2C).
We next determined the IC50 of the BTA-EG6 for inhibition of SEVI-mediated enhancement of HIV-1 infection. To do this, CEM-M7 cells were infected with HIV-1ADA and 15 μg/ml SEVI in the presence of BTA-EG6. Ten different BTA-EG6 concentrations were tested, ranging from 0.4 to 50 μg/ml. The data were fit to an exponential decay curve to calculate the IC50, and results are shown in Fig. 2D. The calculated IC50 was 6.6 μg/ml for BTA-EG6 (equivalent to 13 μm).
We also wanted to confirm whether the effects of SEVI and BTA-EG6 extended to infection in primary cells. Therefore, we infected PBMCs with HIV-1BAL with 15 μg/ml SEVI in the presence or absence of increasing concentrations of BTA-EG6. BTA-EG6 was able to inhibit SEVI-mediated enhancement of HIV-1 infection in PBMCs at similar concentrations to those seen in other cell lines (Fig. 2E). BTA-EG6 had no effect on the infectivity of HIV-1BaL in PBMCs in the absence of SEVI (Fig. 2E). Thus, the effects of BTA-EG6 are neither strain-dependent nor cell type-dependent, and the compound has no effect on HIV-1 infection in the absence of SEVI.
BTA-EG6 Inhibits Semen-mediated Enhancement of HIV-1 Infection
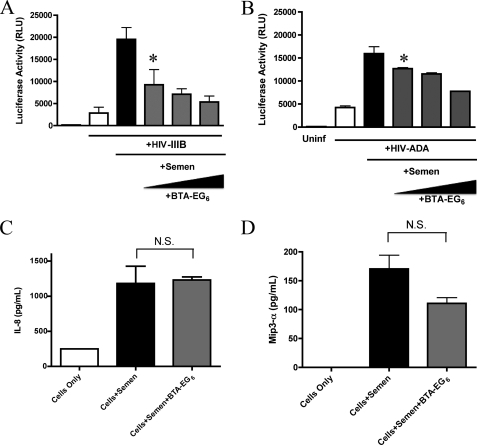
For BTA-EG6 to be a viable microbicide candidate, it must be effective not just against the effects of SEVI but should be able to effectively inhibit the infection-enhancing activity of human semen. Therefore, we examined the effect of this compound on semen-mediated enhancement of HIV-1 infection. As human semen has been reported to be toxic to cultured cells (12), we followed the previously described protocol to minimize this toxicity (2). Pooled human semen was added to HIV-1IIIB virus in a 1:1 dilution. After 15 min, this solution was added to cells at a 1:15 dilution for a final concentration of 3.3%. As shown in Fig. 3A, BTA-EG6 efficiently inhibited the semen-mediated enhancement of HIV-1 infection at similar concentrations to those active against SEVI alone. Fig. 3B shows that this effect extended to infection with an R5 virus, HIVADA as well.
FIGURE 3.
BTA-EG6 inhibits semen-mediated enhancement of HIV-1 infectivity. A, HIV-1IIIB virions were preincubated with 50% pooled human semen, with or without increasing concentrations of BTA-EG6 (5.5, 11, and 22.5 μg/ml). After 10 min, these stocks were diluted 15-fold into CEM-M7 cells. Cells were washed after 1 h, and luciferase expression was measured at 48 h to quantify the extent of infection. Results shown are average values ± S.D. of triplicate measurements from one of three independent experiments that yielded equivalent results. * indicates p < 0.05 when compared with control cells exposed to HIV-1IIIB + semen alone, by ANOVA with Tukey's post test. RLU, relative luciferase units. B, cells were treated as above but with HIV-1ADA and a 50% concentration of an individual semen sample. *, p < 0.05 when compared with control cells exposed to HIV-1ADA + semen alone, by ANOVA with Tukey's post test. C and D, BTA-EG6 does not inhibit semen-mediated cytokine release. SiHa cells were treated with pooled human semen for 6 h, with and without 27 μg/ml BTA-EG6. At 6 h, IL-8 (C) and MIP-3α (D) production in the supernatants was measured by ELISA. Results shown are average values ± S.D. of triplicate measurements from one of three independent experiments that yielded equivalent results. N.S. = not significant when compared with cells treated with semen alone (as determined by ANOVA with Tukey's post test).
We performed a follow-up experiment to test whether the effects of BTA-EG6 on semen were specific to the infection-enhancing components in semen (i.e. SEVI) or due to a more general inhibitory effect against semen. To do this, we examined whether BTA-EG6 inhibited semen-mediated chemokine release. Human semen has been shown to be pro-inflammatory, mediating the release of IL-8 and MIP-3α from cervical endothelial cells (13). This property is thought to be due to the presence of several pro-inflammatory mediators but is not due to the presence of SEVI as we have found that SEVI does not stimulate the release of IL-8 or MIP-3α (data not shown). We treated SiHa cells, a cervical endothelial cell line, with pooled human semen with or without 33 μg/ml BTA-EG6, a dose five times higher than the IC50. After 6 h, supernatants were collected and analyzed for production of IL-8 or MIP-3α. Pooled human semen effectively elicited the production of these chemokines from SiHa cells as expected, whereas BTA-EG6 had no inhibitory effect on semen-stimulated chemokine production (Fig. 3, C and D).
BTA-EG6 Inhibits SEVI-mediated Attachment of HIV-1 to the Cell Surface
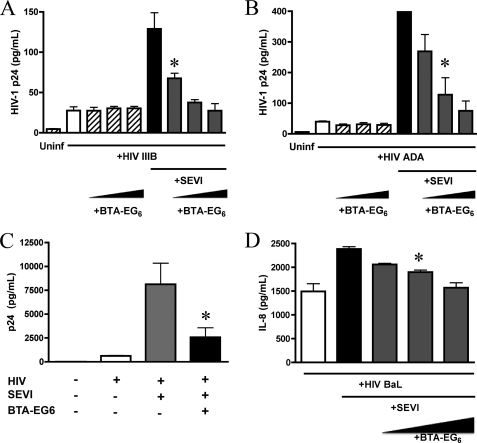
To more closely examine how BTA-EG6 mediates its inhibitory effects on SEVI-mediated HIV-1 infection enhancement, we examined the ability of this compound to interfere with SEVI-enhanced binding of HIV-1 virions to the cell surface. The cationic nature of SEVI has been shown to enhance the binding of virions to the cell surface, which allows it to neutralize the electrostatic repulsion between the negatively charged HIV-1 virion and target cell surface (3). Jurkat T cells were incubated with HIV-1IIIB virions and 15 μg/ml SEVI in the presence or absence of increasing concentrations of BTA-EG6. Surface-bound virions were then measured by p24 ELISA after washing off unbound virus. SEVI was able to strongly enhance the binding of virions to the cell surface, and this effect was efficiently abrogated by BTA-EG6 (Fig. 4A). BTA-EG6 had no effect on the binding of HIV virions to the cell surface in the absence of SEVI (Fig. 4A). Similar results were obtained with an R5 virus, HIV-1ADA (Fig. 4B).
FIGURE 4.
BTA-EG6 inhibits SEVI-mediated attachment of HIV-1 to the cell surface. A, HIV-1IIIB virions were pretreated with or without 10 μg/ml SEVI and added to Jurkat cells with or without increasing concentrations of BTA-EG6 (5.5, 11, and 22.5 μg/ml). After 90 min, cells were washed to remove any unbound virus, and bound virions were detected using a p24 ELISA. The data show that BTA-EG6 efficiently inhibited SEVI-mediated enhancement of HIV-1IIIB attachment to Jurkat cells (* indicates p < 0.01 for cells treated with SEVI plus 5.5, 11, or 22.5 μg/ml BTA-EG6 versus cells treated with SEVI alone; ANOVA with Tukey's post test). BTA-EG6 had no effect on the binding of HIV-1 virions alone to cells. Uninf, uninfected. B, Jurkat cells were treated as above using HIV-1ADA (* indicates p < 0.01 for cells treated with SEVI plus 11 or 22.5 μg/ml BTA-EG6 versus cells treated with SEVI alone; ANOVA with Tukey's post test). C, A2En cells were incubated with HIV-1ADA in the presence or absence of 22.5 μg/ml BTA-EG6 (* indicates p < 0.01 for cells treated with SEVI plus 22.5 μg/ml BTA-EG6 versus cells treated with SEVI alone; ANOVA with Tukey's post test). A–C, all results shown are average values ± S.D. of triplicate measurements from one of three independent experiments that yielded equivalent results. D, A2En cells were treated with HIV-1BaL and 15 μg/ml SEVI with or without increasing concentrations of BTA-EG6 (5.5, 11, and 22.5 μg/ml). At 24 h, supernatants were collected and analyzed by ELISA for the presence of IL-8. (* indicates p < 0.01 for cells treated with SEVI plus 11 or 22.5 μg/ml BTA-EG6 versus cells treated with SEVI alone; ANOVA with Tukey's post test).
Additionally, we performed this experiment using A2En cells, a primary cell-derived endocervical cell line. We found that SEVI also enhanced binding of virions to the surface of these cervical epithelial cells and that this effect was inhibited by BTA-EG6 (Fig. 4C).
We also tested whether SEVI would increase HIV-1-mediated chemokine production and whether BTA-EG6 could inhibit this effect. HIV has previously been shown to stimulate the release of MIP-3α and IL-8 from vaginal and cervical epithelial cells (14). Because SEVI increases the interactions between virions and epithelial cells, we hypothesized that it would likely increase HIV-mediated chemokine release as well. Therefore, we exposed A2En cells to HIV-1BAL virions with and without SEVI, in the presence or absence of BTA-EG6. As seen in Fig. 4D, SEVI modestly increased the release of IL-8 from cells treated with virus, and BTA-EG6 was able to inhibit this release. Similar results were also obtained for MIP-3α (data not shown).
BTA-EG6 Is Not Toxic to Cervical Cells
For a compound to be a legitimate HIV-1 microbicide candidate, it must not have toxic or inflammatory effects on the cervical endothelium. Loss of this protective layer leads to an increased ability for HIV-1 to cross the mucosal barrier, and inflammatory effects drive recruitment of HIV-1 target cells, further decreasing the natural barriers against successful transmission of HIV. Therefore, we examined the effects of BTA-EG6 on cervical endothelial cells. To do this, we used: 1) SiHa cells, a cervical carcinoma cell line; 2) A2En cells, a primary cell-derived line from the endocervical endothelium; and 3) 3EC1 cells, a primary cell-derived line from the ectocervical endothelium.
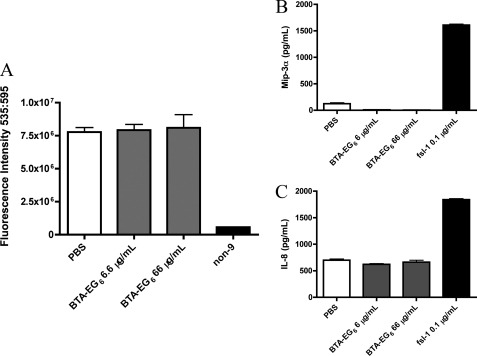
To evaluate the effects of BTA-EG6 on cell viability, the compound was added to cells at concentrations up to 10× the IC50 for up to 24 h. Viability was assessed at 24 h by using the resazurin cytotoxicity assay. Resazurin cytotoxicity data were confirmed by trypan blue counts of viable cells (data not shown). Fig. 5A shows that BTA-EG6 did not have any effects on cell viability, even at the highest concentrations tested. Nonoxynol-9 (non-9), a spermicide, was used as a positive control as it is known to be toxic to cervical epithelial cells (15).
FIGURE 5.
BTA-EG6 is not toxic to cervical cells. A, the cervical endothelial cell lines A2En (endocervical), 3EC1 (ectocervical), and SiHa were treated for 12 h with BTA-EG6 at concentrations up to 10 times greater than the IC50. Control cultures were treated with nonoxynol-9 (non-9) at 0.1% final concentration as a positive control for induction of cell death (33). At 12 h, viability was measured by resazurin cytotoxicity assay (alamarBlue® assay). Representative results from A2En cells are shown; results from 3EC1 and SiHa cells were very similar (not shown). B and C, BTA-EG6 does not induce inflammatory chemokine production in cervical epithelial cells. A2En, 3EC1, and SiHa Cells were treated with BTA-EG6 at varying concentrations for 6 h; control cultures were treated with a well defined TLR2/6 agonist, FSL1 (a synthetic diacylated lipoprotein derived from M. salivarium) at 0.1 μg/ml final concentration as a positive control for chemokine induction (26). At 6 h, supernatants were collected, and production of Mip-3α (B) and IL-8 (C) was determined by ELISA. Representative results from A2En cells are shown; results from 3EC1 and SiHa cells were very similar (not shown). A–C, all results shown are average values ± S.D. of triplicate measurements from one of three independent experiments that yielded equivalent results. No significant difference (p > 0.05) was noted between control cultures treated with PBS and those treated with the highest dose (66 μg/ml) of BTA-EG6, as determined by ANOVA with Tukey's post test.
We also examined whether treatment with BTA-EG6 led to the production of inflammatory cytokines and chemokines from the cervical cell lines. All three cervical cell lines were treated for 6 h with concentrations of BTA-EG6 ranging from 6.6 to 66 μg/ml. Cell culture supernatants were then assessed for the presence of the inflammatory cytokines and chemokines Mip-3α (Fig. 5B), IL-8 (Fig. 5C), IL-1β, and TNF-α (data not shown). These cytokines and chemokines were selected because they have been shown to be up-regulated by other candidate microbicides and because they may play a role in microbicide-mediated enhancement of HIV-1 infection (16–18). BTA-EG6 did not lead to the release of any of these cytokines or chemokines, even at the highest doses tested. Although more in-depth assessment is obviously necessary, these preliminary results suggest that BTA-EG6 is not toxic to cervical endothelial cells.
DISCUSSION
In this study, we demonstrate that an amyloid-binding small molecule is an efficient inhibitor of SEVI- and semen-mediated enhancement of HIV infectivity. We chose to examine the amyloid-binding small molecule BTA-EG6 based on the hypothesis that it would effectively bind and coat SEVI fibrils as it has been previously shown to do for Aβ (6). We found that BTA-EG6 bound to the SEVI fibrils and interfered with their ability to enhance HIV infectivity, suggesting that other amyloid-binding small molecules might also prove to be effective microbicide candidates targeting SEVI. Importantly, BTA-EG6 did not have any direct inhibitory effects on the infectivity of HIV-1 alone.
BTA-EG6 inhibited SEVI-mediated enhancement of infection by both X4 (HIV-1IIIB) and R5 (HIV-1ADA) strains, in a dose-dependent fashion. In the case of HIV-1ADA, we calculated the IC50 to be 13 μm; this value is 100-fold higher than the measured Kd of BTA-EG6 for binding to aggregated SEVI peptides (127 nm). One explanation for this difference is that the ability of BTA-EG6 to compete with virion/fibril or virion/cell interactions requires a greater number of BTA-EG6 molecules than the noncompetitive binding of BTA-EG6 to SEVI alone.
BTA-EG6 also inhibited SEVI-enhanced infection of primary cells (human peripheral blood mononuclear cells) in a dose-dependent fashion, and it blocked SEVI-enhanced binding of X4 (HIV-1IIIB) and R5 (HIV-1ADA) strains to target cells (including both Jurkat T cells and A2En endocervical cells). The ability of SEVI to enhance virus binding to endocervical cells suggests an additional mechanism by which these fibrils might enhance HIV-1 transmission in vivo. The fibrils may enhance the attachment of virus to cells at the cervicovaginal barrier, thereby increasing the likelihood of productive infection of viral target cells in the cervicovaginal mucosa and also eliciting increased production of pro-inflammatory chemokines from cervicovaginal epithelial cells (14, 19). Our data are consistent with this prediction and show that (i) SEVI enhances the ability of HIV-1 virions to elicit IL-8 and MIP-3α from A2En endocervical cells and (ii) this can be inhibited by BTA-EG6. This is important because it suggests that BTA-EG6 and related compounds may have the potential not only to reduce the efficiency of HIV-1 infection of target cells but also to reduce the level of target cell recruitment to virus-exposed genital mucosal tissue.
Our results show that BTA-EG6 effectively prevents semen-mediated enhancement of HIV infectivity, suggesting that this activity of semen can be targeted by specifically inhibiting the SEVI fibrils. BTA-EG6 did not inhibit other properties of semen, such as the ability to elicit pro-inflammatory chemokines (13), further reinforcing the idea that this small molecule specifically targets the fibrils present in semen. These data not only suggest that BTA-EG6 may be an effective microbicide target but also offer proof of principle support for the concept that screening amyloid- or SEVI-binding molecules may allow identification of additional anti-SEVI microbicide candidates. In the case of BTA-EG6, it is likely that the thioflavin-T analog allows it to intercalate into the SEVI fibrils, whereas the hexa(ethylene glycol) moiety extends and inhibits the interactions of SEVI with cells and virions (6). Oligomeric derivatives of BTA-EG6 and related compounds may prove even more effective due to increased avidity and affinity, as may amyloid binders with modified functional groups. Studies to address these hypotheses are presently underway.
Some previous microbicide candidates have not fared well in clinical trials, frequently due to pro-inflammatory properties that damage the cervical mucosa and drive the recruitment of HIV target cells. An instructive example is the nonionic surfactant, nonoxynol-9. Nonoxynol-9 showed strong antiviral activity in preclinical trials but failed to protect against virus transmission in human clinical trials, and in fact, increased the risk of HIV-1 infection by almost 2-fold in women who used the agent several times a day (20, 21). This infection-enhancing activity has been attributed to toxic and pro-inflammatory effects on female reproductive tissue, which increase susceptibility to virus transmission (22). Therefore, it is important to consider toxicity when evaluating candidate microbicides. We have found that BTA-EG6 is not toxic to a variety of cervical cells and that it does not drive the expression of pro-inflammatory cytokines and chemokines that have been shown to be important in recruiting HIV target cells.
An additional consideration for many candidate microbicides is that their properties can, in some cases, be negatively impacted by the presence of semen. The anionic polymer PRO-2000 also showed robust activity in in vitro studies and preclinical models (23–25) but failed to demonstrate a protective effect against HIV-1 transmission in the phase III Microbicide Development Program (MDP) 301 trial.3 Reasons for the futility of PRO-2000 remain unclear but may include inhibition of its antiviral activity by seminal plasma (27); this may contribute to its decreased effectiveness in postcoital cervicovaginal fluid (28). In our experiments, the presence of semen had no effect on the microbicidal activity of BTA-EG6.
It is important to compare the properties of the anti-SEVI small molecules described here with those of previously identified inhibitors of SEVI-enhanced HIV infection activity. Other previously described SEVI inhibitors, such as polyanionic compounds (3) and the heparin antagonist surfen (29), block the actions of SEVI at least in part due to simple electrostatic interactions with the cationic fibrils. Given recent experience with the polyanionic agent PRO-20003 and the presence of small cationic peptides in both semen and cervicovaginal fluid (30, 31), this raises concerns. In contrast, amyloid-binding small molecules, such as BTA-EG6, do not rely on electrostatic properties to bind to SEVI and are therefore expected to offer a new class of SEVI inhibitors whose effects are more specifically targeted at the fibrils themselves. This is expected to reduce potential for off-target effects and increase potential for effectiveness in an in vivo setting. Finally, current microbicide candidates target the HIV virus itself (32), whereas the inhibitor described here does not. We propose that because semen is the vector in the vast majority of transmitted HIV-1 infections, it is reasonable to consider that the addition of a SEVI inhibitor to microbicide formulations might improve the efficacy of antiviral microbicides.
Acknowledgments
We gratefully thank Dr. Stephen Pool of the Fairfax CryoBank (Fairfax, VA) for providing semen samples. Finally, we thank Dr. Jon T. Warren of the National Institutes of Health for support and encouragement, and Dr. Jim Turpin of the National Institutes of Health for advice and discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01AI084111 (to S. D., B. L. N., C. B., and C. F.), T32GM007356 (to J. S. O.), T32DA007232 (to J. S. O.), and P30AI36214 (to C. C. C., M. R., and J. Y.) as well as by a National Science Foundation CAREER award CHE-0847530 (J. Y.) and a DuPont Young Professors Award (B. L. N.).
Global Campaign for Microbicides (2009) Global Campaign for Microbicides, MDP 301, PRO 2000, Announcing the MDP 301 PRO 2000 Trial Results, Global Campaign for Microbicides, Washington, D. C.
- PAP
- prostatic acid phosphatase
- SEVI
- semen-derived enhancer of virus infection
- Aβ
- amyloid-β
- SA-FITC
- covalent conjugate of streptavidin and FITC
- ThT
- thioflavin-T
- BTA
- benzothiazole aniline
- BTA-EG6
- hexa(ethylene glycol) derivative of BTA
- PBMC
- peripheral blood mononuclear cell
- ANOVA
- analysis of variance
- SEVI-Bio
- SEVI-biotin.
REFERENCES
- 1.Royce R. A., Seña A., Cates W., Jr., Cohen M. S. (1997) N. Engl. J. Med. 336, 1072–1078 [DOI] [PubMed] [Google Scholar]
- 2.Münch J., Rücker E., Ständker L., Adermann K., Goffinet C., Schindler M., Wildum S., Chinnadurai R., Rajan D., Specht A., Giménez-Gallego G., Sánchez P. C., Fowler D. M., Koulov A., Kelly J. W., Mothes W., Grivel J. C., Margolis L., Keppler O. T., Forssmann W. G., Kirchhoff F. (2007) Cell 131, 1059–1071 [DOI] [PubMed] [Google Scholar]
- 3.Roan N. R., Münch J., Arhel N., Mothes W., Neidleman J., Kobayashi A., Smith-McCune K., Kirchhoff F., Greene W. C. (2009) J. Virol. 83, 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase A. T. (2005) Nat. Rev. Immunol. 5, 783–792 [DOI] [PubMed] [Google Scholar]
- 5.Chiti F., Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 6.Inbar P., Li C. Q., Takayama S. A., Bautista M. R., Yang J. (2006) Chembiochem 7, 1563–1566 [DOI] [PubMed] [Google Scholar]
- 7.Lockhart A., Ye L., Judd D. B., Merritt A. T., Lowe P. N., Morgenstern J. L., Hong G., Gee A. D., Brown J. (2005) J. Biol. Chem. 280, 7677–7684 [DOI] [PubMed] [Google Scholar]
- 8.Inbar P., Bautista M. R., Takayama S. A., Yang J. (2008) Anal. Chem. 80, 3502–3506 [DOI] [PubMed] [Google Scholar]
- 9.Khurana R., Coleman C., Ionescu-Zanetti C., Carter S. A., Krishna V., Grover R. K., Roy R., Singh S. (2005) J. Struct. Biol. 151, 229–238 [DOI] [PubMed] [Google Scholar]
- 10.LeVine H., 3rd (2005) Amyloid 12, 5–14 [DOI] [PubMed] [Google Scholar]
- 11.Pope M., Haase A. T. (2003) Nat. Med. 9, 847–852 [DOI] [PubMed] [Google Scholar]
- 12.Kiessling A. A. (2005) Methods Mol. Biol. 304, 71–86 [DOI] [PubMed] [Google Scholar]
- 13.Sharkey D. J., Macpherson A. M., Tremellen K. P., Robertson S. A. (2007) Mol. Hum. Reprod. 13, 491–501 [DOI] [PubMed] [Google Scholar]
- 14.Li Q., Estes J. D., Schlievert P. M., Duan L., Brosnahan A. J., Southern P. J., Reilly C. S., Peterson M. L., Schultz-Darken N., Brunner K. G., Nephew K. R., Pambuccian S., Lifson J. D., Carlis J. V., Haase A. T. (2009) Nature 458, 1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer B. E., Doncel G. F., Krebs F. C., Shattock R. J., Fletcher P. S., Buckheit R. W., Jr., Watson K., Dezzutti C. S., Cummins J. E., Bromley E., Richardson-Harman N., Pallansch L. A., Lackman-Smith C., Osterling C., Mankowski M., Miller S. R., Catalone B. J., Welsh P. A., Howett M. K., Wigdahl B., Turpin J. A., Reichelderfer P. (2006) Antimicrob. Agents Chemother. 50, 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alt C., Harrison T., Dousman L., Fujita N., Shew K., Tran T. T., Shayesteh S., Matsukawa A., Mirsalis J., D'Andrea A. (2009) Curr. HIV Res. 7, 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galen B. T., Martin A. P., Hazrati E., Garin A., Guzman E., Wilson S. S., Porter D. D., Lira S. A., Keller M. J., Herold B. C. (2007) J. Infect. Dis. 195, 1332–1339 [DOI] [PubMed] [Google Scholar]
- 18.Cone R. A., Hoen T., Wong X., Abusuwwa R., Anderson D. J., Moench T. R. (2006) BMC Infect Dis. 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGowin C. L., Ma L., Martin D. H., Pyles R. B. (2009) Infect. Immun. 77, 1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Damme L., Ramjee G., Alary M., Vuylsteke B., Chandeying V., Rees H., Sirivongrangson P., Mukenge-Tshibaka L., Ettiègne-Traoré V., Uaheowitchai C., Karim S. S., Mâsse B., Perriëns J., Laga M. (2002) Lancet 360, 971–977 [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson D., Tholandi M., Ramjee G., Rutherford G. W. (2002) Lancet Infect. Dis. 2, 613–617 [DOI] [PubMed] [Google Scholar]
- 22.Fichorova R. N., Tucker L. D., Anderson D. J. (2001) J. Infect. Dis. 184, 418–428 [DOI] [PubMed] [Google Scholar]
- 23.Dezzutti C. S., James V. N., Ramos A., Sullivan S. T., Siddig A., Bush T. J., Grohskopf L. A., Paxton L., Subbarao S., Hart C. E. (2004) Antimicrob. Agents Chemother. 48, 3834–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourne N., Bernstein D. I., Ireland J., Sonderfan A. J., Profy A. T., Stanberry L. R. (1999) J. Infect. Dis. 180, 203–205 [DOI] [PubMed] [Google Scholar]
- 25.Rusconi S., Moonis M., Merrill D. P., Pallai P. V., Neidhardt E. A., Singh S. K., Willis K. J., Osburne M. S., Profy A. T., Jenson J. C., Hirsch M. S. (1996) Antimicrob. Agents Chemother. 40, 234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst-Kralovetz M. M., Quayle A. J., Ficarra M., Greene S., Rose W. A., 2nd, Chesson R., Spagnuolo R. A., Pyles R. B. (2008) Am. J. Reprod. Immunol. 59, 212–224 [DOI] [PubMed] [Google Scholar]
- 27.Patel S., Hazrati E., Cheshenko N., Galen B., Yang H., Guzman E., Wang R., Herold B. C., Keller M. J. (2007) J. Infect. Dis. 196, 1394–1402 [DOI] [PubMed] [Google Scholar]
- 28.Keller M. J., Mesquita P. M., Torres N. M., Cho S., Shust G., Madan R. P., Cohen H. W., Petrie J., Ford T., Soto-Torres L., Profy A. T., Herold B. C. (2010) PLoS ONE 5, e8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roan N. R., Sowinski S., Münch J., Kirchhoff F., Greene W. C. (2010) J. Biol. Chem. 285, 1861–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai S. K., Hida K., Shukair S., Wang Y. Y., Figueiredo A., Cone R., Hope T. J., Hanes J. (2009) J. Virol. 83, 11196–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkataraman N., Cole A. L., Svoboda P., Pohl J., Cole A. M. (2005) J. Immunol. 175, 7560–7567 [DOI] [PubMed] [Google Scholar]
- 32.Cutler B., Justman J. (2008) Lancet Infect. Dis. 8, 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayehunie S., Cannon C., Lamore S., Kubilus J., Anderson D. J., Pudney J., Klausner M. (2006) Toxicol. In Vitro 20, 689–698 [DOI] [PubMed] [Google Scholar]