Abstract
Metabotropic GABAB receptors are abundantly expressed at glutamatergic synapses where they control excitability of the synapse. Here, we tested the hypothesis that glutamatergic neurotransmission may regulate GABAB receptors. We found that application of glutamate to cultured cortical neurons led to rapid down-regulation of GABAB receptors via lysosomal degradation. This effect was mimicked by selective activation of AMPA receptors and further accelerated by coactivation of group I metabotropic glutamate receptors. Inhibition of NMDA receptors, blockade of L-type Ca2+ channels, and removal of extracellular Ca2+ prevented glutamate-induced down-regulation of GABAB receptors, indicating that Ca2+ influx plays a critical role. We further established that glutamate-induced down-regulation depends on the internalization of GABAB receptors. Glutamate did not affect the rate of GABAB receptor endocytosis but led to reduced recycling of the receptors back to the plasma membrane. Blockade of lysosomal activity rescued receptor recycling, indicating that glutamate redirects GABAB receptors from the recycling to the degradation pathway. In conclusion, the data indicate that sustained activation of AMPA receptors down-regulates GABAB receptors by sorting endocytosed GABAB receptors preferentially to lysosomes for degradation on the expense of recycling. This mechanism may relieve glutamatergic synapses from GABAB receptor-mediated inhibition resulting in increased synaptic excitability.
Keywords: Calcium Channels; Endocytosis; G Protein-coupled Receptors (GPCR); GABA Receptors; Glutamate; Glutamate Receptors Metabotropic; Glutamate Receptors Ionotropic (AMPA, NMDA); Lysosomes; Protein Degradation
Introduction
In the mature mammalian nervous system, excitatory neurotransmission mediated by glutamate is balanced by the inhibitory actions of GABA. Slow, prolonged inhibitory neurotransmission is largely mediated by metabotropic GABAB receptors, which are widely expressed in the central nervous system and play an important role in many neurological disorders such as epilepsy, anxiety, depression, addiction, and chronic pain (for a review see Ref. 1). Functional GABAB receptors are heterodimers composed of the two subunits GABAB1 and GABAB2 and are expressed in brain as two receptors subtypes (GABAB(1a,2) and GABAB(1b,2)) based on alternative promoter usage of GABAB1. GABAB receptors are either localized at postsynaptic sites where they induce slow inhibitory postsynaptic potentials by activating K+ channels or at presynaptic sites where they suppress neurotransmitter release by inhibiting Ca2+ channels (1).
GABAB receptors are not only localized at GABAergic synapses but are also abundantly expressed at glutamatergic synapses (2–5). At these synapses, they are activated by GABA spillover derived from sustained GABAergic neurotransmission (6) and control glutamatergic activity at pre- and postsynaptic sites (7–11). There is accumulating evidence that sustained activation of glutamate receptors in turn may affect GABAB receptors. Treatment of cultured cortical neurons with glutamate resulted in the loss of cell-surface GABAB receptors (12), and NMDA-induced excitotoxicity in hippocampal slices led to increased GABAB1 and decreased GABAB2 expression levels (13). In addition, it was reported that glutamate slows the rate of GABAB receptor endocytosis in cultured hippocampal neurons (14). These findings suggest that sustained glutamatergic activity may regulate the availability of GABAB receptors, which in turn is expected to affect excitability of glutamatergic synapses. However, the mechanisms involved in this putative regulation of GABAB receptors by glutamate remain to be established.
Previously, we had shown that endocytosed GABAB receptors are predominantly recycled back to the plasma membrane and are eventually degraded in lysosomes (15). Recycling and degradation of GABAB receptors need to be precisely balanced to maintain the required number of receptors at the cell surface. On the other hand, shifting the balance toward degradation would be a powerful mechanism to down-regulate GABAB receptors. Here, we tested the hypothesis that sustained activation of glutamate receptors might affect GABAB receptor levels by regulating their rate of recycling and degradation. We found that treating cultured cortical neurons with glutamate resulted in a rapid down-regulation of GABAB receptors. The glutamate-induced down-regulation was triggered by sustained activation of AMPA receptors, depended on Ca2+ influx through NMDA receptors as well as L-type Ca2+ channels, and finally resulted in reduced constitutive recycling and increased lysosomal degradation of GABAB receptors.
EXPERIMENTAL PROCEDURES
Antibodies
The following primary antibodies were used: rabbit GABAB1a,b directed against the C terminus of GABAB1 (affinity-purified, 1:500 for in-cell Western assay, immunofluorescence, and Western blotting; for specificity see Ref. 16; custom-made by GenScript); guinea pig GABAB2 (1:1000 for immunofluorescence and 1:2000 for Western blotting, Chemicon International); rabbit GABAB2-F(ab′)2 directed against the N terminus of GABAB2 (1:50 for internalization and recycling assays; for specificity see Ref. 17; custom-made by GenScript); mouse actin (1:1000 for in-cell Western assay and 1:80,000 for Western blotting, Chemicon International); rabbit GABAA receptor directed against the N terminus of the α1 subunit (affinity-purified, 1:100 for in-cell Western assay (18)); mouse PSD95 (1:500 for immunofluorescence, Affinity Bioreagents); rabbit PSD95 (1:2000 for immunofluorescence); guinea pig vGlut1 (1:10,000 for immunofluorescence, Chemicon International); rabbit vGlut1 (1:10,000 for immunofluorescence, Synaptic Systems). Secondary antibodies were coupled either to Alexa Fluor 488 (1:1000, Invitrogen), Cy-3 (1:500, Jackson ImmunoResearch), IRDye®680 (1:800, LI-COR Biosciences), IRDye®800CW (1:200, LI-COR Biosciences), or horseradish peroxidase (1:5000, Jackson ImmunoResearch).
Drugs
The following drugs were used: ω-agatoxin TK (0.5 μm, Tocris Bioscience); AMPA (100 μm, Tocris Bioscience); bafilomycin A1 (0.5 μm, Sigma); chloroquine (100 μm, Sigma); 6-cyano-7-nitroquinoxaline-2,3-dione (20 μm, Tocris Bioscience); ω-conotoxin GVIA (3 μm, Sigma); ω-conotoxin MVIIC (2 μm, Tocris Bioscience); D-AP5 (50 μm, Tocris Bioscience); (2S,1R,2R,3R)-2-(2,3-dicarboxycyclopropyl)glycine (100 μm, Tocris Bioscience); Dynasore (100 μm, Sigma); glycine (10 μm, Sigma); glutamate (50 μm, Sigma); L-AP4 (100 μm, Tocris Bioscience); leupeptin (100 μm, Sigma); MG132 (10 μm, Sigma); monensin (50 μm, Sigma); nifedipine (100 μm, Tocris Bioscience); NASPM (10–250 μm, Sigma); NMDA (100 μm, Tocris Bioscience); tADA (200 μm, Sigma); UBEI-45 (50 μm, Biogenova).
Cell Culture
Primary neuronal cultures of cerebral cortex were prepared from E18 embryos of time-pregnant Wistar rats as described previously (15). Neurons were plated to a density of about 30,000 cells onto poly-l-lysine-coated 96-well plates or 60,000 cells onto poly-l-lysine-coated coverslips, respectively. Neurons were kept in culture at 37 °C and 5% CO2 for 11–21 days. The vast majority of experiments was performed with cultures kept for 11–16 days in vitro, and a few experiments were done with older cultures (up to 21 days in culture). Because the results derived from the older cultures were similar to those obtained from younger cultures, the data were pooled.
Western Blotting
Western blot experiments using GABAB1a,b and GABAB2 antibodies were performed with cortical neurons grown on 6-cm culture dishes (about 2.5 × 106 cells per dish) as described previously (15).
Immunocytochemisty and Confocal Laser Scanning Microscopy
Double labeling immunocytochemistry on cortical neurons cultured on coverslips was performed as described previously (15, 19). Incubation with primary antibodies diluted to the appropriate concentration in PBS containing 10% normal goat serum was done for 1 h at room temperature. After embedding in fluorescence mounting medium (DakoCytomation), neurons were analyzed by confocal laser scanning microscopy (LSM510 Meta; Zeiss) using a 100× plan apochromat oil differential interference contrast objective (1.4 NA). Images were acquired at a resolution of 1024 × 1024 pixels in the sequential mode. For each neuron, 5 optical sections spaced by 0.38 μm were taken. Images were processed using Imaris (version 4.2.0 and 5.7.2, Bitplane, Zurich, Switzerland).
For colocalization studies on dendrites of cortical neurons, raw confocal images were smoothed using the Edge Preserving Filter (filter width 0.636 μm) and then further processed by setting threshold cutoffs for each image individually to only visualize significant signals above background. Individual threshold cutoffs accounted for differences in background staining among distinct neurons and dendrites. A colocalization channel was built (colocalization intensity 255, constant value), and GABAB receptor and synaptic marker protein clusters (>15 pixels) as well as their colocalization were counted within 10 μm of the dendrite beginning at a distance of 10 μm from the soma. In addition, the width of the dendrite was measured to calculate the area analyzed.
Quantification of fluorescence intensities was done using the ImageJ software. For the analysis of GABAB receptor expression, the soma excluding the nucleus of stained neurons was carefully outlined and the integrated density of the fluorescence signals measured. For quantification of cell surface GABAB receptors, the integrated density of the fluorescence signals derived from the surface of living stained neurons was determined. Background fluorescence was determined for each image and subtracted.
In-cell Western Assay
For the in-cell Western assay, neurons were cultured in 96-well plates. On the day of testing the culture medium was removed and replaced by 100 μl of neurobasal medium containing B27 supplement (1:50, Invitrogen), GlutaMAX (1:100, Invitrogen), and the drug to be tested. Cultures were incubated in the presence or absence of the drug for 90 min at 37 °C and 5% CO2. Thereafter, the neurons were rapidly cooled to 4 °C, washed twice with ice-cold PBS, and fixed with 4% paraformaldehyde for 10 min. Subsequently, cells were permeabilized four times for 5 min with 0.1% Triton X-100 (Sigma) in PBS and then incubated with 5% nonfat dry milk in PBS for 2 h at room temperature to block nonspecific antibody-binding sites. Cultures were then incubated for 24–48 h simultaneously with rabbit GABAB1a,b and mouse actin antibodies (in PBS, 5% nonfat dry milk). Nonspecific GABAB1a,b antibody binding was assessed in parallel cultures by competition using the peptide-antigen (10 μg/ml). Subsequently, neurons were extensively washed with 0.1% Tween 20 (Sigma) in PBS and incubated with the appropriate secondary antibodies (IRDye680 goat anti-mouse; IRDye800CW donkey anti-rabbit) in PBS containing 5% nonfat dry milk for 60 min at room temperature and protected from light. After washing the neurons with 0.1% Tween 20 in PBS and once with PBS, the buffer was completely removed, and the fluorescence was measured with the Odyssey infrared imaging system (LI-COR Biosciences).
For data evaluation, integrated fluorescence intensities for nonspecific GABAB1a,b signals (as assessed by competition with the peptide-antigen) were determined in multiple wells, averaged, and subtracted from GABAB1a,b signals. Obtained specific GABAB1a,b signals were then normalized to the actin signal determined in parallel to account for potential cell loss during washing steps or unequal plating of neurons.
Internalization Assay
Cortical neurons grown in 96-well plates were rapidly cooled to 4 °C and washed with ice-cold buffer A (25 mm HEPES, pH 7.4, 119 mm NaCl, 5 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 30 mm glucose). Cell-surface GABAB receptors were then labeled by incubation with GABAB2-F(ab′)2 antibodies in buffer A containing 10% normal donkey serum for 60 min at 4 °C. After extensive washing with buffer A, the neurons were incubated for 1.5–15 min at 37 °C to permit internalization of the receptors in the absence or presence of glutamate (50 μm). Control cultures for determining the total cell surface GABAB receptors were left at 4 °C, a condition that is nonpermissive for internalization. After washing with ice-cold buffer A, the cultures were incubated with IRDye800CW donkey anti-rabbit secondary antibody in buffer A for 90 min at 4 °C. To account for potential cell loss during washing steps or unequal plating of neurons, cultures were incubated in parallel with the cell-permeant nuclear stain DRAQ5 (1:1000, Biostatus Ltd.). After extensive washing, the buffer was completely removed, and the fluorescence of dry cultures was measured with the Odyssey infrared imaging system. Nonspecific GABAB2-F(ab′)2 antibody signals were determined by competition with the peptide-antigen (10 μg/ml) in multiple wells. DRAQ5 signals were used for normalization. For data evaluation see under “In-cell Western Assay.”
Recycling Assay
Cell surface GABAB receptors of cortical neurons grown in 96-well plates were labeled with GABAB2-F(ab′)2 antibodies as described above for the internalization assay. For internalization of labeled receptors, cultures were incubated for 15 min at 37 °C. Thereafter, the neurons were incubated for 60 min at 4 °C with a secondary antibody (Alexa Fluor 488-conjugated, 1:50, Invitrogen) that is not detected by the infrared imaging system and is used to mask primary antibodies that labeled remaining cell surface GABAB receptors. After washing, the neurons were again incubated for 30 min at 37 °C to permit internalized antibody-labeled receptors to recycle back to the cell surface. Recycled receptors were then detected at the cell surface using IRDye800CW donkey anti-rabbit secondary antibodies, and cultures were processed further as described above for the internalization assay.
RESULTS
Sustained Activation of Glutamate Receptors Triggers Lysosomal Degradation of GABAB Receptors
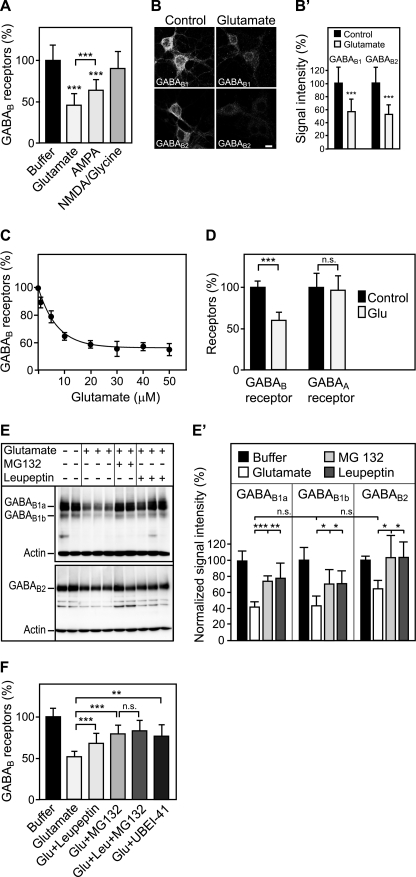
To investigate whether modulation of neuronal activity regulates degradation of GABAB receptors, cultured cortical neurons grown in 96-well plates were incubated for 90 min with agonists and antagonists of the main neurotransmitter systems and were analyzed for changes in GABAB receptor expression using the in-cell Western assay. Among the drugs tested (data not shown), only glutamate and AMPA led to a significant reduction of GABAB receptor signals in cultured neurons with glutamate being more effective than AMPA (90 min of glutamate: 46 ± 14%; AMPA: 64 ± 13% of control; Fig. 1A). Immunofluorescence microscopy showed that levels of GABAB1 and GABAB2 subunits were down-regulated to a similar extent by glutamate (30 min of glutamate: GABAB1, 57 ± 20%; GABAB2, 53 ± 17% of control, n = 33–48 neurons; Fig. 1B). Furthermore, Western blot experiments demonstrated that GABAB1a and GABAB1b receptor subtypes were equally affected by glutamate (90 min of glutamate: GABAB1a, 42 ± 6%; GABAB1b, 43 ± 12% of control, mean ± S.D., n = 5–6 cultures; Fig. 1E). The glutamate-induced down-regulation of GABAB receptors was not due to reduced viability of neurons upon drug exposure because no significant cell death was observed within the time frame of the experiment (control: 0.4 ± 0.6%; 90 min of glutamate: 0.9 ± 0.9%, mean ± S.D., n = 22 cultures; trypan blue dye exclusion assay). Glutamate-induced down-regulation of GABAB receptors was found to be dose-dependent with 5 μm glutamate triggering the half-maximal effect (Fig. 1C). Glutamate did not affect expression of GABAA receptors, indicating that it specifically down-regulates GABAB receptors (Fig. 1D).
FIGURE 1.
Sustained activation of glutamate receptors induces the down-regulation of GABAB receptors via lysosomal degradation. A, down-regulation of GABAB receptors is triggered by glutamate and AMPA. Primary cortical neurons were treated either with 50 μm glutamate, 100 μm AMPA, or 100 μm NMDA + 10 μm glycine for 90 min and tested for GABAB receptor levels using the in-cell Western assay and antibodies directed against the C terminus of GABAB1. Means ± S.D.; n = 20–40 cultures from three to five preparations; ***, p < 0.001, one-way ANOVA; Bonferoni post test. B, glutamate down-regulates GABAB1 and GABAB2 as verified by immunocytochemistry. For immunofluorescence analysis, cells were treated with 50 μm glutamate for 30 min. This incubation time resulted in sufficient remaining fluorescence signals in the glutamate-treated cultures enabling a reliable quantification. After fixation and permeabilization, neurons were stained with antibodies directed against GABAB1 and GABAB2, respectively. Representative confocal images are shown. B′, quantification of fluorescence signals. Scale bar represents 10 μm. Means ± S.D.; n = 33–48 neurons from three preparations; ***, p < 0.0001, t test. C, dose dependence of glutamate-induced down-regulation of GABAB receptors. Neurons were treated with increasing concentrations of glutamate for 90 min and analyzed for GABAB receptor levels using the in-cell Western assay and GABAB1 antibodies. A concentration of 5 μm glutamate led to half-maximal down-regulation of GABAB receptors. Means ± S.D.; n = 20 cultures from three preparations. D, glutamate does not affect expression levels of GABAA receptors. Neurons were subjected to 50 μm glutamate for 90 min and analyzed for GABAB and GABAA receptor levels using the in-cell Western assay and antibodies directed against GABAB1 and the GABAA receptor α1 subunit, respectively. Means ± S.D.; n = 40–60 cultures from five preparations; ***, p < 0.0001, n.s., p = 0.8, t test. E, glutamate-induced down-regulation is prevented by lysosome and proteasome inhibitors. Neurons were treated with 50 μm glutamate in the presence or absence of the lysosome blocker leupeptin (100 μm) or the proteasome blocker MG132 (10 μm), solubilized and analyzed for GABAB receptor levels by Western blotting using GABAB1 and GABAB2 antibodies. GABAB1a and GABA1b isoforms as well as GABAB2 subunits were down-regulated after glutamate treatment. Down-regulation was blocked by the lysosome inhibitor leupeptin and by the proteasome inhibitor MG132. Bands present in the middle of the blot were observed at varying intensities and most likely represent degradation products of GABAB receptors. The experiment shown was repeated once with three cultures for each condition. E′, quantification of the Western blots. Means ± S.D.; n = 4–6 cultures; n.s. = p > 0.05, *, p < 0.05; **, p < 0.01; ***, p < 0.001, one-way ANOVA, Dunnett's post test. F, proteasome is most likely not directly involved in down-regulating GABAB receptors. Neurons were treated for 90 min with 50 μm glutamate in the presence or absence of the lysosome blocker leupeptin (100 μm), the proteasome blocker MG132 (10 μm), or a combination of both. In addition, the involvement of ubiquitination was tested by treating neurons with the ubiquitin-activating enzyme (E1) inhibitor UBEI-45 (50 μm). GABAB receptor levels were determined using the in-cell Western assay and GABAB1 antibodies. Because the effects of leupeptin and MG132 did not add up, it is unlikely that GABAB receptors are degraded by proteasomes. Means ± S.D.; n = 40–48 cultures from four preparations; n.s. = p > 0.05; **, p < 0.01; ***, p < 0.001; one-way ANOVA; Bonferoni post test.
We had previously shown that GABAB receptors are degraded in lysosomes (15, 19). Therefore, we tested the hypothesis that activation of glutamate receptors triggers lysosomal degradation of GABAB receptors. Indeed, blocking lysosomal proteases with leupeptin resulted in a decreased glutamate-induced down-regulation of GABAB receptors, as shown by Western blot analysis (GABAB1a: glutamate, 42 ± 6; glutamate + leupeptin, 79 ± 18%; GABAB1b: glutamate, 43 ± 12%; glutamate + leupeptin, 71 ± 16%; GABAB2: glutamate, 65 ± 10%; glutamate + leupeptin, 105 ± 19% of control; mean ± S.D., n = 5–6 cultures; Fig. 1E). Surprisingly, also the proteasome inhibitor MG132 inhibited glutamate-induced down-regulation of GABAB receptors (GABAB1a: 73 ± 8%; GABAB1b: 70 ± 19%; GABAB2: 103 ± 29% of control; mean ± S.D., n = 5–6 cultures; Fig. 1E). This finding ostensibly suggests that GABAB receptors may also be degraded by proteasomes. However, experiments with proteasome inhibitors are difficult to interpret because blocking proteasomal activity rapidly results in the depletion of free ubiquitin. Ubiquitin not only tags proteins for proteasomal degradation but also serves as sorting signal to lysosomes for many proteins. If the proteasome directly contributes to the degradation of GABAB receptors, it is expected that simultaneous inhibition of proteasomes and lysosomes would block GABAB receptor down-regulation to a greater extent than inhibition of each degradation pathway alone. However, incubation of neurons simultaneously with leupeptin and MG132 did not result in an increased inhibition of glutamate-induced GABAB receptor degradation (Fig. 1F). Therefore, it is unlikely that the proteasome directly contributes to the degradation of GABAB receptors. Instead, this finding suggests that ubiquitin may serve as lysosomal targeting signal for endocytosed GABAB receptors. Blocking ubiquitination using the ubiquitin-activating enzyme (E1) inhibitor UBEI-45 partially inhibited glutamate-induced down-regulation of GABAB receptors (Fig. 1F) indicating that ubiquitination is a critical factor in this process.
Glutamate-induced Down-regulation of GABAB Receptors Is Mediated by AMPA Receptors and Accelerated by Type I Metabotropic Glutamate Receptors
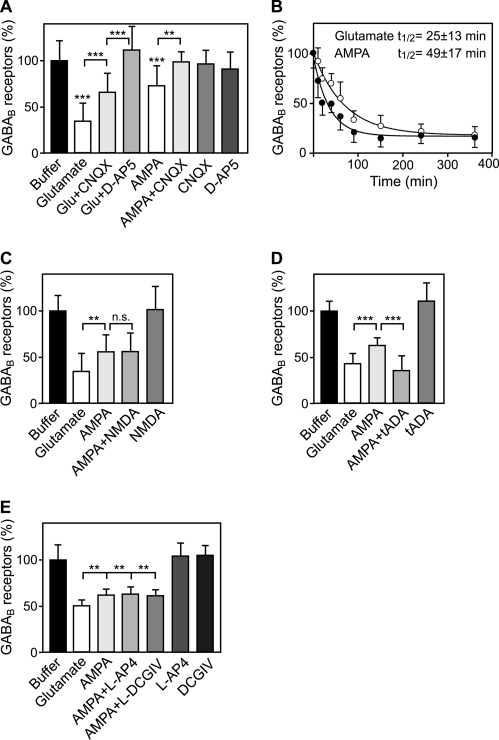
Because AMPA largely mimics the effect of glutamate (Fig. 1A), AMPA receptors appear to primarily trigger the down-regulation of GABAB receptors. However, although the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione fully inhibited AMPA-induced down-regulation of GABAB receptors, it only partially reversed glutamate-induced down-regulation (Fig. 2A). This result also indicates that glutamate receptors other than AMPA receptors may contribute to this effect. Surprisingly, the selective NMDA receptor antagonist D-AP5 completely reversed glutamate-induced GABAB receptor down-regulation (Fig. 2A), although application of NMDA/glycine did not affect GABAB receptor levels (Fig. 1A). Because NMDA receptors are blocked by Mg2+ around resting potential, this apparent discrepancy may be caused by a too low basal AMPA receptor activity in unstimulated cultures to permit sufficient activation of NMDA receptors. In conclusion, the results indicate that in addition to AMPA receptors, NMDA receptors are also involved in glutamate-induced down-regulation of GABAB receptors.
FIGURE 2.
Glutamate-induced down-regulation of GABAB receptors is mediated by AMPA receptors and accelerated by type I mGluRs. A, glutamate-induced down-regulation of GABAB receptors is partially reversed by the AMPA antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and fully reversed by the NMDA antagonist D-AP5. Cells were treated for 90 min either with 50 μm glutamate, 100 μm AMPA, 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione, 50 μm D-AP5, or with the indicated combinations and tested for GABAB receptor levels using the in-cell Western assay, and antibodies, which were directed against the C terminus of GABAB1. Means ± S.D.; n = 27–44 cultures from three to five preparations; **, p < 0.01; ***, p < 0.001, one-way ANOVA; Bonferoni post test. B, selective activation of AMPA receptors mimics the effect of glutamate but is less efficient in down-regulating GABAB receptors. Neurons were incubated with 50 μm glutamate or 100 μm AMPA and were analyzed for GABAB receptor levels at different time points (10 min to 6 h) using the in-cell Western assay. Data were fitted to one-phase exponential decay: glutamate, t½ = 25 ± 13 min; AMPA, t½ = 49 ± 17 min. Mean ± S.D.; n = 12 cultures from three preparations. C–E, combined activation of AMPA and mGluR group I receptors (D), but not AMPA and NMDA (C) or mGluR group II or III receptors (E), mimic glutamate-induced down-regulation of GABAB receptors. Neurons were incubated for 90 min with either 50 μm glutamate, 100 μm AMPA, 100 μm NMDA + 10 μm glycine, 200 μm tADA (mGluR1/5 agonist), 100 μm L-AP4 (mGluR4/6/7/8 agonist), or 100 μm (2S,1R,2R,3R)-2-(2,3-dicarboxycyclopropyl)glycine (DCGIV) (mGluR2/3 agonist) alone or with the indicated combinations. Means ± S.D.; C, n = 24 cultures from three preparations; D, n = 21 cultures from three preparations; E, n = 32 cultures from four preparations. n.s. = p > 0.05, **, p < 0.01; ***, p < 0.001, one-way ANOVA; Bonferoni post test (C and D) or Dunnett's post test (E).
The observation that treatment of neurons for 90 min with AMPA induced less reduction of GABAB receptors than application of glutamate (Fig. 1A) suggests either that activation of AMPA receptors alone is not able to mediate the maximum effect or that glutamate more efficiently down-regulates GABAB receptors than AMPA. A time course of glutamate and AMPA application revealed that both drugs led to a similar maximum reduction of GABAB receptors, but glutamate more rapidly down-regulated GABAB receptors (glutamate t½ = 25 ± 13 min; AMPA t½ = 49 ± 17 min; Fig. 2B).
Because the NMDA receptor antagonist D-AP5 reversed glutamate-induced down-regulation of GABAB receptors (Fig. 2A), we hypothesized that activation of NMDA receptors in addition to AMPA receptors might accelerate this process. However, coapplication of AMPA and NMDA/glycine (or NMDA/d-serine, data not shown) did not result in a further decrease of GABAB receptor levels (Fig. 2C). Next, we tested whether metabotropic glutamate receptors (mGluRs)2 may accelerate AMPA-induced down-regulation of GABAB receptors. Indeed, coapplication of the selective mGluR1/5 agonist tADA with AMPA decreased GABAB receptors to the levels observed after glutamate treatment, whereas tADA was ineffective to down-regulate GABAB receptors in the absence of AMPA (Fig. 2D). In contrast, coactivation of either group II mGluRs with DCG-IV or group III mGluRs with L-AP4 did not enhance AMPA-induced GABAB receptors degradation (Fig. 2E). These results indicate that the sustained activation of AMPA and NMDA receptors is required for the down-regulation of GABAB receptors, which is accelerated by additional activation of mGluR1/5.
Glutamate-induced Down-regulation Depends on Ca2+ Influx through L-type Voltage-gated Ca2+ Channels
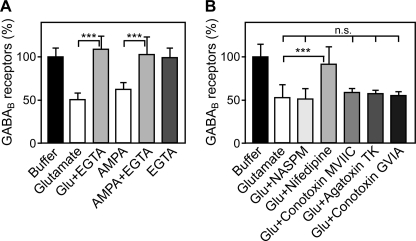
So far our data indicate that glutamate-induced down-regulation of GABAB receptors involves activation of AMPA and NMDA receptors, which both are permeable for Ca2+. Therefore, we hypothesized that Ca2+ influx may be required for this effect. As expected, removal of extracellular Ca2+ using EGTA completely inhibited glutamate- and AMPA-induced down-regulation of GABAB receptors (Fig. 3A). Next we tested whether Ca2+ influx through Ca2+-permeable AMPA receptors (AMPA receptors lacking GluR2) is required for down-regulation of GABAB receptors. NASPM, a selective antagonist for Ca2+-permeable AMPA receptors, did not affect down-regulation of GABAB receptors up to 100 μm (Fig. 3B). However, at 250 μm, NASPM inhibited glutamate-induced down-regulation of GABAB receptors (data not shown). At this high concentration, NASPM has been reported to also block Ca2+-impermeable AMPA receptors (20). Thus, our results suggest that AMPA receptors containing the GluR2 subunit, which are impermeable to Ca2+, mediate the down-regulation of GABAB receptors.
FIGURE 3.
Ca2+ influx through voltage-gated Ca2+ channels is necessary for glutamate-induced down-regulation of GABAB receptors. A, removal of extracellular Ca2+ using EGTA completely inhibited glutamate- and AMPA-induced down-regulation of GABAB receptors. Cells were treated with either 50 μm glutamate, 100 μm AMPA, or 5 mm EGTA or with the indicated combinations for 90 min and tested for GABAB receptor levels using the in-cell Western assay. Means ± S.D.; n = 25–32 cultures from four preparations; ***, p < 0.001, one-way ANOVA; Bonferoni post test. B, inhibition of L-type Ca2+ channels but not P/Q-type and N-type channels prevents down-regulation of GABAB receptors. Neurons were incubated for 90 min either with 50 μm glutamate, 50 μm glutamate +100 μm NASPM (blocker of Ca2+-permeable AMPA receptors) +100 μm nifedipine (L-type Ca2+ channel blocker), +2 μm ω-conotoxin MVIIC (P/Q and N-type Ca2+ channel blocker), +0.5 μm ω-Agatoxin TK (P/Q-type Ca2+ channel blocker), or +3 μm ω-conotoxin GVIA (N-type Ca2+ channel blocker) and subjected to the in-cell Western assay for determination of GABAB receptor levels. Mean ± S.D., n = 16–32 cultures from two to four preparations; n.s. = p > 0.05, ***, p < 0.001, one-way ANOVA; Bonferoni post test.
Because AMPA receptors activate voltage-dependent Ca2+ channels, we tested whether Ca2+ influx through these channels is required for down-regulation of GABAB receptors. Inhibition of L-type Ca2+ channels with nifedipine completely prevented down-regulation of GABAB receptors. In contrast, blocking P/Q-type and N-type Ca2+ channels with ω-conotoxin MVIIC or inhibiting selectively P/Q-type or N-type Ca2+ channels with ω-agatoxin TK and ω-conotoxin GVIA, respectively, was without effect (Fig. 3B). These observations suggest that glutamate-induced down-regulation of GABAB receptors depends on the activity of L-type Ca2+ channels.
Glutamate Affects Recycling but Not Internalization of GABAB Receptors
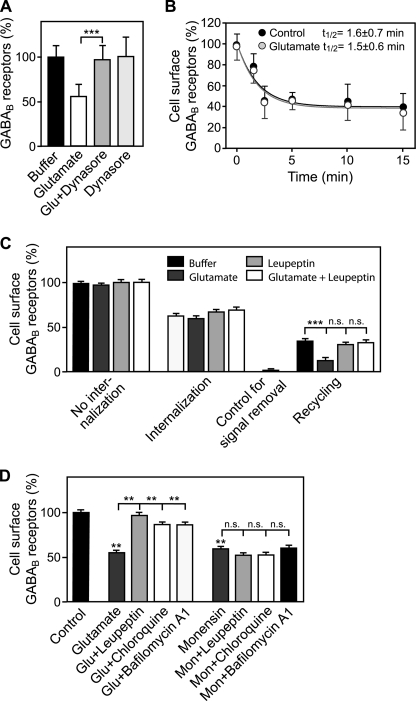
One potential mechanism that may contribute to the down-regulation of GABAB receptors might involve an increased rate of GABAB receptor endocytosis. We therefore tested first whether down-regulation of GABAB receptors depends on the internalization of the receptors. We had previously shown that GABAB receptors internalize via the classical dynamin- and clathrin-dependent pathway (19). As expected, inhibition of dynamin with Dynasore completely blocked the glutamate-induced down-regulation of GABAB receptors, indicating that endocytosis of receptors from the plasma membrane is required for the down-regulation of GABAB receptors (Fig. 4A). However, application of glutamate did not affect the rate of GABAB receptor internalization (control, t½ = 1.6 ± 0.7 min; glutamate, t½ = 1.5 ± 0.6 min; Fig. 4B).
FIGURE 4.
Glutamate affects recycling but not internalization of GABAB receptors. A, inhibition of dynamin with Dynasore completely blocks the glutamate-induced down-regulation of GABAB receptors. Neurons were treated either with 50 μm glutamate, 100 μm Dynasore, or with 50 μm glutamate + 100 μm Dynasore for 90 min and subjected to the in-cell Western assay for determination of GABAB receptor levels. Means ± S.D., n = 40 cultures from five preparations; ***, p < 0.001, one-way ANOVA; Bonferoni post test. B, glutamate did not affect the rate of GABAB receptor internalization. Cell surface receptors of neurons were labeled at 4 °C for 60 min with F(ab′)2 fragments of an antibody directed against the N terminus of GABAB2. Neurons were then incubated for 1.5–15 min at 37 °C in the presence or absence of 50 μm glutamate followed by determination of cell surface GABAB receptor levels. Mean ± S.D.; glutamate: n = 18 cultures, three preparations; AMPA: n = 12 cultures, two preparations. C, glutamate reduces recycling of internalized receptors back to the cell surface. Cell-surface receptors of neurons were labeled as described above, and neurons were incubated for 15 min at 37 °C in the presence or absence of 50 μm glutamate, 100 μm leupeptin, or 50 μm glutamate + 100 μm leupeptin to allow internalization of receptors. After masking remaining antibody-tagged cell-surface receptors with secondary antibodies not detected by the imaging system, neurons were incubated again for 30 min at 37 °C to allow internalized receptors to recycle back to the plasma membrane. Recycled receptors were detected using an appropriately labeled secondary antibody for 90 min at 4 °C. Means ± S.E., n = 30–94 cultures from four to nine preparations; ***, p < 0.001, one-way ANOVA; Bonferoni post test. D, glutamate shifts the balance of GABAB receptor recycling and lysosomal degradation toward degradation without blocking recycling. Neurons were incubated for 1 h with 100 μm leupeptin, 100 μm chloroquine, or 0.5 μm bafilomycin A1 before inducing down-regulation of GABAB receptors by 50 μm glutamate or 50 μm monensin (blocks recycling by preventing fusion of intracellular vesicles with the plasma membrane) for 90 min. Living neurons were subsequently incubated with an antibody directed against the N terminus of GABAB2 to label cell-surface receptors and further processed for immunocytochemistry. Cell-surface fluorescence signals of individual neurons were quantified. Control, no treatment. Means ± S.E., n = 31–81 neurons derived from two to four independent experiments; n.s. = p > 0.05; **, p < 0.01, one-way ANOVA, Dunnett's post test.
Because GABAB receptors are constitutively recycled back to the plasma membrane (15), glutamate may instead interfere with recycling of the receptors. Indeed, treatment of neurons with glutamate significantly reduced recycling of internalized receptors back to the cell surface (Fig. 4C). The reduced recycling was reversed by blocking lysosomal activity with leupeptin, indicating that receptors are preferentially sorted to lysosomes after glutamate application without direct inhibition of the recycling pathway (Fig. 4C). This result was confirmed by directly interfering with receptor recycling using monensin, which blocks fusion of intracellular vesicles with the plasma membrane. Application of monensin resulted in the down-regulation of cell-surface GABAB receptors to the same extent as observed after glutamate treatment (Fig. 4D). However, the down-regulation elicited by glutamate but not the down-regulation induced by monensin was rescued by various blockers of lysosomal activity (leupeptin, chloroquine, and bafilomycin A1; Fig. 4D). This result indicates that sustained glutamate application shifts the balance between recycling and degradation toward receptor degradation.
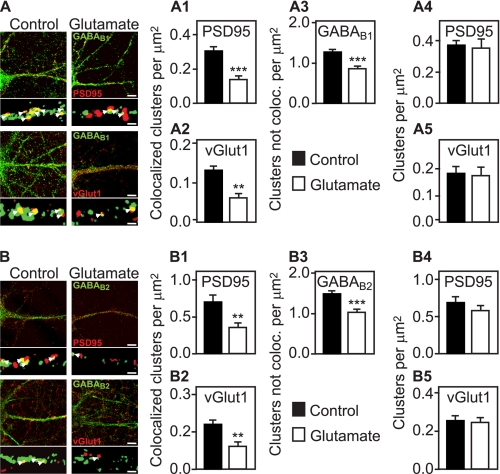
Glutamate Induces the Down-regulation of Synapse-associated GABAB Receptors
To analyze whether GABAB receptors localized at glutamatergic synapses are down-regulated, we performed immunofluorescence colocalization experiments using antibodies directed against GABAB1 (Fig. 5A) or GABAB2 (Fig. 5B) and antibodies against postsynaptic sites (PSD95) or glutamatergic presynaptic sites (vGlut1), respectively. Treatment of neurons for 30 min with glutamate resulted in a dramatic loss (about 50%) of GABAB1 and GABAB2 clusters associated with either PSD95 (Fig. 5, A1 and B1) or vGlut1 (Fig. 5, A2 and B2). GABAB receptor clusters not associated with pre- or postsynaptic marker proteins (which also include intracellular receptors) were found to be reduced to a lesser extent (about 30%, Fig. 5, A3 and B3). The total number of PSD95 (Fig. 5, A4 and B4) and vGlut1 clusters (Fig. 5, A5 and B5) was not affected by glutamate. These results suggest that GABAB receptors associated with synapses may be preferentially down-regulated.
FIGURE 5.
GABAB receptors associated with synapses are down-regulated by activation of glutamate receptors. Neurons were incubated in the presence or absence of 50 μm glutamate for 30 min, fixed, and subjected to double labeling immunocytochemistry using antibodies directed against GABAB1 (A, green) or GABAB2 (B, green) and antibodies against glutamatergic postsynaptic sites (PSD95, red) or glutamatergic presynaptic sites (vGlut1, red), respectively. Large images depict overviews of immunoreactivities in dendrites of lower magnification (scale bars, 5 μm), and the small images below show sections of dendrites after being processed for counting of clusters at high magnification (see “Experimental Procedures” for details; scale bars, 1 μm). In the absence of glutamate, GABAB clusters frequently colocalized (yellow, arrowheads) with either pre- or postsynaptic markers. Glutamate treatment resulted in a significant loss of GABAB1 and GABAB2 clusters associated with either PSD95 (A1 and B1) or vGlut1 (A2 and B2). GABAB receptor clusters not associated with pre- or postsynaptic marker proteins (which also include intracellular receptors) were found to be reduced to a lesser extent (A3 and B3). The number of PSD95 (A4 and B4) and vGlut1 (A5 and B5) clusters was not affected by glutamate. Mean ± S.E., n = 24 neurons derived from two independent experiments. **, p < 0.01; ***, p < 0.001, t test.
DISCUSSION
GABAB receptors are abundantly localized at glutamatergic synapses to regulate excitatory neurotransmission. In this study, we tested the hypothesis that glutamatergic activity may regulate GABAB receptors. Such a mechanism had been suggested by Vargas et al. (12) based on their finding that treating neurons with glutamate resulted in a reduction of plasma membrane GABAB receptors. Our results indicate that sustained activation of AMPA receptors in cultured cortical neurons leads to a rapid and dramatic down-regulation of GABAB receptors by shifting the balance of constitutive recycling and lysosomal degradation toward degradation.
Treatment of neurons with glutamate led to a rapid decrease of the levels of both GABAB receptor subtypes, GABAB(1a,2) and GABAB(1b,2), expressed in neurons. Immunofluorescence analysis showed a slightly stronger loss of GABAB receptor clusters colocalized with marker proteins for glutamatergic synapses than not colocalizing GABAB receptor clusters, suggesting that GABAB receptors associated with synapses might be preferentially down-regulated. The limited resolution of this method permits, however, no conclusion on whether pre- or postsynaptic GABAB receptors are affected to a different extent.
Glutamate activates a variety of distinct receptors, such as AMPA receptors, NMDA receptors, and mGluRs1/5, which all mediate or contribute to down-regulation of GABAB receptors. We found that selective activation of AMPA receptors mimicked the effect of glutamate but was less efficient in down-regulating GABAB receptors, i.e. activating selective AMPA receptors required more time to reach the same level of down-regulation than after glutamate treatment. Simultaneous activation of mGluRs1/5 (group I mGluRs) accelerated AMPA-induced down-regulation of GABAB receptors to the level of glutamate-induced down-regulation, whereas coactivation of group II or group III mGluRs had no effect. Group I mGluRs are predominantly localized postsynaptically and generally promote cell excitability (21). Thus, activation of group I mGluRs in addition to AMPA receptors may further increase excitation of the neuron, accelerating down-regulation of GABAB receptors and explain the difference in time course of GABAB receptor down-regulation by glutamate and AMPA (Fig. 2B). In addition to AMPA receptors and mGluRs1/5, NMDA receptors also play a critical role. Although treatment of neurons with NMDA and glycine (or d-serine) did not induce down-regulation of GABAB receptors, blocking NMDA receptors with D-AP5 completely blocked the effects of glutamate (Fig. 2A) and AMPA (data not shown). Basal AMPA receptor activity in unstimulated cultures may be too low to permit sufficient activation of NMDA receptors (which are blocked by Mg2+ at resting potential) by administration of NMDA and glycine to induce down-regulation of GABAB receptors. Thus, our data indicate that the minimum requirement for glutamate-induced down-regulation of GABAB receptors is the sustained activation of AMPA and NMDA receptors.
So far, the intracellular second messenger pathway(s) linking AMPA and NMDA receptor activation to GABAB receptor down-regulation remains to be established. Because removal of extracellular Ca2+ by EGTA and inhibition of L-type, but not P/Q and N-type, Ca2+ channels blocked GABAB receptor down-regulation, it is very likely that influx and intracellular accumulation of Ca2+ is one component of the intracellular signaling cascade. However, our first attempts to identify further components involved were unsuccessful so far. Activation or inhibition of major classical protein kinases such as protein kinase A, protein kinase C, Ca2+-calmodulin-dependent kinase II, or blocking the Ca2+-dependent phosphatase calcineurin failed to affect glutamate-induced down-regulation of GABAB receptors (data not shown). Thus, further work is required to dissect the intracellular signaling pathway(s) involved.
Endocytosis and sorting mechanisms critically determine the availability of cell surface receptors for signal transduction. We therefore hypothesized that sustained activation of glutamate receptors might affect endocytosis of GABAB receptors. Blocking endocytosis with the dynamin inhibitor Dynasore completely prevented down-regulation of GABAB receptors. This result implies that constant internalization of cell surface GABAB receptors is required for down-regulation. Because GABAB receptors are rapidly endocytosed and constitutively recycled back to the plasma membrane in neurons (12, 15), down-regulation of receptors can principally be achieved by either increasing the rate of internalization or affecting recycling. Our results indicate that the internalization rate of GABAB receptors remained unaffected upon glutamate administration. This is in contrast to a previous report indicating that glutamate administration to hippocampal neurons slowed the internalization rate of GABAB receptors tagged with the α-bungarotoxin-binding site (14). The reason for this discrepancy is currently unknown but may be caused by the different assay systems used (e.g. endogenous versus transfected receptors). However, although glutamate did not impair endocytosis of GABAB receptors, it affected the extent of their recycling in our experimental settings. Impaired receptor recycling may shift the balance between recycling and degradation of the receptors in favor of degradation as recently shown for the transferrin receptor (22). However, this appears not to be the underlying mechanism for the glutamate-induced down-regulation of GABAB receptors. Although directly blocking recycling with monensin resulted in a similar extent of GABAB receptor down-regulation as glutamate treatment, inhibition of lysosomal activity prevented glutamate-induced but not monensin-induced down-regulation of cell-surface receptors. This result suggests that glutamate treatment does not directly block recycling of the receptors but affects the balance of sorting endocytosed GABAB receptors to recycling endosomes and lysosomes.
Regarding the involved degradation pathways, we found that glutamate-induced down-regulation was inhibited by blocking lysosomal as well as proteasomal activity by leupeptin and MG132, respectively. In principle, this observation may suggest that the major protein degradation systems, lysosomes and proteasomes, are involved in down-regulation of GABAB receptors. However, it is rather unlikely that the proteasome is directly involved in glutamate-induced degradation of the receptors because the blocking effects of leupeptin and MG132 did not add up. Inhibition of proteasomes rapidly results in the depletion of free ubiquitin, which is also required as a lysosomal targeting signal for many proteins. Therefore, inhibition of proteasomes with MG132 most likely interferes with ubiquitin-dependent sorting to lysosomes. This interpretation is supported by the observation that sorting of GABAB receptors to lysosomes appears to involve the ESCRT (endosomal sorting complex required for transport) machinery (23) that targets mono- and Lys-63-linked poly-ubiquitinated proteins for degradation to lysosomes (24). Our finding that interfering with protein ubiquitination by blocking the ubiquitin-activating enzyme (E1) reduces glutamate-induced down-regulation of GABAB receptors documents the importance of ubiquitination in this process. However, it is currently unclear at what level ubiquitination may be involved. Ubiquitination may operate at different levels such as endocytosis, sorting, and/or lysosomal targeting and may require direct ubiquitination of the receptors or of proteins involved in these processes or both. This issue needs to be delineated in further studies.
Based on our observations, we propose that sustained activation of AMPA receptors triggers the opening of NMDA receptors and L-type Ca2+ channels, which leads to an intracellular accumulation of Ca2+. A yet to be established and most likely Ca2+-dependent signaling pathway shifts the balance between constitutive recycling and lysosomal degradation of GABAB receptors toward degradation without affecting the rate of receptor internalization. Because all components required for glutamate-induced down-regulation of GABAB receptors are mainly colocalized postsynaptically (25, 26), we propose that glutamate may preferentially down-regulate GABAB receptors associated with postsynaptic sites. In particular, the fact that N-type and P/Q-type Ca2+ channels, which are predominantly associated with presynaptic sites (25, 26), appear not to be involved in down-regulating GABAB receptors supports this hypothesis.
The physiological relevance of glutamate-induced down-regulation of GABAB receptors remains to be determined. Two obvious scenarios can be envisioned. 1) Under pathophysiological conditions, excessive release of glutamate leads to overstimulation of AMPA and NMDA receptors and eventually to neuronal death (27). In this context, glutamate-induced down-regulation of GABAB receptors will result in reduced inhibition of glutamatergic synapses, which is expected to further promote excitotoxicity. 2) Under normal physiological conditions, GABAB receptors associated with glutamatergic synapses control excitability of the synapse and thereby the activity of NMDA receptors (28, 29). Sustained stimulation of individual glutamatergic synapses will down-regulate its associated GABAB receptors and relieves the synapse from inhibition. This is expected to result in locally increased synaptic excitability.
Acknowledgments
We are grateful to Jean-Marc Fritschy for continued support in confocal microscopy and for providing E18 rat cortex for neuronal cultures. We thank Corinne Sidler for preparation of the E18 rat cortex and Hanns Mohler for critical comments on the manuscript.
This work was supported by the Swiss Science Foundation Grant 31003A_121963.
- mGluR
- metabotropic glutamate receptor
- vGlut1
- vesicular glutamate transporter 1
- ANOVA
- analysis of variance
- NASPM
- 1-naphthyl acetyl spermine
- tADA
- trans-azetidine-2,4-dicarboxylic acid.
REFERENCES
- 1.Bettler B., Kaupmann K., Mosbacher J., Gassmann M. (2004) Physiol. Rev. 84, 835–867 [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Boyes J., Yung W. H., Bolam J. P. (2004) J. Comp. Neurol. 474, 340–352 [DOI] [PubMed] [Google Scholar]
- 3.Kulik A., Vida I., Luján R., Haas C. A., López-Bendito G., Shigemoto R., Frotscher M. (2003) J. Neurosci. 23, 11026–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacey C. J., Boyes J., Gerlach O., Chen L., Magill P. J., Bolam J. P. (2005) Neuroscience 136, 1083–1095 [DOI] [PubMed] [Google Scholar]
- 5.Luján R., Shigemoto R., Kulik A., Juiz J. M. (2004) J. Comp. Neurol. 475, 36–46 [DOI] [PubMed] [Google Scholar]
- 6.Scanziani M. (2000) Neuron 25, 673–681 [DOI] [PubMed] [Google Scholar]
- 7.Pan B. X., Dong Y., Ito W., Yanagawa Y., Shigemoto R., Morozov A. (2009) Neuron 61, 917–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaacson J. S., Hille B. (1997) Neuron 18, 143–152 [DOI] [PubMed] [Google Scholar]
- 9.Vigot R., Barbieri S., Bräuner-Osborne H., Turecek R., Shigemoto R., Zhang Y. P., Luján R., Jacobson L. H., Biermann B., Fritschy J. M., Vacher C. M., Müller M., Sansig G., Guetg N., Cryan J. F., Kaupmann K., Gassmann M., Oertner T. G., Bettler B. (2006) Neuron 50, 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guetg N., Seddik R., Vigot R., Turecek R., Gassmann M., Vogt K. E., Bräuner-Osborne H., Shigemoto R., Kretz O., Frotscher M., Kulik A., Bettler B. (2009) J. Neurosci. 29, 1414–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalifoux J. R., Carter A. G. (2010) Neuron 66, 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas K. J., Terunuma M., Tello J. A., Pangalos M. N., Moss S. J., Couve A. (2008) J. Biol. Chem. 283, 24641–24648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimarosti H., Kantamneni S., Henley J. M. (2009) Neuropharmacology 56, 1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilkins M. E., Li X., Smart T. G. (2008) J. Biol. Chem. 283, 34745–34752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grampp T., Notz V., Broll I., Fischer N., Benke D. (2008) Mol. Cell. Neurosci. 39, 628–637 [DOI] [PubMed] [Google Scholar]
- 16.Benke D., Honer M., Michel C., Bettler B., Mohler H. (1999) J. Biol. Chem. 274, 27323–27330 [DOI] [PubMed] [Google Scholar]
- 17.Benke D., Michel C., Mohler H. (2002) J. Recept. Signal Transduct. Res. 22, 253–266 [DOI] [PubMed] [Google Scholar]
- 18.Benke D., Cicin-Sain A., Mertens S., Mohler H. (1991) J. Recept. Res. 11, 407–424 [DOI] [PubMed] [Google Scholar]
- 19.Grampp T., Sauter K., Markovic B., Benke D. (2007) J. Biol. Chem. 282, 24157–24165 [DOI] [PubMed] [Google Scholar]
- 20.Blaschke M., Keller B. U., Rivosecchi R., Hollmann M., Heinemann S., Konnerth A. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 6528–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niswender C. M., Conn P. J. (2010) Annu. Rev. Pharmacol. Toxicol. 50, 295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traer C. J., Rutherford A. C., Palmer K. J., Wassmer T., Oakley J., Attar N., Carlton J. G., Kremerskothen J., Stephens D. J., Cullen P. J. (2007) Nat. Cell Biol. 9, 1370–1380 [DOI] [PubMed] [Google Scholar]
- 23.Kantamneni S., Holman D., Wilkinson K. A., Corrêa S. A., Feligioni M., Ogden S., Fraser W., Nishimune A., Henley J. M. (2008) J. Neurochem. 107, 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raiborg C., Stenmark H. (2009) Nature 458, 445–452 [DOI] [PubMed] [Google Scholar]
- 25.Vacher H., Mohapatra D. P., Trimmer J. S. (2008) Physiol. Rev. 88, 1407–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai S., Hall D. D., Hell J. W. (2009) Physiol. Rev. 89, 411–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arundine M., Tymianski M. (2004) Cell. Mol. Life Sci. 61, 657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziakopoulos Z., Brown M. W., Bashir Z. I. (2000) Eur. J. Neurosci. 12, 803–809 [DOI] [PubMed] [Google Scholar]
- 29.Morrisett R. A., Mott D. D., Lewis D. V., Swartzwelder H. S., Wilson W. A. (1991) J. Neurosci. 11, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]