Abstract
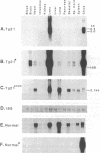
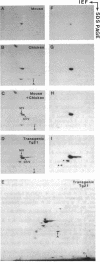
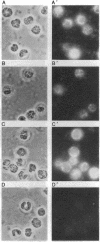
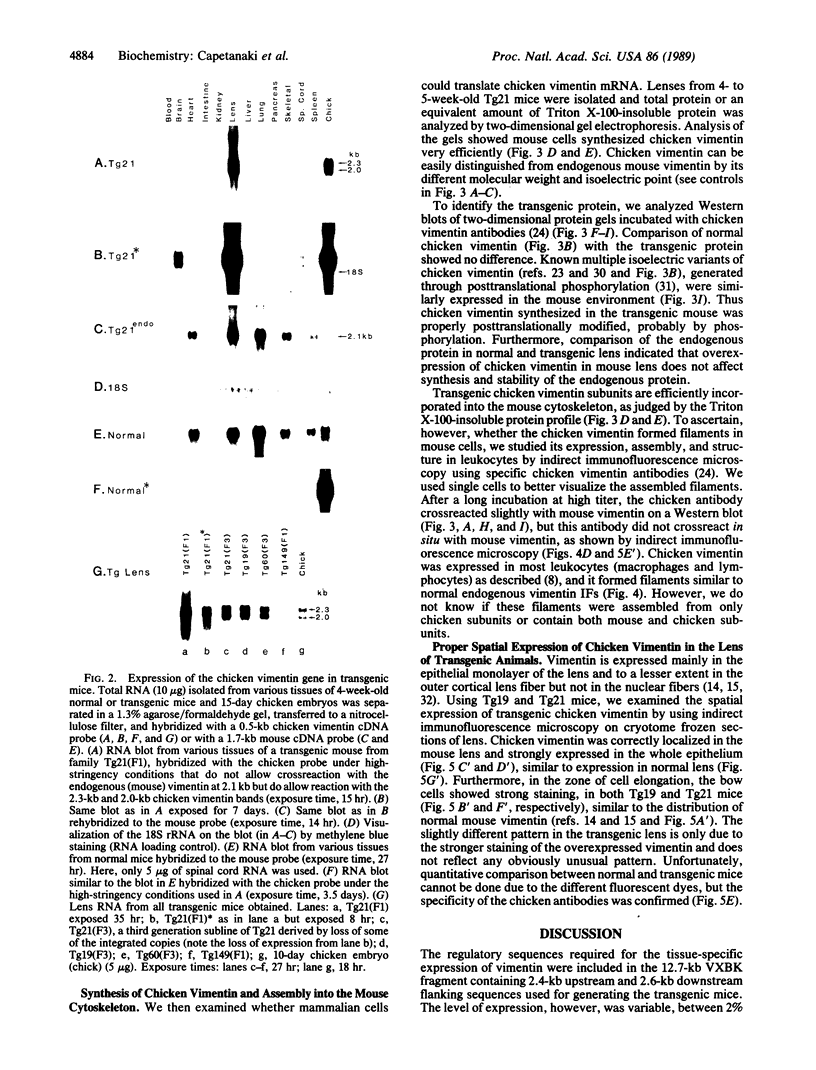
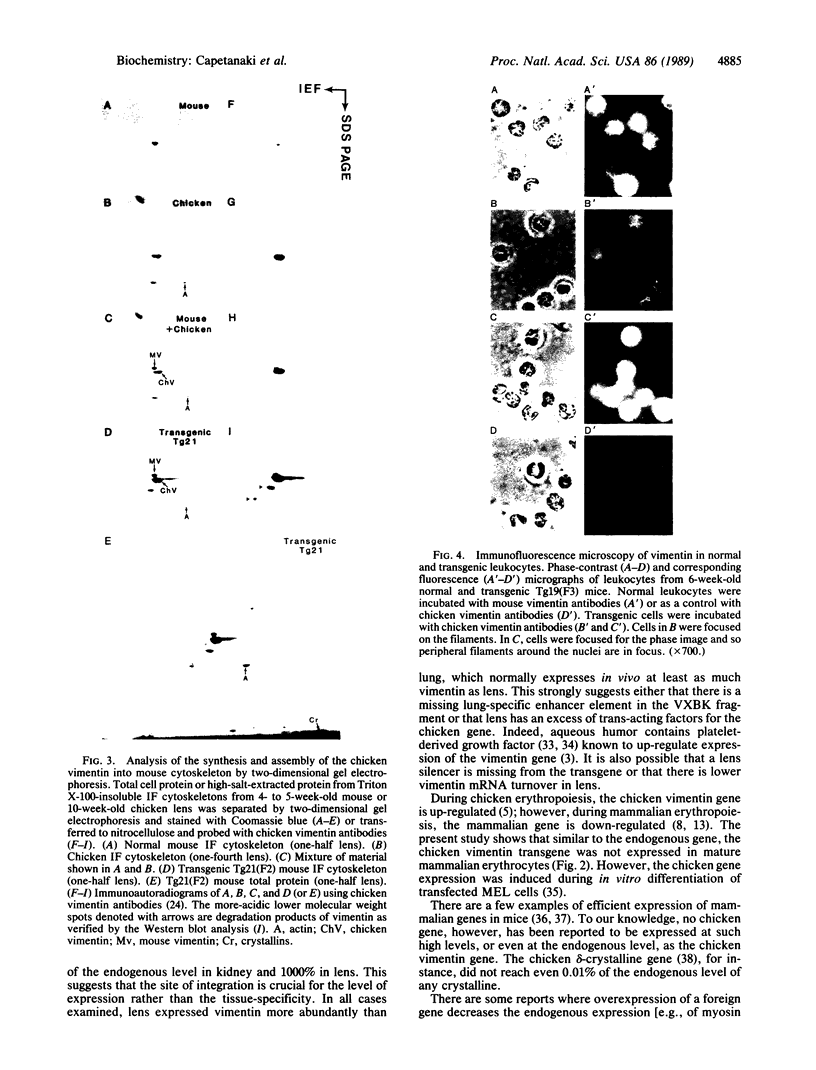
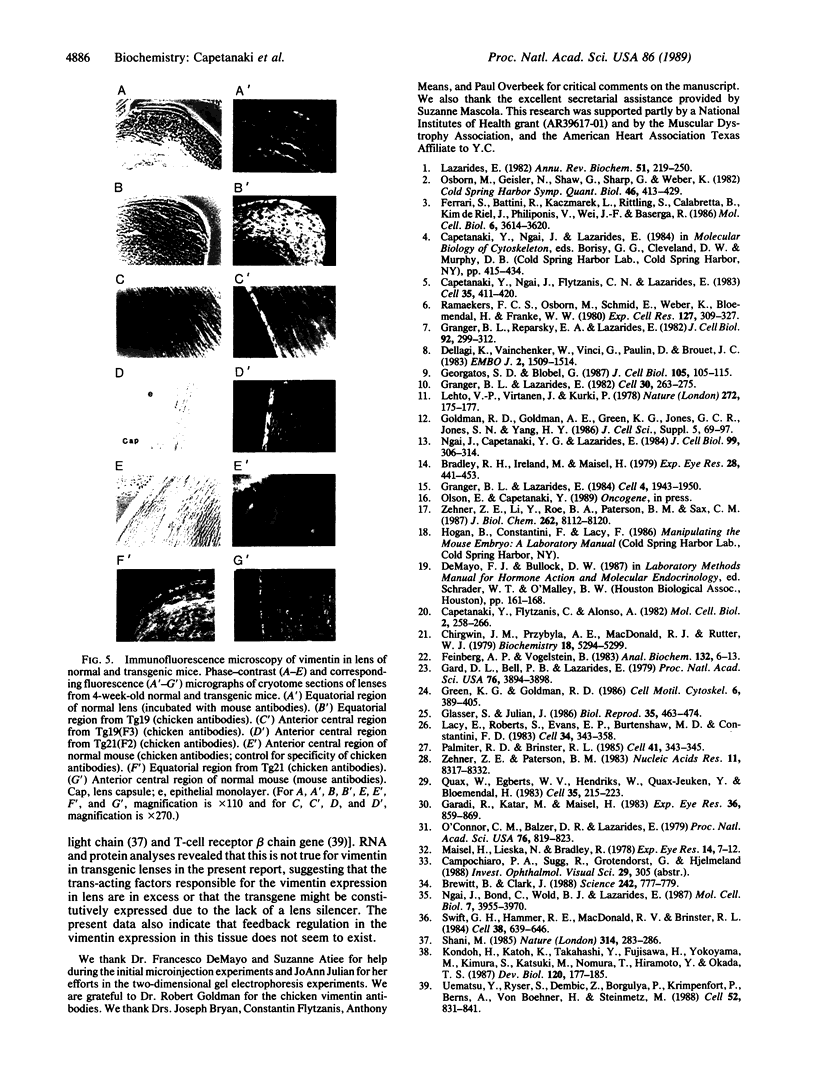
To study expression and function of the vimentin gene, transgenic mice were generated by microinjecting the entire chicken gene plus 2.4 kilobases of 5' and 2.6 kilobases of 3' flanking sequences. All the transgenic mice obtained had incorporated multiple copies of the gene. RNA analyses demonstrated that the chicken vimentin gene was efficiently expressed in an appropriate tissue-specific pattern and that the transcripts were properly processed, as in chicken, giving rise to two RNAs. The vimentin transgene was predominantly expressed in lens at levels of up to 10-fold the endogenous level in every transgenic line studied. The chicken vimentin transcripts were efficiently translated into polypeptides that were modified posttranslationally and could assemble into the mouse cytoskeleton. Overexpression of the chicken vimentin gene did not obviously affect the expression of the endogenous gene at the RNA or the protein level. Immunofluorescence microscopy further demonstrated that the chicken protein was properly expressed spatially in lens. However, the levels were much higher in the transgenic animals.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley R. H., Ireland M., Maisel H. The cytoskeleton of chick lens cells. Exp Eye Res. 1979 Apr;28(4):441–453. doi: 10.1016/0014-4835(79)90119-2. [DOI] [PubMed] [Google Scholar]
- Brewitt B., Clark J. I. Growth and transparency in the lens, an epithelial tissue, stimulated by pulses of PDGF. Science. 1988 Nov 4;242(4879):777–779. doi: 10.1126/science.3187521. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. G., Flytzanis C. N., Alonso A. Repression of the albumin gene in Novikoff hepatoma cells. Mol Cell Biol. 1982 Mar;2(3):258–266. doi: 10.1128/mcb.2.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Flytzanis C. N., Lazarides E. Tissue-specific expression of two mRNA species transcribed from a single vimentin gene. Cell. 1983 Dec;35(2 Pt 1):411–420. doi: 10.1016/0092-8674(83)90174-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dellagi K., Vainchenker W., Vinci G., Paulin D., Brouet J. C. Alteration of vimentin intermediate filament expression during differentiation of human hemopoietic cells. EMBO J. 1983;2(9):1509–1514. doi: 10.1002/j.1460-2075.1983.tb01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garadi R., Katar M., Maisel H. Two-dimensional gel analysis of chick lens proteins. Exp Eye Res. 1983 Jun;36(6):859–869. doi: 10.1016/0014-4835(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Bell P. B., Lazarides E. Coexistence of desmin and the fibroblastic intermediate filament subunit in muscle and nonmuscle cells: identification and comparative peptide analysis. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3894–3898. doi: 10.1073/pnas.76.8.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Two distinct attachment sites for vimentin along the plasma membrane and the nuclear envelope in avian erythrocytes: a basis for a vectorial assembly of intermediate filaments. J Cell Biol. 1987 Jul;105(1):105–115. doi: 10.1083/jcb.105.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser S. R., Julian J. Intermediate filament protein as a marker of uterine stromal cell decidualization. Biol Reprod. 1986 Sep;35(2):463–474. doi: 10.1095/biolreprod35.2.463. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Goldman A. E., Green K. J., Jones J. C., Jones S. M., Yang H. Y. Intermediate filament networks: organization and possible functions of a diverse group of cytoskeletal elements. J Cell Sci Suppl. 1986;5:69–97. doi: 10.1242/jcs.1986.supplement_5.5. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Expression of the intermediate-filament-associated protein synemin in chicken lens cells. Mol Cell Biol. 1984 Oct;4(10):1943–1950. doi: 10.1128/mcb.4.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Structural associations of synemin and vimentin filaments in avian erythrocytes revealed by immunoelectron microscopy. Cell. 1982 Aug;30(1):263–275. doi: 10.1016/0092-8674(82)90032-0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Repasky E. A., Lazarides E. Synemin and vimentin are components of intermediate filaments in avian erythrocytes. J Cell Biol. 1982 Feb;92(2):299–312. doi: 10.1083/jcb.92.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Goldman R. D. Evidence for an interaction between the cell surface and intermediate filaments in cultured fibroblasts. Cell Motil Cytoskeleton. 1986;6(4):389–405. doi: 10.1002/cm.970060405. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Katoh K., Takahashi Y., Fujisawa H., Yokoyama M., Kimura S., Katsuki M., Saito M., Nomura T., Hiramoto Y. Specific expression of the chicken delta-crystallin gene in the lens and the pyramidal neurons of the piriform cortex in transgenic mice. Dev Biol. 1987 Mar;120(1):177–185. doi: 10.1016/0012-1606(87)90116-3. [DOI] [PubMed] [Google Scholar]
- Lacy E., Roberts S., Evans E. P., Burtenshaw M. D., Costantini F. D. A foreign beta-globin gene in transgenic mice: integration at abnormal chromosomal positions and expression in inappropriate tissues. Cell. 1983 Sep;34(2):343–358. doi: 10.1016/0092-8674(83)90369-0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978 Mar 9;272(5649):175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- Maisel H., Perry M. M. Electron microscope observations on some structural proteins of the chick lens. Exp Eye Res. 1972 Jul;14(1):7–12. doi: 10.1016/0014-4835(72)90136-4. [DOI] [PubMed] [Google Scholar]
- Ngai J., Bond V. C., Wold B. J., Lazarides E. Expression of transfected vimentin genes in differentiating murine erythroleukemia cells reveals divergent cis-acting regulation of avian and mammalian vimentin sequences. Mol Cell Biol. 1987 Nov;7(11):3955–3970. doi: 10.1128/mcb.7.11.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai J., Capetanaki Y. G., Lazarides E. Differentiation of murine erythroleukemia cells results in the rapid repression of vimentin gene expression. J Cell Biol. 1984 Jul;99(1 Pt 1):306–314. doi: 10.1083/jcb.99.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. M., Balzer D. R., Jr, Lazarides E. Phosphorylation of subunit proteins of intermediate filaments from chicken muscle and nonmuscle cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):819–823. doi: 10.1073/pnas.76.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Quax W., Egberts W. V., Hendriks W., Quax-Jeuken Y., Bloemendal H. The structure of the vimentin gene. Cell. 1983 Nov;35(1):215–223. doi: 10.1016/0092-8674(83)90224-6. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Osborn M., Schimid E., Weber K., Bloemendal H., Franke W. W. Identification of the cytoskeletal proteins in lens-forming cells, a special epitheloid cell type. Exp Cell Res. 1980 Jun;127(2):309–327. doi: 10.1016/0014-4827(80)90437-1. [DOI] [PubMed] [Google Scholar]
- Shani M. Tissue-specific expression of rat myosin light-chain 2 gene in transgenic mice. Nature. 1985 Mar 21;314(6008):283–286. doi: 10.1038/314283a0. [DOI] [PubMed] [Google Scholar]
- Swift G. H., Hammer R. E., MacDonald R. J., Brinster R. L. Tissue-specific expression of the rat pancreatic elastase I gene in transgenic mice. Cell. 1984 Oct;38(3):639–646. doi: 10.1016/0092-8674(84)90258-7. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Ryser S., Dembić Z., Borgulya P., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988 Mar 25;52(6):831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Li Y., Roe B. A., Paterson B. M., Sax C. M. The chicken vimentin gene. Nucleotide sequence, regulatory elements, and comparison to the hamster gene. J Biol Chem. 1987 Jun 15;262(17):8112–8120. [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Vimentin gene expression during myogenesis: two functional transcripts from a single copy gene. Nucleic Acids Res. 1983 Dec 10;11(23):8317–8332. doi: 10.1093/nar/11.23.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]