Abstract
PTK7 is an essential component of the Wnt/planar cell polarity (PCP) pathway. We provide evidence that the Wnt/PCP pathway converges with pericellular proteolysis in both normal development and cancer. Here, we demonstrate that membrane type-1 matrix metalloproteinase (MT1-MMP), a key proinvasive proteinase, functions as a principal sheddase of PTK7. MT1-MMP directly cleaves the exposed PKP621↓LI sequence of the seventh Ig-like domain of the full-length membrane PTK7 and generates, as a result, an N-terminal, soluble PTK7 fragment (sPTK7). The enforced expression of membrane PTK7 in cancer cells leads to the actin cytoskeleton reorganization and the inhibition of cell invasion. MT1-MMP silencing and the analysis of the uncleavable L622D PTK7 mutant confirm the significance of MT1-MMP proteolysis of PTK7 in cell functions. Our data also demonstrate that a fine balance between the metalloproteinase activity and PTK7 levels is required for normal development of zebrafish (Danio rerio). Aberration of this balance by the proteinase inhibition or PTK7 silencing results in the PCP-dependent convergent extension defects in the zebrafish. Overall, our data suggest that the MT1-MMP-PTK7 axis plays an important role in both cancer cell invasion and normal embryogenesis in vertebrates. Further insight into these novel mechanisms may promote understanding of directional cell motility and lead to the identification of therapeutics to treat PCP-related developmental disorders and malignancy.
Keywords: Breast Cancer, Cell Surface Receptor, Metalloprotease, Proteolytic Enzymes, Wnt Pathway, Zebrafish, MT1-MMP, PTK7
Introduction
Secreted Wnt glycoproteins regulate β-catenin-dependent (canonical) and β-catenin-independent (non-canonical) signaling pathways (1–7). One intriguing and well conserved function of the non-canonical pathway is to control PCP2 and directional cell motility (8). PCP governs the orientation of cells in a monolayer of a tissue plane (front-back orientation) in such a way that all cells within the monolayer are aligned in the same direction. As a result, PCP is important for the directed collective cell movements and orchestrates the synchronized cell arrangements within the tissue plane in the course of a plethora of biological processes (2, 4, 6–11).
The first PCP signaling events occur at a gastrulation stage of embryogenesis to regulate the polarized cell movement and accomplish convergent extension (CE) for the anterior-posterior body axis elongation, neural tube closure, and craniofacial morphogenesis (8, 9, 11, 12). CE failure results in the multiple severe developmental defects, including a shortened body axis (dwarfism), defective neural system, and craniofacial abnormalities. Defects in the non-canonical Wnt/PCP pathway are linked to a broad range of diseases, including cancer (3, 5). Wnt5a, Wnt5b, and Wnt11, which work through the non-canonical pathway, are often up-regulated in cancer and promote cancer cell motility and invasion (6, 13). Evidently, an in depth mechanistic understanding of the PCP mechanism and its aberrant regulation in disease is required to control tumor progression and metastasis in a clinically advantageous manner (6).
Human PTK7 pseudokinase (also known as colon carcinoma kinase-4, CCK-4) is required for PCP and CE (14–16). The full-length membrane PTK7 receptor consists of seven extracellular Ig domains, a transmembrane region, and a catalytically inert cytoplasmic tyrosine kinase (PTK) domain (17–20). PTK7 is evolutionary conserved, and its orthologs include mouse PTK7, chicken KLG, Drosophila Dtrk/Off-track (OTK), and Hydra Lemon (21). PTK7 mutant mice that expressed a 1–114 PTK7 truncation died perinatally with severe defects in neural tube closure, a CE process (16). Overexpression of the mutant PTK7 lacking its cytoplasmic domain resulted in similar abnormalities. An N-terminal, soluble PTK7 fragment (sPTK7) inhibited angiogenesis in vitro and in vivo in a dominant negative fashion by competing with the full-length PTK7 (22). The expression of PTK7 is frequently deregulated in cancers (4, 23–25).
Directional cell locomotion is highly dependent on both well orchestrated actin cytoskeleton dynamics and efficient pericellular proteolysis (26, 27). Proinvasive, promigratory MT1-MMP (MMP-14), a prototypic member of the MMP family, is a major mediator of pericellular proteolytic events in cancer cells (28). MT1-MMP cleaves ECM proteins, initiates activation of soluble MMPs, and controls the functionality of cell adhesion and signaling receptors. MT1-MMP is a prototypic member of a membrane-anchored MMP subfamily and is distinguished from soluble MMPs by a C-terminal transmembrane domain and a cytoplasmic tail (29–31). MT1-MMP is synthesized as a latent zymogen that requires proteolytic processing of the N-terminal inhibitory prodomain (32, 33). Once activated, MT1-MMP can be inhibited by its physiological inhibitors, tissue inhibitors of metalloproteinases-2, -3, and -4 (TIMP-2, -3, and -4). In contrast, TIMP-1 is a poor inhibitor of MT1-MMP (34, 35).
MT1-MMP, as opposed to the soluble MMPs, is ideally positioned to regulate pericellular proteolysis and the functionality of cell receptors (36). In migrating cells, MT1-MMP accumulates predominantly at the leading and trailing edges and, as a result, contributes most efficiently to cell locomotion (37–39).
Knock-out of MT1-MMP has the most significant phenotype among MMP gene knock-out mice; MT1-MMP knock-out mice are dwarfs and die at adulthood (40, 41). Likewise, a loss of the structurally similar primordial At2-MMP induces dwarfism in Arabidopsis plants (42).
Recent studies link MT1-MMP to the non-canonical Wnt/PCP pathway in embryogenesis and cancer (27, 43). Both transcriptional silencing and enforced overexpression of MT1-MMP negatively impacted CE and craniofacial morphogenesis in zebrafish, suggesting that a stringent control of MT1-MMP activity is essential in normal development (27, 44, 45). The molecular mechanisms involved in the MT1-MMP-dependent regulation of the non-canonical Wnt/PCP signaling pathway, however, remain elusive. Intriguingly, co-expression data of 19,777 human and 21,036 mouse genes from the COEXPRESdb database indicate that MT1-MMP and PTK7 are closely co-expressed.
Here, we provide evidence that the full-length membrane PTK7 affects actin cytoskeleton and inhibits cancer cell invasion. Our results demonstrate that MT1-MMP directly cleaves the full-length membrane PTK7, that this cleavage generates the sPTK7 species, and, most importantly, that this proteolytic event is ubiquitous in multiple cell systems. Taken together, our experimental data suggest that the pericellular proteolysis and PCP mechanisms converge in the regulation of directional cell migration and that they work in concert in processes as diverse as embryogenesis and malignancy.
MATERIALS AND METHODS
Antibodies, Reagents, and Cells
A rabbit polyclonal antibody against the N-terminal portion of PTK7 was a kind gift of Dr. Xiaowei Lu (University of Virginia, Charlottesville, VA). A goat polyclonal antibody (catalog no. AF4499) against the N-terminal 31–199 portion of PTK7 was from R&D. A murine monoclonal 3G4 antibody (catalog no. MAB1767) against the catalytic domain of MT1-MMP, a rabbit AB8104 antibody to the hinge domain of MT1-MMP, and the GM6001 hydroxamate inhibitor were from Chemicon. A murine monoclonal antibody to the V5 tag was from Invitrogen. A murine monoclonal FLAG M2 antibody, the FLAG M2 antibody-agarose beads, and a polyclonal rabbit TGN46 antibody (catalog no. T7576) were from Sigma. The phosphomyosin light chain 2 (Ser19) rabbit polyclonal antibody (catalog no. 3671) was from Cell Signaling. Rhotekin-RBD agarose beads and a RhoA monoclonal antibody (catalog no. ARH01) were from Cytoskeleton. EZ-Link sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate was from Pierce. Human fibrosarcoma HT1080, breast carcinoma MCF7, mammary epithelial MCF10A and 184B5 cells, and Madin-Darby canine kidney (MDCK) cells were from ATCC (Manassas, VA). A highly metastatic M4A4 clone of breast carcinoma MDA-MB-435 cells was a gift of Dr. Virginia Urquidi (University of California, San Diego, CA). MCF10A and 184B5 cells were grown in mammary epithelial growth medium (Lonza). Other cell lines were grown in DMEM supplemented with 10% FBS. The recombinant catalytic domain of MT1-MMP was characterized earlier (46). The CD44 (catalog no. 3570) and E-cadherin (catalog no. 610181) monoclonal antibodies were obtained from Cell Signaling and BD Biosciences, respectively.
Cloning and Mutagenesis
MCF7, HT1080, 184B5, MDA-MB-435, and MDCK cells transfected with the full-length MT1-MMP (MCF7-MT1, HT1080-MT1, 184B5-MT1, MDA-MB-435-MT1, and MDCK-MT1 cells, respectively) were established and characterized earlier (47, 48). HT1080 cells with the over 90% transcriptional silencing of MT1-MMP and the required scrambled controls were obtained and extensively characterized earlier (47, 49, 50). The full-length wild-type PTK7 cDNA (OriGene) was amplified by the PCR using the selective PTK7 primers. The construct was subcloned into the pcDNA3.1D/V5-His-TOPO directional TOPO expression vector (Invitrogen). Where indicated, the PTK7 construct was C-terminally tagged with the V5-His and FLAG tags. The full-length PTK7-FLAG template was used to generate the L622D, M641R, and M701D mutants and the sPTK7 1–700 constructs. The primers we used in our experiments are shown in Table 1. The PTK7 constructs were used to stably transfect HT1080, MCF7, MDCK, MDA-MB-435, and MDA-MB-435-MT1 cells using Lipofectamine 2000 (Invitrogen). The full-length and the soluble PTK7 constructs were also transfected into MDCK-MT1 cells to generate the doubly transfected MDCK-MT1-PTK7 and MDCK-MT1-sPTK7 cells, respectively. HT1080 stably transfected with MT6-MMP were used as an additional control in our study (51).
TABLE 1.
Oligonucleotide primers used in our study
| Construct | Forward primer | Reverse primer |
|---|---|---|
| PTK7 | 5′-CACCATGGGAGCTGCGCGGGGATC-3′ | 5′-TCACGGCTTGCTGTCCAC-3′ |
| PTK7-V5-His | 5′-CACCATGGGAGCTGCGCGGGGATC-3′ | 5′-CGGCTTGCTGTCCAC-3′ |
| PTK7-FLAG | 5′-CACCATGGGAGCTGCGCGGGGATC-3′ | 5′-TCACTTGTCATCGTCGTCCTTGTAGTCCGGCTTGCTGTCCACGGTGC-3′ |
| sPTK7 | 5′-CACCATGGGAGCTGCGCGGGGATC-3′ | 5′-TCACTTGTAGGGGGGAGGGCTGCCAG-3′ |
| sPTK7-V5-His | 5′-CACCATGGGAGCTGCGCGGGGATC-3′ | 5′-CTTGTAGGGGGGAGGGCTGCCAG-3′ |
| sPTK7-FLAG | 5′-CACCATGGGAGCTGCGCGGGGATC-3′ | 5′-TCACTTGTCATCGTCGTCCTTGTAGTCCTTGTAGGGGGGAGGGCTGCCAG-3′ |
| L622D | 5′-GGGGACCCCAAGCCGGATATTCAGTGGAAAGGC-3′ | 5′-GCCTTTCCACTGAATATCCGGCTTGGGGTCCCC-3′ |
| M641R | 5′-CCAAGCTGGGACCCAGGCGGCAC-3′ ATCTTCC | 5′-GGAAGATGTGCCGCCTGGGTCCCAGCTTGG |
| M701D | 5′-CCTCCCCCCTACAAGGATATCCAGACCATTGGG-3′ | 5′-CCCAATGGTCTGGATATCCTTGTAGGGGGGAGG-3′ |
| Zebrafish PKT7 | 5′-GCGACCACAACATCACACTC-3′ | 5′-TCCATCACTCAGCTCAGCAC-3′ |
| Zebrafish PKT7 | 5′-GGATCAACAGTGCTGAGCTG-3′ | 5′-CAGACTCTTGACCAGCACCA-3′ |
Cell Surface Biotinylation, Two-dimensional PAGE, and Protein Identification by LC/MS/MS
Cell surface proteins were biotinylated by incubating cells for 1 h on ice in PBS containing 0.1 mg/ml EZ-Link sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate. Cells were lysed in 20 mm Tris-HCl, 150 mm NaCl, 1% deoxycholate, 1% IGEPAL, pH 7.4, supplemented with a protease inhibitor mixture set III (Sigma), 1 mm phenylmethylsulfonyl fluoride, and 10 mm EDTA. Biotinylated proteins were precipitated from cell lysates with streptavidin-agarose beads (Sigma). Biotinylated proteins were eluted from the beads using 50 mm DTT. The samples were then alkylated using a ReadyPrep reduction-alkylation kit (Bio-Rad) and separated by two-dimensional PAGE using a PROTEAN II xi Cell (Bio-Rad). The gels were stained with SimplyBlue SafeStain (Invitrogen). The gel images were analyzed with ImageJ software (National Institutes of Health). The individual stained spots were excised from the gel and subjected to in-gel trypsin digestion with Trypsin Gold, mass spectrometry grade (Promega). The digest samples were analyzed by LC/MS/MS using an LTQ XL linear ion trap mass spectrometer (Thermo Scientific). MS/MS spectra were searched against the Swiss-Prot data base using SEQUEST Sorcerer software. The peptides with a probability score of >0.95 and a cross-correlation (Xcorr) value of >2.0 were further analyzed and annotated.
MT1-MMP Proteolysis of PTK7 in Vitro
The biotinylated plasma membrane proteins from MCF7 cells (1 × 106) were captured on streptavidin-agarose beads (20 μl of a 50% slurry) and co-incubated for the indicated time at 37 °C with the recombinant catalytic domain of MT1-MMP (20 nm) in 100 μl of 50 mm HEPES, pH 6.8, containing 10 mm CaCl2, 0.5 mm MgCl2, and 50 μm ZnCl2. The digests were analyzed by Western blotting with the PTK7, CD44, and E-cadherin antibodies.
Modeling of the PTK7 Structure
The three-dimensional structure of PTK7 was modeled by threading its sequence on the known structures of the homologues using the program MODWEB (52, 53). The first six Ig domains of PTK7 (residues 28–588) were built using Protein Data Bank entry 3B43 (titin) as a template. The seventh Ig domain of PTK7 (residues 594–684) was built from the fragment of Protein Data Bank entry 2DM7 (Kiaa1556, residues 22–105, sequence identity 38%). The transmembrane region of PTK7 (residues 703–778) was built using Protein Data Bank entry 1SYS (Hla), and the PTK7 kinase domain (residues 789–1072) was built using a fragment of Protein Data Bank entry 2BDF (Src, residues 258–525, sequence identity 38%). The modeled fragments of PTK7 were merged together and visualized by using PyMOL software (DeLano Scientific).
Immunofluorescence
Cells grown on a glass coverslip were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, and then blocked in 1% casein for 1 h. Cells were stained with the primary antibodies (dilution 1:1,000) for 16 h at 4 °C, followed by staining with the secondary antibodies conjugated with AlexaFluor 488 or AlexaFluor 594 (Molecular Probes; dilution 1:500) for 2 h at ambient temperature. The coverslips were mounted in the Vectashield mounting medium with DAPI (Vector Laboratories). Images were acquired on an Olympus BX51 fluorescence microscope equipped with a MagnaFire digital camera and MagnaFire 2.1C software (Olympus).
Rho Activation Assay
The Rho activation assay kit (Cytoskeleton) was used to estimate the cellular GTP-bound RhoA. Briefly, the active, GTP-bound RhoA was precipitated from the cell lysate using rhotekin-RBD beads. The precipitated RhoA was analyzed by Western blotting with a RhoA monoclonal antibody.
Collagen Gel Contraction Assay
Cells (1 × 105) were mixed with 0.2 ml of type I collagen in DMEM (0.3 mg/ml) on ice and placed in wells of a 24-well low adhesion cell culture plate. After gel polymerization at 37 °C for 30 min, the growth medium (0.5 ml) was added to the wells. Following a 24-h incubation, the gel images were taken using a digital camera.
Invasion Assay
The invasion assay was performed in wells of a 24-well Transwell plate with an 8-μm pore size membrane (48). The membranes of Transwell inserts were coated with type I collagen (2.5 μg/well; BD Bioscience). Cells (1 × 105/well) were placed in serum-free DMEM (0.1 ml) into the upper chamber. The 10% FBS-containing DMEM (used as a chemoattractant, 0.6 ml) was placed in the lower chamber. Serum-free DMEM (0.6 ml) was used as a control. Cells were allowed to invade for 6 h. The cells were then stained for 10 min with 0.2% crystal violet in 20% methanol (0.3 ml). The cells on the upper membrane surface were removed with a cotton swab. The dye from the cells that migrated onto the membrane's lower surface was extracted with 1% SDS (0.25 ml). The resulting A570 nm was measured using a SpectraFluor Plus plate reader (Tecan). The assays were run in triplicate in three independent experiments.
Immunohistochemistry
Breast cancer tissue arrays BR962 were from US Biomax. After deparaffinization and antigen retrieval, the arrays were stained using the goat PTK7 polyclonal antibody and the MT1-MMP 3G4 monoclonal antibody, followed by the staining with the secondary antibodies conjugated with AlexaFluor 488 or AlexaFluor 594.
Zebrafish Maintenance, Treatment with Inhibitors and Morpholino Injection
The zebrafish (AB strain) were maintained under the standard laboratory conditions at 28.5 °C. One-cell stage embryos were collected from natural matings. GM6001 (100 μm) was added to the one-cell stage embryos and replaced twice daily. MT1-MMP (MMP14a and MMP14b) and PTK7 morpholinos 5′-GACGGTACTCAAGTCGGGACACAAA-3′ and 5′-GAACCCGCTCCAGATCATTTTTCGC-3′ (MT1-MMP) and 5′-GCTTGCTCTTGCTCTCTCCCGGCAT-3′ (PTK7) were from Gene Tools. The morpholinos were microinjected at a one-cell stage (2–10 ng/embryo). To examine protein expression, the embryos were lysed in 20 mm Tris-HCl, 150 mm NaCl, 1% deoxycholate, 1% IGEPAL, pH 7.4, supplemented with a protease inhibitor mixture set III (Sigma), 1 mm phenylmethylsulfonyl fluoride, and 10 mm EDTA. The lysates were analyzed by Western blotting with the goat PTK7 antibody (R&D Systems).
Whole Mount in Situ Hybridization
The in situ hybridization of zebrafish embryos using the antisense RNA probe myoD was performed following the protocol described previously (54).
The Zebrafish PTK7 cDNA Sequence and RT-PCR
Five 48-h-old embryos were homogenized in 1 ml of TRIzol (Invitrogen) by passing through a 20-gauge needle. Total RNA samples were extracted from the lysates and then purified using the RNA miniprep columns (Zymo Research). First-strand cDNA was synthesized using the purified RNA samples (1 μg), SuperScript II reverse transcriptase (Invitrogen), and a random primer (100 ng). The forward and reverse primers for the PCR amplification of the zebrafish Ptk7 cDNA fragments (5′-GCGACCACAACATCACACTC-3′ and 5′-TCCATCACTCAGCTCAGCAC-3′, respectively, and 5′-GGATCAACAGTGCTGAGCTG-3′ and 5′-CAGACTCTTGACCAGCACCA-3′, respectively) were designed using Primer 3 software (55). These primer sets were also used for the RT-PCR. The amplification reactions (25 μl) included the cDNA (50 ng) and the respective primers (0.6 μm). PCRs (30 cycles) were performed using denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 1 min. The products were separated by 2% agarose gel-electrophoresis. Specific PCR products were purified from the gels using a gel extraction kit (Qiagen). Both the sense and antisense cDNA strands were sequenced to obtain the nucleotide sequence of PTK7. As a result, the 1782-bp cDNA sequence of zebrafish PTK7 was assembled and deposited in GenBankTM (accession number GU211905).
RESULTS
PTK7 Is a Proteolytic Target of MT1-MMP
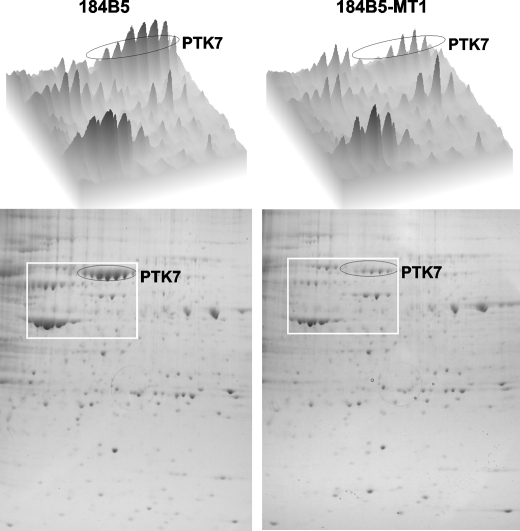
To search for novel cell surface cleavage targets of MT1-MMP, we compared two-dimensional gel profiles of biotin-labeled plasma membrane proteins from normal mammary 184B5 epithelial cells with those from 184B5 cells stably transfected with MT1-MMP (184B5-MT1 cells) (Fig. 1). The identity of the protein spots was determined using LC/MS/MS. We readily detected reduced levels of PTK7 in 184B5-MT1 cells. We then determined PTK7 levels in several normal and cancer cell lines of a diversified tissue origin (namely 184B5, MCF10A, MCF7, MDA-MB-435, HT1080, and MDCK) (Fig. 2A).
FIGURE 1.
MT1-MMP cleaves PTK7. Biotin-labeled plasma membrane proteins from normal mammary epithelial 184B5 cells transfected with MT1-MMP (184B5-MT1 cells) and untransfected control cells were separated by two-dimensional gel electrophoresis (bottom panels). The densitometry profile of the selected region (square) is shown in the top panels. The identity of PTK7 (circled) was confirmed by LC/MS/MS.
FIGURE 2.
MT1-MMP directly cleaves the full-length membrane PTK7 and generates sPTK7. A, biotin-labeled plasma membrane proteins from normal mammary 184B5 and MCF10A epithelial cells, non-invasive breast carcinoma MCF-7, metastatic breast carcinoma MDA-MB-435, and MT1-MMP-transfected cells (184B5-MT1 and MDA-MB-435-MT1) were analyzed by immunoblotting with the PTK7 antibody. B, GM6001 and TIMP-2 (but not TIMP-1) inhibit MT1-MMP proteolysis of membrane PTK7. Biotin-labeled plasma membrane proteins from MCF7 and MCF7-MT1 cells were analyzed by immunoblotting with the PTK7 antibody. C, sPTK7 is released by MT1-MMP-transfected cells (HT1080-MT1) but not by MT6-MMP-transfected HT1080-MT6 cells. D, MT1-MMP cleaves PTK7 more efficiently than it does E-cadherin and CD44. Biotin-labeled plasma membrane proteins from MCF7 cells were co-incubated with the recombinant catalytic domain of MT1-MMP. Samples were analyzed by immunoblotting with PTK7, CD44, and E-cadherin antibodies. The right lanes show full-length PTK7 and soluble, N-terminal sPTK7 fragment controls (from MCF7-PTK7 and MCF7-sPTK7 cells, respectively). E, the N-terminal PTK7 fragment is present in both MDCK cells that co-express full-length PTK7 and MT1-MMP constructs (MDCK-MT1-PTK7 cells) and MDCK cells that co-express MT1-MMP with the soluble PTK7 construct (MDCK-MT1-sPTK7 cells). Where indicated, GM6001 was added to the cells.
Normal 184B5 and MCF10A mammary cells as well as non-invasive breast carcinoma MCF7 cells exhibited high levels of full-length membrane PTK7. In contrast, PTK7 levels were low in highly metastatic, invasive breast carcinoma MDA-MB-435 cells and in 184B5 and MCF7 cells in which MT1-MMP had been overexpressed (184B5-MT1 and MCF7-MT1 cells, respectively). Treatment of cells with GM6001 (a wide range hydroxamate MMP inhibitor) or tissue inhibitor of metalloproteinases-2 (TIMP-2; a potent MT1-MMP inhibitor) increased levels of full-length PTK7 in MCF7-MT1 cells to those observed in MT1-MMP-deficient MCF7 cells. TIMP-1 (an inefficient MT1-MMP inhibitor) had no effect (Fig. 2B). MT1-MMP activity was correlated with the presence of sPTK7 in the medium. Expression of the lipid raft-associated MT6-MMP in cells did not promote a similar effect (Fig. 2C). sPTK7 was also detected in plasma membrane samples, suggesting that sPTK7 and full-length PTK7 interact (Fig. 2E).
In agreement with our two-dimensional gel profiling data, PTK7 was highly sensitive to MT1-MMP proteolysis in vitro, especially when compared with well known targets of MT1-MMP, such as CD44 and E-cadherin (56, 57) (Fig. 2D). Taken together, our results indicate that PTK7 is a major target of MT1-MMP.
MT1-MMP Directly Cleaves the PKP621↓LI Site of PTK7
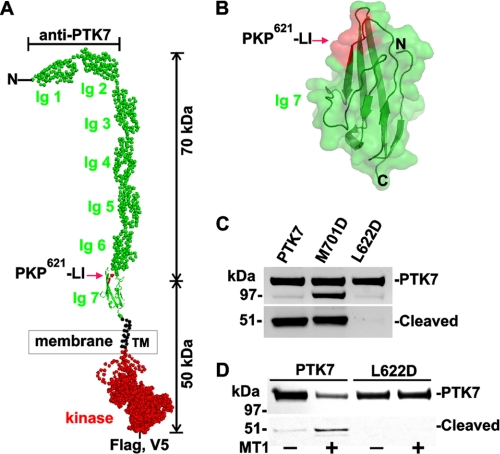
We next determined the identity of the cleavage site and the cleavage events that result in sPTK7. To predict PTK7 cleavage site(s) that could lead to sPTK7 generation, we analyzed the region encompassing PTK7 amino acids 600–710 for potential MMP cleavage sites using software we developed (58) (Table 2). We identified three potential MT1-MMP cleavage sites (PKP621↓LI, PRM641↓HI, and PYK700↓MI) in this region. Corresponding L622D, M641R, and M701D PTK7 point mutants with inactivated cleavage sites were generated, and the constructs were expressed in fibrosarcoma HT1080 cells, which express significant levels of endogenous MT1-MMP (59).
TABLE 2.
Potential MT1-MMP cleavage sites in the 600–710 PTK7 sequence region as predicted by the positional weight matrix approach
The high score directly correlates with the high cleavage probability. PWM, positional weight matrix.
| Cleavage site | Residues | PWM score | Mutant site | PWM score |
|---|---|---|---|---|
| PKP↓LI | 621–622 | 4.463 | PKP↓DI | −1.32 |
| PRM↓HI | 641–642 | 3.006 | PRR↓HI | −2.08 |
| PYK↓MI | 700–701 | 2.731 | PYK↓DI | −1.14 |
To support our results, mutants were also transfected into MDA-MB-435 cells and MT1-MMP-expressing MDA-MB-435-MT1 cells. Our analysis determined that the L622D mutant was fully resistant to MT1-MMP proteolysis in both HT1080 and MDA-MB-435-MT1 cells. Structural modeling suggests that the PKP621↓LI cleavage site is localized in the exposed region of the seventh Ig-like domain of PTK7, and, as a result, it is probably accessible to proteolysis (Fig. 3).
FIGURE 3.
MT1-MMP cleaves the PKP621↓LI site in the full-length PTK7 sequence. A, a structure model of full-length PTK7. Ig domains 1–7 and the transmembrane and kinase domains are shown in green, black, and red, respectively. Cleavage of the PKP621↓LI site (MMP, red arrow) generates the N-terminal, 70-kDa sPTK7 and the C-terminal, 50-kDa membrane-tethered fragment. Anti-PTK7, -FLAG, and -V5 staining shows localization of the respective epitopes. B, a surface model of the Ig 7 domain. The PKP621↓LI site (red) is in the exposed region and is therefore accessible to proteolysis. C, the L622D PTK7 mutant is resistant to MT1-MMP proteolysis in HT1080 cells, which express endogenous MT1-MMP. D, the L622D PTK7 mutant is resistant to MT1-MMP proteolysis in MDA-MB-435-MT1 cells in which MT1-MMP is overexpressed.
MT1-MMP and PTK7 Co-localize in the Cells
The observed proteolysis of PTK7 by MT1-MMP suggests that the proteinase and the kinase are proximal to each other in cells. As predicted, MT1-MMP and PTK7 co-localized at cell-cell junctions in all cell types analyzed, including 184B5 cells, which co-express endogenous MT1-MMP and PTK7. Enforced expression of MT1-MMP in MDCK epithelial cells (MDCK-MT1 cells) resulted in reduced levels of full-length membrane PTK7 both at cell-cell junctions and at the leading edge of migrating cells (Fig. 4, A–C). The C-terminal fragment of PTK7 (anti-V5 staining) but not full-length PTK7 (anti-PTK7 staining), however, accumulates at the leading edge in migrating MDCK-MT1 cells. These observations indicate the enhanced proteolysis of membrane PTK7 by MT1-MMP which, normally, redistributes to the leading edge, and, as a result, MT1-MMP proteolysis contributes to the polarized localization of membrane PTK7 in migrating cells. Our findings support the role of PTK7 in cell migration and correlate with earlier observations by others who showed that PTK7 contributes to neural crest migration in Xenopus by recruiting Dishevelled (14). In agreement, Van Gogh-like 2, another important regulator of the non-canonical Wnt pathway, also co-localizes with MT1-MMP and redistributes toward the leading edge of the polarized human cancer cells (27).
FIGURE 4.
PTK7 co-localizes with MT1-MMP in cells. A, endogenous PTK7 (red) is localized at cell-cell junctions (arrowheads) in normal 184B5 mammary cells. Endogenous MT1-MMP (green) is seen at cell-cell junctions and at the cell leading edge (arrows). B, endogenous PTK7 immunoreactivity is reduced at cell-cell junctions in MDCK-MT1 cells. C, in MDCK-MT1 cells, levels of PTK7 with a C-terminal V5 tag are reduced at cell-cell junctions (arrows). The C-terminal fragment of PTK7 (anti-V5 staining) but not full-length PTK7 (anti-PTK7 staining) accumulates at the leading edge in migrating MDCK-MT1 cells (arrows). D, PTK7 immunoreactivity co-localizes with MT1-MMP and is reduced in breast cancer tissue relative to normal mammary tissue.
Previous reports indicate that PTK7 levels are inversely correlated with melanoma tumorigenicity (25). To further investigate the relationship between PTK7 and cancer, we analyzed PTK7 in breast cancer biopsies by immunostaining and observed reduced membrane PTK7 immunoreactivity in tumor lesions compared with normal mammary tissue. In the analyzed tumors, MT1-MMP and PTK7 were co-localized at cell-cell junctions (Fig. 4D).
MT1-MMP/PTK7 Axis Regulates the Actomyosin Contractility and Cell Invasion
Although previous reports indicate that PTK7 regulates PCP, the precise role of PTK7 in the non-canonical Wnt/PCP signaling is not known (14–17, 19). To determine such a role, we analyzed actin cytoskeleton organization, RhoA GTPase activation, and myosin light chain (MLC) phosphorylation (pMLC) in HT1080 cells transfected with MT1-MMP siRNA and PTK7 expression constructs. Expression of the full-length PTK7 and the uncleavable L622D PTK7 mutant altered the actin cytoskeleton, particularly in membrane ruffles, and reduced levels of pMLC compared with those seen in the parental HT1080 cells (Fig. 5, A and B). Similarly, silencing of MT1-MMP using siRNA decreased pMLC levels. Conversely, expression of sPTK7 alone dramatically stimulated RhoA activity in cells (Fig. 5C).
FIGURE 5.
PTK7 regulates the cytoskeleton and cell invasion. A, HT1080 cells transiently transfected with full-length PTK7 (green; asterisk indicates PTK7-expressing cells) rearrange the actin cytoskeleton (red). DNA, DAPI nuclear staining. B, phalloidin staining of the actin cytoskeleton in HT1080 cells transfected with the indicated PTK7 constructs. C, pMLC, activated RhoA (GTP-RhoA), MT1-MMP, and α-actin (a loading control). D, collagen gel contraction. E, cell invasion through a type 1 collagen matrix in HT1080 cells transfected with the indicated MT1-MMP and PTK7 constructs. FBS (10%) was used as a chemoattractant. The dotted line shows cell invasion without chemoattractant in serum-free medium (SF). *, p < 0.05 when compared with HT1080 cells in the presence of FBS. To facilitate the direct comparison of the resulting pMLC bands, equal amounts of the total protein (50 μg/lane) were analyzed in C. Similarly, equal amounts (1 mg of total protein) were used in the GTP-RhoA pull-down experiments shown in C. Error bars, S.D.
MLC phosphorylation is critical for actomyosin contractility, which, in turn, correlates with the ability of cells to contract three-dimensional collagen gels (60–62). In agreement, pMLC levels were positively correlated with the ability of cells to contract three-dimensional collagen gels in our cell system (Fig. 5D). Because MT1-MMP expression and alterations in actin cytoskeleton dynamics are known to be associated with cell invasion (63, 64), we next analyzed the invasive capacity of HT1080 cells that had been transfected with MT1-MMP siRNA and PTK7 constructs. Cells transfected with full-length PTK7 and, particularly, with the uncleavable L622D construct were significantly less invasive compared with HT1080 control cells, whereas overexpression of sPTK7 did not promote any significant effect. Conversely, PTK7 proteolysis by MT1-MMP reversed this inhibitory effect (Fig. 5E). We concluded that full-length PTK7 strongly represses cell invasion and that PTK7 levels might be inversely correlated with tumor aggressiveness and metastatic potential.
MT1-MMP/PTK7 Axis in the Zebrafish Embryogenesis
To confirm whether MT1-MMP regulates Wnt/PCP in diverse organisms, we used the zebrafish (Danio rerio) developmental model to analyze the role of the MT1-MMP/PTK7 axis in regulating CE movements, a non-canonical Wnt/PCP-dependent process, in embryos. PTK7 is reportedly required for PCP and CE in mouse and frog development (15, 16). Studies of non-canonical Wnt/PCP signaling in the zebrafish model are also well established (26, 65). Zebrafish PTK7 is already expressed at an early, 6 h postfertilization, stage of embryogenesis, and, therefore, it is present at the right time during gastrulation to govern CE (Fig. 6A). To evaluate whether human and zebrafish proteins could be similarly proteolytically processed, we sequenced zebrafish PTK7 cDNA (deposited in GenBankTM; accession number GU211905) and compared it with the human gene. We observed a high level of sequence homology between the zebrafish and human genes, including in the region encoding the human PKP621↓LI cleavage site (Fig. 6C), suggesting that PTK7 could be cleaved by MT1-MMP in both species.
FIGURE 6.
PTK7 expression in zebrafish embryos. A, RT-PCR analysis of PTK7 in embryo extracts using two selective primer pairs. beta-actin, loading control. B, treatment with specific morpholinos silenced PTK7 expression in zebrafish. Equal amount of total protein (50 μg) was loaded per lane. C, alignment of human and zebrafish PTK7 sequence. The MT1-MMP cleavage site (PKP621↓LI) is underlined in the human sequence.
Using morpholino knockdown approaches, we observed that PTK7 silencing (Fig. 6B) induced characteristic CE abnormalities in zebrafish embryos. These characteristic abnormalities included a short anterior-posterior body axis and a wide lateral axis in the zebrafish (Fig. 7, A and C). Treatment of embryos with the MMP hydroxamate inhibitors (GM6001 and AG3340) induced similar CE phenotypes (Fig. 7A) accompanied by accumulation of full-length PTK7 in the 2–3-day embryos (Fig. 7B). The developmental defects caused by GM6001 and AG3340 were similar to PCP and CE phenotypes reported by others following silencing of MT1-MMP in zebrafish (27). We also observed that MT1-MMP and PTK7 interact genetically; injections of low, subthreshold dosages of MT1-MMP and PTK7 morpholinos together caused a synergistic effect on the CE phenotype (Fig. 7D).
FIGURE 7.
MT1-MMP and PTK7 interact in zebrafish embryogenesis. A, MMP hydroxamate inhibitors or PTK7 morpholino (PTK MO) induce a CE phenotype in zebrafish. Day 5 embryos are shown. B, GM6001 causes accumulation of full-length PTK7 in embryos. An equal amount of total protein (50 μg) was loaded per lane. C, PTK7 regulates CE in zebrafish. Control and PTK7 morpholino embryos (10 h postfertilization) were stained with a myoD RNA probe to identify mesodermal tissues. The distance between bilateral adaxial cells is presented as a percentage relative to the intact control. D, synergistic effect of MT1-MMP and PTK7 in embryo development. Embryos received low doses of MT1-MMP (MMP-14a+b) and PTK7 morpholinos (2 ng/embryo) on Day 0. Day 3 embryos are shown. Error bars, S.D.
DISCUSSION
The importance of receptor shedding by MT1-MMP is well documented in cancer (56, 66–73). Our proteomics studies identified cellular PTK7 pseudokinase as a primary cleavage target of MT1-MMP and as a link to the Wnt/PCP pathway. Our subsequent studies confirmed that the full-length membrane PTK7 is most efficiently targeted by MT1-MMP, especially when compared with other receptors, including CD44. Cellular MT1-MMP functions as a principal sheddase of PTK7 and directly cleaves the exposed PKP621↓LI sequence of the seventh Ig-like domain of the full-length membrane PTK7. MT1-MMP proteolysis generates the C-terminal, membrane-tethered (50-kDa) and the N-terminal, soluble (70-kDa) fragments of PTK7. In turn, inactivation of the cleavage site generates the uncleavable PTK7 mutant (L622D) that is resistant to MT1-MMP proteolysis.
Because PTK7 is an essential component of the Wnt/PCP pathway, we analyzed the effect of the full-length membrane PTK7 and sPTK7 on the actin cytoskeleton, a downstream target of the Wnt/PCP signaling. The precise molecular mechanism of PTK7 signaling leading to the regulation of the actomyosin contraction is not yet understood; however, either Dishevelled (Dsh) or plexins may be interacting partners of PTK7 (14, 74, 75).
The availability of the cells transfected with the full-length, soluble, and uncleavable L622D PTK7 constructs allowed us to demonstrate that PTK7 affects the downstream events of the Wnt/PCP pathway and that the membrane full-length PTK7 and especially the uncleavable L622D mutant reorganize the actin cytoskeleton, repress the MLC phosphorylation, alter the actomyosin contraction, and inhibit cancer cell invasion. MT1-MMP silencing recapitulates the effects that are observed in the cells with the enforced expression of the L622D PTK7 mutant. Consistently, MT1-MMP proteolysis reverses the effects of the full-length PTK7 on cell functions.
The enforced expression of sPTK7 in HT1080 cells, however, significantly up-regulated RhoA activation, the upstream event of the Wnt/PCP pathway, rather than the downstream pMLC and actin reorganization. It is likely that the high preexisting levels of pMLC in the highly migratory HT1080 cells make any further increase of these parameters nearly impossible. As a result, the expression of MT1-MMP alone does not cause a noticeable effect on RhoA. In agreement, Rho-ROCK-myosin signaling mediates MT1-MMP-induced cellular aggregation of keratinocytes, but the overexpression of MT1-MMP itself does not result in a readily detectable increase of RhoA activation (76). MT1-MMP and Rho-ROCK activity and MLC phosphorylation were also demonstrated to play an important role in podosome formation and cell migration (16, 60, 76–78) and embryogenesis (26, 27, 79). Conversely, MT1-MMP silencing affected pMLC more noticeably, especially if compared with RhoA. Evidently, the effects of the multifunctional MT1-MMP on the net levels of pMLC and RhoA in the highly migratory HT1080 cells are more complex (80) than the MT1-MMP/PTK7 interactions alone and involve multiple parameters that are additional and distinct from PTK7.
Because of the presence of the link between PTK7 and MT1-MMP that we detected in cancer cells, we investigated the role of the PTK7/MT1-MMP axis in embryogenesis using the zebrafish (D. rerio) developmental model. Our results suggest that zebrafish PTK7 is already expressed as early as 6 h postfertilization and that PTK7 is present at the right time during gastrulation to govern CE in the zebrafish embryo. Both transcriptional silencing of PTK7 and inhibition of MT1-MMP activity, either by small molecule inhibitors or by transcriptional silencing, led to characteristic CE abnormalities, including a short anterior-posterior body axis (dwarfism) and a wide lateral axis in the zebrafish. In agreement, subthreshold dosages of MT1-MMP and PTK7 morpholinos together caused a synergistic effect on the CE phenotype and the developmental abnormalities in zebrafish.
Regardless of the opposing effect of PTK7 morpholino and small molecule MMP inhibitors on the levels of the full-length membrane PTK7, these treatments resulted in similar CE defects in the zebrafish embryos. It appears that both in cancer cells and normal embryogenesis, not the level of membrane PTK7 alone but also the well balanced ratio of the full-length PTK7 to sPTK7 plays an important role in regulating the overall effect of this pseudokinase on cell functions. In agreement, according to the findings of others, both overexpression and silencing of MT1-MMP resulted in a similar CE phenotype in zebrafish (27, 45). Taken together, our data suggest that the MT1-MMP/PTK7 axis plays an important role in normal embryogenesis in the course of gastrulation in zebrafish.
Our data in zebrafish correlate well with the role PTK7 plays in polarized cell motility and CE during mouse gastrulation (15). Thus, in embryos mutant for PTK7, the CE is severely affected. Although there is no alternative splicing in the murine PTK7 gene (18), the presence of the full-length, 140-kDa PTK7 and a PTK7 fragment that is similar to the sPTK7 species was reported in mice (16). Most excitingly, very recently an N-ethyl-N-nitrosourea-induced mutant, named chuzhoi (chz), has been reported in mice (44). The chz mutation resulted in the insertion of three amino acids (Ala-Asn-Pro) into the junction region between the fifth and the sixth Ig-like domains of PTK7. The Ala-Asn-Pro insertion did not change the membrane PTK7 levels in mice but led to the degradation of the sPTK7 species. The resulting imbalance between membrane PTK7 and sPTK7 led to characteristics consistent with defective CE, including a shortened body axis and multiple defects in heart, lung, and inner ear development. These observations agree very well with our results that we generated in cancer cells and zebrafish.
As a result, we believe that a fine balance between the protease and PTK7 is required for normal embryo development. In general, it is now highly likely that the MT1-MMP/PTK7 axis plays an essential role in embryogenesis in the course of gastrulation in vertebrates. Conversely, aberrations of the MT1-MMP/PTK7 axis seem to be the cause of abnormal CE during gastrulation in vertebrates.
Conclusions
Overall, we established that the full-length PTK7 down-regulates myosin light chain phosphorylation, actin cytoskeleton organization, and actomyosin contraction (all downstream events in the Wnt/PCP pathway) and that it strongly inhibits cell invasion. PTK7 is a major cleavage target of MT1-MMP in the plasma membrane. MT1-MMP directly cleaves the PKP621↓LI sequence in an exposed region of PTK7, generating the N-terminal, soluble PTK7 ectodomain. The latter forms a complex with the full-length membrane PTK7. MT1-MMP proteolysis reverses the inhibitory action of the full-length PTK on cell locomotion. MT1-MMP silencing and the analysis of the uncleavable L622D PTK7 mutant also confirm the significance of MT1-MMP proteolysis of PTK7 in cell functions. Our novel data suggest that the MT1-MMP/PTK7 axis plays an important role in the regulation of the non-canonical Wnt/PCP pathway and polarized cell motility both in malignancy and vertebrate embryo development. Our results bring us a step closer to the development of selective therapeutics to target the MT1-MMP/PTK7 axis and the Wnt/PCP pathway in a clinically beneficial manner.
This work was supported, in whole or in part, by National Institutes of Health Grants CA83017 and CA77470 (to A. Y. S.).
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-BankTM/EBI Data Bank with accession number(s) GU211905.
- PCP
- planar cell polarity
- CE
- convergent extension
- MLC
- myosin light chain
- pMLC
- phosphorylated MLC
- MMP
- matrix metalloproteinase
- MT1-MMP
- membrane type-1 matrix metalloproteinase
- PTK
- protein-tyrosine kinase
- sPTK7
- N-terminal, soluble PTK7 fragment
- TIMP
- tissue inhibitor of metalloproteinases
- MDCK
- Madin-Darby canine kinase.
REFERENCES
- 1.Gao C., Chen Y. G. (2010) Cell. Signal. 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 2.Schlessinger K., Hall A., Tolwinski N. (2009) Genes Dev. 23, 265–277 [DOI] [PubMed] [Google Scholar]
- 3.Simons M., Mlodzik M. (2008) Annu. Rev. Genet. 42, 517–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katoh M. (2005) Oncol. Rep. 14, 1583–1588 [PubMed] [Google Scholar]
- 5.Jessen J. R. (2009) Zebrafish 6, 21–28 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y. (2009) Mol. Cancer Ther. 8, 2103–2109 [DOI] [PubMed] [Google Scholar]
- 7.Vladar E. K., Antic D., Axelrod J. D. (2009) Cold Spring Harb. Perspect. Biol. 1, a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodrich L. V. (2008) Neuron 60, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale R. M., Sisson B. E., Topczewski J. (2009) Zebrafish 6, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montcouquiol M., Crenshaw E. B., 3rd, Kelley M. W. (2006) Annu. Rev. Neurosci. 29, 363–386 [DOI] [PubMed] [Google Scholar]
- 11.Yin C., Ciruna B., Solnica-Krezel L. (2009) Curr. Top. Dev. Biol. 89, 163–192 [DOI] [PubMed] [Google Scholar]
- 12.Roszko I., Sawada A., Solnica-Krezel L. (2009) Semin. Cell Dev. Biol. 20, 986–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weeraratna A. T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J. M. (2002) Cancer Cell 1, 279–288 [DOI] [PubMed] [Google Scholar]
- 14.Shnitsar I., Borchers A. (2008) Development 135, 4015–4024 [DOI] [PubMed] [Google Scholar]
- 15.Yen W. W., Williams M., Periasamy A., Conaway M., Burdsal C., Keller R., Lu X., Sutherland A. (2009) Development 136, 2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X., Borchers A. G., Jolicoeur C., Rayburn H., Baker J. C., Tessier-Lavigne M. (2004) Nature 430, 93–98 [DOI] [PubMed] [Google Scholar]
- 17.Jung J. W., Ji A. R., Lee J., Kim U. J., Lee S. T. (2002) Biochim. Biophys. Acta 1579, 153–163 [DOI] [PubMed] [Google Scholar]
- 18.Jung J. W., Shin W. S., Song J., Lee S. T. (2004) Gene 328, 75–84 [DOI] [PubMed] [Google Scholar]
- 19.Park S. K., Lee H. S., Lee S. T. (1996) J. Biochem. 119, 235–239 [DOI] [PubMed] [Google Scholar]
- 20.Mossie K., Jallal B., Alves F., Sures I., Plowman G. D., Ullrich A. (1995) Oncogene 11, 2179–2184 [PubMed] [Google Scholar]
- 21.Grassot J., Gouy M., Perrière G., Mouchiroud G. (2006) Mol. Biol. Evol. 23, 1232–1241 [DOI] [PubMed] [Google Scholar]
- 22.Shin W. S., Maeng Y. S., Jung J. W., Min J. K., Kwon Y. G., Lee S. T. (2008) Biochem. Biophys. Res. Commun. 371, 793–798 [DOI] [PubMed] [Google Scholar]
- 23.Boudeau J., Miranda-Saavedra D., Barton G. J., Alessi D. R. (2006) Trends Cell Biol. 16, 443–452 [DOI] [PubMed] [Google Scholar]
- 24.Müller-Tidow C., Schwäble J., Steffen B., Tidow N., Brandt B., Becker K., Schulze-Bahr E., Halfter H., Vogt U., Metzger R., Schneider P. M., Büchner T., Brandts C., Berdel W. E., Serve H. (2004) Clin. Cancer Res. 10, 1241–1249 [DOI] [PubMed] [Google Scholar]
- 25.Easty D. J., Mitchell P. J., Patel K., Flørenes V. A., Spritz R. A., Bennett D. C. (1997) Int. J. Cancer 71, 1061–1065 [DOI] [PubMed] [Google Scholar]
- 26.Zhu S., Liu L., Korzh V., Gong Z., Low B. C. (2006) Cell. Signal. 18, 359–372 [DOI] [PubMed] [Google Scholar]
- 27.Coyle R. C., Latimer A., Jessen J. R. (2008) Exp. Cell Res. 314, 2150–2162 [DOI] [PubMed] [Google Scholar]
- 28.Barbolina M. V., Stack M. S. (2008) Semin. Cell Dev. Biol. 19, 24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egeblad M., Werb Z. (2002) Nat. Rev. Cancer 2, 161–174 [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y., Seiki M. (2006) J. Cell. Physiol. 206, 1–8 [DOI] [PubMed] [Google Scholar]
- 31.Seiki M. (2003) Cancer Lett. 194, 1–11 [DOI] [PubMed] [Google Scholar]
- 32.Roghi C., Jones L., Gratian M., English W. R., Murphy G. (2010) FEBS J. 277, 3158–3175 [DOI] [PubMed] [Google Scholar]
- 33.Golubkov V. S., Cieplak P., Chekanov A. V., Ratnikov B. I., Aleshin A. E., Golubkova N. V., Postnova T. I., Radichev I. A., Rozanov D. V., Zhu W., Motamedchaboki K., Strongin A. Y. (2010) J. Biol. Chem. 285, 27726–27736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Will H., Atkinson S. J., Butler G. S., Smith B., Murphy G. (1996) J. Biol. Chem. 271, 17119–17123 [DOI] [PubMed] [Google Scholar]
- 35.Brew K., Nagase H. (2010) Biochim. Biophys. Acta 1803, 55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kessenbrock K., Plaks V., Werb Z. (2010) Cell 141, 52–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bravo-Cordero J. J., Marrero-Diaz R., Megías D., Genís L., García-Grande A., García M. A., Arroyo A. G., Montoya M. C. (2007) EMBO J. 26, 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf K., Friedl P. (2009) Clin. Exp. Metastasis 26, 289–298 [DOI] [PubMed] [Google Scholar]
- 39.Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., Friedl P. (2007) Nat. Cell Biol. 9, 893–904 [DOI] [PubMed] [Google Scholar]
- 40.Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S. A., Mankani M., Robey P. G., Poole A. R., Pidoux I., Ward J. M., Birkedal-Hansen H. (1999) Cell 99, 81–92 [DOI] [PubMed] [Google Scholar]
- 41.Holmbeck K., Bianco P., Yamada S., Birkedal-Hansen H. (2004) J. Cell. Physiol. 200, 11–19 [DOI] [PubMed] [Google Scholar]
- 42.Golldack D., Popova O. V., Dietz K. J. (2002) J. Biol. Chem. 277, 5541–5547 [DOI] [PubMed] [Google Scholar]
- 43.Cantrell V. A., Jessen J. R. (2010) Cancer Lett. 287, 54–61 [DOI] [PubMed] [Google Scholar]
- 44.Paudyal A., Damrau C., Patterson V. L., Ermakov A., Formstone C., Lalanne Z., Wells S., Lu X., Norris D. P., Dean C. H., Henderson D. J., Murdoch J. N. (2010) BMC Dev. Biol. 10, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J., Bai S., Zhang X., Nagase H., Sarras M. P., Jr. (2003) Matrix Biol. 22, 279–293 [DOI] [PubMed] [Google Scholar]
- 46.Ratnikov B., Deryugina E., Leng J., Marchenko G., Dembrow D., Strongin A. (2000) Anal. Biochem. 286, 149–155 [DOI] [PubMed] [Google Scholar]
- 47.Golubkov V. S., Boyd S., Savinov A. Y., Chekanov A. V., Osterman A. L., Remacle A., Rozanov D. V., Doxsey S. J., Strongin A. Y. (2005) J. Biol. Chem. 280, 25079–25086 [DOI] [PubMed] [Google Scholar]
- 48.Golubkov V. S., Chekanov A. V., Savinov A. Y., Rozanov D. V., Golubkova N. V., Strongin A. Y. (2006) Cancer Res. 66, 10460–10465 [DOI] [PubMed] [Google Scholar]
- 49.Rozanov D. V., Savinov A. Y., Williams R., Liu K., Golubkov V. S., Krajewski S., Strongin A. Y. (2008) Cancer Res. 68, 4086–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sounni N. E., Rozanov D. V., Remacle A. G., Golubkov V. S., Noel A., Strongin A. Y. (2010) Int. J. Cancer 126, 1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radichev I. A., Remacle A. G., Shiryaev S. A., Purves A. N., Johnson S. L., Pellecchia M., Strongin A. Y. (2010) J. Biol. Chem. 285, 16076–16086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eswar N., Eramian D., Webb B., Shen M. Y., Sali A. (2008) Methods Mol. Biol. 426, 145–159 [DOI] [PubMed] [Google Scholar]
- 53.Pieper U., Eswar N., Webb B. M., Eramian D., Kelly L., Barkan D. T., Carter H., Mankoo P., Karchin R., Marti-Renom M. A., Davis F. P., Sali A. (2009) Nucleic Acids Res. 37, D347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westerfield M. (2000) The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th Ed., University of Oregon Press, Eugene, OR [Google Scholar]
- 55.Rozen S., Skaletsky H. (2000) Methods Mol. Biol. 132, 365–386 [DOI] [PubMed] [Google Scholar]
- 56.Suenaga N., Mori H., Itoh Y., Seiki M. (2005) Oncogene 24, 859–868 [DOI] [PubMed] [Google Scholar]
- 57.Rozanov D. V., Deryugina E. I., Monosov E. Z., Marchenko N. D., Strongin A. Y. (2004) Exp. Cell Res. 293, 81–95 [DOI] [PubMed] [Google Scholar]
- 58.Shiryaev S. A., Savinov A. Y., Cieplak P., Ratnikov B. I., Motamedchaboki K., Smith J. W., Strongin A. Y. (2009) PLoS ONE 4, e4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez-Barrantes S., Toth M., Bernardo M. M., Yurkova M., Gervasi D. C., Raz Y., Sang Q. A., Fridman R. (2000) J. Biol. Chem. 275, 12080–12089 [DOI] [PubMed] [Google Scholar]
- 60.Wyckoff J. B., Pinner S. E., Gschmeissner S., Condeelis J. S., Sahai E. (2006) Curr. Biol. 16, 1515–1523 [DOI] [PubMed] [Google Scholar]
- 61.Kita T., Hata Y., Arita R., Kawahara S., Miura M., Nakao S., Mochizuki Y., Enaida H., Goto Y., Shimokawa H., Hafezi-Moghadam A., Ishibashi T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17504–17509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirayama K., Hata Y., Noda Y., Miura M., Yamanaka I., Shimokawa H., Ishibashi T. (2004) Invest. Ophthalmol. Vis. Sci. 45, 3896–3903 [DOI] [PubMed] [Google Scholar]
- 63.Liu S., Goldstein R. H., Scepansky E. M., Rosenblatt M. (2009) Cancer Res. 69, 8742–8751 [DOI] [PubMed] [Google Scholar]
- 64.Hotary K. B., Allen E. D., Brooks P. C., Datta N. S., Long M. W., Weiss S. J. (2003) Cell 114, 33–45 [DOI] [PubMed] [Google Scholar]
- 65.Tada M., Kai M. (2009) Zebrafish 6, 29–40 [DOI] [PubMed] [Google Scholar]
- 66.Belkin A. M., Akimov S. S., Zaritskaya L. S., Ratnikov B. I., Deryugina E. I., Strongin A. Y. (2001) J. Biol. Chem. 276, 18415–18422 [DOI] [PubMed] [Google Scholar]
- 67.Deryugina E. I., Ratnikov B. I., Postnova T. I., Rozanov D. V., Strongin A. Y. (2002) J. Biol. Chem. 277, 9749–9756 [DOI] [PubMed] [Google Scholar]
- 68.Deryugina E. I., Ratnikov B. I., Yu Q., Baciu P. C., Rozanov D. V., Strongin A. Y. (2004) Traffic 5, 627–641 [DOI] [PubMed] [Google Scholar]
- 69.Kajita M., Itoh Y., Chiba T., Mori H., Okada A., Kinoh H., Seiki M. (2001) J. Cell Biol. 153, 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori H., Tomari T., Koshikawa N., Kajita M., Itoh Y., Sato H., Tojo H., Yana I., Seiki M. (2002) EMBO J. 21, 3949–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murai S., Umemiya T., Seiki M., Harigaya K. (2004) Virchows Arch. 445, 271–278 [DOI] [PubMed] [Google Scholar]
- 72.Nakamura H., Suenaga N., Taniwaki K., Matsuki H., Yonezawa K., Fujii M., Okada Y., Seiki M. (2004) Cancer Res. 64, 876–882 [DOI] [PubMed] [Google Scholar]
- 73.Rozanov D. V., Hahn-Dantona E., Strickland D. K., Strongin A. Y. (2004) J. Biol. Chem. 279, 4260–4268 [DOI] [PubMed] [Google Scholar]
- 74.Whitford K. L., Ghosh A. (2001) Neuron 32, 1–3 [DOI] [PubMed] [Google Scholar]
- 75.Winberg M. L., Tamagnone L., Bai J., Comoglio P. M., Montell D., Goodman C. S. (2001) Neuron 32, 53–62 [DOI] [PubMed] [Google Scholar]
- 76.Dangi-Garimella S., Redig A. J., Shields M. A., Siddiqui M. A., Munshi H. G. (2010) J. Biol. Chem. 285, 28363–28372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gawden-Bone C., Zhou Z., King E., Prescott A., Watts C., Lucocq J. (2010) J. Cell Sci. 123, 1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrie R. J., Doyle A. D., Yamada K. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marlow F., Topczewski J., Sepich D., Solnica-Krezel L. (2002) Curr. Biol. 12, 876–884 [DOI] [PubMed] [Google Scholar]
- 80.Lincoln T. M. (2007) Circ. Res. 100, 10–12 [DOI] [PubMed] [Google Scholar]