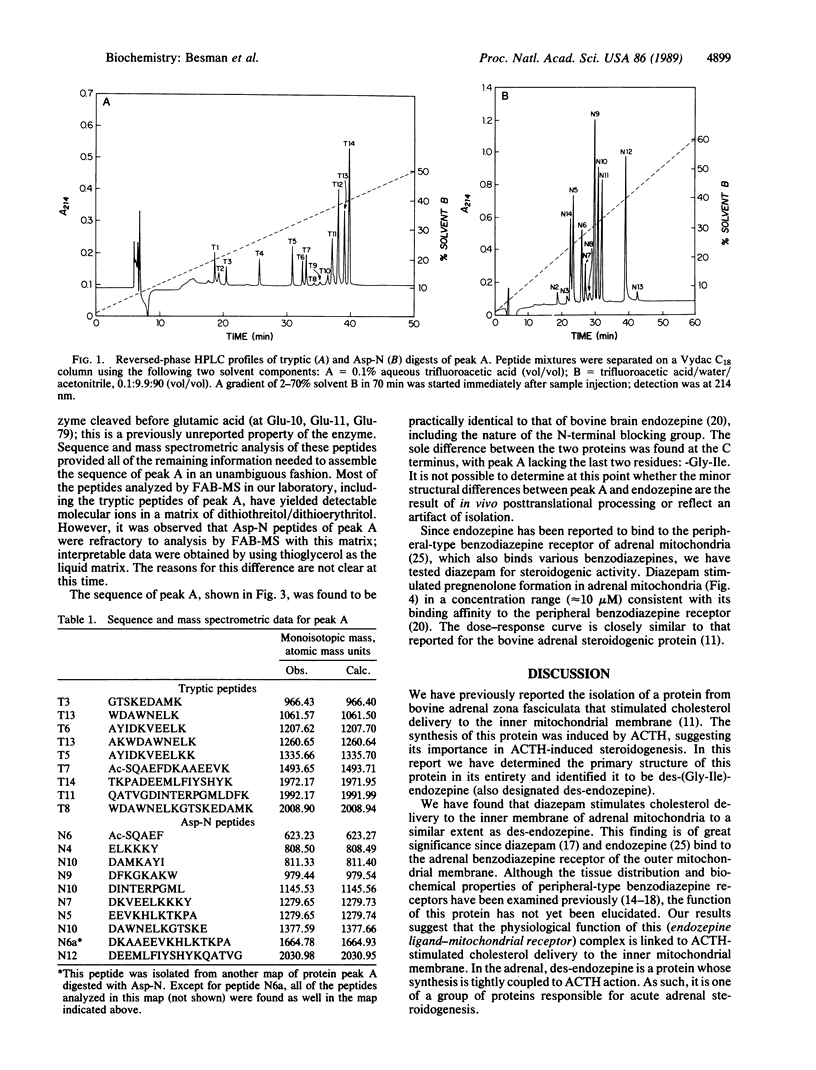
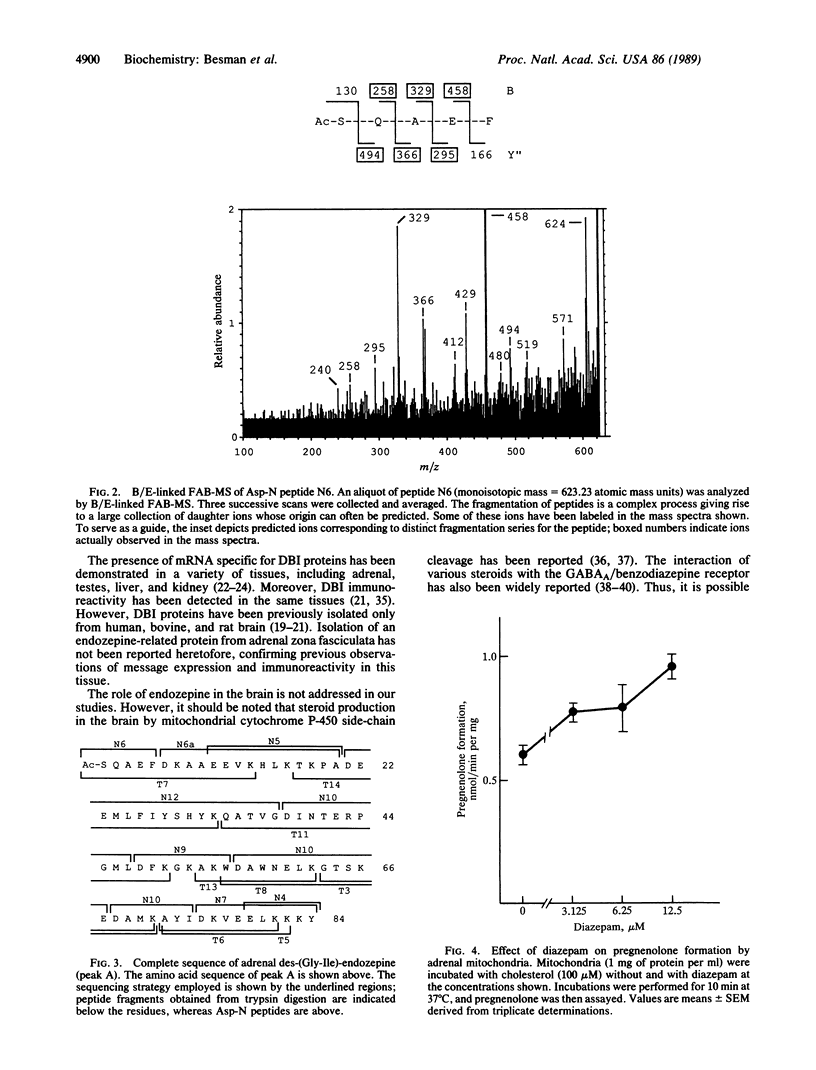
Abstract
Delivery of cholesterol to inner mitochondrial membranes is rate-limiting for steroidogenesis in the zona fasciculata of adrenal cortex. A protein that stimulates this process was isolated to homogeneity from bovine adrenal tissue. This protein's primary structure has been determined in its entirety by a combination of automated Edman microsequencing, fast-atom bombardment mass spectrometry (FAB-MS). The sequence was identical to that previously reported for bovine brain endozepine, except that it lacks the last two residues, -Gly-Ile, at the C terminus. To our knowledge, isolation of an endozepine-related protein from a tissue other than brain has not been reported previously. Endozepine competes with benzodiazepines for saturable binding sites in synaptosomes and in mitochondria of specific peripheral tissues. Previous reports have localized the adrenal benzodiazepine receptor to the outer mitochondrial membrane. In this report, we show that the prototypic benzodiazepine, diazepam, effects a stimulation of adrenal mitochondrial cholesterol delivery similar to that observed for endozepine. The effective diazepam concentration was consistent with that previously shown to displace a high-affinity ligand of the mitochondrial benzodiazepine receptor. The action of diazepam in adrenal mitochondria suggests that the mediation of corticotropin-induced steroidogenesis may be the physiological function of the peripheral-type benzodiazepine receptor. These studies provide new insights into the previously unknown function of peripheral benzodiazepine receptors and should allow new investigations into the stimulation of steroidogenesis by endozepines and benzodiazepines in the brain and in certain peripheral tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho H., Fremeau R. T., Jr, Tiedge H., Wilcox J., Bovolin P., Brosius J., Roberts J. L., Costa E. Diazepam binding inhibitor gene expression: location in brain and peripheral tissues of rat. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7018–7022. doi: 10.1073/pnas.85.18.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt R. R., Aebi U., Pedersen P. L., Snyder S. H. Solubilization and reassembly of the mitochondrial benzodiazepine receptor. Biochemistry. 1986 Apr 22;25(8):2120–2125. doi: 10.1021/bi00356a041. [DOI] [PubMed] [Google Scholar]
- Anholt R. R., Pedersen P. L., De Souza E. B., Snyder S. H. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986 Jan 15;261(2):576–583. [PubMed] [Google Scholar]
- Ball J. A., Burnet P. W., Fountain B. A., Ghatei M. A., Bloom S. R. Octadecaneuropeptide, benzodiazepine ligand, -like immunoreactivity in rat central nervous system, plasma and peripheral tissues. Neurosci Lett. 1986 Dec 12;72(2):183–188. doi: 10.1016/0304-3940(86)90077-7. [DOI] [PubMed] [Google Scholar]
- De Souza E. B., Anholt R. R., Murphy K. M., Snyder S. H., Kuhar M. J. Peripheral-type benzodiazepine receptors in endocrine organs: autoradiographic localization in rat pituitary, adrenal, and testis. Endocrinology. 1985 Feb;116(2):567–573. doi: 10.1210/endo-116-2-567. [DOI] [PubMed] [Google Scholar]
- Gee K. W. Steroid modulation of the GABA/benzodiazepine receptor-linked chloride ionophore. Mol Neurobiol. 1988 Winter;2(4):291–317. doi: 10.1007/BF02935636. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Glaister D., Seeburg P. H., Guidotti A., Costa E. Cloning and expression of cDNA for human diazepam binding inhibitor, a natural ligand of an allosteric regulatory site of the gamma-aminobutyric acid type A receptor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7547–7551. doi: 10.1073/pnas.83.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. F. Cellular organization for steroidogenesis. Int Rev Cytol. 1984;86:53–95. doi: 10.1016/s0074-7696(08)60177-1. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Charpponnier C., Nakamura M., Gabbiani G. The role of microfilaments in the response of adrenal tumor cells to adrenocorticotropic hormone. J Biol Chem. 1979 Sep 25;254(18):9080–9084. [PubMed] [Google Scholar]
- Hawke D. H., Harris D. C., Shively J. E. Microsequence analysis of peptides and proteins. V. Design and performance of a novel gas-liquid-solid phase instrument. Anal Biochem. 1985 Jun;147(2):315–330. doi: 10.1016/0003-2697(85)90278-7. [DOI] [PubMed] [Google Scholar]
- Hefta S. A., Hefta L. J., Lee T. D., Paxton R. J., Shively J. E. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4648–4652. doi: 10.1073/pnas.85.13.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z. Y., Bourreau E., Jung-Testas I., Robel P., Baulieu E. E. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goascogne C., Robel P., Gouézou M., Sananès N., Baulieu E. E., Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987 Sep 4;237(4819):1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- Lehman W. Hybrid troponin reconstituted from vertebrate and arthropod subunits. Nature. 1975 May 29;255(5507):424–426. doi: 10.1038/255424a0. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Mienville J. M., Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988 Aug 1;90(3):279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Schwartz R. D. Pregnenolone-sulfate: an endogenous antagonist of the gamma-aminobutyric acid receptor complex in brain? Brain Res. 1987 Feb 24;404(1-2):355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J., Shoyab M. Complete amino acid sequences of bovine and human endozepines. Homology with rat diazepam binding inhibitor. J Biol Chem. 1986 Jul 25;261(21):9727–9731. [PubMed] [Google Scholar]
- Mocchetti I., Einstein R., Brosius J. Putative diazepam binding inhibitor peptide: cDNA clones from rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7221–7225. doi: 10.1073/pnas.83.19.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrotek J. J., Hall P. F. Response of adrenal tumor cells to adrenocorticotropin: site of inhibition by cytochalasin B. Biochemistry. 1977 Jul 12;16(14):3177–3181. doi: 10.1021/bi00633a021. [DOI] [PubMed] [Google Scholar]
- Ohno Y., Yanagibashi K., Yonezawa Y., Ishiwatari S., Matsuba M. A possible role of "steroidogenic factor" in the corticoidogenic response to ACTH; effect of ACTH, cycloheximide and aminoglutethimide on the content of cholesterol in the outer and inner mitochondrial membrane of rat adrenal cortex. Endocrinol Jpn. 1983 Jun;30(3):335–338. doi: 10.1507/endocrj1954.30.335. [DOI] [PubMed] [Google Scholar]
- Osawa S., Betz G., Hall P. F. Role of actin in the responses of adrenal cells to ACTH and cyclic AMP: inhibition by DNase I. J Cell Biol. 1984 Oct;99(4 Pt 1):1335–1342. doi: 10.1083/jcb.99.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Cholesterol side-chain cleavage in the rat adrenal cortex: isolation of a cycloheximide-sensitive activator peptide. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1882–1886. doi: 10.1073/pnas.80.7.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen R. C., Brownie A. C. Steroidogenesis-activator polypeptide isolated from a rat Leydig cell tumor. Science. 1987 Apr 10;236(4798):188–190. doi: 10.1126/science.3563495. [DOI] [PubMed] [Google Scholar]
- Pedersen R. C. Polypeptide activators of cholesterol side-chain cleavage. Endocr Res. 1984;10(3-4):533–561. doi: 10.1080/07435808409036515. [DOI] [PubMed] [Google Scholar]
- Pon L. A., Hartigan J. A., Orme-Johnson N. R. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986 Oct 5;261(28):13309–13316. [PubMed] [Google Scholar]
- Privalle C. T., Crivello J. F., Jefcoate C. R. Regulation of intramitochondrial cholesterol transfer to side-chain cleavage cytochrome P-450 in rat adrenal gland. Proc Natl Acad Sci U S A. 1983 Feb;80(3):702–706. doi: 10.1073/pnas.80.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Roepstorff P., Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984 Nov;11(11):601–601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- Scallen T. J., Noland B. J., Gavey K. L., Bass N. M., Ockner R. K., Chanderbhan R., Vahouny G. V. Sterol carrier protein 2 and fatty acid-binding protein. Separate and distinct physiological functions. J Biol Chem. 1985 Apr 25;260(8):4733–4739. [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Miller P., Ronk M. Microsequence analysis of peptides and proteins. VI. A continuous flow reactor for sample concentration and sequence analysis. Anal Biochem. 1987 Jun;163(2):517–529. doi: 10.1016/0003-2697(87)90257-0. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Gentry L. E., Marquardt H., Todaro G. J. Isolation and characterization of a putative endogenous benzodiazepineoid (endozepine) from bovine and human brain. J Biol Chem. 1986 Sep 15;261(26):11968–11973. [PubMed] [Google Scholar]
- Wang J. K., Morgan J. I., Spector S. Benzodiazepines that bind at peripheral sites inhibit cell proliferation. Proc Natl Acad Sci U S A. 1984 Feb;81(3):753–756. doi: 10.1073/pnas.81.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb N. R., Rose T. M., Malik N., Marquardt H., Shoyab M., Todaro G. J., Lee D. C. Bovine and human cDNA sequences encoding a putative benzodiazepine receptor ligand. DNA. 1987 Feb;6(1):71–79. doi: 10.1089/dna.1987.6.71. [DOI] [PubMed] [Google Scholar]
- Weissman B. A., Cott J., Hommer D., Quirion R., Paul S., Skolnick P. Pharmacological, electrophysiological, and neurochemical actions of the convulsant benzodiazepine Ro 5-4864 (4'-chlordiazepam). Adv Biochem Psychopharmacol. 1983;38:139–151. [PubMed] [Google Scholar]
- Yanagibashi K., Ohno Y., Kawamura M., Hall P. F. The regulation of intracellular transport of cholesterol in bovine adrenal cells: purification of a novel protein. Endocrinology. 1988 Oct;123(4):2075–2082. doi: 10.1210/endo-123-4-2075. [DOI] [PubMed] [Google Scholar]


