Abstract
Immunoglobulin G (IgG) dependent activities are important in host defense and autoimmune diseases. Various cell types including macrophages and neutrophils contribute to pathogen destruction and tissue damage through binding of IgG to Fcγ receptors (FcγR). One member of this family, FcγRIIA, is a transmembrane glycoprotein known to mediate binding and internalization of IgG-containing targets. FcγRIIA has been observed to translocate into lipids rafts upon binding IgG-containing targets. We hypothesize that lipid rafts participate to different extents in binding and internalizing targets of different sizes. We demonstrate that disruption of lipid rafts with 8mM methyl-β-cyclodextrin (MβCD) nearly abolishes binding (91% reduction) and phagocytosis (60% reduction) of large IgG-coated targets. Conversely, binding and internalization of small IgG-complexes is less dependent on lipid rafts (49% and 17% inhibition at 8mM MβCD respectively). These observations suggest that differences between phagocytosis and endocytosis may arise as early as the initial stages of ligand recognition.
Keywords: methy1-β-cyclodextrin, endocytosis, phagocytosis, Fc receptors
Introduction
The mammalian immune system has developed various means of defense from foreign pathogens. One of the most important of these, phagocytosis, involves the cellular internalization of particles and their sequestering into a phagosome. Recognition of a particle for internalization is the primary step in phagocytosis requiring receptor-ligand interactions. Phagocytic cells of the immune system express a variety of receptors specific for this purpose. For example, innate recognition by mannose receptors of non-self sugar moieties and scavenger receptor recognition of specific pathogen recognition motifs and low-density lipoprotein allow phagocytes to detect and degrade pathogens.
Another receptor found on phagocytes, the Fc receptor (FcR) is able to recognize the Fc portion of immunoglobulin (Ig) in the context of pathogens opsonized with antibody [1; 2]. FcRs are designated by the class of Ig they bind. For example, FcγRs recognize and bind IgG, while FcαR binds IgA, and FcεR binds IgE [3; 4]. The recognition of the appropriate Ig triggers signaling cascades through the clustering of FcRs on the cell surface. This accelerates the engulfment process and can lead to the generation of microbicidal oxygen and nitrogen species [5; 6; 7; 8].
Three classes of FcR are responsible for binding IgG: FcγRI (CD64), FcγRII (CD32) and FcγRIII (CD16), each of which can be further differentiated by structure [9]. Both activating (FcγRI, FcγRIIA, FcγRIII) and inhibitory (FcγRIIB) FcγRs participate in phagocytosis of IgG-coated particles, allowing for strict self-regulation among the FcR classes through cytoplasmic activation and inhibition motifs [10; 11]. FcγRIIA is a 40 kDa transmembrane receptor found on neutrophils, monocytes, macrophages, dendritic cells and platelets [12]. FcγRIIA can initiate phagocytosis of large IgG-coated targets or endocytosis of small IgG containing immune complexes. Most signaling is initiated by the immunoreceptor tyrosine based activation motif (ITAM) sequence located in the cytoplasmic domain. The ITAM of FcγRIIA is slightly different than traditional ITAM sequences due to the extra intervening residues between the dual YxxL motifs (Y-M-T-L-12aa -Y-L-T-L) and is therefore referred to as an ITAM-like region (reviewed in[12]).
Our laboratory has recently shown that specific domains of the receptor are differentially involved in phagocytosis and endocytosis[13; 14; 15]. For instance, phosphorylation of the tyrosine residues in the ITAM-like sequence is required for phagocytic signaling. However, the first leucine residue has been shown to be necessary for the endocytic mechanisms of FcγRIIA, while the L-T-L motif is required for phagolysosome fusion[16; 17; 18; 19; 20].
Activation of FcγRIIA by IgG-coated pathogens results in lipid raft localization and cross-linking of receptors. FcγRIIA is then phosphorylated on tyrosine residues in the ITAM-like sequence by members of the Src family of tyrosine kinases [21; 22; 23; 24]. Phosphorylation of the ITAM like region creates two SH2 (Src Homology 2) binding sites, together being able to bind spleen tyrosine kinase (Syk) [25]. This signaling leads to reorganization of actin filaments, driving the formation of a phagosome and the initiation of signaling events which result in the destruction of pathogens and the release of pro-inflammatory mediators such as cytokines and reactive oxygen species [2; 26; 27].
In addition to phagocytosis, FcγRIIA can also mediate the uptake of small immune complexes composed of IgG through endocytic (clathrin-dependent) mechanisms [28; 29]. Mutational analysis has revealed that the YMTL sequence of FcγRIIA is important for internalization of IgG complexes. Furthermore, this process has also recently been shown to require ubiquitylation, which is not required for phagocytosis of large targets [15].
Residues outside of the ITAM have also been shown to be necessary for FcγRIIA activity. Recent studies have elucidated functionality in FcR translocation to lipid rafts. Recruitment of FcγRIIA to lipid rafts has been shown to be dependent on the palmitoylation of a juxtamembrane cysteine (cysteine 208) or on transmembrane residues [30; 31]. Studies with FcγRIIB2 have suggested a role for lipid rafts in the temporal regulation of functions associated with endocytic mechanisms [32]. While the actual translocation to lipid rafts and the effect of lipid rafts on binding is well documented, the functional significance leading to phagocytosis or endocytosis is still a matter of much speculation[33].
To ascertain the role lipid rafts play in FcγRIIA-mediated internalization mechanisms, we have investigated the necessity for such membrane domains in the binding and internalization of IgG coated targets and heat-aggregated IgG-complexes. By disrupting lipid rafts and observing FcγRIIA mediated binding and internalization, we report that lipid rafts are required for binding and internalization of large IgG coated targets but may inhibit endocytosis of IgG complexes.
Materials & Methods
Cell Culture
Chinese hamster ovary (CHO) cells expressing human FcγRIIA containing a MYC/His epitope tag or CR3 were generated as previously described and maintained in Ham’s F-12 (Biowhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (Summit Biotechnology, Ft. Collins, CO) and expression was maintained by selection in G-418 (HyClone, Logan, UT)[16]. THP-1 (ATCC) cells were grown in RPMI-1640 media supplemented with 10% FCS, streptomycin and leucine per ATCC recommendations. THP-1 cell differentiation was induced by the addition of 100nM phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis MO) for three days at 37°C. Surface expression and blocking of FcγRIIA was accomplished using anti-FcγRII mAb IV.3 or isotype matched control antibodies at 2.5µg/ml.
Measurement of cellular cholesterol
Total cellular cholesterol was measured following MβCD treatment either using an Amplex Red Cholesterol Assay Kit (Invitrogen, Carlsbad, CA) or labeling with Filipin complex from Streptomyces filipinensis (Sigma, St. Louis, MO). Filipin staining was performed at a working concentration of 0.5mg/ml on ice for 30 minutes, then washed away and remaining filipin bound to cholesterol was read (355nm excitation, 460nm emission) using a FLUOstar Omega microplate reader (BMG Labtech, Offenburg, Germany).
Lipid Raft Disruption
Disruption of lipid rafts by plasma-membrane cholesterol depletion was accomplished through treatment with a range of Methyl-β Cyclodextrin (MβCD) concentrations (0mM, 1mM, 2mM, 5mM and 8mM) (Sigma, St. Louis, MO). To test the effect of lipid raft disruption on binding, cells were pre-treated with MβCD for 45 minutes at 37°C and binding experiments were performed with opsonized beads or heat-aggregated IgG. To determine the effect of lipid raft disruption on internalization, binding in the absence of MβCD occurred on ice, followed by incubation with MβCD for 45 minutes on ice prior to warming cells to 37°C for stimulation of internalization.
Membrane fractionation and Western Blot
FcγRIIA expressing CHO cells were treated with MβCD (5mM) for 30 minutes then exposed to opsonized targets or heat-aggregated IgG for an additional 30 minutes. Cells were washed twice with cold PBS to remove unbound targets and incubated in TNE buffer (0.05% TX-100 and protease inhibitor) for 30 minutes on ice for lysis. Lysates were processed as previously described[23; 34]. Briefly, lysates were suspended in a final concentration of 40% sucrose, loaded onto a sucrose step gradient (10–80%) and centrifuged overnight at 38k RPM using a Beckman SW41 rotor. 1ml fractions were collected, sucrose was removed by MeOH/Chloroform precipitation and protein loaded onto an SDS-PAGE gel. Following gel electrophoresis, samples were transferred to a PVDF membrane and blotted with an anti-MYC FcγRIIA antibody (Santa Cruz, Santa Cruz, CA) or with horseradish peroxidase conjugated Cholera toxin B (Invitrogen) which recognizes the lipid raft associated sphingolipid, GM-1. Factions were compared for the presence of FcγRIIA following exposure to either target or MβCD drug treatment. Samples were blotted for GM1 using cholera toxin B (Sigma, St. Louis, MO) to confirm rafts in lanes 7–9. Blots were imaged and quantitated on an Omega 12iC (Ultra-Lum, Claremont, CA) using UltraQuant 6.0 software (Ultra-Lum).
IgG coated targets
Opsonized latex beads were used to investigate FcγRIIA mediated phagocytosis. 4.5µm polystyrene beads (OB) (Polysciences, Warrington, PA) were opsonized by incubation in a 10mg/ml solution of human IgG (MP, Aurora, OH) for 2 hours at room temperature. OBs were added to effector cells (CHO-IIA or THP-1) at a ratio of 10:1 targets to cells. Binding was allowed for 45 minutes on ice, and non-bound targets were removed with buffered saline washes. For internalization assays, six-well plates were floated on the surface of a 37°C water bath and allowed to internalize for 30 minutes. Following internalization, the cells were returned to the ice, cold PBS was added to each well, and non-internalized beads were labeled with goat anti-human IgG F(ab’)2 fragments conjugated with phycoerythrin (Jackson Immunoresearch, Gilbertsville, PA) in a 7.5µg/ml solution for 20 minutes. Cells were washed again with ice-cold buffered saline and fixed in 2% paraformaldehyde. Binding and phagocytosis were assessed by counting the number of bound beads per cell as well as the number internalized determined by lack of staining with the secondary antibody. Percent total binding and internalization are calculated by comparing the number of beads bound or internalized per cell in untreated samples with treated samples. Each experiment was repeated at least three times, assessing 300+ cells for each test sample.
Heat-aggregated IgG Complex
Solutions of 10mg/ml FITC-conjugated human IgG (Sigma, St. Louis, MO) were complexed by aggregation at 62°C for 20 minutes. Large aggregates were cleared by centrifugation (10,000xg for 10 minutes). The remaining soluble complexes have been reported to contain a mixture of IgG aggregates of 2 to 6 molecules per complex[35]. Assuming the size of individual IgG molecules to be ∼12nm in length along the heavy chain, the maximum size aggregate could not exceed 72nm.
The IgG-complexes (concentration 100µg/ml) were allowed to bind to cells for 45 minutes on ice. Excess IgG-complex was removed by washing twice with PBS (containing calcium and magnesium). Internalization assays were performed by floating six-well plates containing the cells on the surface of a 37°C water bath and allowed to internalize for 30 minutes. Following internalization, the cells were returned to ice and non-internalized IgG complexes were labeled with a 2.5µg/ml solution of goat anti-human IgG F(ab )2 fragments conjugated with phycoerythrin (PE) (Jackson Immunoresearch, Gilbertsville, PA) for 20 minutes. Excess secondary antibody was removed through buffered saline washes and the cells were detached and fixed in 2% paraformaldehyde overnight. The samples were filtered through 100µm nylon mesh and analyzed on a BD-FACSCalibur (Becton Dickinson, San Jose, CA) using Cell Quest software.
Percent binding and internalization are calculated by comparing the Mean Fluorescence Intensity (MFI) of either FITC (total cell-associated immune complex, binding value) or PE (external immune complex only, internalization value) at each concentration of MβCD and normalizing to untreated control samples. Each experiment was repeated at least three times, and 10,000 cellular events were assessed for each test sample.
Fluorescence Microscopy
After treatments were complete, coverslips (No 1, 25mm, Corning Life Sciences, Lowell, MA) were mounted on glass microscope slides (Corning Life Sciences) in phosphate buffered saline and placed on an Axiovert 200 fluorescence microscope (Carl Zeiss, Thornwood, NY) utilizing mercury illumination. Cells were visualized using differential interference contrast or fluorescence. Optical filters for fluorescein excitation and emission were 480DF22 and 530DF30, respectively (Chroma, Rockingham, VT). Images were observed using an Orca ER-AG (Hamamatsu, Japan) CCD camera connected to a Dell Optiplex 620 Workstation (Round Rock, TX). Metamorph software (Molecular Devices, Downingtown, PA) was used to acquire and process images.
Confocal Microscopy
Treated samples were mounted on glass coverslips and imaged on an inverted Leica SP5 laser scanning confocal microscope (Leica Microsystems, Mannheim, Germany). Excitation was accomplished using laser lines at 488nm for FITC and 561nm for TRITC and fluorescence emission was collected at 530nm and 580nm respectively. Acquisition and processing of confocal images was accomplished using Leica Applications Suite Advanced Fluorescence (LAS AF) v. 2.0.2 software (Leica Microsystems).
Statistics
Significance values were determined by Students t-test.
Results
FcγRIIA translocates to lipid rafts in response to target binding
In order to determine the level to which lipid rafts play a role in FcγRIIA-mediated binding and internalization of targets, cell fractionation experiments were performed to determine the membrane raft localization of FcγRIIA following binding in Chinese hamster ovary cells expressing human FcγRIIA (CHO-IIA). To verify the cell fractionation procedure, slot blots were performed on individual fractions using HRP-conjugated cholera toxin B which binds the sphingolipid GM1, known to be enriched in lipid rafts. As demonstrated in Figure 1A, row 1, GM1 is found primarily in fractions 6–9.
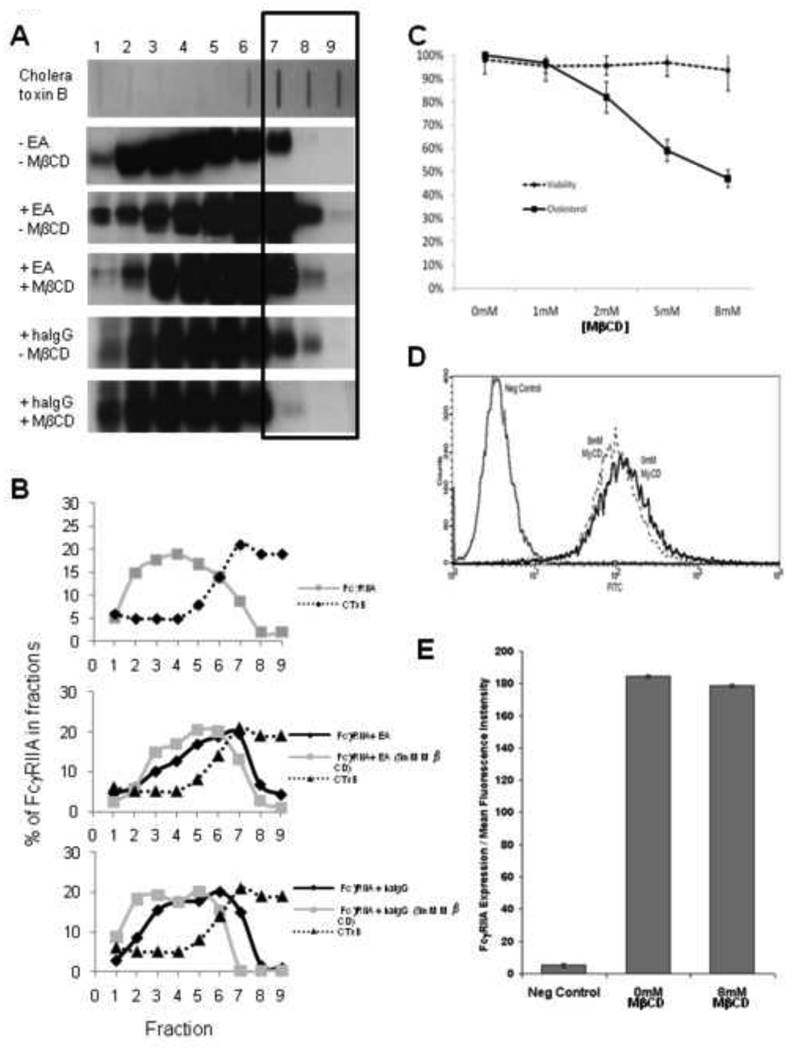
Figure 1. FcγRIIA migrates into lipid rafts following activation, and this migration can be attenuated by treatment with MβCD.
(A) Blot data from FcγRIIA expressing CHO cells treated with IgG coated erythrocytes (EA) (phagocytosis) or heat aggregated IgG (endocytosis). Detection of the sphingolipid GM-1 by cholera toxin B demonstrates that lipid rafts are located predominantly in fraction 6–8 (Top Row). Cross-linking with EA induces FcγRIIA to migrate into detergent insoluble fractions as shown by a shift in location toward larger numbered fractions (lanes 6–8). Treatment with MβCD inhibits the translocation. (B) The blot in A was quantitated for Cholera Toxin B binding and FcγRIIA expression using UltraQuant software. Band size and density of each lane were determined for normal cells at rest (top), the cross-linking of FcγRIIA with EA (middle), and the cross-linking of FcγRIIA with heat aggregated IgG (bottom). (C) Cell viability was assessed by Trypan blue exclusion and plasma membrane cholesterol composition via fluorescent filipin binding. As the concentration of MβCD is increased, the amount of filipin binding decreases nearly 50% while over 95% of cell remain viable. (D) Flow cytometry analysis after labeling CHO-IIA cells with anti-FcγRIIA mAB IV.3 verifies no loss of receptor expression following treatment with 8mM MβCD. (E) Data compiled from 3 experiments analyzing 10,000 cells each by flow cytometry.
In non-stimulated cells, FcγRIIA is evenly dispersed throughout non-raft layers following fractionation by a sucrose gradient (Figure 1A (row 2), Figure 1B (top)). These data are consistent with fluorescence microscopy experiments showing that FcγRIIA is evenly distributed across cell membranes. Exposure to IgG-opsonized sheep erythrocytes (EA) induces FcγRIIA to translocate to cell fractions associated with lipid rafts, as evidenced by the increased presence in fractions 6–9 (Figure 1A (row 3), Figure 1B (middle)). Translocation of FcγRIIA to lipid rafts is reduced by ∼48% upon treatment with the cholesterol chelator MβCD which disrupts lipid raft domains by sequestering cholesterol and other lipophiles from the plasma membrane (Figure 1A (row 4), Figure 1B (middle)) (reviewed in [36; 37; 38]). Because MβCD has been shown to dissolve lipid rafts by chelating membrane cholesterol, total cellular cholesterol was measured by filipin staining. As shown in Figure 1C, exposing cells to 8mM MβCD depleted nearly 50% of the total plasma membrane cholesterol content while not affecting cell viability. There is also no effect on the surface expression of FcγRIIA following treatment with 8mM MβCD (Figure 1D and 1E).
To explore whether this lipid raft co-localization is standard for different FcγRIIA functions, human IgG was heat-aggregated into small IgG-complexes (ha-IgG) and exposed to CHO-IIA cells. Similar fractionation experiments were conducted and a similar proportion of FcγRIIA translocated into lipd rafts (Figure 1A (rows 5 and 6), Figure 1B (bottom)). Ha-IgG translocation was also inhibited by 5mM MβCD exposure demonstrating that FcγRIIA translocates into cholesterol rich microdomains (lipid rafts) upon binding both large (EA) and small (IC) targets.
FcγRIIA binding is inhibited by MβCD
To investigate potential differences in FcγRIIA function, binding assays using IgG-opsonized beads (OB) and FITC-tagged heat-aggregated immune complexes (FITC-HaIgG) were performed. In order to measure the effect of lipid raft disruption on FcγRIIA binding, Chinese Hamster Ovary (CHO) cells expressing human FcγRIIA (CHO-IIA) were pretreated with varying concentrations of MβCD for 45 minutes at 37°C. Concentrations of MβCD up to 8mM had no significant effect on cell viability (Fig. 1B). Total cellular cholesterol was reduced by ∼50% following treatment with 8mM MβCD, similar to previous studies (Fig. 1B)[39]. To evaluate the dependency of FcγRIIA binding on lipid rafts, cells were treated with MβCD, cooled to 0°C and exposed to either OB or FITC-HaIgG for 45 minutes and then unbound targets were removed by washing the cells with an isotonic phosphate-buffered saline solution. Microscopic observation (Fig. 2A) revealed a gradual decrease of bound OB as MβCD concentrations increased. Likewise, microscopic observations and flow cytometry measured a decrease in FITC-tagged HaIgG binding as MβCD concentration increased (Fig. 2B). Following treatment with 8mM MβCD, binding of OB was reduced by ∼90% while FITC-Ha-IgG binding was only inhibited by ∼50% versus control (Figure 2C). Pre-treating cells with anti-FcγRIIA mAb IV.3 blocked binding of both FITC-HaIgG and OB, demonstrating that the effects observed are FcγRIIA-mediated (Figure 2C).
Figure 2. Disruption of lipid rafts inhibits binding of both large IgG coated targets and small IgG complexes.
(A) Representative micrographs displaying the concentration-dependent loss of binding following MβCD exposure. Small dark objects clustered around the cells are IgG-opsonized beads (OB). Blocking with IV.3 (last panel) verifies observed results are due to FcγRIIA-related activity. (B) (Top) Representative histogram demonstrating a loss in FITC-IgG signal in response to 8mM MβCD treatment, suggesting a loss in the number of FITC-conjugated IgG complexes bound to each cell. Blocking of the receptor with IV.3 completely diminished binding ability. (Bottom) Fluorescence micrographs depicting loss of fluorescence represented by less FITC-conjugated HaIgG bound to the cell surface following 8mM MβCD treatment or blocking with IV.3 mAb. (C) Binding of both IgG opsonized polystyrene beads and IgG complexes are inhibited by MβCD. Percent binding values are normalized to the 0mM control sample and represent percent of control binding after treatment. (Flow Cytometry: N=7; 10,000 cells/sample/condition. Microscopy: N=5, 300+ cells/sample/condition) (* p<0.05 compared to control, ** p<0.005 compared to control)
Based on these data, it is apparent that FcγRIIA-mediated binding of large targets is significantly more dependent on lipid rafts than small IgG complexes. This variation in raft requirement suggests distinct differences in receptor function at the initial binding stages of phagocyte effector activity. Therefore, we next examined the effect of lipid rafts on different modes of internalization.
Lipid rafts participate in FcγRIIA-mediated internalization
To elucidate the differences in lipid raft requirement related to FcγRIIA function, internalization assays were performed using OB and HaIgG. As binding of both OB and HaIgG are dependent on lipid rafts, binding was allowed to occur on ice prior to MβCD treatment and samples were evaluated by microscopy and flow cytometry to be certain that displacement of targets did not occur. Following binding, CHO-IIA cells were treated with variable concentrations of MβCD on ice for 45 minutes. After phosphate-buffered saline washes (0°C) to remove any unbound target, the samples were then heated to 37°C for 30 minutes to trigger internalization. Phagocytic and endocytic function was arrested by returning the cells to ice. In order to discriminate between internal and external targets, phycoerythrin (PE) tagged secondary antibody against OB (Fig. 3A) or HaIgG (Fig. 3B) was employed. Fluorescence microscopy was performed and the total number of internalized OB was determined by subtracting the PE-labeled (external) targets from the total OB associated with the cell.
Figure 3. Disruption of lipid rafts inhibits phagocytosis of IgG opsonized beads but not endocytosis of IgG complexes.
(A) CHO-IIA cells internalizing opsonized beads. Following internalization, extracellular beads were labeled with PE-conjugated goat anti-human IgG antibody to discriminate between internalized and non-internalized (right panel) beads (arrows pointing to external beads), with those fluorescing being located on the outside of the cell. (B) CHO-IIA cells internalizing FITC-conjugated HaIgG. As before, following internalization, extracellular HaIgG were labeled with PE-conjugated goat anti-human IgG antibody. Row 1 displays binding on ice with no internalization, as can be observed in the merged image, showing near complete colocalization with the secondary Ab. Row 2 exhibits changes in HaIgG location after allowing for internalization, as evidenced by an increased amount on FITC signal inside the cells, a lesser degree of PE staining, and very little colocalization in the merged image. Row 3 displays the effects of 8mM MβCD treatment prior to internalization, exhibiting a slight loss of internalization (increased PE colocalization), though some internalization is still taking place (arrows in merged image, FITC signal inside cell). (C) Representative histogram displaying measurement of IgG complex internalization. Non-labeled CHO-IIA cells exhibited no PE signal (grey line). A positive control kept on ice to inhibit internalization is included (bold black line). Samples treated with 8mM MβCD exhibited a higher level of PE labeling than untreated samples following 30 minutes of internalization at 37°C (dashed and dotted lines, respectively). This increase in PE following treatment represents more external IgG following endocytosis, thus signifying a decrease in the total amount of IgG complexes internalized. (D) Comparative graph displaying variances in lipid raft dependency for large and small targets. Percent total internalization is calculated as the loss of internalized targets normalized to control. (Flow Cytometry: N=6, 10,000 cells each sample condition. Microscopy: N=5, 300+ cells each sample condition)(* p<0.05 compared to control, ** p<0.005 compared to control)
To measure FITC-HaIgG internalization we employed both confocal fluorescence microscopy and flow cytometry, using two colors to monitor both the FITC-HaIgG and its relative location (inside – PE negative vs. outside – PE positive). As opposed to the binding assay, higher PE signals represent less internalization (Figure 3C). FITC-haIgG complexes were used to verify that MβCD treatment did not result in loss of complex bound to FcγRIIA (Figure 3B).
An even more drastic difference in the necessity for cholesterol-containing lipid rafts was observed for internalization of the two target sizes mediated by FcγRIIA (Figure 3C). Treatment with 2mM MβCD diminished internalization of OB by ∼ 30% while FITC-Ha-IgG was only inhibited by ∼ 10%. Remarkably, while phagocytosis was inhibited by > 60% after 8mM MβCD treatment, endocytosis of FITC-HAIgG was only slightly reduced (<20%) in response to increased MβCD concentrations.
Lipid rafts are not required for phagocytosis by all receptors
To determine if MβCD treatment was responsible for inhibition of phagocytosis due to dissolving lipid rafts or by inhibiting other cellular process responsible for phagocytosis, the experiments were repeated in CHO cells expressing human Complement Receptor 3 (CR3). CR3 is capable of triggering internalization by Type 1 (non-opsonized, lipid raft dependent) or Type 2 (opsonized, lipid raft independent) phagocytosis [40; 41; 42]. To investigate if MβCD treatment inhibits all forms of phagocytosis, we chose to observe CHO-CR3 cells exposed to human serum-opsonized zymosan particles (SOZ) to initiate Type 2 (Lipid raft independent) CR3-mediated phagocytosis. Binding of SOZ to CR3 was inhibited by MβCD similar to FcγRIIA (Figure 4A). However, no effect was observed on the ability of CR3 to internalize targets, suggesting that MβCD is not inhibiting cellular processes leading to phagocytosis but has a more direct effect on lipid raft-dependent processes (Figure 4B). Together, these data suggest that while lipid rafts may play an important role in binding by various receptors, there appears to be a unique role for lipid rafts in FcγRIIA-mediated phagocytosis.
Figure 4. Lipid rafts participate in binding but not phagocytosis triggered by CR3.
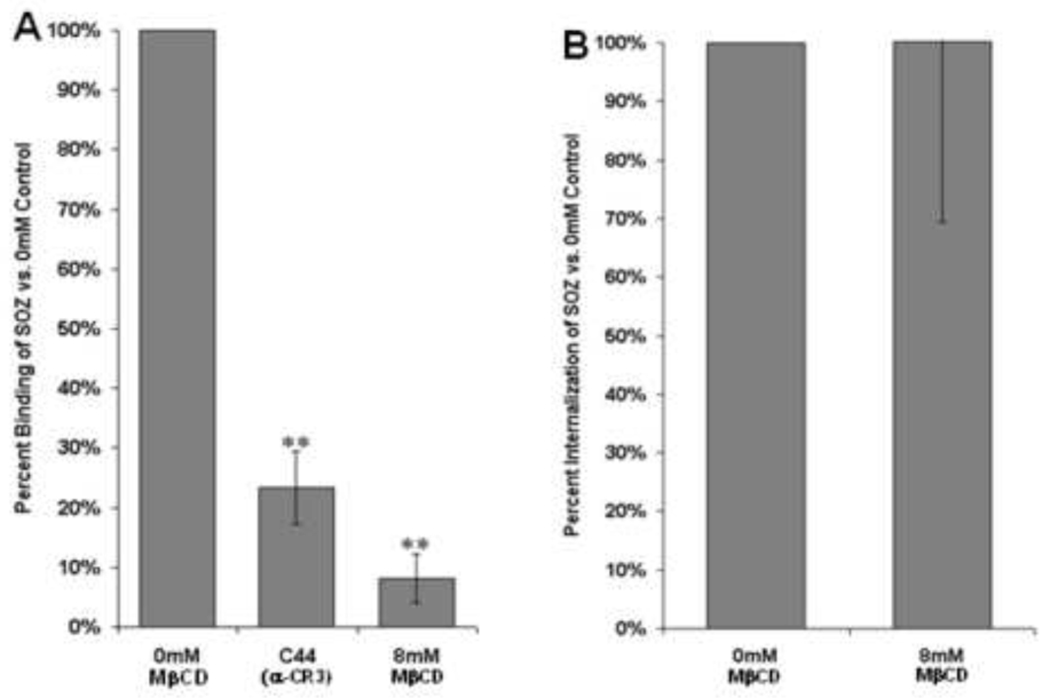
(A) Binding of serum-opsonized zymosan particles (OZ) is decreased by lipid raft disruption, similar to what is observed for FcγRIIA (N=3, 300+ cells/sample/condition). (B) Internalization of serum opsonized zymosan particles determined by trypan blue staining is not affected by disrupting lipid raft with MβCD (N=3, 300+ cells/sample/condition). (* p<0.05 compared to control, ** p<0.005 compared to control)
Results observed in the CHO-IIA model system are similar in THP-1 human monocyte/macrophage-like cells
In order to test the physiological relevance of these observations using a model system, similar experiments were performed in a model human macrophage cell line (THP-1). THP-1 cells have been used as a model for observing the role of lipid rafts and their association with various surface receptors [43; 44; 45]. Interestingly, similar results were obtained for binding and internalization as those observed in CHO-IIA cells at each MβCD concentration tested (Figure 5, A and B). Values for loss of binding mimicked the differential lipid raft requirement for large and small targets (OB and FITC-Ha-IgG respectively) as in FcγRIIA-expressing CHO cells (Figure 5A). Internalization studies yield a similar trend, though both the loss of phagocytosis of OB and endocytosis of FITC-HAIgG were dampened compared to the CHO-IIA data. Compared to blocking with IV.3, similar levels of inhibition are achieved suggesting that loss of binding and internalization is most likely due to FcγRIIA activity. However, additional differences may be due to interference from other FcγR such as FcγRI and FcγRIII. Furthermore, since FcγRIIA and CR3 interact and can trigger internalization signals on behalf of one another, these buffeted observations are not surprising [46].
Figure 5. Differentiated THP-1 cells display similar characteristics in lipid raft utilization as CHO cells.

Dependency on lipid rafts for the binding (A) and internalization (B) of large opsonized beads and small IgG complexes in THP-1 cells mimicked results observed in our model CHO-IIA cells. Binding (Flow Cytometry: N=4, 10,000 cells each sample condition. Microscopy: N=3, 300+ cells/sample/condition). Internalization (Flow Cytometry: N=7, 10,000 cells each sample condition. Microscopy: N=6, 300+ cells/sample/condition). (* p<0.05 compared to control, ** p<0.005 compared to control)
Discussion
Together, these data suggests two very different mechanisms for FcγRIIA function based on the size of the target itself. While the differences between phagocytic and endocytic mechanisms are well characterized [15; 47; 48; 49], there is little data suggesting the rationale behind initiation of either of these processes. We propose that the mode of internalization is determined very early in the process of engulfment. The necessity for lipid rafts suggests they may contribute to the direction and ability of FcRs to facilitate the engulfment of particles of drastically different sizes. While being crucial for phagocytosis of large particles, lipid rafts appear to be non-essential for FcγRIIA mediated endocytosis.
Interestingly, fractionation experiments revealed that ∼50% of the total cellular FcγRIIA is associated with lipid rafts following exposure of CHO-IIA cells to opsonized erythrocytes (EA). Given this fraction of the receptor exhibited to translocate to lipid rafts, it raises the question of how removal of roughly 50% of cellular cholesterol by 8mM MβCD is capable of nearly abolishing the ability of the receptor to bind phagocytic targets and significantly disrupt its ability to internalize them. Considering that the size of the large targets used in this study is 4.5µm in diameter, combined with the average size of our CHO-IIA cells (20µm diameter) suggests roughly 5% of the total cell membrane being necessary to form a phagosome around an individual target. As we added target to the cells at a ratio of 10:1 respectively, and generally observed 3–5 targets per cell bound under normal conditions, the 50% translocation of FcγRIIA to lipid rafts is more than enough to facilitate phagocytosis. This is verified by our findings that the affects of lipid raft disruption on FcγRIIA are concentration-dependent, suggesting that activation of the receptor and translocation to lipid rafts is a localized, not a global event.
FcγRs utilize detergent resistant microdomains or lipid rafts to mediate effector activities [23; 33; 50; 51; 52; 53; 54; 55]. Work by numerous laboratories have investigated essential components of the receptor needed to translocate to lipid rafts including a cytoplasmic cysteine residue (C208A) and the transmembrane domain (A224S)[30; 31; 33]. Consistent with both of these studies, we have shown that lipid rafts are essential for receptor mediated events prior to signaling. For example, we have shown that binding of both small IgG complexes and larger IgG coated targets are inhibited by the addition of MβCD similar to previous reports[33]. This observation suggests that some form of receptor organization needs to be present in order for efficient target binding. However, disruption of lipid rafts after ligand binding drastically inhibits only internalization of IgG coated targets such as erythrocytes or latex beads but not small IgG complexes. These results are consistent with previous observations suggesting that entry of small ligands is through clathrin coated pits versus phagocytosis which is actin dependent[13; 56]. Interestingly, lipid rafts do not play a significant role in phagocytosis by all receptors. As shown in this study, binding but not phagocytosis of SOZ by CR3 is dependent on lipid rafts which is consistent with previous finding [41].
Similar observation of descrepancies between FcγRIIA mediated endocytosis and phagocytosis have implicated differential use of kinases[14]. FcγRIIA mediated phagocytosis is triggered by phosphorylation of ITAM tyrosines by members of the src family of tyrosine kinases. Phosphorylation of the ITAM creates a SH2 binding site required for syk binding and activation. Inhibition of this process by mutating ITAM tyrosines, treating cells with the src inhibitors PP1/2 or treatment with the syk inhibitor piceatannol results in loss of phagocytic capacity but has limited if any affect on FcγRIIA triggered endocytosis[14].
Eloquent studies by Grinstein et al. have suggested that many proteins are targeted to the plasma membrane through interaction of the cationic C-2 domain with the negatively charged inner leaflet of the plasma membrane[57; 58]. This negative charge is largely contributed to by phosphatidylserine[57; 58]. Additional studies claim that PS prefers liquid ordered domains which are commonly referred to as lipid rafts[59]. These two observations suggest that lipid rafts, composed of PS on the inner leaflet, attract C-2 containing src family members and thus contribute to the lipid raft dependency of phagocytic signaling.
Further studies will be needed to determine the molecular mechanism(s) by which lipid rafts participate in discrimination between endocytosis and phagocytosis.
Acknowledgments
This work is supported by grants from NHLBI (A.D.S.) and an Arthritis Foundation Investigator Award (R.G.W.). The authors would like to thank the Advanced Microscopy and Imaging Center (AMIC) and Flow Cytometry Core facility at the University of Toledo for technical assistance and use of the equipment.
Abbreviations
- CR3
complement receptor type 3
- EA
IgG coated erythrocyte
- FcγRIIA
IgG receptor type 2A
- haIgG
heat-aggregated IgG
- IC
immune complex
- OB
IgG coated latex bead
- MβCD
methyl-β-cyclodextrin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship
J.A.V. performed experiments and wrote the manuscript, M.K.K., X.Q.P. performed experiments, A.D.S. contributed crucial reagents, R.G.W. designed the project, analyzed data and edited the manuscript.
References
- 1.Gavin AL, Barnes N, Dijstelbloem HM, Hogarth PM. Identification of the mouse IgG3 receptor: implications for antibody effector function at the interface between innate and adaptive immunity. J Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 2.Joshi T, Butchar JP, Tridandapani S. Fcgamma receptor signaling in phagocytes. Int J Hematol. 2006;84:210–216. doi: 10.1532/IJH97.06140. [DOI] [PubMed] [Google Scholar]
- 3.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 4.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 5.Ganesan LP, Joshi T, Fang H, Kutala VK, Roda J, Trotta R, Lehman A, Kuppusamy P, Byrd JC, Carson WE, Caligiuri MA, Tridandapani S. FcgammaR-induced production of superoxide and inflammatory cytokines is differentially regulated by SHIP through its influence on PI3K and/or Ras/Erk pathways. Blood. 2006;108:718–725. doi: 10.1182/blood-2005-09-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamichi T, Koyama J. Two distinct types of Fc receptor for IgG on guinea pig macrophages activate the NADPH oxidase through different signal-transduction pathways. Biochem Biophys Res Commun. 1990;172:223–228. doi: 10.1016/s0006-291x(05)80197-4. [DOI] [PubMed] [Google Scholar]
- 7.Tamoto K, Koyama J. Superoxide anion production from guinea pig macrophages stimulated with immune complexes of different IgG subclasses. J Biochem. 1980;87:1649–1657. doi: 10.1093/oxfordjournals.jbchem.a132909. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi V, Zhang SC, Castoreno AB, Stockinger W, Shieh EC, Vyas JM, Frickel EM, Nohturfft A. Immunoglobulin G signaling activates lysosome/phagosome docking. Proc Natl Acad Sci U S A. 2006;103:18226–18231. doi: 10.1073/pnas.0609182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 10.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 11.Van den Herik-Oudijk IE, Ter Bekke MW, Tempelman MJ, Capel PJ, Van de Winkel JG. Functional differences between two Fc receptor ITAM signaling motifs. Blood. 1995;86:3302–3307. [PubMed] [Google Scholar]
- 12.Worth RG, Schreiber AD. Fc Receptor Phagocytosis. Springer. 2005 [Google Scholar]
- 13.Tse SM, Furuya W, Gold E, Schreiber AD, Sandvig K, Inman RD, Grinstein S. Differential role of actin clathrinand dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003;278:3331–3338. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- 14.Huang ZY, Barreda DR, Worth RG, Indik ZK, Kim MK, Chien P, Schreiber AD. Differential kinase requirements in human and mouse Fc-gamma receptor phagocytosis and endocytosis. J Leukoc Biol. 2006;80:1553–1562. doi: 10.1189/jlb.0106019. [DOI] [PubMed] [Google Scholar]
- 15.Booth JW, Kim MK, Jankowski A, Schreiber AD, Grinstein S. Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. Embo J. 2002;21:251–258. doi: 10.1093/emboj/21.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worth RG, Mayo-Bond L, Kim MK, van de Winkel JG, Todd RF, 3rd, Petty HR, Schreiber AD. The cytoplasmic domain of FcgammaRIIA (CD32) participates in phagolysosome formation. Blood. 2001;98:3429–3434. doi: 10.1182/blood.v98.12.3429. [DOI] [PubMed] [Google Scholar]
- 17.Indik ZK, Park JG, Hunter S, Mantaring M, Schreiber AD. Molecular dissection of Fc gamma receptor-mediated phagocytosis. Immunol Lett. 1995;44:133–138. doi: 10.1016/0165-2478(94)00204-5. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell MA, Huang MM, Chien P, Indik ZK, Pan XQ, Schreiber AD. Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor Fc gamma RIIA: effect on receptor tyrosine phosphorylation and phagocytosis. Blood. 1994;84:1753–1759. [PubMed] [Google Scholar]
- 19.Indik ZK, Mitchell MA, Chien P, Schreiber AD. Structural requirements for phagocytosis by the human Fc receptor Fc gamma RIIA. Trans Assoc Am Physicians. 1993;106:77–85. [PubMed] [Google Scholar]
- 20.Worth RG, Kim MK, Kindzelskii AL, Petty HR, Schreiber AD. Signal sequence within Fc{gamma}RIIA controls calcium wave propagation patterns: Apparent role in phagolysosome fusion. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.0836650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alijani A, Hanna GB, Cuschieri A. Abdominal wall lift versus positive-pressure capnoperitoneum for laparoscopic cholecystectomy: randomized controlled trial. Ann Surg. 2004;239:388–394. doi: 10.1097/01.sla.0000114226.31773.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoessli DC, Ilangumaran S, Soltermann A, Robinson PJ, Borisch B, Nasir Ud D. Signaling through sphingolipid microdomains of the plasma membrane: the concept of signaling platform. Glycoconj J. 2000;17:191–197. doi: 10.1023/a:1026585006064. [DOI] [PubMed] [Google Scholar]
- 23.Kono H, Suzuki T, Yamamoto K, Okada M, Yamamoto T, Honda Z. Spatial raft coalescence represents an initial step in Fc gamma R signaling. J Immunol. 2002;169:193–203. doi: 10.4049/jimmunol.169.1.193. [DOI] [PubMed] [Google Scholar]
- 24.Flaswinkel H, Barner M, Reth M. The tyrosine activation motif as a target of protein tyrosine kinases and SH2 domains. Semin Immunol. 1995;7:21–27. doi: 10.1016/1044-5323(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 25.Ghazizadeh S, Bolen JB, Fleit HB. Tyrosine phosphorylation and association of Syk with Fc gamma RII in monocytic THP-1 cells. Biochem J. 1995;305(Pt 2):669–674. doi: 10.1042/bj3050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg S. Modular components of phagocytosis. J Leukoc Biol. 1999;66:712–717. doi: 10.1002/jlb.66.5.712. [DOI] [PubMed] [Google Scholar]
- 27.Hunter S, Kamoun M, Schreiber AD. Transfection of an Fc gamma receptor cDNA induces T cells to become phagocytic. Proc Natl Acad Sci U S A. 1994;91:10232–10236. doi: 10.1073/pnas.91.21.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt W, Gorczytza H, Butterweck A, Monkemann H, Frey J. Structural requirements of the cytoplasmic domains of the human macrophage Fc gamma receptor IIa and B cell Fc gamma receptor IIb2 for the endocytosis of immune complexes. Eur J Immunol. 1991;21:2227–2238. doi: 10.1002/eji.1830210934. [DOI] [PubMed] [Google Scholar]
- 29.Ukkonen P, Lewis V, Marsh M, Helenius A, Mellman I. Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J Exp Med. 1986;163:952–971. doi: 10.1084/jem.163.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Garcia E, Brown EJ, Rosales C. Transmembrane mutations to FcgammaRIIA alter its association with lipid rafts: implications for receptor signaling. J Immunol. 2007;178:3048–3058. doi: 10.4049/jimmunol.178.5.3048. [DOI] [PubMed] [Google Scholar]
- 31.Barnes NC, Powell MS, Trist HM, Gavin AL, Wines BD, Hogarth PM. Raft localisation of FcgammaRIIa and efficient signaling are dependent on palmitoylation of cysteine 208. Immunol Lett. 2006;104:118–123. doi: 10.1016/j.imlet.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Mousavi SA, Sporstol M, Fladeby C, Kjeken R, Barois N, Berg T. Receptor-mediated endocytosis of immune complexes in rat liver sinusoidal endothelial cells is mediated by FcgammaRIIb2. Hepatology. 2007;46:871–884. doi: 10.1002/hep.21748. [DOI] [PubMed] [Google Scholar]
- 33.Bournazos S, Hart SP, Chamberlain LH, Glennie MJ, Dransfield I. Association of FcgammaRIIa (CD32a) with lipid rafts regulates ligand binding activity. J Immunol. 2009;182:8026–8036. doi: 10.4049/jimmunol.0900107. [DOI] [PubMed] [Google Scholar]
- 34.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 35.Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp Hematol. 2006;34:1490–1495. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Barenholz Y. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell Biochem. 2004;37:167–215. doi: 10.1007/978-1-4757-5806-1_5. [DOI] [PubMed] [Google Scholar]
- 37.Ostrom RS, Liu X. Detergent and detergent-free methods to define lipid rafts and caveolae. Methods Mol Biol. 2007;400:459–468. doi: 10.1007/978-1-59745-519-0_30. [DOI] [PubMed] [Google Scholar]
- 38.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romanenko VG, Fang Y, Byfield F, Travis AJ, Vandenberg CA, Rothblat GH, Levitan I. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys J. 2004;87:3850–3861. doi: 10.1529/biophysj.104.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh FK, Chalut C, Lopez A, Guilhot C. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 2009;5:1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Cabec V, Carreno S, Moisand A, Bordier C, Maridonneau-Parini I. Complement receptor 3 (CD11b/CD18) mediates type I type IIphagocytosis during nonopsonic, opsonic phagocytosis respectively. J Immunol. 2002;169:2003–2009. doi: 10.4049/jimmunol.169.4.2003. [DOI] [PubMed] [Google Scholar]
- 42.Peyron P, Bordier C, N’Diaye EN, Maridonneau-Parini I. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J Immunol. 2000;165:5186–5191. doi: 10.4049/jimmunol.165.9.5186. [DOI] [PubMed] [Google Scholar]
- 43.Cuschieri J. Implications of lipid raft disintegration: enhanced anti-inflammatory macrophage phenotype. Surgery. 2004;136:169–175. doi: 10.1016/j.surg.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Cuschieri J, Bulmus V, Gourlay D, Garcia I, Hoffman A, Stayton P, Maier RV. Modulation of macrophage responsiveness to lipopolysaccharide by IRAK-1 manipulation. Shock. 2004;21:182–188. doi: 10.1097/01.shk.0000111828.07309.26. [DOI] [PubMed] [Google Scholar]
- 45.Cuschieri J, Umanskiy K, Solomkin J. PKC-zeta is essential for endotoxin-induced macrophage activation. J Surg Res. 2004;121:76–83. doi: 10.1016/j.jss.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Worth RG, Mayo-Bond L, van de Winkel JG, Todd RF, 3rd, Petty HR. CR3 (alphaM beta2; CD11b/CD18) restores IgG-dependent phagocytosis in transfectants expressing a phagocytosis-defective Fc gammaRIIA (CD32) tail-minus mutant. J Immunol. 1996;157:5660–5665. [PubMed] [Google Scholar]
- 47.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 48.Allen LA, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40. doi: 10.1016/s0952-7915(96)80102-6. [DOI] [PubMed] [Google Scholar]
- 49.Walters MN, Papadimitriou JM. Phagocytosis: a review. CRC Crit Rev Toxicol. 1978;5:377–421. doi: 10.3109/10408447809081012. [DOI] [PubMed] [Google Scholar]
- 50.Hinkovska-Galcheva V, Boxer LA, Kindzelskii A, Hiraoka M, Abe A, Goparju S, Spiegel S, Petty HR, Shayman JA, Ceramide 1-phosphate. a mediator of phagocytosis. J Biol Chem. 2005;280:26612–26621. doi: 10.1074/jbc.M501359200. [DOI] [PubMed] [Google Scholar]
- 51.Mansfield PJ, Hinkovska-Galcheva V, Borofsky MS, Shayman JA, Boxer LA. Phagocytic signaling molecules in lipid rafts of COS-1 cells transfected with FcgammaRIIA. Biochem Biophys Res Commun. 2005;331:132–138. doi: 10.1016/j.bbrc.2005.02.191. [DOI] [PubMed] [Google Scholar]
- 52.Kwiatkowska K, Frey J, Sobota A. Phosphorylation of FcgammaRIIA is required for the receptor-induced actin rearrangement and capping: the role of membrane rafts. J Cell Sci. 2003;116:537–550. doi: 10.1242/jcs.00254. [DOI] [PubMed] [Google Scholar]
- 53.Korzeniowski M, Kwiatkowska K, Sobota A. Insights into the association of FcgammaRII and TCR with detergent-resistant membrane domains: isolation of the domains in detergent-free density gradients facilitates membrane fragment reconstitution. Biochemistry. 2003;42:5358–5367. doi: 10.1021/bi027135x. [DOI] [PubMed] [Google Scholar]
- 54.Abdel Shakor AB, Kwiatkowska K, Sobota A. Cell surface ceramide generation precedes and controls FcgammaRII clustering and phosphorylation in rafts. J Biol Chem. 2004;279:36778–36787. doi: 10.1074/jbc.M402170200. [DOI] [PubMed] [Google Scholar]
- 55.Strzelecka-Kiliszek A, Korzeniowski M, Kwiatkowska K, Mrozinska K, Sobota A. Activated FcgammaRII and signalling molecules revealed in rafts by ultra-structural observations of plasma-membrane sheets. Mol Membr Biol. 2004;21:101–108. doi: 10.1080/09687680310001639094. [DOI] [PubMed] [Google Scholar]
- 56.Mero P, Zhang CY, Huang ZY, Kim MK, Schreiber AD, Grinstein S, Booth JW. Phosphorylation-independent ubiquitylation and endocytosis of Fc gammaRIIA. J Biol Chem. 2006;281:33242–33249. doi: 10.1074/jbc.M605372200. [DOI] [PubMed] [Google Scholar]
- 57.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 58.Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, Levine T, Kapus A, Grinstein S. Receptor activation alters inner surface potential during phagocytosis. Science. 2006;313:347–351. doi: 10.1126/science.1129551. [DOI] [PubMed] [Google Scholar]
- 59.Bakht O, Pathak P, London E. Effect of the structure of lipids favoring disordered domain formation on the stability of cholesterol-containing ordered domains (lipid rafts): identification of multiple raft-stabilization mechanisms. Biophys J. 2007;93:4307–4318. doi: 10.1529/biophysj.107.114967. [DOI] [PMC free article] [PubMed] [Google Scholar]