Abstract
The cJun NH2-terminal kinase isoform JNK1 is implicated in the mechanism of obesity-induced insulin resistance. Feeding a high fat diet causes activation of the JNK1 signaling pathway, insulin resistance, and obesity in mice. Germ-line ablation of Jnk1 prevents both diet-induced obesity and insulin resistance. Genetic analysis indicates that the effects of JNK1 on insulin resistance can be separated from effects of JNK1 on obesity. Emerging research indicates that JNK1 plays multiple roles in the regulation of insulin resistance, including altered gene expression, hormone/cytokine production, and lipid metabolism. Together, these studies establish JNK1 as a potential pharmacological target for the development drugs that might be useful for the treatment of insulin resistance, metabolic syndrome, and type 2 diabetes.
JNK1 plays a central role in obesity-induced insulin resistance
Obesity is a critical risk factor in the development of insulin resistance [1]. Importantly, insulin resistance can cause hyperinsulinemia and β-cell hypertrophy that precede β-cell failure and type 2 diabetes. Risk factors that cause obesity, including diet and a sedentary life-style, represent major threats to human health in the 21st century [2]. Although the mechanisms that account for insulin resistance are incompletely understood, roles for inflammation and altered lipid metabolism have been identified [3]. Indeed, obesity causes chronic low-grade inflammatory responses that lead to activation of stress pathways (including the cJun NH2-terminal kinase JNK1) that play critical roles in the etiology of obesity-induced insulin resistance [4–7].
The JNK group of signaling proteins is encoded by three genes [8]. Jnk1 and Jnk2 are expressed ubiquitously whereas Jnk3 is expressed in a more limited number of tissues, including brain and heart [8]. It is the JNK1 isoform that has primarily been implicated in the development of obesity and insulin resistance [9], although JNK2 might also play a contributing role [10]. Disruption of JNK1 function in mice by targeted ablation of the Jnk1 gene, knockdown of Jnk1 gene expression with short hairpin RNA (shRNA) or antisense oligonucleotides, or inhibition of JNK activity using pharmacological inhibitors, protect against obesity-induced insulin resistance [9, 11–15]. These studies demonstrate that obesity causes chronic JNK1 activation and that JNK1 might be a direct cause of insulin resistance.
Studies of mice with tissue-specific Jnk1-deficiency have provided important new insight into the mechanism of JNK1 function. In addition, mechanisms that mediate JNK1 activation caused by metabolic stress have been defined. Here we review recent progress towards understanding the role of JNK1 in diet-induced obesity and insulin resistance.
Diet-induced JNK1 activation
Feeding mice a high fat diet (HFD) causes activation of JNK1 in insulin responsive tissues, including fat, muscle, and liver [9]. JNK1 is activated by the mitogen-activated protein kinase kinases MKK4 and MKK7 [16], but the mechanism that triggers this pathway in response to a HFD is incompletely understood. Four potential mechanisms of obesity-induced JNK1 activation have been reported. First, feeding a HFD (or exposure of cells to saturated fatty acids in vitro) causes endoplasmic reticulum stress [17] and induction of the unfolded protein response (UPR) pathway leading to JNK1 activation [18] by a mechanism that requires the double-stranded RNA-dependent protein kinase (PKR) [19]. Second, saturated fatty acids might act as ligands for Toll-like receptors that activate the JNK pathway [20, 21]. Third, saturated fatty acids can activate the JNK pathway by a mechanism that involves protein kinase C-mediated activation of the mixed-lineage protein kinase (MLK) group of MAP kinase kinase kinases [22] and subsequent JNK activation mediated by the JIP1 scaffold protein [23]. Fourth, HFD-induced insulin resistance is associated with chronic low-grade inflammation and expression of inflammatory cytokines that can cause JNK activation [8], including tumor necrosis factor [24]. The relative contribution and mechanistic relationships between these four pathways remains to be determined.
Role of obesity in the regulation of JNK1-mediated insulin resistance
The JNK1 protein kinase is implicated in the development of insulin resistance. Indeed, Jnk1−/− mice are protected against HFD-induced insulin resistance [9]. However, Jnk1−/− mice are also resistant to HFD-induced obesity [9]. This failure to become obese might be sufficient to account for the effects of germ-line Jnk1 gene ablation on insulin resistance. However, recent studies have confirmed the conclusion that JNK1 can contribute to HFD-induced insulin resistance independently of the effects of JNK1 on obesity. Specifically, feeding a HFD to mice with tissue-specific Jnk1-deficiency in insulin target tissues (e.g. fat and muscle) causes normal levels of obesity, yet these mice exhibit increased insulin sensitivity compared with control mice [25, 26].
The mechanism that accounts for JNK1-mediated insulin resistance was initially considered to be mediated by phosphorylation of the insulin receptor adapter protein IRS1 on Ser-307, a phosphorylation site that disrupts the interaction of the IRS1 phosphotyrosine binding (PTB) domain with the tyrosine phosphorylated insulin receptor [27–29]. JNK1-mediated phosphorylation of IRS1 could therefore be a direct cause of insulin resistance. This conclusion is strongly supported by in vitro analysis and studies using cell culture models [27–29]. However, in vivo analysis demonstrates that Ser-307 of IRS1 is not essential for the development of insulin resistance [30]. Nevertheless, it remains possible that JNK-mediated phosphorylation of IRS1 might be a contributing factor during the development of insulin resistance in the context of tissue-specific and multi-site regulatory phosphorylation of IRS proteins [31–35]. Further studies of the role of IRS phosphorylation are warranted. However, it has become clear that IRS-independent mechanisms also play important roles as mediators of insulin resistance [36]. These considerations indicate that JNK targets multiple nodes within the insulin signaling network to cause insulin resistance.
JNK1 in myeloid cells and diet-induced insulin resistance
JNK1 might play a critical role in macrophages during the development of insulin resistance. Thus, JNK1 could influence the infiltration of adipose tissue by macrophages and could also alter the expression of inflammatory cytokines (e.g. tumor necrosis factor [37]) that are implicated as mediators of insulin resistance. Two different approaches have been employed to test the role of JNK1 in macrophages. First, bone marrow transplantation assays have been used to create chimeric mice with a Jnk1-deficient hematopoietic system. This approach has not led to definitive conclusions because inconsistent results were obtained by studies performed by three independent groups: two studies reported that Jnk1-deficient hematopoietic cells caused no change in HFD-induced insulin resistance [25, 38]; and one study reported that Jnk1-deficient hematopoietic cells protected mice against HFD-induced insulin resistance [39]. Further studies will be required to resolve these different conclusions. A second approach to address this question required the generation of mice with myeloid-specific Jnk1 ablation [25]. Feeding a HFD caused similar insulin resistance in control mice and mice with Jnk1-deficient myeloid cells [25]. Together, these studies do not support a major role for JNK1 in macrophages during HFD-induced insulin resistance, but further studies are warranted to confirm this conclusion.
Adipose tissue JNK1 regulates insulin resistance
Adipose tissue is an important insulin target tissue that functions as a depot for the storage of fuel molecules in the form of triglycerides. In addition, adipocytes serve a critical endocrine function for the regulation of metabolism because they secrete hormones (adipokines) and bioactive lipids that can regulate insulin resistance and other physiological processes, including satiety. Feeding a HFD to mice causes increased adiposity – an expansion of adipocyte mass and also infiltration of adipose tissue by macrophages. Interactions between adipocytes and macrophages contribute to the development of obesity-induced insulin resistance [40]. Indeed, adipocytes play a key role in obesity-induced activation of adipose tissue macrophages [41, 42].
The role of JNK1 in adipocytes has been examined using mice with tissue-specific Jnk1-deficiency [25]. These mice were protected against the development of insulin resistance when fed a HFD [25]. The increased insulin sensitivity of Jnk1-deficient adipocytes was demonstrated by analysis of glucose uptake and measurement of insulin-stimulated tyrosine phosphorylation of IRS1 and increased insulin-stimulated activation of AKT [25]. This effect was tissue-specific because Jnk1-deficiency in adipocytes caused no change in insulin sensitivity of muscle [25]. These in vivo results confirm previous studies using RNAi-mediated knockdown of JNK expression in 3T3-L1 adipocytes in vitro [43, 44]. The mechanism that accounts for the increased insulin sensitivity of Jnk1-deficient adipocytes remains unclear. One contributing factor might be a reduction in IRS1 phosphorylation [25]. However, altered triglyceride metabolism, including lipolysis and re-esterification, might also contribute to the effects of JNK1 on insulin resistance [45]. Further studies are required to define the mechanism of Jnk1-mediated insulin resistance in adipocytes.
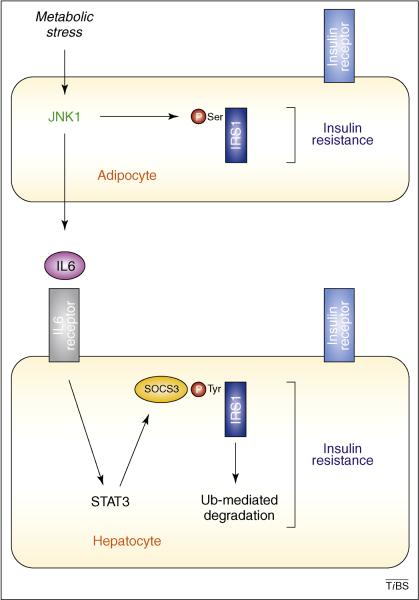
Unexpectedly, mice with Jnk1-deficient adipocytes exhibit dramatically improved hepatic insulin sensitivity [25]. This effect of JNK1 on the liver is non-cell autonomous and is mediated by loss of JNK1 in adipocytes. The mechanism, in part, reflects a requirement of JNK1 for expression of the adipokine interleukin 6 (IL6) in response to feeding a HFD [25]. Mice with adipocyte Jnk1-deficiency failed to show increased amounts of IL6 in the blood when fed a HFD. Moreover, these HFD-fed mice exhibited decreased IL6 target gene expression in the liver compared with HFD-fed control mice. Reconstitution experiments demonstrated that IL6 treatment complemented the effects of adipocyte Jnk1-deficiency on hepatic insulin resistance [25]. Importantly, IL6 can cause hepatic insulin resistance by increasing the expression of SOCS3 [46], an adapter protein that can bind IRS1 and the insulin receptor, and can target IRS1 for ubiquitin-mediated degradation [46–50].
The metabolic role of IL6 is controversial because this cytokine has multiple functions, including tissue-specific effects on glucose metabolism and insulin signaling in different peripheral tissues [51, 52]. IL6 can mediate some central actions of insulin signaling in the brain [53], and it also regulates the hypothalamic-pituitary-adrenal axis [54]. Nevertheless, the concentration of IL6 in the blood of obese, diabetic subjects is increased [1], and changes in the circulating concentration of IL6 in the blood can regulate hepatic insulin sensitivity [55–57]. These observations correlate with the finding that ablation of the IL6 target gene Socs3 in the liver of young mice causes improved hepatic insulin sensitivity [58]. Together, these findings support the conclusion that IL6 mediates, in part, the effect of adipocyte JNK1 on hepatic insulin resistance (Figure 1). A key test of this hypothesis will be to compare the hepatic phenotype of mice with adipocyte-specific ablation of the Il6 and Jnk1 genes. Moreover, it will be important to evaluate possible roles of other JNK1-dependent adipokines which might contribute to the hepatic phenotype of adipose tissue-specific Jnk1-deficient mice.
Figure 1. Role of JNK1 in adipocytes.
Feeding a high fat diet (HFD) causes JNK1 activation in adipocytes, leading to insulin resistance in adipocytes and expression of the adipokine IL6. JNK-mediated phosphorylation of IRS1 might contribute to insulin resistance in adipocytes. The increased concentration of IL6 in the blood up-regulates expression of SOCS3, a protein that targets IRS1 for ubiquitin-mediated degradation and hepatic insulin resistance.
Muscle JNK1 regulates insulin resistance
Glucose uptake by skeletal muscle represents an important mechanism of insulin-stimulated glucose disposal [59]. In obese mice, muscle could therefore be a site of JNK1 function, regulating insulin sensitivity and glucose homeostasis. Indeed, shRNA-mediated knock-down of JNK in cultured myotubes causes increased fatty acid oxidation and increased conversion of glucose to the storage form glycogen [60].
To test the hypothesis that muscle JNK1 plays a key role in vivo, mice were created with Jnk1-deficient muscle [26]. Feeding a HFD caused similar obesity in control and muscle-specific Jnk1-deficient mice [26]. Muscle Jnk1-deficiency selectively protected muscle, but not liver or adipose tissue, against HFD-induced insulin resistance [26]. The mechanism of protection against muscle insulin resistance is unclear, but might reflect decreased phosphorylation of IRS1 [26]. This idea is consistent with a study showing improved muscle insulin sensitivity of transgenic mice expressing a phosphorylation-defective IRS1 protein in muscle [61]. Further studies are required to more fully define the role of IRS1 phosphorylation in Jnk1-mediated muscle insulin resistance.
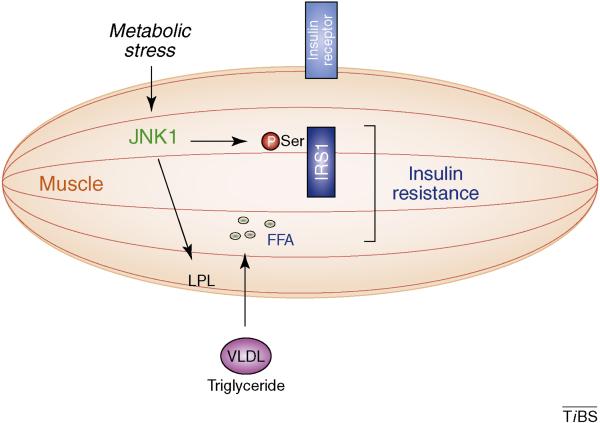
Muscle Jnk1-deficiency causes a large increase in the concentration of triglyceride in the blood [26], a change which is associated with hepatic steatosis and adipose tissue inflammation [26]. This increased blood triglyceride concentration is mediated, in part, by reduced expression of muscle lipoprotein lipase (LPL) [26]. Indeed, muscle Lpl-deficiency causes increased blood triglyceride levels and redistribution of triglyceride to non-muscle tissues [62]. Redistribution of triglycerides in these muscle Lpl-deficient mice results in increased insulin sensitivity in muscle and decreased insulin sensitivity in other tissues, including liver and adipose tissue [62]. The phenotype is similar to muscle-specific Jnk1-deficient mice. Together, these studies suggest that reduced muscle LPL expression might contribute to the increased muscle insulin sensitivity detected in mice with muscle-specific Jnk1-deficiency (Figure 2).
Figure 2. Role of JNK1 in muscle.
Feeding a high fat diet (HFD) causes JNK1 activation in muscle and insulin resistance. Loss of JNK1 causes decreased expression of lipoprotein lipase (LPL), an enzyme that promotes absorption of triglycerides (e.g. VLDL; very low density lipoprotein). Increased accumulation of fatty acids (FFA) and increased phosphorylation of IRS1 might contribute to JNK1-mediated insulin resistance.
Hepatic JNK1 does not contribute to insulin resistance
The liver is an organ that makes critical contributions to the normal regulation of glucose homeostasis. Indeed, the ability of insulin to inhibit hepatic gluconeogenesis is an important aspect of insulin-stimulated clearance of blood glucose. The liver is therefore a potential site of metabolic regulation by JNK1. Studies using adenoviral vectors to disrupt JNK1 signaling in the liver using dominant-negative JNK [12] or Jnk shRNA [63] suggest that hepatic JNK1 also participates in negative regulation of hepatic insulin signalling. Furthermore, transgenic expression of the MAP kinase phosphatase DUSP-9 in the liver suppresses MAP kinase activation, including JNK, and increases insulin sensitivity [64]. These data indicate that JNK1 might play a critical role in the development of hepatic insulin resistance. However, hepatocyte-specific Jnk1 ablation does not protect mice against HFD-induced insulin resistance [65]. Contrary to predictions, these mice show glucose intolerance, insulin resistance, and hepatic steatosis. These phenotypes are similar to those caused by the IRS1 Ser307Ala substitution in vivo [30]. It is therefore possible that loss of IRS1 phosphorylation on Ser-307 in the liver of hepatocyte-specific Jnk1-deficient mice contributes to the observed insulin resistance [65]. Increased hepatic insulin clearance, associated with elevated expression of the insulin receptor and ceacam-1, represents a second mechanism that contributes to the insulin resistance caused by hepatocyte-specific Jnk1-deficiency [65]. This effect of Jnk1-deficiency in hepatocytes to promote insulin resistance contrasts with the ability of Jnk1-deficiency in muscle and adipose tissue to promote insulin sensitivity.
JNK1 in the central nervous system regulates diet-induced obesity
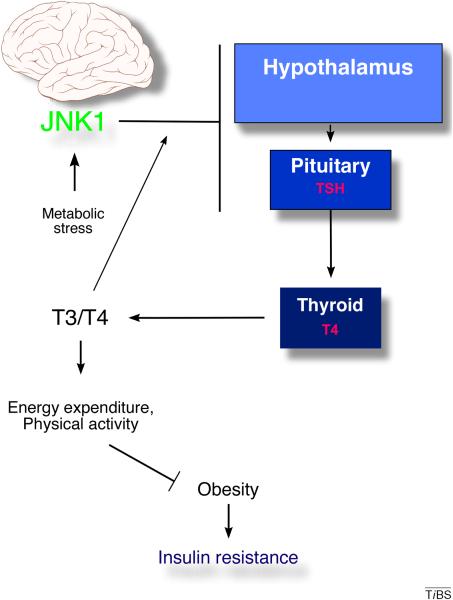
Germ-line ablation of Jnk1 protects mice against HFD-induced obesity [9]. However, mice with tissue-specific Jnk1-deficiency in adipocytes, muscle, hepatocytes, and myeloid cells do not exhibit defects in HFD-induced obesity [25, 26, 65]. The brain is a possible site of JNK1 function in the regulation of obesity owing to the role of the hypothalamus in regulating feeding behavior, physical activity, and energy expenditure [66]. Indeed, Jnk1 ablation in the mouse nervous system prevents HFD-induced obesity via slightly reduced food intake and markedly increased physical activity and energy expenditure [67, 68]. JNK1 function in the nervous system appears to be complex, but a major factor that contributes to metabolic regulation is increased signaling mediated by the hypothalamic-pituitary-thyroid axis (Figure 3). Nervous system Jnk1-deficiency caused increased expression of thyrotropin releasing hormone (TRH) in the hypothalamus, thyroid stimulating hormone (TSH) by the pituitary gland, and increased circulating levels of thyroid hormones (T3 and T4) in the blood [67, 68]. Nervous system JNK1 appears to be required for negative feed-back regulation of TRH and TSH by T3/4. Pharmacological inhibition of thyroid hormone secretion blocked the effect of nervous system Jnk1-deficiency on HFD-induced obesity [67]. The hypothalamic-pituitary-thyroid axis (and subsequent changes in energy expenditure) is therefore an important target of JNK1-mediated metabolic regulation [67, 68].
Figure 3. Role of JNK1 in the central nervous system.
Feeding a high fat diet (HFD) causes activation of JNK1 in the hypothalamus and pituitary gland. In the hypothalamus, JNK1 suppresses expression of thyrotropin releasing hormone (TRH); in the pituitary gland, JNK1 activity reduces the expression of thyroid stimulating hormone (TSH). Increased thyroid hormones (T3 and T4) in the blood act to suppress expression of TRH and TSH. Together, these changes regulate energy expenditure, physical activity and food intake. The resulting obesity contributes to insulin resistance in peripheral organs.
It should be noted that Jnk1-deficiency in the nervous system also causes defects in the expression of other pituitary hormones (e.g. growth hormone and adrenocorticotrophic hormone) that might also contribute to the metabolic phenotype of Jnk1-deficient mice [67, 68]. Moreover, defects in the regulation of hypothalamic leptin receptor expression and increased central insulin sensitivity might contribute to the altered feeding behaviour of nervous system-specific Jnk1-deficient mice [67, 68]. Further studies are required to fully establish the complete repertoire of roles of JNK1 in the brain that contribute to metabolic regulation.
Studies of mice with a Jnk1-deficient nervous system demonstrate that the hypothalamic-pituitary-thyroid axis is a critical target of JNK1 that controls obesity [67, 68]. These studies have also demonstrated that nervous system Jnk1-deficiency protects mice against HFD-induced insulin resistance [67, 68]. Interestingly, a HFD does not elicit JNK1 activation in peripheral tissues of mice with nervous system-specific Jnk1-deficiency [67, 68]. This observation suggests that the effects of nervous system Jnk1-deficiency on insulin resistance are most likely a secondary consequence of the failure of these mice to become obese.
Role of JNK1 in pancreatic β cells
Mice with selective Jnk1-deficiency in pancreatic β cells have been described, but the physiological consequence of β cell-specific Jnk1-deficiency has not been reported [67]. Nevertheless, pharmacological studies and examination of Jnk1−/− mice have demonstrated that JNK1 plays a key role in the inhibition of insulin gene transcription and β cell death observed during the development of type 2 diabetes [69]. Moreover, JNK1 mediates β cell death during islet transplantation therapy [69]. Further studies are required to identify β cell-specific roles of JNK1, and the molecular mechanisms that account for JNK1 function in the β cell, that contribute to the etiology of type 2 diabetes.
How does JNK1 regulate insulin resistance?
Germ-line ablation of Jnk1 protects mice against insulin resistance caused by feeding a HFD [9]. A major contributing factor that accounts for the protection of HFD-fed Jnk1−/− mice against insulin resistance is the failure of these mice to become obese. Indeed, obesity is a critical determinant for the development of insulin resistance [1]. This effect of Jnk1-deficiency to prevent obesity is mediated, in part, by actions of JNK1 on the hypothalamic-pituitary-thyroid axis that cause increased energy expenditure [67, 68].
Although the function of JNK1 in the nervous system largely accounts for both the obesity and insulin resistance phenotypes of Jnk1−/− mice [67, 68], other studies show that JNK1 can regulate insulin resistance independently of obesity [25, 26, 65]. Thus, HFD-induced insulin resistance in adipose tissue and liver requires JNK1 in adipocytes [25]. Moreover, JNK1 in muscle contributes to HFD-induced muscle insulin resistance [26]. By contrast, JNK1 in hepatocytes is not required for the development of hepatic insulin resistance; indeed, JNK1 in hepatocytes might actually promote hepatic insulin sensitivity [65]. Together, these data indicate that multiple mechanisms mediate the effects of JNK1 on insulin resistance, including altered cytokine/adipokine expression [25], LPL expression [26], insulin clearance [65], and phosphorylation of IRS proteins [25, 26]. It is likely that additional mechanisms of Jnk1-regulated insulin resistance remain to be identified.
A key question that remains to be fully addressed is the role of IRS phosphorylation in Jnk1-mediated insulin resistance. Germ-line mutational analysis in mice demonstrates that the JNK phosphorylation site Ser-307 on IRS1 is not essential for insulin resistance in vivo [30]. Indeed, reconstitution studies using adenoviral delivery of IRS1 to Irs1; Irs2-deficient liver indicate that decreased phosphorylation on Ser-307 inhibits hepatic insulin sensitivity [30]. This finding is consistent with the observation that Jnk1-deficiency in hepatocytes decreases IRS1 phosphorylation on Ser-307 and causes hepatic insulin resistance [65]. However, this conclusion contrasts with previous observations concerning IRS1 phosphorylation on Ser-307 [27–29] and raises questions concerning possible tissue-specific roles of IRS1 Ser-307 phosphorylation. Could phosphorylation of Ser-307 on IRS1 cause insulin sensitivity in liver and insulin resistance in other tissues? Cell type-specific patterns of IRS1 phosphorylation could account for differences in IRS1 regulatory phosphorylation, including roles of multi-site IRS1 phosphorylation [31–35]. Thus, it is intriguing that simultaneous amino acid substitutions (leading to replacement with Ala) of the IRS1 phosphorylation sites Ser-302, Ser-307, and Ser-612 in muscle can protect mice against muscle insulin resistance [61]. Further studies are warranted to test whether tissue-specific and multi-site phosphorylation of IRS proteins might cause different effects on insulin resistance in individual tissues.
Concluding remarks
Significant progress towards understanding the roles of JNK1 in diet-induced insulin resistance has been achieved. However, molecular aspects of the mechanisms of Jnk1-mediated regulation of insulin resistance, including the significance of IRS1 phosphorylation, remain to be fully established. Moreover, the analysis of mouse mutants is subject to caveats concerning possible contributions of developmental defects and long-term (vs short-term) loss of JNK1 function. Nevertheless, it is now clear that JNK1 plays a critical role during the development of insulin resistance. JNK1 therefore represents a pharmacological target for the development drugs that might be useful for the treatment of insulin resistance, metabolic syndrome, and type 2 diabetes. Indeed, treatment of mice with JNK inhibitors has been shown to reduce hyperglycemia and to improve insulin sensitivity [13–15]. A better mechanistic understanding of JNK1-regulated insulin resistance will assist the design of JNK1-based therapeutics. Specifically, knowledge of mechanism could be used to reduce possible toxic effects of JNK1 inhibition [70]. The potential for translation of basic research on JNK to the treatment of human disease provides an exciting goal for future studies.
Acknowledgments
We thank Kathy Gemme and Catherine Mark for expert administrative assistance. The authors' studies were supported by grants from the National Institutes of Health (DK080665, CA065861 and NS054948). Roger Davis is a member of the NIDDK Diabetes and Endocrinology Research Center (P30 DK32520) at the University of Massachusetts Medical School and is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bastard JP, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 2.Zimmet P, et al. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 4.Shoelson SE, et al. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 7.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 9.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 10.Tuncman G, et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneto H, et al. Oxidative stress and the JNK pathway as a potential therapeutic target for diabetes. Drug News Perspect. 2004;17:447–453. doi: 10.1358/dnp.2004.17.7.863704. [DOI] [PubMed] [Google Scholar]
- 12.Nakatani Y, et al. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 13.Bennett BL, et al. JNK: a new therapeutic target for diabetes. Curr Opin Pharmacol. 2003;3:420–425. doi: 10.1016/s1471-4892(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 14.Kaneto H, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 15.Stebbins JL, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci U S A. 2008;105:16809–16813. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tournier C, et al. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl 7):S52–54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, et al. Double-Stranded RNA-Dependent Protein Kinase Links Pathogen Sensing with Stress and Metabolic Homeostasis. Cell. 140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukumo DM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 21.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaeschke A, et al. An essential role of the JIP1 scaffold protein for JNK activation in adipose tissue. Genes Dev. 2004;18:1976–1980. doi: 10.1101/gad.1216504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uysal KT, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 25.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabio G, et al. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguirre V, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 28.Lee YH, et al. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 29.Aguirre V, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 30.Copps KD, et al. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2009;11:84–92. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanti JF, Jager J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol. 2009;9:753–762. doi: 10.1016/j.coph.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Giraud J, et al. Phosphorylation of Irs1 at SER-522 inhibits insulin signaling. Mol Endocrinol. 2007;21:2294–2302. doi: 10.1210/me.2007-0159. [DOI] [PubMed] [Google Scholar]
- 33.Luo M, et al. Phosphorylation of human insulin receptor substrate-1 at Serine 629 plays a positive role in insulin signaling. Endocrinology. 2007;148:4895–4905. doi: 10.1210/en.2007-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo M, et al. Identification of insulin receptor substrate 1 serine/threonine phosphorylation sites using mass spectrometry analysis: regulatory role of serine 1223. Endocrinology. 2005;146:4410–4416. doi: 10.1210/en.2005-0260. [DOI] [PubMed] [Google Scholar]
- 35.Yi Z, et al. Global assessment of regulation of phosphorylation of insulin receptor substrate-1 by insulin in vivo in human muscle. Diabetes. 2007;56:1508–1516. doi: 10.2337/db06-1355. [DOI] [PubMed] [Google Scholar]
- 36.Hoehn KL, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das M, et al. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallerie SN, et al. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa W, Kasuga M. Cell signaling. Fat stress and liver resistance. Science. 2008;322:1483–1484. doi: 10.1126/science.1167571. [DOI] [PubMed] [Google Scholar]
- 41.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen MT, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 44.Kim T, et al. Knockdown of JNK rescues 3T3-L1 adipocytes from insulin resistance induced by mitochondrial dysfunction. Biochem Biophys Res Commun. 2009;378:772–776. doi: 10.1016/j.bbrc.2008.11.121. [DOI] [PubMed] [Google Scholar]
- 45.Rozo AV, et al. Silencing Jnk1 and Jnk2 accelerates basal lipolysis and promotes fatty acid re-esterification in mouse adipocytes. Diabetologia. 2008;51:1493–1504. doi: 10.1007/s00125-008-1036-6. [DOI] [PubMed] [Google Scholar]
- 46.Emanuelli B, et al. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 47.Rui L, et al. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 48.Mooney RA, et al. Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance. J Biol Chem. 2001;276:25889–25893. doi: 10.1074/jbc.M010579200. [DOI] [PubMed] [Google Scholar]
- 49.Senn JJ, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–13746. doi: 10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 50.Emanuelli B, et al. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 51.Mooney RA. Counterpoint: Interleukin-6 does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol. 2007;102:816–818. doi: 10.1152/japplphysiol.01208a.2006. discussion 818–819. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen BK, Febbraio MA. Point: Interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol. 2007;102:814–816. doi: 10.1152/japplphysiol.01208.2006. [DOI] [PubMed] [Google Scholar]
- 53.Inoue H, et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006;3:267–275. doi: 10.1016/j.cmet.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Wallenius V, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 55.Klover PJ, et al. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 56.Kim HJ, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 57.Klover PJ, et al. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417–3427. doi: 10.1210/en.2004-1468. [DOI] [PubMed] [Google Scholar]
- 58.Torisu T, et al. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells. 2007;12:143–154. doi: 10.1111/j.1365-2443.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 59.Savage DB, et al. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijayvargia R, et al. JNK Deficiency Enhances Fatty Acid Utilization and Diverts Glucose From Oxidation to Glycogen Storage in Cultured Myotubes. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2009.501. [DOI] [PubMed] [Google Scholar]
- 61.Morino K, et al. Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes. 2008;57:2644–2651. doi: 10.2337/db06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang R, et al. Liver-specific knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. J Biol Chem. 2007;282:22765–22774. doi: 10.1074/jbc.M700790200. [DOI] [PubMed] [Google Scholar]
- 64.Emanuelli B, et al. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:3545–3550. doi: 10.1073/pnas.0712275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabio G, et al. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabio G, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belgardt BF, et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci U S A. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneto H, et al. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid Redox Signal. 2007;9:355–366. doi: 10.1089/ars.2006.1465. [DOI] [PubMed] [Google Scholar]
- 70.Ijaz A, et al. Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney Int. 2009;75:381–388. doi: 10.1038/ki.2008.559. [DOI] [PubMed] [Google Scholar]