Abstract
Tuberous sclerosis complex 1 (TSC1) inhibits mammalian target of rapamycin (mTOR), a central promotor of cell growth and proliferation. The protein product of the TSC1 gene, hamartin (referred to as TSC1) is known to interact with Polo-like kinase 1 (Plk1) in a cell cycle regulated, phosphorylation-dependent manner. We hypothesized that the p53 target gene, Plk2, is a tumor suppressor, mediating its tumor suppressor function through interactions with TSC1 that facilitate TSC1/2 restraint of mTOR under hypoxic stress. We found that human lung tumor cells deficient in Plk2 grew larger than control tumors, and that Plk2 interacts with endogenous TSC1 protein. Additionally, C-terminal Plk2-GST fusion protein bound both TSC1 and TSC2 proteins. TSC1 levels were elevated in response to Adriamycin and cells transiently overexpressing Plk2 demonstrated decreased phosphorylation of the downstream target of mTOR, ribosomal protein p70S6 kinase during hypoxia. Plk2 levels were inversely correlated with cytoplasmic p70S6K phosphorylation. Plk2 levels did not increase in response to DNA damage (Adriamycin, CPT-11) when HCT 116 and H460 cells were exposed to hypoxia. TSC1-deficient mouse embryonic fibroblasts with TSC1 added back demonstrated decreased S6K phosphorylation, which was further decreased when Plk2 was transiently overexpressed. Interestingly, under normoxia, Plk2 deficient tumor cells demonstrated increased apoptosis in response to various chemotherapeutic agents including CPT-11 but increased resistance to apoptotic death after CPT-11 treatment under hypoxia, and tumor xenografts comprised of these Plk2-deficient cells were resistant to CPT-11. Our results point to a novel Plk2-TSC1 interaction with effects on mTOR signaling during hypoxia, and tumor growth that may enable targeting Plk2 signaling in cancer therapy.
Keywords: polo-like kinase (Plk2), p53, tuberous sclerosis complex 1 (TSC1), mammalian target of rapamycin (mTOR), hypoxia, p70S6 kinase (p70S6K), tuberous sclerosis complex 2 (TSC2)
Introduction
Disregulated cell growth and proliferation can promote tumori-genesis. The mammalian target of rapamycin (mTOR), often activated in neoplasias, is a central promotor of cell growth. Two multiprotein complexes are formed by mTOR, mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. The two most extensively described downstream targets of mTOR, ribosomal protein S6 kinase (p70S6K/S6K) and 4E-BP1, are regulators of mRNA translation. Tuberous sclerosis complex 1 (TSC1) and TSC2 form a heterodimer that negatively regulates mTOR, preventing the phosphorylation of S6K. Phosphorylation of S6K is routinely utilized in both research and the clinic as a biomarker of mTOR activity.1 Although the upstream regulators of TSC2 are well-characterized, much less is known about the function and regulation of TSC1. However, mutations in either TSC1 or TSC2 cause the same disease (tuberous sclerosis complex).1,2 TSC1 has been demonstrated to interact with Polo-like kinase 1 (Plk1) in cell cycle regulated, phosphorylation-dependent manner.3 We previously identified Polo-like Kinase 2 (Plk2/Snk) as a direct target for transcriptional regulation by the tumor suppressor p53 protein.4 Evidence for multiple links between the p53 and mTOR pathways has been emerging. p21 accumulation after genotoxic stress appears to be mTORC1 dependent.5 Genotoxic stress may inhibit mTOR activity through p53-dependent upregulation of known negative regulators PTEN, TSC2 and AMPKβ1 (AMP-responsive protein kinase) β1 and AMPKα.6,7 The products of two p53 target genes, Sestrin 1 and 2 activate AMPK, which phosphorylates TSC2 and stimulates its GAP activity enabling mTOR inhibition.7 Hypoxia inhibits mTOR by promoting TSC1/2 activation.1 Specifically, it has been demonstrated that it is by releasing TSC2 from growth factor-induced association with inhibitory 14-3-3 protein that hypoxia and REDD1 suppress mammalian TORC1 activity.8
Hypoxia induces expression of the p53 protein9,10 and acts as a selective pressure for the elimination of cells with wild-type p53 and expansion of cells with mutant or inactive p53 protein.10,11 p53 induced by hypoxic conditions is unable to upregulate its established transcriptional targets, due to a failure to recruit transcriptional coactivators p300/CBP.10 p53 was instead found to be complexed with the corepressor molecule mSin3a.10 Although p53 was found to be bound to the promotors of known activated target genes during hypoxia, it is a deficiency in p300/CBP that limits transactivation.12
The polo-like kinases (Plks) are a family of serine/threonine kinases that regulate both normal cell cycle progression and the cellular response to DNA damage. The polo-like kinases are characterized by an N-terminal serine/threonine kinase domain and a C-terminal polo-box domain. The four Plks that have been identified in mammalian cells are Plk1, Plk2/Snk, Plk3/Fnk/Prk and Plk4/Sak3. Plk1, the best characterized of the polo like kinases plays multiple roles in mitosis.13 Plk2, the least characterized of the polo like kinases, has been shown to play a role in centrosome duplication,14 which takes place during S-phase. Both the Plk2 and Plk3 genes were identified as serum-inducible early growth response genes.15,16 Plk1 and Plk3 genes were found to be overexpressed in Burkitt’s lymphoma cell lines lacking Plk2, consistent with possible functional degeneracy among the polo kinases.17 Functional degeneracy among the polo like kinases may account for the observation that Plk2-null mice survive with phenotypic abnormalities such as delayed S-phase entry.18
The Polo-like Kinase 2 gene (Plk2/Snk) is a direct target for transcriptional regulation by p53.4 Following stable knock-down of Plk2 in wild-type p53 expressing H460 human non-small cell lung cancer cells there was a significant increase in cell death observed in aphidicolin-treated cells and a further increase after release from aphidicolin-block. The highest levels of cell death were observed when Plk2-deficient cells were released from both aphidicolin and etoposide treatment. These results suggested that a defective S-phase checkpoint may contribute to enhanced sensitivity of Plk2-deficient cells to replication stress. Consistent with this hypothesis, we observed higher levels of Serine 139 H2AX phosphorylation in Plk2-deficient as compared to control cells before and after aphidicolin treatment indicating that there is more DNA damage when Plk2 is depleted. Plk2-deficient cells had higher levels of Chk1 protein associated with reduced levels of Serine 317-phosphorylated Chk1. In aphidicolin-treated cells, there were lower levels of Serine 317-phosphorylated Chk1 when Plk2 was knocked-down. Plk2 was demonstrated to interact with Chk2, Chk1, Serine 317-phoshorylated Chk1 and p53. Thus, increased cell death observed after aphidicolin treatment and release in Plk2-deficient cells may result from both higher levels of replication stress-induced DNA damage and a dysfunctional S-phase checkpoint.19
Plk2 transcripts were initially detected in mouse brain, heart and lung, but not in thymus, spleen, liver or kidney.20,21 A more comprehensive study determined that Plk2 mRNA levels were high in the testis, mammary gland and spleen while intermediate in the brain, heart, uterus and trachea.21,22 In response to serum stimulation Plk2 mRNA levels were found to transiently increase in various fibroblasts types and Plk2 protein levels were similarly transiently increased in NIH 3T3 cells. Although, Plk2 protein was observed to be expressed and catalytically active in the G1 phase of the cell cycle with a half-life of approximately 15 minutes,22,23 little is known about the regulation of Plk2 protein stability. Alterations in the expression of polo-like family members, Plk1 and Plk3 in human tumors have been linked to cancer prognosis.24 Plk1 has been demonstrated to be elevated in prostate25 and ovarian cancer, and clearly linked to patient prognosis in prostate,25 ovarian,24 breast,26 lung, head-neck squamous cell,27 esophageal and gastric carcinoma.28 Elevated Plk1 has been linked to cell proliferation. Plk2 loss as a result of methylation-dependent silencing of the Plk2 gene has been discovered in patients with Burkitt’s lymphoma.17 Loss of Plk2 protein in tumors may result from p53 mutations or alterations in Plk2 stability.
In the present studies we identify a novel connection between the p53 target Plk2 and signaling through the mTOR pathway. Our studies support a role for p53-regulated Plk2 as a candidate tumor suppressor gene whose encoded protein physically associates with TSC proteins to regulate checkpoint signaling and tumor growth. This work adds to the emerging complexity of links between the p53 pathway and mTOR pathway and has implications for new strategies for cancer therapy.
Results
Plk2 knock-down enhances tumor growth in vivo
To further our understanding of the function of Plk2, a target of p53, as it relates to mTOR signaling and tumor suppression, we investigated the consequences of Plk2 deficiency in human tumor cells. After stably knocking down Plk2 in H460 non-small cell lung carcinoma cells,19 six million of these cells in Matrigel per tumor site were injected subcutanteously into nude mice, (Fig. 1A). One week after inoculation, the mice were either untreated or injected intraperitoneally with 40 mg/kg of camptothecin (CPT-11), three times a week for one week, and sacrificed at day 20 (Figs. 1 and 2A). As determined by visual assessment, final tumor weight (data not shown), and caliper measurements (Fig. 2A), Plk2-deficient tumors grew larger than control tumors in both treated and untreated groups. These findings point to a role for Plk2 as a candidate tumor suppressor.
Figure 1.
Plk2-deficiency stimulates tumor growth. (A) Mice were inoculated with Plk2 deficient (or control) H460 non-small cell lung carcinoma cells. One week after inoculation, mice were treated with 40 mg/kg camptothecin, three times per week (or untreated) and sacrificed at day 20 post-inoculation. (B) Representative growth curves. (C) Representative mice with control (left flank of mouse) and Plk2 deficient (right flank of mouse) tumors at day 14.
Figure 2.
Plk2-deficiency stimulates tumor growth and camptothecin resistance in vivo not in vitro. (A) Mice bearing Plk2 deficient H460 non-small cell lung carcinoma cell xenografts (Fig. 1) were measured for tumor volume at day 20. (B) Two H460 Plk2 knock-down clones (Snk9; Snk7) and control (pSuper1) were plated at equal densities and counted every 24 hours for 7 days. Cell numbers were plotted on a log scale.
Plk2-TSC interactions implicate crosstalk between the p53 and mTOR pathways
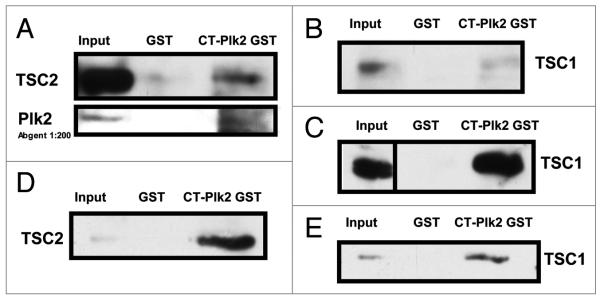
TSC1 has been demonstrated to interact with Polo-like kinase 1 (Plk1) in cell cycle-regulated, phosphorylation-dependent manner.3 It is not known whether any of the other polo-like kinases interact with TSC1 or signal through the mTOR pathway. H460 cell lysates were used for endogenous co-immunoprecipitation of TSC1 and Plk2. Using TSC1 as the immunoprecipitating antibody, both Plk2 and TSC1 were detected by immunoblotting (Fig. 3A). TSC1 and TSC2 are known to form a complex. A Carboxy-terminal Plk2 GST construct was able to pull-down both TSC1 and TSC2 in H460 cells, HCT116 cells (TSC2 data not shown) and mouse embryonic fibroblasts rescued with TSC1 (Fig. 4). Because Plk2 is a p53 target gene, the observed interaction between TSC1 and Plk2 indicates possible cross-talk between the p53 and mTOR pathways. Although TSC1 is not a known target of p53, TSC1 levels increased in response to 0.2 μg/mL Adriamycin for 20 hrs (Fig. 3B) and 10 hrs (Fig. 3C). We suggest that Plk2 is a tumor suppressor, possibly mediating its tumor suppressor function through interactions with TSC1 that facilitate TSC1/2 restraint of mTOR.
Figure 3.
p53 and mTOR pathways cross-talk: Plk2 interacts with TSC1 and inhibits down-stream mTOR signaling and DNA damage stimulates TSC1 expression. (A) Plk2-TSC1 interaction. H460 cell lysates were precleared, half the cell lysate was incubated with Anti-TSC1 antibody and the other half with equal μgrams of whole mouse IgG. Protein-antibody complexes were immunoprecipitated with protein agarose A/G beads, run on an 11% SDS-PAGE gel and immunoblotted for TSC1 and Plk2. (B) DNA Damage: TSC1. HCT 116 cells were treated with Adriamycin (0.2 μg/mL, 20 hours). Cell lysates were run on an 7.5% SDS-PAGE gel and immunoblotted for hamartin (TSC1), phosphorylated p70S6K and β-actin (loading control). (C) DNA Damage: TSC1 HCT 116 cells were untreated or treated with 0.2 μg/mL of Adriamycin for 10 hours. Cell lysates were run on an SDS-PAGE gel and immunoblotted for hamartin (TSC1) and β-actin (loading control). (D) Hypoxia: Plk2 overexpression and mTOR signaling. HCT 116 cells were transfected with pcDNA3.1V5His-Plk2 (Plk2) or pcDNAV5His (Control = Cntrl). Forty hours post-transfection, cells were placed in hypoxia (0.2% oxygen) for 12 hours, harvested and the lysates run on a 4–12% Bis-Tris gel and immunoblotted for V5, phospho-p70 S6K and β-actin.
Figure 4.
Plk2 interactions with TSC1 and TSC2 are cell-type independent. pGEX4T3 Empty (GST) or pGEX4T3 expressing Carboxy-Terminal Plk2 (CT-Plk2 GST) were run over glutathione-Sepharose beads followed by PBS wash (2x), untreated H460 (A and B), HCT 116 (C) or T3 MEF (D and E) cell lysates and PBS wash (2x). Glutathione elution buffer was used to elute or the beads were harvested directly. Equal volume Laemmli was added to eluate or beads, boiled and loaded in duplicate with input onto an SDS-PAGE gel. After transfer and blocking (5% NFDM), membranes were probed with TSC1, TSC2 and Plk2.
Plk2 inhibits p70S6K phosphorylation under hypoxic conditions
Tumors comprised of Plk2-deficient H460 cells grew larger than control tumors. However, in culture prior to injection into mice, these Plk2-deficient cells grew at the same rate as control (Fig. 2B). Therefore, when Plk2 is deficient, factors within the tumor microenvironment such as hypoxia may be signaling through the mTOR pathway to regulate the growth of these tumors. In order to evaluate the relationship between Plk2 and mTOR signaling during hypoxia, Plk2 levels were correlated with phosphorylation of the downstream target of mTOR, p70S6K. In HCT116 cells transiently overexpressing Plk2 (pcDNA3.1V5His-Plk2), an inverse correlation between Plk2 and p70S6K phosphorylation was observed after hypoxia (0.2%, 12 hrs; Fig. 3D). During hypoxia, Plk2 interactions with TSC1 may act to restrain mTOR leading to less p70S6K phosphorylation. Null mouse embryonic fibroblasts rescued with hTSC1 (T3) demonstrated decreased phosphorylation of S6K, which was further decreased when Plk2 was overexpressed (Fig. 5). p53 induced by hypoxic conditions is unable to upregulate its established targets. This appears to be the case for Plk2. During hypoxia (0.2%, 19 hrs) Plk2 levels in H460 cells failed to increase in response to the DNA damaging agent, campthothecin (100 μg/mL, 22 hrs total includes 2.5 hrs pre-hypoxia) (Fig. 6A). Similarly, in HCT116 cells exposed to hypoxia (0.2%, 20 hrs), Plk2 levels failed to increase in response to Adriamycin (0.2 μg/mL, for 20 hrs; Fig. 6B). Plk2 was demonstrated to be a p53 target after DNA damage.
Figure 5.

Plk2 facilitates TSC1 inhibition of mTOR: decreased phosphorylation of p70S6K. TSC1−/− MEFs with empty vector (P2) or hTSC1 stably re-introduced (T3) were transiently transfected with pcD-NAV5His (V) or pcDNAV5His-Plk2 (S). Forty hours post-transfection, cells were kept in normoxia or exposed to hypoxia (0.5%, 8 hrs), harvested on ice, washed with PBS and lysed in PTY buffer with protease and phosphatase inhibitors. Cell lysates were mixed with equal volume 2x sample buffer, boiled and loaded on an 11% SDS-PAGE gel.
Figure 6.
Plk2 levels are not increased by DNA damage under hypoxia. (A) H460 cells were treated with CPT-11 (100 μg/mL) for 22 hours. After the first 2.5 hours of CPT-11, cells were placed in 0.2% hypoxia for 19.5 hrs, harvested, run on a 7.5% SDS-PAGE gel and immunoblotted for Plk2 and Ran (loading control). (B) HCT 116 cells were treated with Adriamycin (0.2 μg/mL, 20 hrs). Cell lysates were run on a 7.5% SDS-PAGE gel and immunoblotted for Plk2 and β-actin (loading control).
Chemoresistance of Plk 2-deficient xenografted tumors or tumor cells cultured under hypoxia
Plk2-deficient cells demonstrate increased cell death after aphidicolin,19 etoposide, camptothecin (Fig. 7), perhaps as a result of an abrogated S-phase checkpoint during Plk2-deficiency.19 However, tumor xenografts comprised of these Plk2-deficient cells were resistant to camptothecin (Fig. 2A). It was hypothesized that a tumor environmental factor such as hypoxia may be regulating Plk2 sensitivity to chemotherapy. In fact, Plk2-deficient cells exposed to hypoxia demonstrated resistance to cell death by camptothecin (Fig. 8). A connection between the observed chemosensitivity-chemoresistance and Plk2 signaling through the mTOR pathway has not been established. These results have implications for the development of anti-tumor therapy.
Figure 7.
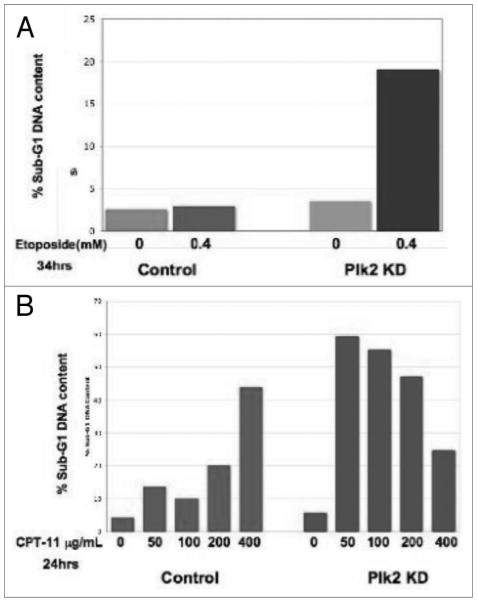
Plk2-deficient cells demonstrated increased sensitivity to DNA damage in vitro under normoxic conditions. H460 cells with Plk2 stably knocked-down were treated with either (A) Etoposide (4 mM, 34 hrs) or (B) Camptothecin (24 hrs) at increasing concentrations. The percentage of cells with sub-G1 DNA content was determined through propidium iodide staining and flow cytometry. Control, pSuper empty vector; Plk2 KD, Plk2 Knock Down.
Figure 8.
Plk2-deficient cells are chemoresistant under hypoxia and chemosensitive under normoxia in vitro. Plk2 deficient or control H460 cells were treated with camptothecin (0, 100 or 200 μg; 22 hrs includes 2.5 hrs pre-hypoxia) and hypoxia (0.2% oxygen, 19 hrs) or normoxia. Cells were harvested, fixed and stained with propidium iodide and analyzed by flow cytometry for sub-G1 DNA content.
In summary, Plk2-deficient tumors grew larger than control tumors pointing to a possible tumor suppressor function for Plk2. Plk2 was demonstrated to interact with both TSC1 and TSC2 linking the p53 pathway through regulation of Plk2 to the mTOR pathway through TSC1/2 function. Although clearly demonstrated to be a p53 target, Plk2 levels did not increase in response DNA damage during hypoxia. Exogenous expression of Plk2 decreased downstream mTOR signaling during hypoxia. TSC1 rescue of null mouse embryonic fibroblasts restored restraint of mTOR signaling that was further restrained with the introduction of exogenous Plk2. These findings are consistent with our hypothesis that the Plk2-TSC1 interaction facilitates TSC1/2 restraint of mTOR and this cross-talk between the p53 and mTOR pathways is regulated during hypoxia, during DNA damage and perhaps during tumor progression (Fig. 9). Plk2-deficient H460 non-small cell lung carcinoma cells did not demonstrate differences in growth in culture, indicating that tumor microenvironmental factors including hypoxia may regulate the growth of tumors deficient in Plk2. Although Plk2-deficient cells demonstrate chemosensitivity in normoxic culture conditions, they were chemoresistant under hypoxia. Tumor xenografts comprising Plk2-deficient cells were chemoresistant to camptothecin in vivo. Whether cross-talk between the p53 and mTOR pathways regulates Plk2-dependent chemosensitivity-chemoresistance under hypoxia remains to be elucidated.
Figure 9.

Schematic model for regulation of mTOR pathway signaling through interaction of the p53 target Plk2 with TSC proteins. Our model proposes that Plk2 and TSC1 interact to facilitate TSC1/TSC2 restraint of mTOR. Decreased mTOR activity leads to decreased phosphorylation of p70S6K and consequent decrease in protein translation, cell growth and proliferation. These events are regulated by hypoxia and DNA damage.
Discussion
Our in vivo studies indicate that the least characterized of the polo-like kinases, Plk2 may act as a tumor suppressor and its tumor suppressor functions may be regulated by the tumor microenvironment. As a target of tumor suppressor p53 after genotoxic stress,4 it is not surprising that Plk2 itself may act as a tumor suppressor. The prior finding that Plk1 interacts with TSC1 in a phosphorylation dependent manner3 raised the possibility that Plk2 may itself interact with TSC1 and effect downstream mTOR signaling, cell growth and proliferation, thereby accounting for the inhibitory effect of Plk2 on tumor growth. We have demonstrated that Plk2 interacts with both TSC1 (endogenous, CT-Plk2) and TSC2 (CT-Plk2) in various cell types.
Our observation that Plk2 levels do not increase in response to DNA damaging agents during hypoxia maybe a result of the inability of p53 to upregulate Plk2 under hypoxia as is the case with other established p53 targets. Nevertheless, Plk2 appears to facilitate restraint of mTOR under hypoxia as overexpression of Plk2 decreases downstream mTOR signaling (phospho-p70S6K) in HCT116 cells and TSC1-mediated reductions in phospho-S6K levels are further decreased when Plk2 is overexpressed. Our novel observation that TSC1 levels increased in response to DNA damaging agents that activate p53 provides further evidence for cross-talk between the p53 and mTOR pathways. The finding that Plk2-deficient tumors are completely resistant to in vivo camptothecin therapy despite the fact that Plk2-deficient cancer cells in culture demonstrate increased sensitivity to chemotherapeutic agents further points to the possibility that Plk2 is regulated by factors within the tumor microenvironment. When we evaluated the effect of one such factor, hypoxia on sensitivity to chemotherapeutic agents in Plk2-deficient cells, our findings were consistent with our tumor studies. Specifically, Plk2-deficient cells demonstrate increased resistant to chemotherapy induced apoptotic death during hypoxia but increased sensitivity during normoxia.
Genotoxic stress may inhibit mTOR activity through p53-dependent upregulation of known negative regulators PTEN, TSC2 and AMPKβ1 (AMP-responsive protein kinase) β1 and AMPKα.6,7 p53 is a genotoxic stress sensor which activates cell cycle arrest thereby protecting cells from DNA damage. Without a mechanism for metabolic arrest, these arrested cells will continue metabolic activity at a high rate. As Hay further points out, by inhibiting mTOR, a major regulator of protein synthesis, p53 is able to couple metabolic arrest and cell cycle arrest. During energy stress, AMPK, the sensor of intracellular ATP levels is activated, thereby phosphorylating and activating TSC2, which inhibits mTORC1.30 Although it was previously known that p53 inhibits mTOR by elevating AMPK activity,30 the Karin laboratory demonstrated that this may be mediated through the sestrins.7 Our studies provide further evidence for inhibition of mTOR by the p53 signaling pathway through another one of its targets, Plk2. As indicated above, determining whether Plk2 is acting together with AMPKα and the Sestrins to restrain mTOR through TSC2 will further illuminate our understanding of this mechanism, providing an improved platform for developing therapeutics. Plk2 may inhibit mTOR through TSC1/2 complex by a mechanism that is independent of AMPK and sestrins1/2. Targeting parallel pathways simultaneously may yield an enhanced therapeutic outcome.
There are numerous questions that remain for future study. This includes a determination of the effects of the Plk2-TSC1 interaction on TSC1/2 and mTOR activity after DNA damage. Further study is required to evaluate whether Plk2 kinase activity is necessary to modulate mTOR signaling. It is important to determine whether TSC1 (or TSC2) is a substrate for Plk2 kinase activity. As mentioned above, recent work from the Karin laboratory has demonstrated that the products of two p53 target genes, Sestrin 1 and 2 activate AMPK, which phosphorylates TSC2 and stimulates GAP activity enabling mTOR inhibition. Mice treated with a p53 activating alkylating agent were used to demonstrate the functional importance of Sestrin 2 in inhibiting mTOR signaling.7 Evaluating the possible presence of AMPKα, or Sestrin 2 in the complex that Plk2 forms with TSC1/2 will be necessary to understanding this system. The role of Plk2 and its interaction with TSC1 on mTOR signaling during both hypoxia and tumor development remains to be evaluated. Future studies will need to further investigate the effects of hypoxia on chemosensitivity and chemoresistance in Plk2-deficient cancer cells and determine whether these effects are mediated by signaling through the mTOR pathway.
Our findings have far reaching clinical implications. Inhibitors of Plk1 and Plk3 are in clinical trials. Some of these inhibitors demonstrate specificity for Plk2. Understanding the effects of Plk2 inhibition on tumor development and growth and mTOR signaling is critical to the development of these new therapies. The impact of hypoxia and the tumor microenvironment is also extremely relevant to the signaling and the effects of therapeutic agents. mTOR inhibitors are already in use for renal cell carcinomas and other tumors. Until now, the p53 and mTOR pathways were individually targeted in many studies, but not targeted together. Elucidating the connection between these two pathways holds promise for effective combination therapies.
Material and Methods
Mouse tumor studies
Female nude (Nu/nu, Taconic) mice were inoculated subcutaneously with Plk2-deficient (or control) H460 non-small cell lung carcinoma cells. One week after tumor cell inoculation, mice were treated with 40 mg/kg (intraperitoneal) camptothecin three times per week (or untreated) and sacrificed at day 20 post-inoculation. Construction of stable Plk2 knock-down H460 cells has been previously described.19 Tumors were measured using calipers and tumor volume calculated using the following formula: volume = 0.52 x (width2) x length, expressed in mm3.
Immunoprecipitation and western blot
Immediately after removing growth media, cells were placed on ice, washed in cold PBS and harvested with PTY buffer29 containing freshly added 1x EDTA-free protease inhibitor cocktail (Roche) and 1 mM sodium orthovanadate (Sigma). After the addition of Laemmli buffer, cell lysates were boiled, spun and run on an SDS-PAGE gel. Proteins were transferred to membranes. Membranes were blocked in 5% non-fat dry milk (NFDM)/TTBS (Tris-buffered saline with Tween) for 45 minutes, incubated overnight with primary antibody dissolved in 5% NFDM/TTBS, washed 3x in TTBS, incubated in horseradish peroxidase conjugated secondary antibody (1:2,000) in 5% NFDM/TTBS for 2 hours at room temperature and washed 3x in TTBS. Membranes were subsequently incubated in ECL or ECL Plus reagent and exposed to film. Membrane exposed to β-actin or ran antibodies (loading controls) were incubated in fluorescence secondary antibody (Alexa-fluor 680, Invitrogen) for 2 hours at room temperature and scanned using Li-COR Odyssey. Primary antibodies included TSC1 (1:1,000 in 2.5% bovine serum albumin and 2.5% NFDM in TTBS, Zymed Laboratories), TSC2 (1:1,000, Santa Cruz Biotechnology), Plk2 (1:200, Abgent), Phospho-p70S6K (1:1,000, Cell Signaling), V5 (1:300, Invitrogen), Ran (1:10,000, BD Biosciences), β-actin (1:5,000, Sigma).
For endogenous immunoprecipitation, an aliquot of cell lysates harvested as described above was set aside for input. The remaining cell lysate was pre-cleared with mouse IgG2a and protein agarose A/G beads. After spinning to remove the beads, the supernatant was divided in half. Half of this lysate was incubated in IgG2a and half in TSC1 at 4°C with gentle rotation over-night. The next day protein agarose A/G beads were added for 2 hr at 4°C with gentle rotation. The samples were spun at 3,000 RPM at 4°C to harvest the beads, discarding the supernatant and adding fresh cold PTY buffer. This was repeated three times. After the final spin, Laemmli buffer was added to the beads. The samples were boiled, electrophoresed on and SDS-PAGE gels and immunoblotted as described above.
For GST-pull down assays, a C-Terminal Plk2-GST was generated by cloning a carboxy-terminal portion of the Polo-like kinase gene (nucleotides 1153 to 2058) into the pGEX4T3.19 After bacterial expression, IPTG induction, sonication in lysis buffer and centrifugation, the supernatant containing the CT-Plk2-GST (or GST) was purified. Glutathione beads packed in a column at 4°C were incubated with CT-Plk2-GST (or GST), washed with PBS 3x, incubated with cell lysates and washed 3x with PBS. The beads were boiled for 5 minutes with 1x Laemmli buffer, the supernatant loaded onto an SDS-PAGE gel, electrophoresed and immunoblotted as described above.
Sub-G1 DNA analysis
Cells were plated into six-well plates, using one well per sample/condition. After first harvesting the cells floating in the media, adherent cells were washed in PBS and digested with 1 mL of trypsin and washed with 3 mL of fresh media/10% FBS. Cells were washed in 2 mL PBS/1% fetal bovine serum (FBS) and then in 1 mL of PBS. Cells were fixed by adding 9 mL of cold 70% ethanol with gentle vortexing. After storing at 4°C overnight, cells were spun down to remove ethanol and resuspended in 4 mL PBS/1% FBS. The cells were spun and resuspended in 1 mL of PBS + 0.5 mL phosphate citric acid buffer, incubated for 5 minutes, spun and resuspended in PBS/1% FBS/15 μg propidium iodide/3KU RNAse (USB). Samples were stored at room temperature in the dark for 30 minutes and subsequently analyzed by flow cytometry.
Hypoxia
To induce hypoxia, cells were placed in an In Vivo2 hypoxia chamber (Ruskinn) flushed with a gas mixture comprised of 0.2% or 0.5% oxygen levels, 5% carbon dioxide and balance nitrogen.
Cell lines
H460 non-small cell lung carcinoma cells (RPMI) and HCT116 colon cancer cells (Mc Coy’s) were grown in medium containing 10% FBS and 1% penicillin/streptomycin (P/S). TSC1 null mouse embryonic fibroblasts before (P2) and after rescue with hTSC1 (T3) were grown in DMEM with 10% FBS, 1% P/S, 1 mg/mL of Geneticin (G418, Invitrogen), and 1x MEM non-essential amino acids (Invitrogen). P2 and T3 cells were previously described.3 Empty vector (pcDNA3.1V5His) and Plk2 overexpressing (pcDNA3.1V5His-hSNK) vectors4 were transiently transfected into P2 and T3 cells or HCT116 cells using the Lipofectamine 2000 (Invitrogen) protocol. Adriamycin (Ben Venue Labs), camptothecin (irinotecan, Dabur Oncology, Plc) and etoposide (Ben Venue Labs) were obtained from the Hospital of the University of Pennsylvania Pharmacy.
Acknowledgements
This work was supported in part by NIH grants CA105008, CA123258 and CA135273 to W.S.E.-D. and by the Littlefield-AACR grant for metastatic colon cancer research. This work was presented in part at the 99th annual AACR meeting in San Diego, CA in April 2008, and at the 100th annual AACR meeting in Denver, CO in April 2009. W.S.E.-D. is an American Cancer Society Research Professor.
Abbreviations
- Plk2
polo-like kinase
- mTOR
mammalian target of rapamycin
- TSC1
hamartin/tuberous sclerosis complex 1
- p70S6K/S6K
p70S6 kinase
- TSC2
tuberin/tuberous sclerosis complex 2
References
- 1.Guertin D, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Crino PB, Hathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 3.Astrinidis A, Senapedis W, Henske EP. Hamartin, the tuberous sclerosis complex 1 gene product, interacts with polo-like kinase 1 in a phosphorylation-dependent manner. Human Mol Genet. 2006;15:287–97. doi: 10.1093/hmg/ddi444. [DOI] [PubMed] [Google Scholar]
- 4.Burns TF, Fei P, Scata KA, Dicker DT, El-Deiry WS. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–71. doi: 10.1128/MCB.23.16.5556-5571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Reutz S, O’Reilly T, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damage induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 6.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, et al. The regulation of AMPKbeta1, TSC2 and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–53. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 7.Budanov A, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–60. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeYoung MP, Horak P, Sofer A, Sgrol D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–51. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graeber T, Peterson JF, Tsai M, Monica K, Fornace AJ, Jr, Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–77. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koumenis C, Larcon R, Hammond E, Sutphin P, Hoffman W, Murphy M, et al. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol. 2001;21:1297–310. doi: 10.1128/MCB.21.4.1297-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graeber T, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 12.Krieg A, Hammond EM, Giaccia AJ. Functional analysis of p53 binding under differential stresses. Mol Cell Biol. 2006;26:7030–45. doi: 10.1128/MCB.00322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Vugt MA, Medema RH. Getting in and out of mitosis with Polo-like kinase-1. Oncogene. 2005;24:2844–59. doi: 10.1038/sj.onc.1208617. [DOI] [PubMed] [Google Scholar]
- 14.Warnke S, Kemmler S, Hames RS, Tsai HL, Hoffmann-Rohrer U, Fry AM, et al. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr Biol. 2004;14:1200–7. doi: 10.1016/j.cub.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 15.Chase D, Feng Y, Hanshew B, Winkles JA, Longo DL, Ferris DK. Expression and phosphorylation of fibroblast-growth-factor-inducible kinase (Fnk) during cell cycle progression. Biochem J. 1998;333:655–60. doi: 10.1042/bj3330655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–7. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 17.Syed N, Smith P, Sullivan A, Spender LC, Dyer M, Karran L, et al. Transcriptional silencing of Polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood. 2006;107:250–6. doi: 10.1182/blood-2005-03-1194. [DOI] [PubMed] [Google Scholar]
- 18.Ma S, Charron J, Erikson RL. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol Cell Biol. 2003;23:6936–43. doi: 10.1128/MCB.23.19.6936-6943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthew EM, Yen TJ, Dicker DT, Dorsey JF, Yang W, Navaraj A, et al. Replication stress, defective S-phase checkpoint and increased death in Plk2-deficient human cancer cells. Cell Cycle. 2007;6:2571–8. doi: 10.4161/cc.6.20.5079. [DOI] [PubMed] [Google Scholar]
- 20.Simmons DL, Neel BG, Stevens R, Evett G, Erikson RL. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–9. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkles J, Alberts GF. Differential regulation of pololike kinase 1, 2, 3 and 4 gene expression in mammalian cells and tissues. Oncogene. 2005;24:260–6. doi: 10.1038/sj.onc.1208219. [DOI] [PubMed] [Google Scholar]
- 22.Liby K, Wu H, Ouyang B, Wu S, Chen J, Dai W. Identification of the human homologue of the early-growth response gene Snk, encoding a serum-inducible kinase. DNA Seq. 2001;11:527–33. doi: 10.3109/10425170109041337. [DOI] [PubMed] [Google Scholar]
- 23.Ma S, Liu MA, Yuan YL, Erikson RL. The serum-inducible protein kinase Snk is a G1 phase polo-like kinase that is inhibited by the calcium-integrin-binding protein CIB. Mol Cancer Res. 2003;1:376–84. [PubMed] [Google Scholar]
- 24.Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Kobel M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004;90:815–20. doi: 10.1038/sj.bjc.6601610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weichert W, Schmidt M, Gekeler V, Denkert C, Stephan C, Jung K, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate. 2004;60:240–5. doi: 10.1002/pros.20050. [DOI] [PubMed] [Google Scholar]
- 26.Weichert W, Kristiansen G, Winzer KJ, Schmidt M, Gekeler V, Noske A, et al. Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch. 2005;446:442–50. doi: 10.1007/s00428-005-1212-8. [DOI] [PubMed] [Google Scholar]
- 27.Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (Plk) expression in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:2794–7. [PubMed] [Google Scholar]
- 28.Kanaji S, Saito H, Tsujitani S, Matsumoto S, Tatebe S, Kondo A, et al. Expression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinoma. Oncology. 2006;70:126–33. doi: 10.1159/000093003. [DOI] [PubMed] [Google Scholar]
- 29.O’Neill GM, Golemis EA. Proteolysis of the docking protein HEF1 and implications for focal adhesion dynamics. Mol Cell Biol. 2001;21:5094–108. doi: 10.1128/MCB.21.15.5094-5108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hay N. p53 strikes mTORC1 by employing sestrins. Cell Metab. 2008;8:184–5. doi: 10.1016/j.cmet.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]