Abstract
The organs of animal embryos are typically covered with an extracellular matrix (ECM) that must be carefully remodeled as these organs enlarge during post-embryonic growth; otherwise, their shape and functions may be compromised. We previously described the twisting of the Caenorhabditis elegans pharynx (here called the Twp phenotype) as a quantitative mutant phenotype that worsens as that organ enlarges during growth. Mutations previously known to cause pharyngeal twist affect membrane proteins with large extracellular domains (DIG-1 and SAX-7), as well as a C. elegans septin (UNC-61). Here we show that two novel alleles of the C. elegans papilin gene, mig-6(et4) and mig-6(sa580), can also cause the Twp phenotype. We also show that overexpression of the ADAMTS protease gene mig-17 can suppress the pharyngeal twist in mig-6 mutants and identify several alleles of other ECM-related genes that can cause or influence the Twp phenotype, including alleles of fibulin (fbl-1), perlecan (unc-52), collagens (cle-1, dpy-7), laminins (lam-1, lam-3), one ADAM protease (sup-17), and one ADAMTS protease (adt-1). The Twp phenotype in C. elegans is easily monitored using light microscopy, is quantitative via measurements of the torsion angle, and reveals that ECM components, metalloproteinases, and ECM attachment molecules are important for this organ to retain its correct shape during post-embryonic growth. The Twp phenotype is therefore a promising experimental system to study ECM remodeling and diseases.
EVERY tissue and organ of multicellular animals is surrounded by a specialized extracellular matrix (ECM) that supports their shapes and mechanical properties and contributes adhesive substrates while coordinating the sequestering and availability of guidance cues and growth factors during development (Rozario and Desimone 2010; Tsang et al. 2010). The composition of the ECM varies with the needs of the tissues and organs, but typically includes networks of collagen, laminin, or fibronectin, which are interconnected by molecules such as fibulin and the proteoglycans perlecan and nidogen.
Organs usually complete their basic assembly during embryogenesis and later development mostly consists of the enlargement of these organs. This must be accompanied by remodeling of the ECM that surrounds the organs and tissues, a phenomenon that is further complicated by allometry, i.e., the fact that different organs grow at different rates, and by the varying mechanical requirements of the different organs. There are dozens of human genetic disorders where defects in ECM remodeling during growth are the likely causes of disease (Carter and Raggio 2009). Unfortunately, except for the Drosophila tracheal system, there are few established genetic models to study ECM remodeling during organ growth. In Drosophila, the regulation of a luminal ECM matrix containing chitin determines tracheal tube diameter (Tonning et al. 2005; Laprise et al. 2010), and matrix metalloproteinases that can degrade ECM components are essential for the growth-related remodeling of the tracheal tubes themselves (Glasheen et al. 2010).
The Caenorhabditis elegans pharynx is a straight feeding tube responsible for rhythmically pumping, trapping, and grinding bacterial food before passing it on to the intestine (Albertson and Thomson 1976; Avery and Shtonda 2003). The pharynx, which is surrounded by a thick basement membrane (C. C. Huang et al. 2003; Kramer 2005), is composed of 80 cells (some fuse post-embryonically to produce an organ with 63 cells but 80 nuclei) and exhibits threefold symmetry, with one dorsal and two subventral muscular sectors containing radially directed contractile arrays. It is attached to the mouth via hemidesmosomes between pharyngeal epithelial cells and the arcade cells of the mouth and to the intestine via the pharyngeal-intestinal valve composed of six cells (Albertson and Thomson 1976). Four rows of 12–15 tendons composed of hemicentin and fibulin also secure the anterior part of the pharynx to the body wall of the worm (Vogel and Hedgecock 2001; Muriel et al. 2005). The pharynx develops during embryogenesis from a semispherical primordium that elongates as cells differentiate to form the mature elongated structure (Portereiko and Mango 2001), and this process is under the control of the forkhead transcription factor PHA-4 that seems to regulate most pharyngeal genes (Mango et al. 1994; Horner et al. 1998; Kalb et al. 1998; Gaudet and Mango 2002).
We previously described the twisted pharynx (Twp) phenotype as being easily monitored and quantifiable by DIC microscopy (Axäng et al. 2007). Mutants with the Twp phenotype have fully functional pharynges with normal pumping rates. The twisted phenotype itself is likely growth-related: the pharyngeal twist becomes visible post-embryonically, worsens as the organ enlarges during post-embryonic growth, is not enhanced by pharyngeal pumping in the absence of food or once adult size is reached, and affects more severely the parts of the pharynx that are thinner, while mutants with a thickened pharynx show less twist (Axäng et al. 2007). In a survey of candidate mutants, we identified alleles in four genes that could cause the Twp phenotype: unc-61, which encodes a septin (Nguyen et al. 2000); dig-1 and sax-7, which encode transmembrane proteins with very large extracellular domains (Sasakura et al. 2005; Wang et al. 2005; Bénard et al. 2006; Burket et al. 2006); and mig-4, which affects a hitherto unidentified gene. Another mutant allele, mnm-4(et4), was isolated in a forward genetics screen for abnormal pharynges and also remained unidentified (Axäng et al. 2007). Here we show that et4 and the noncomplementing allele sa580 are actually novel alleles of mig-6, which encodes the C. elegans homolog of papilin, a component of the ECM that contains several domains homologous to ADAMTS-like proteins. We also identify genes for collagen XVIII (cle-1), cuticle collagen (dpy-7), fibulin, laminin, perlecan, and metalloproteinases as participating in ECM remodeling during organ growth.
MATERIALS AND METHODS
Strains and C. elegans maintenance:
The wild-type C. elegans strain used in this project was Bristol N2 (Brenner 1974; Sulston and Hodgkin 1988). C. elegans worms were maintained at 20° using standard methods (Sulston and Hodgkin 1988). All C. elegans strains were obtained from the C. elegans Genetics Center in Minneapolis, Minnesota (http://www.cbs.umn.edu/CGC/), except for strains containing the following previously described alleles of mig-6 (Kawano et al. 2009): ev700, ev701, ev788, e1931, k177, and oz113. The mig-6(sa580) allele was isolated in a screen for high-temperature dauer formation, where all candidate mutant animals were also cross-examined for anatomical defects of the amphid neurons (Ailion and Thomas 2003). Later analysis showed that sa580 was a background mutation and does not itself have a dauer phenotype. The mig-6(et4) allele was isolated in a screen for abnormal pharyngeal neurons (Mörck et al. 2003).
Nomarski (DIC) microscopy and scoring of the Twp phenotype:
Animals were placed on 2% agarose pads (0.04 g agarose, 2 ml 0.05% phenoxypropanol) on glass slides in a drop of 0.02% phenoxypropanol as anesthetic and overlaid with a cover slip. Animals were observed with a Zeiss Axiophot microscope using a GFP filter or Nomarski optics. Images were taken using the Axiovision 4.5 program (Zeiss) and further processed using Photoshop (Adobe). To measure the torsion angle, L4 larvae were picked and 24 hr later the pharynx of each young adult was photographed under Nomarski optics on a focal plane that showed the strongest twist within the upper half of the worm. The angle formed by the torsion lines in each twisted pharynx was measured twice using the public domain software ImageJ (National Institutes of Health) and the mean was recorded as one entry. In cases where there was obviously no twist as determined by microscopic examination, the pharynx was scored as having a torsion angle of 0° without recourse to ImageJ. In Tables 1–3, the minimum and maximum torsion angles observed for each genotype scored are provided, together with the mean ± SEM of the torsion angles of all the worms photographed and the fraction of twisted pharynges that twisted to the left.
TABLE 1.
Pharyngeal torsion angles in animals with various mig-6 genotypes
| Strains | Minimum angle(°) | Maximum angle (°) | Mean angle ± SEM(°) | n |
|---|---|---|---|---|
| Wild type | ||||
| N2 | 0 | 0 | 0 | 20 |
| Homozygotes and heterozygotes of mig-6(et4) and mig-6(sa580) | ||||
| mig-6(et4) | 17.9 | 43.3 | 28.9 ± 1.2 | 20 |
| mig-6(sa580) | 13.9 | 33.3 | 23.8 ± 1.2 | 20 |
| mig-6(et4)/+ | 4.1 | 29 | 14 ± 1.2 | 20 |
| mig-6(sa580)/+ | 2.3 | 18 | 8.3 ± 1.1 | 20 |
| mig-6(et4)/mig-6(sa580) | 12.4 | 32.6 | 21.9 ± 1.1 | 20 |
| mig-6 alleles other than et4 and sa580 | ||||
| mig-6(k177) | 0 | 0 | 0 | 20 |
| mig-6(ev701) | 0 | 0 | 0 | 20 |
| mig-6(ev700) | 0 | 0 | 0 | 20 |
| mig-6(e1931) | 0 | 0 | 0 | 20 |
| mig-6(oz113) | 0 | 0 | 0 | 20 |
| mig-6(ev788)/eT1a | 0 | 0 | 0 | 20 |
| Rescue of mig-6(sa580) using cosmids ZK742 + C37C3 | ||||
| Line 1, ZK742 + C37C3 in mig-6(sa580) | 3.2 | 17.9 | 12.6 ± 1.0 | 17 |
| Line 2, ZK742 + C37C3 in mig-6(sa580) | 5.8 | 17.5 | 12.2 ± 1.3 | 11 |
| Line 3, ZK742 + C37C3 in mig-6(sa580) | 2.1 | 12.8 | 7.9 ± 0.6 | 25 |
| Line 4, ZK742 + C37C3 in mig-6(sa580) | 1.4 | 11.9 | 5.3 ± 0.6 | 27 |
| Line 5, ZK742 + C37C3 in mig-6(sa580) | 1.4 | 19.5 | 8.7 ± 1.9 | 11 |
| Rescue of mig-6(et4) using minigenes mig-6S and mig-6L | ||||
| Line 1, pZH125 (mig-6S) in mig-6(et4) | 0.1 | 16.1 | 3.8 ± 0.9 | 20 |
| Line 2, pZH125 (mig-6S) in mig-6(et4) | 0.1 | 23 | 8.2 ± 1.2 | 20 |
| Line 3, pZH125 (mig-6S) in mig-6(et4) | 0.9 | 25.5 | 8.1 ± 1.4 | 20 |
| Line 4, pZH125 (mig-6S) in mig-6(et4) | 0.2 | 25.5 | 6.3 ± 1.2 | 20 |
| Line 1, pZH117 (mig-6L) in mig-6(et4) | 0.0 | 29.5 | 11.9 ± 2.2 | 20 |
| Line 2, pZH117 (mig-6L) in mig-6(et4) | 2.2 | 28.1 | 14.1 ± 1.5 | 20 |
| Rescue of mig-6(sa580) using minigenes mig-6S and mig-6L | ||||
| Line 1, pZH125 (mig-6S) in mig-6(sa580) | 2.6 | 27.9 | 12.4 ± 1.9 | 20 |
| Line 2, pZH125 (mig-6S) in mig-6(sa580) | 0.9 | 25.9 | 11.2 ± 1.5 | 20 |
| Line 1, pZH117 (mig-6L) in mig-6(sa580) | 2.8 | 25.2 | 11.1 ± 1.9 | 11 |
| Rescue of mig-6(et4) using a mig-17∷GFP translational reporter | ||||
| Line 1, mig-17∷GFP in N2 | 0 | 0 | 0 | 20 |
| Line 1, mig-17∷GFP in mig-6(et4) | 5.2 | 21.7 | 12.5 ± 1.1 | 20 |
| Phenocopies of et4 and sa580 phenotypes in wild-type N2 | ||||
| Line 1, pQC10.1 (pZH125_et4) in N2 | 0.6 | 14.7 | 7.2 ± 1.0 | 20 |
| Line 2, pQC10.1 (pZH125_et4) in N2 | 4.5 | 21.9 | 12.8 ± 1.3 | 15 |
| Line 3, pQC10.1 (pZH125_et4) in N2 | 7.1 | 29 | 16.8 ± 1.7 | 15 |
| Line 1, pQC10.2 (pZH125_sa580) in N2 | 2.9 | 17.7 | 10.8 ± 0.9 | 22 |
ev788 is a putative null allele, and homozygotes are lethal; eT1 is used as a balancer.
Second harmonic generation microscopy:
Second harmonic generation (SHG) microscopy was performed on pharyngeal muscles using a specially adapted confocal microscope (Mathew et al. 2009) that facilitates nonlinear microscopy. Light with a wavelength of 868 nm from a tuneable, 160-fsec pulse duration, 76-MHz repetition rate ultrashort pulsed laser (Coherent MIRA 900f) was employed. The laser beam with 15–18 mW average power (on the sample plane) was scanned across the sample. The SHG signal was collected using an oil immersion condenser with a high numerical aperture (1.4) and detected using a photo multiplier tube that accommodates a 434-nm (half of 868 nm) narrow band pass optical filter. The SHG signal is produced intrinsically in ordered structures such as muscle, and the SHG signal detected during the laser scanning process is converted to an image exactly as with a confocal microscope.
Fluorescent dye-filling assay:
1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate (DiI) preferentially fills some amphid head neurons and phasmid tail neurons (Perkins et al. 1986; Starich et al. 1995). Stock solutions of the fluorescent Vybrant DiI cell-labeling solution (Invitrogen) were stored at −20°, and DiI dye-filling assays were performed by incubating worms for 2 hr at 20° in 10 mg/ml of DiI in M9 buffer solution. After incubation, the animals were washed three times with M9 and observed using a fluorescence microscope (Axiophot, Zeiss).
Plasmid constructs:
pZH125 and pZH117 carry mig-6S and mig-6L minigenes, respectively, and have been described previously (Kawano et al. 2009). The pQC10.1 and pQC10.2 plasmids were derived from pZH125 and carry mig-6S minigenes with the mig-6(et4) and mig-6(sa580) mutations, respectively. A fragment of 1540 bp spanning parts of mig-6 exons 8 and 9 was amplified from genomic DNA of mig-6(et4) or mig-6(sa580) mutant worms using Pfu Ultra DNA polymerase and the following primers: PstI_mig-6 F (5′-ctgcagcgtcacaaacgag-3′) and PstI_mig-6 R (5′-ctgcagatgtcttccattg-3′). The PCR product was subcloned into pCR_Blunt_II_TOPO (Invitrogen), and the resulting construct was cut with PstI. The released 1540-bp fragment was subcloned into the PstI site of pZH125 to generate pQC10.1 or pQC10.2. Restriction enzyme mapping and sequencing confirmed the correct orientation and structure. The mig-17∷GFP fusion construct was a kind gift from K. Nishiwaki (Nishiwaki et al. 2000).
Transgenic worms:
Where applicable, 20 ng/μl of pZH125, pZH117, pQC10.1, and pQC10.2 were coinjected into the gonad syncytium of adult hermaphrodites (Mello et al. 1991) with 48 ng/μl of pCC∷gfp, a coelomocyte transformation marker (Miyabayashi et al. 1999), and transgenic worms were identified by their GFP-positive coelomocytes.
RNA interference:
RNA interference (RNAi) by feeding was done using a published RNAi library and as previously described (Kamath et al. 2001). The following genes, with the open reading frame name given in parentheses, were knocked down by RNAi: adm-2 (C04A11.4), adt-1 (C02B4.1), agr-1 (F41G3.12), cle-1 (C36B1.1), dgn-1 (T21B6.1), dpy-7 (F46C8.6), emb-9 (K04H4.1), epi-1 (K08C7.3), fbl-1 (F56H11.1), gon-1 (F25H8.3), lam-1 (W03F8.5), lam-3 (T22A3.8), let-2 (F01G12.5), mig-6 (C37C3.6), mig-17 (F57B7.4), mig-23 (R07E4.4), sup-17 (DY3.7), unc-22 (ZK617.1), unc-52 (ZC101.2), and unc-71 (Y37D8A.13). For each of the genes of interest, two plates were seeded with 500 μl liquid bacteria and then allowed to dry overnight at 20°. The following day, four L3 stage hermaphrodite worms were transferred onto the first plate containing seeded bacteria expressing double-stranded RNA and incubated for 72 hr at 20°. Then, L4 animals (F1) were transferred onto the second plate, seeded with the same bacterial clone, and incubated for 24 hr at 20°. Young adults were then microscopically examined to analyze the RNAi effects. unc-22 was used as a positive control for the RNAi method and always produced a typical twitching phenotype. For the RNAi experiments, IPTG (1 mm) and carbenicillin (25 μg/ml) were added to the NGM agar worm plates, and tetracycline (12.5 μg/ml) was included in the bacterial liquid cultures to maintain the RNAse-deficient clones.
RESULTS
The pharyngeal twist (Twp) phenotype:
We previously characterized the Twp phenotype and described a method to quantify the degree of twist using the equation:
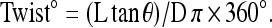 |
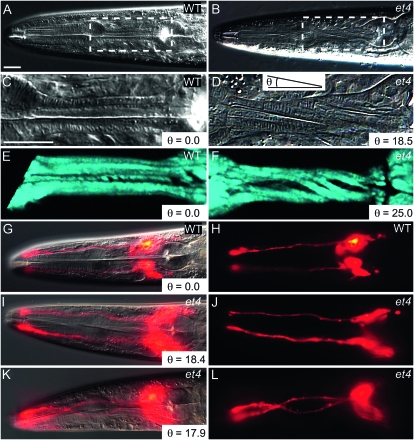
The equation is based on the measurements of the isthmus length (L), diameter (D), and torsion angle θ formed between the torsion lines and the pharyngeal longitudinal axis (Axäng et al. 2007). In age-matched worms of similar pharynx sizes, it is more expedient to quantify the Twp phenotype by measuring only the torsion angle θ. In our earlier report, we also described the et4 mutant allele and showed that it exhibits a post-embryonic pharyngeal left-handed Twp phenotype that worsens during larval growth (Axäng et al. 2007). The pharyngeal twist is easily imaged using Nomarski optics (Figure 1, A–D), and the torsion angle θ is highly reproducible among age-matched animals; for example, et4 homozygous animals have a torsion angle of 28.9° ± 1.2°. Imaging of wild-type and twisted pharynges using SHG microscopy, which is a high-resolution and dye-independent method to generate three-dimensional images of noncentrosymmetric quasi-crystalline structures, such as are formed by the contractile arrays within muscle cells (Psilodimitrakopoulos et al. 2009), shows that the pharyngeal muscles are also twisted but not grossly compromised in their subcellular architecture (Figure 1, E and F). This is consistent with the observation that et4 mutants are not feeding-defective and exhibit perfectly normal pharyngeal contraction cycles (Axäng et al. 2007).
Figure 1.—
The Twp phenotype in the mig-6(et4) mutant. (A) The head of a wild-type animal, with the boxed area enlarged in C. Note that the pharyngeal structures are perfectly straight along the anterior–posterior axis. (B) The head of a mig-6(et4) mutant, with the boxed area enlarged in D. Note the torsion lines indicative of a twisted pharynx. The angle θ is defined here as that formed by the torsion lines of the muscles and the anterior–posterior axis, as shown in D. (E and F) A wild-type and mig-6(et4) mutant pharynx, respectively, imaged using SHG microscopy at successive focal planes and rendered three dimensionally. Note the twisted appearance of the muscles in the mutant. A wild-type worm stained with DiI is shown as an overlay of DIC and epifluorescence in G or epifluorescence only in H. Note that the DiI-filled amphid neurons are straight. (I–L) mig-6(et4) mutants similarly stained with DiI, with DIC and epifluorescence overlays in I and K and epifluorescence only in J and L. Note that one worm displays straight amphid neurons (I and J) while the other has twisted amphid neurons (K and L), even though both have twisted pharynges as indicated by the angle θ in I and K. Bars, 20 μm, with the bar in panel C applying to panels C through F, and the bar in panel A applying to all other panels.
An obvious question is whether other structures in addition to the pharynx are twisted within the head in et4 mutant worms. We addressed this by visualizing the dendrites of amphid neurons that run along the side of the pharynx and terminate around the mouth. Amphid neurons can be visualized using the fluorescent dye DiI (Perkins et al. 1986; Starich et al. 1995). In wild-type worms, the DiI-filled amphid neurons run straight along the pharynx surface in 100% of the animals (Figure 1, G and H). In et4 mutants, we noted that, while 100% of the animals have the Twp phenotype, the amphid neurons are either straight (75% of cases) or conspicuously twisted (25% of cases) (n = 20; Figure 1, I–L). This result suggests that the degree of adhesion between amphid neuron dendrites and the pharynx is variable and that strong adhesions may cause the amphid dendrites to twist together with the pharynx during growth. The twist is likely an intrinsic property of the pharynx in et4 mutant animals, and any twisting of extrapharyngeal structures is likely a secondary consequence.
The et4 and sa580 mutations are novel alleles of mig-6:
The et4 allele is semidominant (Axäng et al. 2007). A second allele of the same locus, sa580, came to our attention during the course of this study (cf. materials and methods) (Ailion and Thomas 2003) and, upon characterization, turned out to be slightly less semidominant than et4: while heterozygotes of et4 exhibit an average torsion angle of 14° ± 1.2°, sa580 heterozygotes have an average angle of 8.3° ± 1.1°. Doubly heterozygous animals have average twist angles of 21.9° ± 1.1°, consistent with noncomplementation of the two alleles. As with the et4 allele, only the pharynx and a fraction of the amphid neurons embedded in the pharyngeal ECM are twisted in sa580 mutant animals. For example, while pharyngeal muscles and neurons are clearly twisted, body neurons and amphid socket cells are not (supporting information, Figure S1).
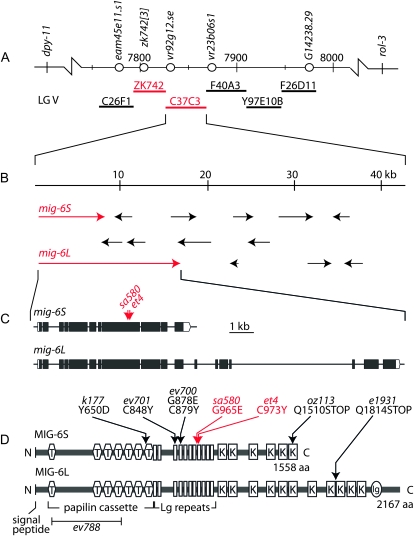
By genetic mapping using recombinants between visible genetic markers and then between single nucleotide polymorphisms that distinguish the parental N2 strain from the Hawaiian strain CB4856, we defined the position of the et4 and sa580 mutations to a narrow region of chromosome V (Figure 2A). We then tested six cosmids for their ability to rescue the Twp phenotype (Table 1 and Figure 2A). Two cosmids, ZK742 and C37C3, scored positive in this assay but only when co-introduced. Upon examination of the sequences, we noted the presence of the mig-6 gene at the left end of the C37C3 cosmid, with the upstream regulatory 5′ region lying within cosmid ZK742 (Figure 2, A and B). Genomic DNA covering the mig-6 locus was amplified from et4 and sa580 mutants, and sequencing revealed the presence of single point mutations: the et4 allele carries a G-to-A transition that changes a cysteine at position 973 to a tyrosine, and sa580 carries a G-to-A transition that changes a glycine at position 965 to a glutamate (Figure 2, C and D). The et4 and sa580 alleles are therefore novel variants of the mig-6 gene and will be referred to as mig-6(et4) and mig-6(sa580).
Figure 2.—
Mapping of the mig-6(et4) and mig-6(sa580) mutant alleles. (A) Part of the map for chromosome V, including the positions of the genetic markers used to map et4 and sa580. Single nucleotide polymorphisms that distinguish the wild-type N2 genome from the Hawaiian strain CB4856 are shown as open circles and the numbered positions refer to chromosomal locations expressed in kilobases. The locations of six cosmids that were tested for their ability to rescue the sa580 mutant are also shown (thick horizontal lines). The ZK742 and C37C3 cosmids could rescue the sa580 pharynx only when co-injected. (B) Enlarged view of the cosmid C37C3 showing genes as arrows and the two variants of the mig-6 gene in red. (C) Structure of the mig-6 gene showing the positions of the et4 and sa580 mutant alleles, which are both G-to-A transitions. (D) Domain structure of the MIG-6S and MIG-6L proteins, with the et4 and sa580 amino acid substitutions indicated by red arrows, and other mutant alleles by black arrows. T, thrombospondin domain; Lg repeats, lagrin repeats; K, Kunitz domain; and Ig, immunoglobulin domain. A signal peptide is also present at the N terminus of each protein.
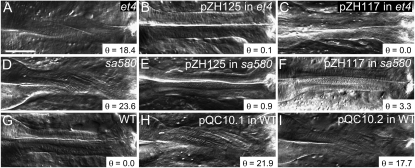
The mig-6 mRNA can be spliced in alternative ways to produce a short protein, MIG-6S, and a long protein, MIG-6L (Figure 2, C and D) (Kawano et al. 2009). Introduction of either the mig-6S or the mig-6L minigenes rescued the twisted pharynx in both the et4 and sa580 mutants (Table 1 and Figure 3). Conversely, introduction into wild-type worms of mutant forms of the mig-6 minigene, harboring either the et4 or the sa580 mutation, caused the left-handed Twp phenotype (Table 1 and Figure 3). The ability of the mutant minigenes to cause Twp is consistent with the fact that both alleles are semidominant.
Figure 3.—
Rescue and phenocopy of the mig-6(et4) and mig-6(sa580) twisted pharynges. (A-I) The pharynges of the indicated genotypes were photographed to emphasize the presence or absence of a pharyngeal twist in the isthmus. pZH125 is a plasmid bearing a mig-6S minigene, pZH117 is a plasmid bearing a mig-6L minigene, and pQC10.1 and pQC10.2 are modified versions of pZH125 in which the et4 and sa580 mutations, respectively, were introduced. Bar, 20 μm. The angle θ is that formed by the torsion lines and the anterior–posterior axis.
The MIG-6 proteins are homologous to the Drosophila protein Papilin and the Manduca sexta protein lacunin (Nardi et al. 1999) and include a domain structure similar to the ancillary domains of ADAMTS metalloproteinases (Figure 2D). In Drosophila, Papilin is abundantly present in most ECM and is essential for development (Campbell et al. 1987; Kramerova et al. 2000, 2003; Fessler et al. 2004). In C. elegans, MIG-6S and MIG-6L are secreted proteins that contribute to the ECM and genetically interact with the ADAMTS protease MIG-17 and with collagen IV (Kawano et al. 2009). Interestingly, none of six other alleles of mig-6 that we tested showed the Twp phenotype (Table 1), although all do display typical defects in distal tip cell migration during gonad development either during the longitudinal phase of distal tip cell migration (class -l alleles) or during the second migration phase (class -s alleles) (Kawano et al. 2009). The mig-6(et4) and mig-6(sa580) alleles have by themselves no defects in distal tip cell migration and therefore represent a novel allele class, i.e., class -twp. These results uncouple the pharyngeal and gonad phenotypes and suggest the possibility of a pharynx-specific function for the eighth lagrin repeat (Nardi et al. 1999), where the et4 and sa580 mutations are located.
mig-6 is present in pharyngeal basement membranes, but not expressed by pharyngeal cells:
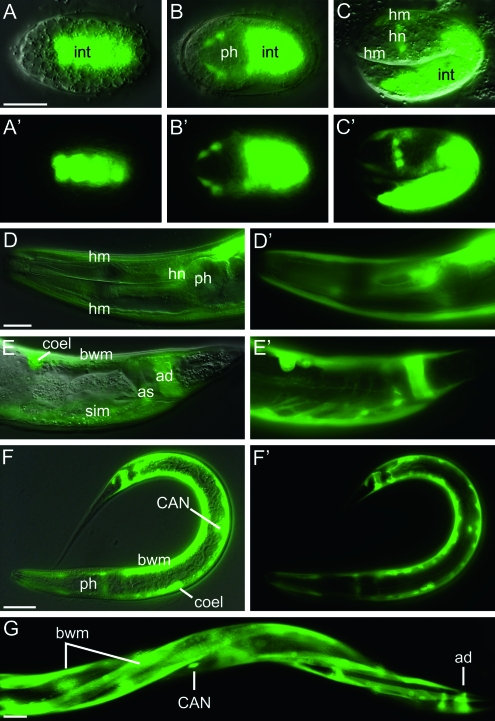
We previously showed that mig-6 transcripts are present in body-wall muscles, distal tip cells, and other unidentified cells and that the MIG-6 protein is present in body-wall muscles of embryos and then becomes enriched in the basement membranes of the pharynx, gonad, and intestine in larvae and adults (Kawano et al. 2009). For this study, we re-examined the expression profile of a mig-6∷gfp transcriptional reporter construct throughout embryonic and larval development and in adults with the aim of detecting possible pharyngeal expression. The following novel observations were made. During embryogenesis, the first expression is in the intestinal primordium followed at the comma and 1.5-fold stages by expression also in future head neurons and head muscle cells (Figure 4, A–C). Post-embryonic expression persists in head neurons and muscles, as well as in coelomocytes, body-wall muscles, anal depressor and sphincter, stomatointestinal muscle, and CAN neurons (Figure 4, D–G). Expression of the mig-6∷gfp transcriptional reporter is never detected in pharyngeal cells at any stage during development or in adults. The MIG-6 protein that accumulates in the pharyngeal basement membrane (Kawano et al. 2009) therefore likely originates from other cells, e.g., from the body-wall muscle cells.
Figure 4.—
Expression profile of a mig-6 transcriptional GFP reporter. Worms transgenic for a mig-6 promoter∷gfp construct were photographed under DIC illumination or epifluorescence using GFP filters. Each panel is provided as a DIC/GFP image overlay and as a GFP image alone, except for G, where only the GFP image is shown. The following developmental stages are shown: (A) an embryo at 270 min post-fertilization at the E16 stage; (B) dorsal aspect of an embryo at the end of gastrulation, mid-plane; (C) 1.5-fold stage embryo; (D, E, and G) young adults; and (F) an L1 larva. int, intestine; ph, pharynx; hm, head muscle; hn, head neuron; bwm, body-wall muscle; coel, coelomocyte; ad, anal depressor; as, anal sphincter; sim, stomatointestinal muscle; CAN, CAN neuron. Each scale bar represents 20 μm; the bar in A applies to panels A through C while the bar in D applies to panels D and E.
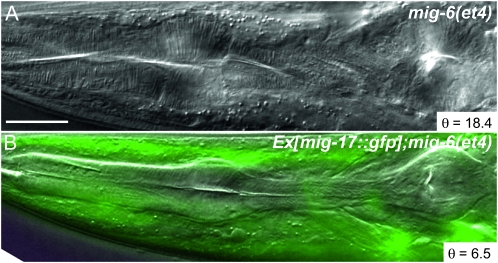
Overexpression of the metalloproteinase gene mig-17 rescues the mig-6(et4) twisted pharynx phenotype:
We previously showed that mig-6 genetically interacts with mig-17, which encodes an ADAMTS protease, and that some mig-6 mutant alleles cause MIG-17 protein to be abnormally distributed (Kawano et al. 2009). To investigate if this could also be the case for the mig-6(et4) allele, we introduced a mig-17∷GFP translational reporter construct where GFP is fused to the C-terminal end of the functional MIG-17 protein (Nishiwaki et al. 2000) into a mig-6(et4) mutant background. No discernible changes in GFP distributions in the head or elsewhere in the body were observed, indicating that the mig-6(et4) allele does not cause dramatic changes in MIG-17 protein localization. To our surprise, we also observed that expression of the mig-17∷GFP construct rescued pharynges in the mig-6(et4) mutant: the transgenic progeny had much straighter pharynges than the nontransgenic parental strain, with average twist angles of 12.5° ± 1.1° and 28.9° ± 1.2°, respectively (Figure 5 and Table 1). The same transgene introduced into wild-type worms did not produce any pharyngeal twist (Table 1). Overexpression of mig-17 can therefore compensate for defects in mig-6, suggesting that the twisting force can be weakened by the proteolytic degradation of ECM components around the pharynx. Alternatively, MIG-17 activity could also lead to changes in the composition of the ECM that surrounds the pharynx, as occurs during distal tip cell migration where the MIG-17 protein helps recruit fibulin and nidogen to the gonadal ECM (Kubota et al. 2004, 2008).
Figure 5.—
mig-17 rescues the Twp phenotype of mig-6(et4) mutants. (A) Twisted pharynx of a mig-6(et4) mutant and (B) straight pharynx of a mig-6(et4) worm rescued by a transgenic mig-17∷GFP translational reporter construct. Bar, 20 μm. The angle θ is that formed by the torsion lines and the anterior–posterior axis.
Relationship between the Twp phenotype and ECM components/regulators:
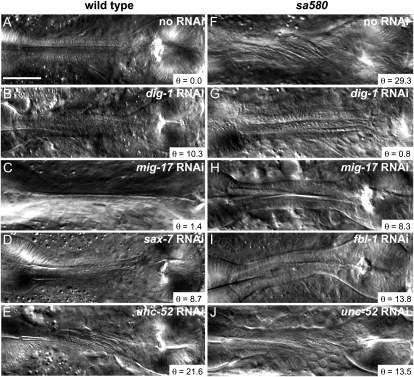
The fact that both mig-6 and mig-17 can influence the ECM to cause pharyngeal twist during growth led us to test other ECM components or regulators. The results are presented in Table 2, with representative images shown in Figure 6. Among ∼20 genes tested by RNAi, only three induced pharyngeal twists: dig-1 (encodes a transmembrane protein with a large extracellular domain embedded in the ECM), sax-7 (another gene encoding a transmembrane protein with a large extracellular domain), and unc-52 (encodes perlecan).
TABLE 2.
Genes that showed pharyngeal twist when mutated or depleted by RNAi in a wild-type background
| Gene | Molecular identity | RNAi or allele | Mutation type | Minimum angle(°) | Maximum angle (°) | Mean angle ± SEM(°) | Twist incidencea | Twist directionb |
|---|---|---|---|---|---|---|---|---|
| N2 (wild type) | — | None | 0 | 0 | 0 | 0% | — | |
| adt-1 | ADAMTS | cn30 | Deletion, no enzymatic activity | 0 | 14.9 | 2.2 ± 0.6 | 23% | 78% left |
| adt-1 | ADAMTS | RNAi | 0 | 0 | 0 | 0 | — | |
| cle-1 | Collagen XVIII | cg120 | Deletion of exons 18–20, truncation | 0 | 24.0 | 2.8 ± 0.8 | 30% | 12% left |
| cle-1 | Collagen XVIII | gk364 | Internal deletion | 0 | 22.4 | 2.6 ± 0.7 | 30% | 0% left |
| cle-1 | Collagen XVIII | gk421 | Internal deletion | 0 | 18.1 | 8.9 ± 0.7 | 95% | 36% left |
| cle-1 | Collagen XVIII | RNAi | 0 | 0 | 0 | 0 | — | |
| dig-1 | Adhesion molecule | RNAi | 0 | 16.7 | 6.0 ± 1.5 | 45% | 40% left | |
| dpy-7 | Cuticle collagen | e1324 | Missense G189D | 0 | 18.0 | 5.5 ± 1.0 | 50% | 28% left |
| dpy-7 | Cuticle collagen | e88 | Missense G156R | 0 | 21.4 | 8.6 ± 0.8 | 88% | 20% left |
| dpy-7 | Cuticle collagen | sc27 | Missense G101R | 0 | 18.4 | 5.1 ± 0.9 | 53% | 0% left |
| dpy-7 | Cuticle collagen | m38 | Missense G201R | 0 | 0 | 0 | 0 | — |
| dpy-7 | Cuticle collagen | RNAi | 0 | 0 | 0 | 0 | — | |
| lam-1 | Laminin β | ok3139 | Internal deletion | 0 | 20.6 | 3.7 ± 1.0 | 28% | 0% left |
| lam-1 | Laminin β | ok3221 | Internal deletion | 0 | 14.1 | 4.1 ± 0.8 | 43% | 0% left |
| lam-3 | Laminin α and γ | ok2030 | Internal deletion | 0 | 11.5 | 3.2 ± 0.6 | 60% | 13% left |
| mig-6 | Papilin | et4 | Missense C973Y | 17.9 | 43.3 | 28.9 ± 1.2 | 100% | 100% left |
| mig-6 | Papilin | sa580 | Missense G965E | 13.9 | 33.3 | 23.8 ± 1.0 | 100% | 100% left |
| mig-6 | Papilin | RNAi | 0 | 0 | 0 | 0 | — | |
| sax-7 | Adhesion molecule | RNAi | 0 | 18.5 | 4.2 ± 1.2 | 50% | 26% left | |
| sup-17 | ADAM | n1258 | Missense V473D | 0 | 13.3 | 1.0 ± 0.4 | 13% | 0% left |
| sup-17 | ADAM | n1260 | Unknown | 0 | 0 | 0 | 0 | — |
| sup-17 | ADAM | n316 | Splicing mutant? | 0 | 0 | 0 | 0 | — |
| unc-52 | Perlecan | e444 | Truncated protein, V1811STOP | 0 | 22.1 | 2.6 ± 0.8 | 33% | 64% left |
| unc-52 | Perlecan | RNAi | 0 | 26.5 | 5.2 ± 1.7 | 40% | 80% left |
Only genes for which RNAi or at least one allele showed a significant effect (P < 0.05; t-test) are listed. Other genes examined by RNAi that showed no twist are the following: adm-2, agr-1, dgn-1, gon-1, lam-3, mig-17, mig-23, and unc-71; emb-9, epi-1, and lam-1 RNAi were also tested but could not be scored due to lethality or sterility. Other mutant alleles that were examined but showed no twist are the following: adm-2(ok3178)X, emb-9(g34)III, emb-9(b117)III, emb-9(b189)III, emb-9(g23)III, emb-9(hc70)III, epi-1(gm57)IV, fbl-1(k201)IV, fbl-1(k206)IV, gon-1 (e1254)/eDf18 IV, gon-1(q518) IV/nT1[unc(n754) let-?](IV;V), let-2(b246)X, let-2(g25)X, let-2(g30)X, let-2(g37)X, mig-17(k113)V, mig-17(k174)V, mig-23(k180)X, and unc-71(e541)III.
Indicates what fraction of scored pharynges exhibited a twist. For RNAi, n = 20 in all cases, except agr-1 (n = 10) and adm-2 (n = 9); for mutants, n = 40 in all cases.
Indicates the percentage of the twisted pharynges that twisted to the left; the others twisted to the right.
Figure 6.—
RNAi knockdown of several genes affects the Twp phenotype. (A–E) The effects of RNAi against the indicated genes in wild-type N2 worms. (F–J) The effects of RNAi against the indicated genes in mig-6(sa580) mutant worms. Bar, 20 μm. The angle θ is that formed by the torsion lines and the anterior–posterior axis.
Of the mutants examined, we found that three alleles of the gene cle-1, which encodes collagen XVIII, and three alleles of dpy-7, which encodes a cuticle collagen, produced the Twp phenotype (Table 2). All cle-1 alleles that caused Twp represent partial deletions or late truncations of the amino acid sequence, and all of the dpy-7 alleles that caused pharyngeal twists represent missense mutations that convert one amino acid to another. Two alleles of lam-1, which encodes laminin β, and one allele of lam-3, which encodes laminin α and γ, also produced the Twp phenotype. All the laminin mutations that cause twists introduce internal deletions. A nonsense mutation (e444) that introduces a stop codon at position 1811 of the perlecan protein encoded by unc-52 also resulted in twisted pharynges. Several splice variants of perlecan are expressed from unc-52, with the longest isoform consisting of 3375 amino acids, and e444 is not a null allele (Rogalski et al. 2001). Finally, mutations in the metalloproteinases sup-17 and adt-1 also caused pharyngeal twists.
Of all the mutants currently known to produce pharyngeal twists, only the mig-6(et4) and mig-6(sa580) alleles are 100% penetrant and always twist to the left (Table 2). RNAi against unc-52, dig-1, or sax-7 caused the Twp phenotype with penetrances of 40–50% and produced a mixture of left- and right-handed pharyngeal twists (80%, 40%, and 26% of left-handed twisted pharynges, respectively). Similarly, specific mutations in some genes cause only left-handed pharyngeal twists, while others produced a mixture of left- or right-handed twists.
Genetic interactions with mig-6(sa580):
To explore possible epistatic interactions, we tested RNAi against ECM-related genes for their ability to reduce or increase the pharyngeal twist caused by the mig-6(sa580) allele (Table 3 and Figure 6). Of the ∼20 genes tested, only RNAi against the genes dpy-7, dig-1, fbl-1 (encodes fibulin), and unc-52 reduced significantly and reproducibly the severity of the Twp phenotype in the sa580 mutant background (P < 0.05). RNAi against some of the other genes tested occasionally inhibited the Twp phenotype of sa580 mutants, as judged by the minimal twist angles observed, even if their mean torsion angle did not differ significantly from the sa580 mutant. These were the genes mig-17 and unc-71, encoding metalloproteinases, and mig-23, which encodes a nucleotide diphosphatase required for glycosylation and proper localization of MIG-17, as well as lam-3, which encodes laminin α and γ. The pharynx of mig-6(sa580) mutants combined with RNAi always twisted to the left irrespective of which RNAi was applied.
TABLE 3.
Effect of RNAi or overexpression of ECM-related genes on the pharyngeal twist caused by the mig-6(sa580) mutant allele
| Gene | Molecular identity | RNAi/overexpression | Mutant Background | Minimum angle (°) | Maximum angle (°) | Mean angle ± SEM (°) | Twist incidenceb | Twist directionc |
|---|---|---|---|---|---|---|---|---|
| — | — | None | mig-6(sa580) | 13.4 | 32.1 | 23.7 ± 1.0 | 100% | 100% left |
| adt-1 | ADAMTS | RNAi | mig-6(sa580) | 17.0 | 34.4 | 26.2 ± 1.0 | 100% | 100% left |
| cle-1 | Collagen XVIII | RNAi | mig-6(sa580) | 11.1 | 31.5 | 21.8 ± 1.4 | 100% | 100% left |
| dig-1 | Adhesion molecule | RNAi | mig-6(sa580) | 0.8 | 13.8 | 5.3 ± 0.9a | 100% | 100% left |
| dpy-7 | Cuticle collagen | RNAi | mig-6(sa580) | 11.0 | 28.6 | 19.5 ± 1.1a | 100% | 100% left |
| fbl-1 | Fibulin | RNAi | mig-6(sa580) | 0.6 | 18.3 | 9.6 ± 1.0a | 100% | 100% left |
| lam-3 | Laminin α and γ | RNAi | mig-6(sa580) | 5.8 | 36.0 | 20.7 ± 1.0 | 100% | 100% left |
| mig-17 | ADAMTS | RNAi | mig-6(sa580) | 5.9 | 33.1 | 21.4 ± 1.6 | 100% | 100% left |
| mig-17∷GFP | ADAMTS | Overexpression | mig-6(et4) | 5.2 | 21.7 | 12.5 ± 1.1a | 100% | 100% left |
| mig-23 | Nucleotide diphosphatase | RNAi | mig-6(sa580) | 8.9 | 36.5 | 23.1 ± 1.6 | 100% | 100% left |
| sax-7 | Adhesion molecule | RNAi | mig-6(sa580) | 12.0 | 41.2 | 25.1 ± 1.7 | 100% | 100% left |
| unc-52 | Perlecan | RNAi | mig-6(sa580) | 6.0 | 28.4 | 19.4 ± 1.3a | 100% | 100% left |
| unc-71 | ADAM | RNAi | mig-6(sa580) | 4.7 | 32.3 | 20.8 ± 1.6 | 100% | 100% left |
Indicates treatments that showed a significant difference in mean torsion angle compared to the reference strain mig-6(sa580) (P < 0.05; t-test). lam-1 RNAi were tested but could not be scored due to lethality and sterility.
Indicates what fraction of scored pharynges exhibited a twist. n = 20 in all cases.
Indicates the percentage of twisted pharynges that twisted to the left; the others twisted to the right.
DISCUSSION
Mechanical basis of the pharyngeal twist (Twp phenotype):
We have identified many genes that, when inhibited or mutated, can cause or inhibit the Twp phenotype in C. elegans (Table 4). These genes encode proteins related to the ECM either as intrinsic components (e.g., collagens, laminins, fibulin, perlecan, papilin) or as ECM proteases of the ADAM and ADAMTS families (e.g., ADT-1, MIG-17, SUP-17). A previous study had also identified mutant alleles of four other genes that caused pharyngeal twists: two encode transmembrane proteins with large extracellular domains (dig-1 and sax-7) and one encodes a C. elegans septin (unc-61) (Axäng et al. 2007); the fourth, mig-4, remains unidentified. The nature of the mutations indicates that the Twp phenotype results from defects in the ECM or in ECM remodeling as the pharynx increases in size during larval growth. We showed previously that the radial contractile arrays within the pharyngeal muscle cells are bent within the twisted pharynges (Axäng et al. 2007). This is exactly what one would expect if these arrays elongated at their normal rate during larval growth but were constrained into bending by a recalcitrant ECM that is too rigid or otherwise flawed to expand appropriately. An over-rigid ECM can be expected if proteases responsible for its softening during growth are inhibited either by RNAi or genetic mutations or, if ECM components are mutated in such a way as to impair their malleability or regulation, by proteases or other factors. A general but limited model consistent with our results for some of the genes studied could be that MIG-6 and some metalloproteinases (e.g., MIG-17) may promote softening of the ECM during growth, while other genes promote stiffening (e.g., DIG-1 and FBL-1). This sort of model would explain why overexpressing mig-17 or RNAi against dig-1 and fbl-1 can rescue Twp in the mig-6(sa580) mutant.
TABLE 4.
Description of the genes that influenced the Twp phenotype
| Gene | Protein type | Expression, function, and mutant phenotypes | References |
|---|---|---|---|
| adt-1 | ADAMTS protease | Expressed in the head ganglion, ventral nerve cord, vulva, and rays of the male tail. The cn30 allele lacking the protease activity exhibits defects in the formation of the male tail rays. | Kuno et al. (2002) |
| cle-1 | Collagen type XVIII | Present at low levels in all basement membranes but enriched on neurons at synaptic contacts. The cg120 mutant lacks a terminal NC1/endostatin domain and exhibits cell migration and axon guidance defects as well as defective neuromuscular junctions. gk364 and gk421 are superficially wild type according to WormBase. | Ackley et al. (2001),Ackley et al. (2003) |
| dig-1 | Large integral membrane protein | Expressed in muscles, hypodermal cells, and coelomocytes. Mutants exhibit defects in the maintenance of fasciculation and cell-body position, including defects in the positioning of several neurons and of the gonad primordium. | Bénard et al. (2006),Burket et al. (2006) |
| dpy-7 | Cuticle collagen | Expressed by hypodermal cells and forms part of the cuticle that forms the exoskeleton. Mutants have abnormal body form and are typically short and fat. | Johnstone et al. (1992),Gilleard et al. (1997) |
| fbl-1 | Fibulin | Expressed by head muscle cells and intestine, but accumulates on ECM of the pharynx, gonad, and other cells. The mutant exhibits enlarged gonads and has body-shape defects. FBL-1 antagonizes the ADAMTS protease GON-1 during gonad development, and its localization to ECM depends on MIG-17 activity. | Kubota et al. (2004),Hesselson et al. (2004),Kubota et al. (2008) |
| lam-1 | Laminin β | Present in all ECM in C. elegans. Null mutants are lethal. RNAi treatment is also lethal, with embryos failing to properly separate various tissues and pharyngeal muscle cells separating into surrounding tissues. | Kao et al. (2006) |
| lam-3 | Laminin α and γ | Present in all ECM in C. elegans. Null mutants are lethal, with missing or abnormal ECM and defective cell migration and differentiation. | C. C. Huang et al. (2003) |
| mig-6 | Papilin | Transcript is expressed in distal tip cell, body-wall muscles, CAN neurons, and some other cells. Protein is enriched in the ECM of the pharynx, gonad, and intestine. Mutants fall into three allelic classes: mig-6l with defects in the longitudinal migration of the DTCs, mig-6s with defects in the second migration of the DTCs, and mig-6Twp that exhibit twisted pharynges (this study). | Kawano et al. (2009) This study |
| mig-17 | ADAMTS protease | Transcript expressed by body-wall muscles. Protein accumulates on the basement membranes of gonad and pharynx and requires glycosylation for its recruitment to gonad surfaces. Its activity leads to the recruitment of nidogen to the ECM. Mutants have defects in some cell migrations, including DTC migration. | Nishiwaki et al. (2000),Kubota et al. (2004),Nishiwaki et al. (2004),Kubota et al. (2008),Kawano et al. (2009) |
| mig-23 | Membrane-bound nucleoside diphosphatase | Expressed in the pharynx and intestine. It is required for glycosylation and proper localization of MIG-17. The mutant exhibits DTC migration defects similar to mig-17 mutants. | Nishiwaki et al. (2004) |
| sax-7 | L1 CAM homolog | The protein is present in regions of cell–cell contacts during early embryogenesis and later in the nerve ring, nerve cord, pharynx, and gonad. It likely acts as a homo- and heterophilic adhesion molecule. Mutants have defects in blastomere compaction during gastrulation and exhibit embryonic lethality and abnormal neuronal distribution and trajectories. | Sasakura et al. (2005),Wang et al. (2005),Grana et al. (2010) |
| sup-17 | ADAM proteinase orthologous to Drosophila and mouse KUZBANIAN | Activates signaling of NOTCH homolog LIN-12 by cleavage of the ectodomain. Mutants have defects in vulva development as well as in body morphology and male tail development; null alleles are embryonic lethal. | Jarriault and Greenwald (2005),Wen et al. (1997) |
| unc-52 | Basement membrane heparan sulfate proteoglycan Perlecan | Three different isoforms (short, medium, and long) expressed differentially. All isoforms are expressed on muscle surfaces during embryogenesis, and, during late embryogenesis, the short form is enriched in the pharyngeal ECM while the medium form is enriched on body-wall muscles. Null mutants are lethal with defects in the formation of muscle filament lattices. Certain viable alleles have defects in distal tip cell migrations and/or defects in pharyngeal neuron development. | Rogalski et al. (1993),Mullen et al. (1999),Rogalski et al. (2001),Merz et al. (2003),Mörck et al. (2010) |
| unc-71 | ADAM protease | Expressed by the excretory canal cell, the excretory gland, head neurons, and in epidermal cells of the vulva. The protein accumulates on membranes. Mutants have defects in motor axon guidance and sex myoblast migrations. | X. Huang et al. (2003) |
DTC, distal tip cell
Most of the alleles so far identified to cause the Twp phenotype produce abnormal proteins, rather than being null alleles, and often cause a Twp phenotype that cannot be replicated by RNAi. For example, the mig-6(et4) and mig-6(sa580) alleles described here are missense mutations impacting the lagrin repeats of the MIG-6 protein. Specific functions for these domains have not been established, but they are likely involved in protein–protein interactions (Nardi et al. 1999; Kawano et al. 2009). Other mig-6 alleles or RNAi against mig-6 did not cause the Twp phenotype. Similarly, the three dpy-7 alleles that cause Twp are missense mutations, while a fourth allele and RNAi caused no pharyngeal twist. The three cle-1 alleles that cause Twp are either internal deletions or express a moderately truncated protein as is the case for cle-1(cg120), while cle-1 RNAi did not cause Twp. In the case of the metalloproteinases adt-1 and sup-17, it is possible that the alleles that can cause a twisted pharynx phenotype have lost enzymatic activity. This is certainly true for the adt-1(cn30) allele, where the enzymatic domain is compromised by a deletion. Non-enzymatic effects of protease genes may relate to their ancillary domains that may sequester substrates, occupy binding sites within the ECM, or even compete with other proteases. Thus, it appears that the twisted pharynx is often the result of defective (as produced by the alleles of collagen, laminin, papilin, or perlecan that cause twists), depleted (as would result from some RNAi), or misregulated ECM components (as would result from the metalloproteinase mutations).
An intriguing observation relates to the semidominance of the et4 and sa580 alleles of mig-6, which appear to behave with near-precise additivity (Table 1), suggesting the possibility of a linear dose-dependent effect on the twist. Also intriguing was the fact that several alleles of dpy-7, which encodes a cuticle collagen, could cause the Twp phenotype. This suggests either that DPY-7 is present in the pharyngeal ECM or that defects in the cuticle lining the pharyngeal lumen (Albertson and Thomson 1976) may contribute to a Twp phenotype.
Another mechanistic consideration relates to the handedness of the pharyngeal twists: some mutations [e.g., mig-6(sa580), cle-1(gk364), or dpy-7(sc27)] always produce left-handed twists, while other mutations produce mixtures of left- and right-handed twists [e.g., cle-1(gk421), dpy-7(e88), or unc-52(e444)]. Clearly, some alleles have an impact on the ordering of the molecules that make up the ECM such that a directional flaw is introduced while other alleles introduce no such bias. ECM molecules can also affect each other's ordering, as when COL-19 defects result in abnormal distribution of DPY-7 in the cuticle (Thein et al. 2003), which further complicates interpretations. Molecules known to organize themselves in ordered structures include laminins and collagens, for which we have now identified mutant alleles with twisted pharynges. In the future, it should be informative to determine how protein–protein interactions are altered in the mutant proteins that cause the Twp phenotype. Defects such as detachment and disorganization in the basement membrane surrounding the pharynx have already been documented for dig-1 mutants by using electron microscopy (Bénard et al. 2006), and it will be interesting to examine the ultrastructure of other Twp mutants. It will also be valuable to examine double mutants among the genes that cause the Twp phenotype to establish genetic pathways.
Comparison with the Roller phenotype:
The Twp phenotype is reminiscent of the Roller phenotype that results from missense or regulatory mutations, rather than from null mutations, in cuticular collagen genes. Kramer and Johnson (1993) carefully studied several alleles of the sqt-1 (also known as rol-5) and rol-6 genes, which encode subunits of a heterotrimeric collagen complex. As with the Twp phenotype, the Roller phenotype first appears at the L2 stage and worsens during growth. The sqt-1 and rol-6 genes are coordinately expressed during development (Park and Kramer 1994), and some mutant alleles cause left-handed Roller phenotypes, while others cause right-handed Roller phenotypes (Kramer and Johnson 1993). The nature of the mutations, typically affecting conserved motifs implicated in disulfide bridges, suggests a model where too much or too little covalent interaction between subunits could cause cuticle alterations with opposite handedness. Ultrastructural analysis also helped provide a mechanical explanation for the Roller phenotype: a repetitive pentagonal pattern in the right-handed rol-6(su1006) mutant ectopically filled a space within the cuticle (Peixoto et al. 1998).
Why the pharynx?
While the emphasis of this article is on the Twp phenotype, all of the genes studied here play important roles in other processes (see Table 4) ranging from maintenance of axon fasciculation [e.g., sax-7 (Sasakura et al. 2005; Wang et al. 2005)], to distal tip cell migration [e.g., fbl-1 (Kubota et al. 2004), mig-6 (Kawano et al. 2009), mig-17 (Kubota et al. 2008), and unc-52 (Merz et al. 2003)], or male tail morphogenesis [e.g., adt-1 (Kuno et al. 2002)], muscle development [e.g., unc-52 (Rogalski et al. 1993)], cuticle development [e.g. dpy-7 (Johnstone et al. 1992)], and embryonic morphogenesis [e.g., lam-1 (Kao et al. 2006) and lam-3 (C. C. Huang et al. 2003)]. However, mutations in these genes do not cause Roller or Squat phenotypes and share no other mutant phenotype in common in addition to Twp, although many also do exhibit cell migration defects, suggesting that both phenotypes are particularly sensitive to ECM remodeling defects. The pharynx is unique within the worm in the frequency and vigor with which it experiences muscular contractions at the pace of three to four pump cycles per second (Avery and Shtonda 2003), which probably also calls for special structural requirements that explain the thickness, 50–100 nm, of the basement membrane that surrounds it (C. C. Huang et al. 2003; Kramer 2005).
Future prospects:
In humans, the sensitivity of muscle contraction to ECM defects probably explains why these are often implicated in congenital muscular dystrophies (Schessl et al. 2006). Similarly, there is a long list of genetic disorders, ranging from Marfan syndrome to deafness, myopathies and brain small-vessel disease, that have their roots in mutations that affect collagen genes, again drawing attention to the importance of ECM structure and regulation in human health (Carter and Raggio 2009). The sensitivity of the Twp phenotype to ECM defects represents a great opportunity to uncover, via forward genetics and with RNAi screens, many new genes and mechanisms important for ECM integrity and remodeling during organ growth. Such efforts will help elucidate the molecular mechanisms underlying ECM-related human diseases.
Acknowledgments
The mig-17∷GFP fusion construct was a kind gift from K. Nishiwaki. The authors thank the technical assistance given by Avelino Javer in getting SHG images using the multimodal optical workstation at Institut de Ciències Fotòniques, Spain. This research was supported by grants from the following agencies: Vetenskapsrådet, Cancerfonden, Åhlén Stiftelse, Magnus Bergvalls Stiftelse, Carl Tryggers Stiftelse, and Erik Philip-Sörensens Stiftelse. J.G.C. also received grant support from the Canadian Institutes of Health Research (MOP-77722) and a Canada Research Chair (950-202224). M.A. was supported by a Howard Hughes Medical Institute Predoctoral Fellowship. Parts of this work were supported by a Public Health Service Grant to J.H.T. (R01 GM48700). M.M. thanks Generalitat de Catalunya, Spain, for an FI fellowship and a BE-Agaur-2008 fellowship, as well as the Sven and Lilly Lawski Foundation for a postdoctoral fellowship. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Insitutes of Health National Center for Research Resources.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.120519/DC1.
References
- Ackley, B. D., J. R. Crew, H. Elamaa, T. Pihlajaniemi, C. J. Kuo et al., 2001. The NC1/Endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 152 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackley, B. D., S. H. Kang, J. R. Crew, C. Suh, Y. Jin et al., 2003. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J. Neurosci. 23 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailion, M., and J. H. Thomas, 2003. Isolation and characterization of high-temperature-induced Dauer formation mutants in Caenorhabditis elegans. Genetics 165 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson, D. G., and J. N. Thomson, 1976. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275 299–325. [DOI] [PubMed] [Google Scholar]
- Avery, L., and B. S. Shtonda, 2003. Food transport in the C. elegans pharynx. J. Exp. Biol. 206 2441–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axäng, C., M. Rauthan, D. H. Hall and M. Pilon, 2007. The twisted pharynx phenotype in C. elegans. BMC Dev. Biol. 7 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénard, C. Y., A. Boyanov, D. H. Hall and O. Hobert, 2006. DIG-1, a novel giant protein non-autonomously mediates maintenance of nervous system architecture. Development 133 3329–3340. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burket, C. T., C. E. Higgins, L. C. Hull, P. M. Berninsone and E. F. Ryder, 2006. The C. elegans gene dig-1 encodes a giant member of the immunoglobulin superfamily that promotes fasciculation of neuronal processes. Dev. Biol. 299 193–205. [DOI] [PubMed] [Google Scholar]
- Campbell, A. G., L. I. Fessler, T. Salo and J. H. Fessler, 1987. Papilin: a Drosophila proteoglycan-like sulfated glycoprotein from basement membranes. J. Biol. Chem. 262 17605–17612. [PubMed] [Google Scholar]
- Carter, E. M., and C. L. Raggio, 2009. Genetic and orthopedic aspects of collagen disorders. Curr. Opin. Pediatr. 21 46–54. [DOI] [PubMed] [Google Scholar]
- Fessler, J. H., I. Kramerova, A. Kramerov, Y. Chen and L. I. Fessler, 2004. Papilin, a novel component of basement membranes, in relation to ADAMTS metalloproteases and ECM development. Int. J. Biochem. Cell Biol. 36 1079–1084. [DOI] [PubMed] [Google Scholar]
- Gaudet, J., and S. E. Mango, 2002. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science 295 821–825. [DOI] [PubMed] [Google Scholar]
- Gilleard, J. S., J. D. Barry and I. L. Johnstone, 1997. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol. Cell. Biol. 17 2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasheen, B. M., R. M. Robbins, C. Piette, G. J. Beitel and A. Page-McCaw, 2010. A matrix metalloproteinase mediates airway remodeling in Drosophila. Dev. Biol. 344 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana, T. M., E. A. Cox, A. M. Lynch and J. Hardin, 2010. SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation. Dev. Biol. 344 731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselson, D., C. Newman, K. W. Kim and J. Kimble, 2004. GON-1 and fibulin have antagonistic roles in control of organ shape. Curr. Biol. 14 2005–2010. [DOI] [PubMed] [Google Scholar]
- Horner, M. A., S. Qhintin, M. E. Domeier, J. Kimble, M. Labouesse et al., 1998. pha-4, an HNF-3 homolog, specifies pharyngeal organ identity in Caenorhabditis elegans. Genes Dev. 12 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. C., D. H. Hall, E. M. Hedgecock, G. Kao, V. Karantza et al., 2003. Laminin alpha subunits and their role in C. elegans development. Development 130 3343–3358. [DOI] [PubMed] [Google Scholar]
- Huang, X., P. Huang, M. K. Robinson, M. J. Stern and Y. Jin, 2003. UNC-71, a disintegrin and metalloprotease (ADAM) protein, regulates motor axon guidance and sex myoblast migration in C. elegans. Development 130 3147–3161. [DOI] [PubMed] [Google Scholar]
- Jarriault, S., and I. Greenwald, 2005. Evidence for functional redundancy between C. elegans ADAM proteins SUP-17/Kuzbanian and ADM-4/TACE. Dev. Biol. 287 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, I. L., Y. Shafi and J. D. Barry, 1992. Molecular analysis of mutations in the Caenorhabditis elegans collagen gene dpy-7. EMBO J. 11 3857–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb, J. M., K. K. Lau, B. Goszczynski, T. Fukushige, D. Moons et al., 1998. pha-4 is Ce-fkh-1, a fork head/HNF-3α, β, γ homolog that functions in organogenesis of the C. elegans pharynx. Development 125 2171–2180. [DOI] [PubMed] [Google Scholar]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser and J. Ahringer, 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2 RESEARCH0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, G., C. C. Huang, E. M. Hedgecock, D. H. Hall and W. G. Wadsworth, 2006. The role of the laminin beta subunit in laminin heterotrimer assembly and basement membrane function and development in C. elegans. Dev. Biol. 290 211–219. [DOI] [PubMed] [Google Scholar]
- Kawano, T., H. Zheng, D. C. Merz, Y. Kohara, K. K. Tamai et al., 2009. C. elegans mig-6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig-17 and collagen IV. Development 136 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, J. M., 2005. Basement membranes (September 1, 2005), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.16.1, http://www.wormbook.org.
- Kramer, J. M., and J. J. Johnson, 1993. Analysis of mutations in the sqt-1 and rol-6 collagen genes of Caenorhabditis elegans. Genetics 135 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerova, I. A., N. Kawaguchi, L. I. Fessler, R. E. Nelson, Y. Chen et al., 2000. Papilin in development; a pericellular protein with a homology to the ADAMTS metalloproteinases. Development 127 5475–5485. [DOI] [PubMed] [Google Scholar]
- Kramerova, I. A., A. A. Kramerov and J. H. Fessler, 2003. Alternative splicing of papilin and the diversity of Drosophila extracellular matrix during embryonic morphogenesis. Dev. Dyn. 226 634–642. [DOI] [PubMed] [Google Scholar]
- Kubota, Y., R. Kuroki and K. Nishiwaki, 2004. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr. Biol. 14 2011–2018. [DOI] [PubMed] [Google Scholar]
- Kubota, Y., K. Ohkura, K. K. Tamai, K. Nagata and K. Nishiwaki, 2008. MIG-17/ADAMTS controls cell migration by recruiting nidogen to the basement membrane in C. elegans. Proc. Natl. Acad. Sci. USA 105 20804–20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno, K., C. Baba, A. Asaka, C. Matsushima, K. Matsushima et al., 2002. The Caenorhabditis elegans ADAMTS family gene adt-1 is necessary for morphogenesis of the male copulatory organs. J. Biol. Chem. 277 12228–12236. [DOI] [PubMed] [Google Scholar]
- Laprise, P., S. M. Paul, J. Boulanger, R. M. Robbins, G. J. Beitel et al., 2010. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr. Biol. 20 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mango, S. E., E. J. Lambie and J. Kimble, 1994. The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development 120 3019–3031. [DOI] [PubMed] [Google Scholar]
- Mathew, M., S. I. Santos, D. Zalvidea and P. Loza-Alvarez, 2009. Multimodal optical workstation for simultaneous linear, nonlinear microscopy and nanomanipulation: upgrading a commercial confocal inverted microscope. Rev. Sci. Instrum. 80 073701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, D. C., G. Alves, T. Kawano, H. Zheng and J. G. Culotti, 2003. UNC-52/Perlecan affects gonadal leader cell migrations in C. elegans hermaphrodites through alterations in growth factor signaling. Dev. Biol. 256 173–186. [DOI] [PubMed] [Google Scholar]
- Miyabayashi, T., M. T. Palfreyman, A. E. Sluder, F. Slack and P. Sengupta, 1999. Expression and function of members of a divergent nuclear receptor family in Caenorhabditis elegans. Dev. Biol. 215 314–331. [DOI] [PubMed] [Google Scholar]
- Mörck, C., C. Axäng and M. Pilon, 2003. A genetic analysis of axon guidance in the C. elegans pharynx. Dev. Biol. 260 158–175. [DOI] [PubMed] [Google Scholar]
- Mörck, C., V. Vivekanand, G. Jafari and M. Pilon, 2010. C. elegans ten-1 is synthetic lethal with mutations in cytoskeleton regulators, and enhances many axon guidance defective mutants. BMC Dev. Biol. 10 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, G. P., T. M. Rogalski, J. A. Bush, P. R. Gorji and D. G. Moerman, 1999. Complex patterns of alternative splicing mediate the spatial and temporal distribution of perlecan/UNC-52 in Caenorhabditis elegans. Mol. Biol. Cell 10 3205–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel, J. M., C. Dong, H. Hutter and B. E. Vogel, 2005. Fibulin-1C and fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development 132 4223–4234. [DOI] [PubMed] [Google Scholar]
- Nardi, J. B., R. Martos, K. K. Walden, D. J. Lampe and H. M. Robertson, 1999. Expression of lacunin, a large multidomain extracellular matrix protein, accompanies morphogenesis of epithelial monolayers in Manduca sexta. Insect Biochem. Mol. Biol. 29 883–897. [DOI] [PubMed] [Google Scholar]
- Nguyen, T. Q., H. Sawa, H. Okano and J. G. White, 2000. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J. Cell Sci. 113 3825–3837. [DOI] [PubMed] [Google Scholar]
- Nishiwaki, K., N. Hisamoto and K. Matsumoto, 2000. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science 288 2205–2208. [DOI] [PubMed] [Google Scholar]
- Nishiwaki, K., Y. Kubota, Y. Chigira, S. K. Roy, M. Suzuki et al., 2004. An NDPase links ADAM protease glycosylation with organ morphogenesis in C. elegans. Nat. Cell Biol. 6 31–37. [DOI] [PubMed] [Google Scholar]
- Park, Y. S., and J. M. Kramer, 1994. The C. elegans sqt-1 and rol-6 collagen genes are coordinately expressed during development, but not at all stages that display mutant phenotypes. Dev. Biol. 163 112–124. [DOI] [PubMed] [Google Scholar]
- Peixoto, C. A., J. V. de Melo, J. M. Kramer and W. de Souza, 1998. Ultrastructural analyses of the Caenorhabditis elegans rol-6 (su1006) mutant, which produces abnormal cuticle collagen. J. Parasitol. 84 45–49. [PubMed] [Google Scholar]
- Perkins, L. A., E. M. Hedgecock, J. N. Thomson and J. G. Culotti, 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117 456–487. [DOI] [PubMed] [Google Scholar]
- Portereiko, M. F., and S. E. Mango, 2001. Early morphogenesis of the Caenorhabditis elegans pharynx. Dev. Biol. 233 482–494. [DOI] [PubMed] [Google Scholar]
- Psilodimitrakopoulos, S., S. I. Santos, I. Amat-Roldan, A. K. Thayil, D. Artigas et al., 2009. In vivo, pixel-resolution mapping of thick filaments' orientation in nonfibrilar muscle using polarization-sensitive second harmonic generation microscopy. J. Biomed. Opt. 14 014001. [DOI] [PubMed] [Google Scholar]
- Rogalski, T. M., B. D. Williams, G. P. Mullen and D. G. Moerman, 1993. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev. 7 1471–1484. [DOI] [PubMed] [Google Scholar]
- Rogalski, T. M., G. P. Mullen, J. A. Bush, E. J. Gilchrist and D. G. Moerman, 2001. UNC-52/perlecan isoform diversity and function in Caenorhabditis elegans. Biochem. Soc. Trans. 29 171–176. [DOI] [PubMed] [Google Scholar]
- Rozario, T., and D. W. DeSimone, 2010. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura, H., H. Inada, A. Kuhara, E. Fusaoka, D. Takemoto et al., 2005. Maintenance of neuronal positions in organized ganglia by SAX-7, a Caenorhabditis elegans homologue of L1. EMBO J. 24 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schessl, J., Y. Zou and C. G. Bonnemann, 2006. Congenital muscular dystrophies and the extracellular matrix. Semin. Pediatr. Neurol. 13 80–89. [DOI] [PubMed] [Google Scholar]
- Starich, T. A., R. K. Herman, C. K. Kari, W. H. Yeh, W. S. Schackwitz et al., 1995. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics 139 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., and J. A. Hodgkin, 1988. Methods, pp. 587–606 in The Nematode Caernorhabditis elegans, edited by W. B. Wood. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Thein, M. C., G. McCormack, A. D. Winter, I. L. Johnstone, C. B. Shoemaker et al., 2003. Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev. Dyn. 226 523–539. [DOI] [PubMed] [Google Scholar]
- Tonning, A., J. Hemphala, E. Tang, U. Nannmark, C. Samakovlis et al., 2005. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 9 423–430. [DOI] [PubMed] [Google Scholar]
- Tsang, K. Y., M. C. Cheung, D. Chan and K. S. Cheah, 2010. The developmental roles of the extracellular matrix: beyond structure to regulation. Cell Tissue Res. 339 93–110. [DOI] [PubMed] [Google Scholar]
- Vogel, B. E., and E. M. Hedgecock, 2001. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 128 883–894. [DOI] [PubMed] [Google Scholar]
- Wang, X., J. Kweon, S. Larson and L. Chen, 2005. A role for the C. elegans L1CAM homologue lad-1/sax-7 in maintaining tissue attachment. Dev. Biol. 284 273–291. [DOI] [PubMed] [Google Scholar]
- Wen, C., M. M. Metzstein and I. Greenwald, 1997. SUP-17, a Caenorhabditis elegans ADAM protein related to Drosophila KUZBANIAN, and its role in LIN-12/NOTCH signalling. Development 124 4759–4767. [DOI] [PubMed] [Google Scholar]