Abstract
Evolution through natural selection suggests unnecessary genes are lost. We observed that the yeast Candida glabrata lost the gene encoding a phosphate-repressible acid phosphatase (PHO5) present in many yeasts including Saccharomyces cerevisiae. However, C. glabrata still had phosphate starvation-inducible phosphatase activity. Screening a C. glabrata genomic library, we identified CgPMU2, a member of a three-gene family that contains a phosphomutase-like domain. This small-scale gene duplication event could allow for sub- or neofunctionalization. On the basis of phylogenetic and biochemical characterizations, CgPMU2 has neofunctionalized to become a broad range, phosphate starvation-regulated acid phosphatase, which functionally replaces PHO5 in this pathogenic yeast. We determined that CgPmu2, unlike ScPho5, is not able to hydrolyze phytic acid (inositol hexakisphosphate). Phytic acid is present in fruits and seeds where S. cerevisiae grows, but is not abundant in mammalian tissues where C. glabrata grows. We demonstrated that C. glabrata is limited from an environment where phytic acid is the only source of phosphate. Our work suggests that during evolutionary time, the selection for the ancestral PHO5 was lost and that C. glabrata neofunctionalized a weak phosphatase to replace PHO5. Convergent evolution of a phosphate starvation-inducible acid phosphatase in C. glabrata relative to most yeast species provides an example of how small changes in signal transduction pathways can mediate genetic isolation and uncovers a potential speciation gene.
THE phosphate signal transduction (PHO) pathway in Candida glabrata is similar to the PHO pathway in Saccharomyces cerevisiae and serves as a model to examine species-specific changes to a signal transduction pathway (Kerwin and Wykoff 2009). C. glabrata is a mammalian pathogen that can cause candidiasis and is relatively closely related to budding yeast, S. cerevisiae (Redondo-Lopez et al. 1990; Cormack and Falkow 1999; Domergue et al. 2005). Thus, identifying differences in the PHO pathway between the two species may shed light on their environmental niche. In both species, the transcription factor Pho4 is critical to the phosphate starvation response, and it appears to be regulated similarly in both species—through phosphorylation by the Pho80/Pho85/Pho81 complex (Schneider et al. 1994; O'Neill et al. 1996; Kerwin and Wykoff 2009). However, another transcription factor, Pho2, which is essential for the starvation response in budding yeast, appears to be unimportant in C. glabrata (Barbaric et al. 1998; Springer et al. 2003; Kerwin and Wykoff 2009).
Activation of the PHO pathway in S. cerevisiae often is measured by the transcription of PHO5, which encodes a phosphate starvation-inducible acid phosphatase (Huang and O'Shea 2005). Assays can quantitatively or qualitatively measure the extent of Pho5 activity and serve as a proxy for Pho4 activity. In studying C. glabrata, we observe strong acid phosphatase activity that is regulated by Pho4; however, the sequenced genome does not appear to contain a PHO5 homolog (Kerwin and Wykoff 2009). Through complementation of a Scpho5Δ strain with a C. glabrata genomic library (Sanglard et al. 1999), we identified a three-gene cluster (which we have named CgPMU1, CgPMU2, and CgPMU3) that contains the functional analog of PHO5. This cluster, present in only C. glabrata and in none of the other sequenced yeast genomes, provides an interesting model system to understand how gene duplication impacts the species-specific features of the PHO pathway in C. glabrata.
Gene duplication is a major evolutionary force through which new functions can appear (He and Zhang 2005; Conant and Wolfe 2008). Duplication of genes can allow one gene to maintain its ancestral function while the duplicate is relieved from stringent selection (Zhang 2003; Beisswanger and Stephan 2008). The duplicated gene can be lost through pseudogenization, can partition functions between the paralogs (subfunctionalize), or can acquire new, but often related functions through neofunctionalization (Conant and Wolfe 2008). A new function can generate a species-specific feature that might isolate one species from another (Ting et al. 2004). Generation of a new function in one pathway is unlikely to be sufficient for genetic isolation; however, many small changes to signal transduction pathways through duplication and drift or selection may reinforce isolated genetic populations and drive speciation.
We studied the function of three homologs of the ScPMU1 gene in C. glabrata to establish which paralog is regulated by phosphate starvation and Pho4. Upon identifying CgPMU2 as the ScPHO5 analog in C. glabrata, we determined that CgPMU2 has neofunctionalized to hydrolyze phosphate from organic phosphate sources that CgPMU1 and CgPMU3 are unable to hydrolyze. Finally, we determined that loss of PHO5 and its replacement with the analog PMU2 has restricted C. glabrata from an environmental niche that contains phytic acid as the sole phosphate source.
MATERIALS AND METHODS
Strains and media:
Yeast strains (Table 1) were grown in synthetic dextrose (SD) media with complete supplement mixture (CSM) (Sunrise Science Products, San Diego, CA) with or without histidine. For all experiments, cells were grown at 30° until logarithmic growth phase (OD600 ∼0.5). For phosphate starvation experiments, cells grown to logarithmic phase were harvested by centrifugation, washed three times in media without phosphate, and then transferred to media without phosphate (no-phosphate conditions) or to media with 10 mm KH2PO4 added (high-phosphate conditions) and grown at 30° for 3 hr (Kerwin and Wykoff 2009).
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| C. glabrata | ||
| BG99 | his3Δ (1 + 631) | Cormack and Falkow (1999) |
| DG2 | pho4Δ∷ KANMX6 in BG99 | Kerwin and Wykoff (2009) |
| DG66 | pmu1Δ∷ KANMX6 in BG99 | This study |
| DG29 | pmu2Δ∷ NATMX6 in BG99 | This study |
| DG30 | pmu3Δ∷ NATMX6 in BG99 | This study |
| DG87 | pmu3Δ∷ NATMX6 in BG99 | This study |
| S. cerevisiae | ||
| EY57 | K699 with MATa | Wykoff and O'Shea (2001) |
| EY132 | pho5∷TRP1 | Wykoff et al. (2007) |
The genotype of K699 is ade2-1 trp1-1 can1-100 leu2-3, 112 his3-11, 15 ura3.
C. glabrata mutants were generated using the antibiotic resistance genes KANMX6 or NATMX6 (Kerwin and Wykoff 2009) and homologous recombination to inactivate PMU1, PMU2, or PMU3 in a C. glabrata his3− background strain (Cormack and Falkow 1999; Hentges et al. 2005; Kerwin and Wykoff 2009). Refer to supporting information, Table S1 for a list of primers used to inactivate genes. Deletion of genes was confirmed by PCR and a phosphatase plate assay of multiple isolates.
Identification of PMU2 genomic clone:
To screen for the C. glabrata phosphatase gene, we transformed by electroporation a C. glabrata genomic DNA library (Sanglard et al. 1999) into a Scpho5Δ strain. Cells were plated on SD−Ura plates, at a density of ∼1000 colonies per plate. Approximately 38,000 transformants were replica plated onto SD −Ura plates lacking phosphate. After ∼16 hr, the colonies were assayed for the presence of a secreted acid phosphatase using the semiquantitative phosphatase assay described previously (Wykoff and O'Shea 2001). Red colonies were reassayed in high-phosphate and no-phosphate conditions for phosphatase activity. From yeast genomic DNA preparations of the selected colonies, plasmids were isolated by chemical transformation of Escherichia coli cells, and ∼500 nucleotides were sequenced on each side of the plasmid insert.
Assays for phosphatase activity:
For the semiquantitative assay, agar plates were overlaid with Fast Blue Salt B stain, 1-naphthyl phosphate (1-NP), and 0.1 m sodium acetate (pH 4.2) (Wykoff et al. 2007). For quantitative measurements of hydrolysis of p-nitrophenylphosphate phosphate (PNPP), 1 ml of cells (OD600 ∼0.5) was pelleted by centrifugation and resuspended in sterile water. Because PNPP does not cross the plasma membrane and <5% of total phosphatase activity can be washed away from the cells (data not shown), we conclude that we are measuring periplasmic phosphatase activity. Cells were incubated with 10 mm PNPP at pH 4.2 at 25° for 10 min or 20 min. The reaction was quenched with saturated Na2CO3. Phosphatase activity was measured in units expressed as OD400/OD600 (Huang and O'Shea 2005).
Quantitative reverse-transcription PCR:
RNA was extracted using a phenol–chloroform protocol (Huang and O'Shea 2005) and converted to cDNA by a reverse-transcription reaction (Bio-Rad iScript cDNA synthesis kit). Quantitative PCR was performed with a Chromo-4 PCR machine (Bio-Rad) using SyberGreen I dye (Sigma-Aldrich, St. Louis, MO) with a 50-μl PCR reaction (Kerwin and Wykoff 2009). Primers (Table S1) were designed for C. glabrata PMU1, PMU2, and PMU3 and for S. cerevisiae PMU1 and ACT1, and data were normalized to expression of ACT1 (Kerwin and Wykoff 2009). Each gene was equally amplified with the designed primers using 10-fold genomic DNA dilutions.
Bacterial expression and purification of Pmu1, Pmu2, and Pmu3:
Using primers O218–O223 (Table S1), PMU1, PMU2, and PMU3 were amplified with PCR, digested with BamHI and/or XhoI and ligated into pET16b vector. Sequence confirmed clones were transformed into C3013H E. coli cells (New England Biolabs). Pooled transformants were grown at 30° and induced with 1mm IPTG for 2 hr. Cells were lysed by sonication in 10% glycerol, 50 mm Tris (pH 8), 250 mm NaCl, 0.1% NP-40 (or Tween 20), 10 mm imidazole (pH 8), 1 mm 2-mercaptoethanol, and protease inhibitors (G-Biosciences, St. Louis, MO). IDA resin (Sigma-Aldrich) was charged with cobalt chloride, loaded with cell extract, and washed with the same buffer until no protein was detected in the flow through. The tagged proteins were eluted with 20 mm EDTA and dialyzed overnight in a 500-fold volume lysis buffer. Because additional bands were observed in each extract along with the His10-tagged protein, densitometry was used to estimate the purity of the extract. When assaying against various phosphate substrates, this percentage was multiplied by the total concentration of protein in the extract (determined by measuring the absorbance at 280 nm) to calculate the concentration of pET16b-tagged protein in each reaction.
Detection of phosphatase activity—phosphate released:
To ascertain the optimum pH of each enzyme, the His10-purified proteins were incubated with solutions ranging in pH from 3.4 to 8.0, all at 0.1 m final osmolarity. Solutions were made from combining different amounts of 100 mm acetate and 100 mm Tris. Final pH readings were measured using a pH meter. To determine the specificity of each enzyme, the His10-purified proteins were incubated with various phosphate-containing substrates. Using twofold dilutions, the kinetics of each reaction were observed. The substrates glycerol-1 phosphate and glycerol-2-phosphate were tested at concentrations ranging from 100 mm to 0.78 mm. Inosine-5′-monophosphate was tested at concentrations ranging from 25 mm to 0.195 mm, trehalose-6-phosphate from concentrations ranging from 10 mm to 0.078 mm, and PNPP from concentrations ranging from 14.85 mm to 0.116 mm. Reactions were performed with and without enzyme, using the optimum pH buffer for each enzyme and differing concentrations of phosphate substrate. The reactions were terminated by heating at 99° for 5 min. The amount of phosphate released was quantified by incubating the reactions with 300 μl Brilliant Green phosphate dye in a final volume of 1 ml at 30° for 30 min (Chen et al. 1956). Phosphate released was measured in units of OD639, and reactions with and without enzyme were compared. Data were normalized using a standard curve with known amounts of inorganic phosphate (KH2PO4). Vmax and KM values were calculated using either Hanes–Woolf or Lineweaver–Burk plots. All calculations were normalized by subtracting a sample with non-His10–tagged protein added. When comparing substrate specificity, we used the same purification batches to eliminate any error in the estimation of protein concentration, which could impact Vmax measurements. This prevented us from performing three replicates of all of the assays. A linear regression with at least four points (R2 > 0.8) was required for a determination of Vmax and KM values, and if we did not observe consistent Michaelis–Menton kinetics then the measurement was considered not detected (ND). We were unable to measure 1-naphthyl phosphatase activity because of interfering levels of inorganic phosphate.
For measuring phytase activity of whole cells, cells were grown overnight in SD + CSM + 10 mm KH2PO4, washed three times with no-phosphate media, and starved in no-phosphate media for ∼6 hr. Two independent cultures of S. cerevisiae wild type, S. cerevisiae pho5Δ, and C. glabrata wild type were assayed for phytase activity. Aliquots were thawed at 4° with lysis buffer (see above). The cell density was measured by OD600 and normalized on the basis of the values that 1 OD of S. cerevisiae is equivalent to 5 × 107 cells/ml/OD and that 1 -OD of C. glabrata is equivalent to 2.5 × 108 cells/ml/OD. Approximately, the same optical density of cells (5 μl) was added to 100 μl of 0.125 mm potassium phytate pH 3.4 and incubated for 10 min. The concentration of phytate was chosen to minimize the amount of contaminating inorganic phosphate. After 10 min, the cells were centrifuged away from the reaction, and 80 μl of the clarified reaction was boiled at 99° for 5 min. To detect the amount of inorganic phosphate liberated from phytate, 40 μl of the reactions was incubated with Brilliant Green phosphate dye (protocol described above). It is worth noting that whether this reaction is normalized to optical density or to cell density, the results have similar trends.
Generation of regulated expression of the PMU genes in a Cgpmu2Δ strain:
Using primers to place a gene under the control of the ScPHO5 promoter (Table S1), ScPMU1, CgPMU1, CgPMU2, and CgPMU3 were amplified using PCR. The template for these PCR reactions was either the pET16b plasmid containing a PMU gene or S. cerevisiae genomic DNA. A vector (DB146) containing 1000 bp of the ScPHO5 promoter and YFP separated by a PacI site was digested, combined with each PCR product, and transformed into the pmu2Δ strain. When translationally fused to YFP, CgPmu2 was not functional; however, inclusion of a stop codon at the end of CgPMU2 allowed for full complementation of the Cgpmu2Δ strain. All four constructs included a stop codon, were rescued and subjected to diagnostic digests and PCR to confirm their identity, and retransformed into the pmu2Δ strain. We also generated plasmids using the same scheme as above with the ScADH1 promoter and the CgPMU2 promoter using primers described in Table S1. The CgPMU2 promoter contained ∼1040 bp upstream of the CgPMU2 start codon and did not include any portion of the CgPMU1 ORF. Genomic clones of each PMU gene were generated with primers indicated in Table S1 and cloned by homologous recombination into pRS313.
RESULTS
Identification of PMU region in C. glabrata as containing inducible phosphatase:
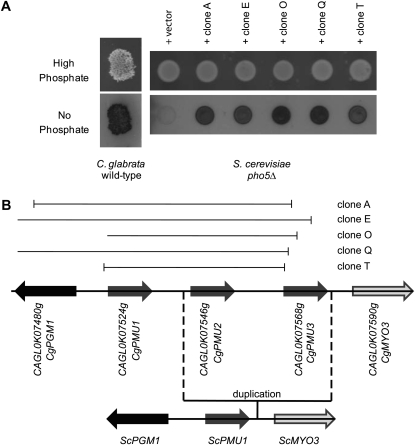
Whereas the C. glabrata genome does not contain a homolog of the ScPHO5 gene, C. glabrata exhibits phosphatase activity during phosphate starvation conditions (Figure 1A), suggesting the presence of a cryptic gene in C. glabrata that encodes a phosphate starvation-inducible acid phosphatase. To identify this gene, we transformed a C. glabrata genomic DNA library (Sanglard et al. 1999) into a Scpho5Δ strain. We hypothesized that the library contained genomic clones capable of restoring phosphate starvation-inducible phosphatase activity and some clones would functionally complement the Scpho5Δ strain. Five colonies out of ∼38,000 colonies exhibited significant phosphatase activity during phosphate starvation and no significant activity in high-phosphate conditions (Figure 1A). When the ends of the genomic clones were sequenced, all 5 contained genes within an ∼13-kb region on chromosome 11 in C. glabrata (Figure 1B). This region contains three paralogs of PMU1, a gene that in S. cerevisiae encodes a phosphomutase-like protein (Elliott et al. 1996; Rebora et al. 2005; Byrne and Wolfe 2006). Because phosphomutase binds to phosphoglycerate and isomerizes carbon phosphate bonds, it seemed plausible that these proteins might bind to and hydrolyze organic phosphate compounds. We named these three paralogs CgPMU1 (CAGL0K07524g), which has the most similarity to ScPMU1, CgPMU2 (CAGL0K07546g), and CgPMU3 (CAGL0K07568g). CgPMU2 and CgPMU3 appear to have derived from small-scale duplication events as the genes surrounding this cluster have conserved synteny in other Ascomycetes, including S. cerevisiae (Figure 1B).
Figure 1.—
Complementation of Scpho5Δ by C. glabrata genomic library. (A) A semiquantitative phosphatase plate assay was performed on wild-type C. glabrata grown in high-phosphate media and in no-phosphate media with the dark color indicating secreted acid phosphatase activity. Five genomic clones conferred phosphatase activity in no-phosphate conditions and repressed phosphatase activity in high-phosphate conditions. (B) The genomic clones with phosphatase activity were further analyzed. Clone A spans nucleotides 740743–747471, clone E 748278–738994, clone Q 747445–735219, and clone T 743041–747455. Clone O ends at nucleotide 747642 (numbering is based on chromosome K sequence NC_006034). Using CgPMU1 primers designed to amplify the ORF, it was determined that clone O contains CgPMU1; however, one end was not refractory to sequencing. The same region of chromosome 11 from S. cerevisiae is below the C. glabrata schematic showing the conserved synteny. The direction of the arrows indicates the direction of transcription.
Relatively little is known about ScPMU1 in S. cerevisiae. When ScPMU1 is overexpressed, it suppresses a histidine auxotrophy of the ade3ade16ade17 triple mutant (Rebora et al. 2005). The histidine requirement is probably suppressed by detoxifying AICAR, a monophosphate nucleotide derivative (Rebora et al. 2005). Because of its potential phosphomutase activity, ScPMU1 might remove the phosphate group of AICAR (Rebora et al. 2005). ScPMU1 has also been isolated as a high-copy suppressor of the temperature sensitivity of a tps2 mutant that lacks trehalose-6-phosphatase activity (Elliott et al. 1996). In this case, ScPmu1 likely removes the phosphate from trehalose-6-phosphate, preventing the accumulation of trehalose-6-phosphate (Elliott et al. 1996). These previous studies, in combination with PMU1 containing a domain that is related to phosphomutases, suggest that PMU1 may function to manipulate or hydrolyze phosphate groups from organic compounds (Elliott et al. 1996). Because PMU1 contains a potential phosphomutase domain, it may have some affinity for phosphate; therefore, all three close relatives in C. glabrata may also have the ability to bind and hydrolyze organic phosphate compounds.
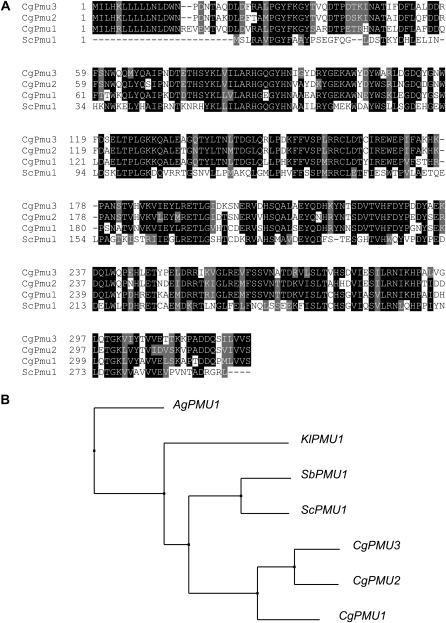
To determine the similarity and possible evolutionary history between ScPMU1 and these three paralogs, we performed a ClustalW alignment with the predicted protein sequences (Figure 2A). ScPmu1 is more similar to CgPmu1 (51% identical) than to CgPmu2 (45%) or CgPmu3 (46% identical). We hypothesize CgPMU1 is likely an ortholog of ScPMU1 because CgPMU1 is most closely related to the ancestral PMU1 gene in other Ascomycetes. It is noteworthy that there are 22–24 amino acids on the N terminus of all three paralogs in C. glabrata that are not present in S. cerevisiae or most other Ascomycetes (Figure 2A). This likely is a signal sequence allowing for secretion of all three products into the periplasm, much like ScPho5 or Kluyveromyces lactis Pho5 (Ferminan and Dominguez 1997). Our assumption is supported by analysis of the sequences for a putative signal peptide with SignalP (Bendtsen et al. 2004). On the basis of similarity, we hypothesize that the ancestral PMU1 acquired a signal sequence altering its ancestral function or localization (but not eliminating it) and then duplicated twice, because all three PMU genes are located in tandem on chromosome 11 in C. glabrata. This tandem triplet of PMU1-like genes is only observed in C. glabrata and not in the other sequenced Ascomycetes genomes (Wapinski et al. 2007), suggesting positive selection on this gene family in C. glabrata (Figure 2B).
Figure 2.—
(A) ClustalW alignment of CgPmu proteins with ScPmu1. After alignment, the ALN file was entered into BOXSHADE with a cutoff of identity of 0.7. Solid boxes indicate identity and shaded boxes indicate similarity. (B) Tree of relationships generated from JalView 2.4 using a neighbor joining tree with BLOSSUM62. The sequences used were: Ashbya gossypii AEL304C, Kluyveromyces lactis KLLA0B12628g, Saccharomyces bayanus sbayc559-g5.1, and previously identified protein sequences. This tree is representative of trees generated by other methods as well.
Identification of CgPMU2 as inducible phosphatase:
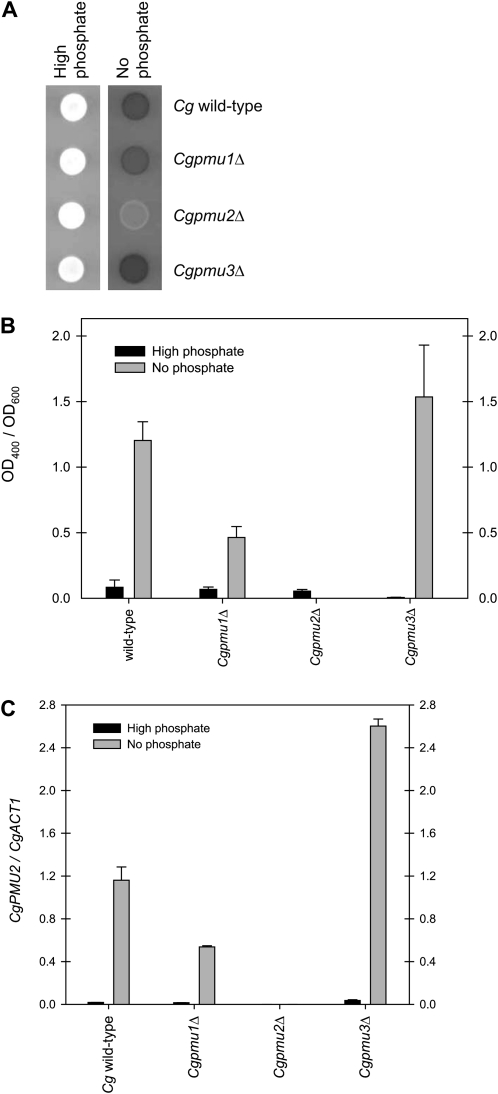
To determine which of these duplicate genes has phosphate starvation-inducible secreted acid phosphatase activity, each gene was individually inactivated with a KANMX6 or NATMX6 cassette (Longtine et al. 1998; Kerwin and Wykoff 2009). Each deletion strain was assayed for phosphatase activity with a qualitative phosphatase plate assay (Kerwin and Wykoff 2009), where colonies remain white if there is little phosphatase activity and turn red through a diazo coupling reaction when phosphate is cleaved from 1-NP. We expected CgPMU2 to be the phosphate starvation-inducible secreted acid phosphatase in C. glabrata because it is the only gene with both the ORF and promoter region present in all five genomic clones (Figure 1B). In a Cgpmu2Δ strain, there is essentially no secreted acid phosphatase activity in C. glabrata (Figure 3A). However, if either CgPMU1 or CgPMU3 is deleted, there is still phosphatase activity in phosphate starvation conditions. Pmu1 and Pmu3 are not critical for phosphatase activity in C. glabrata because when either is inactivated, there is still detectable acid phosphatase activity. We quantified phosphatase activity using PNPP during phosphate starvation and confirmed these qualitative results (Figure 3B) (Huang and O'Shea 2005). Additionally, we quantified levels of PMU2 transcript through quantitative (q) PCR on reverse transcribed RNA of each of the strains (Kerwin and Wykoff 2009) and observed that the levels of PMU2 were very similar to the levels of phosphatase activity, consistent with PMU2 encoding the phosphate starvation-inducible phosphatase activity.
Figure 3.—
Pmu2 is phosphate starvation-inducible acid phosphatase in C. glabrata. (A) Phosphatase plate assay in high- and no-phosphate conditions with only the pmu2Δ strain having a major defect in phosphatase activity. (B) The phosphatase activity (PNPP hydrolysis) of the deletion strains was quantified to determine activity normalized to cell density. Data in this figure and all following figures are expressed as mean ± SEM, n = 3 for each strain unless noted. Generation of new Cgpmu1Δ strain (Figure S1), which did not disrupt CgPMU2 promoter, prevented this effect. (C) Quantitative real-time PCR was performed on the same strains and CgPMU2 was quantified and normalized to the expression level of CgACT1 because the expression of CgACT1 does not change in response to phosphate starvation (Kerwin and Wykoff 2009). Deletion of CgPMU3 appears to lead to increased phosphatase activity and increased levels of CgPMU2 transcript. This 1.5- to 2-fold increase is consistently observed, but was not tractable enough to pursue in this study.
It is worth noting that there is a significant decline in phosphatase activity in the Cgpmu1Δ strain and this could be explained in one of two ways—either CgPMU1 is encoding approximately half of phosphatase activity or disruption of CgPMU1 compromises the function of the CgPMU2 promoter, preventing full expression of the phosphatase. To differentiate between these two possibilities, we deleted PMU1 again, but deleted only the promoter and start codon of the PMU1 ORF, maintaining more of the PMU2 promoter. This strain had higher levels of PNPP hydrolysis and expressed PMU2 near wild-type levels (Figure S1, A and B), consistent with PMU1 not being directly required for phosphatase expression. Additionally, we introduced genomic clones of each PMU gene (1 kbp of promoter and the ORF) into the Cgpmu1Δ, Cgpmu2Δ, or Cgpmu3Δ strains (Figure S2A). Only when the PMU2 gene is inserted into a pmu2Δ strain, phosphatase activity is restored, as characterized with a quantitative PNPP hydrolysis assay (Figure S2B). Genomic clones of PMU1 and PMU3 had no effect on phosphatase activity when measured with 1-NP or PNPP. Therefore, Pmu2 is responsible for the majority of phosphatase activity during phosphate starvation conditions in C. glabrata and the defect observed in the Cgpmu1Δ strain is likely a consequence of promoter disruption.
Regulation of CgPMU2 by CgPho4:
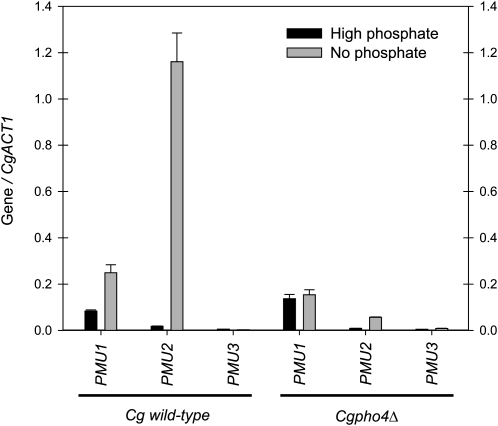
To determine whether the transcription of the three paralogs was regulated by phosphate starvation and by the transcription factor CgPho4, we utilized qPCR on reverse transcribed RNA to measure gene expression levels (Kerwin and Wykoff 2009). Expression levels of each PMU gene (Figure 4) were first examined in wild-type C. glabrata to determine which genes are upregulated in the absence of phosphate. Primers were verified to be specific to each PMU gene by analyzing expression levels in each appropriate mutant (see Figure S3). Assuming equal amplification with primers (which appears to occur with control samples) and normalization to CgACT1, CgPMU1 is an abundant transcript and is induced approximately twofold in response to phosphate starvation; CgPMU3 is expressed at a low level and does not change abundance in response to starvation (Figure 4). CgPMU2 is expressed at a low level in high-phosphate conditions and is ∼20-fold induced during phosphate starvation. To determine whether this induction is ancestral, we determined that in S. cerevisiae, ScPMU1 is not regulated by phosphate starvation (data not shown), suggesting regulation by phosphate starvation is derived. We confirmed that the upregulation of CgPMU2 during phosphate starvation was regulated by CgPho4 because there was not a significant increase in transcript abundance during phosphate starvation in a Cgpho4Δ strain (Figure 4). The twofold increase in CgPMU1 is also Pho4 dependent, but on the basis of Figure S2, it seems unlikely that CgPMU1 plays a large role in the induction of phosphatase activity during phosphate starvation. It is worth noting that disruption of CgPMU3 appears to lead to an increase in phosphatase activity; however, the difference is under twofold and often this difference is within the standard error of the experiment. We cannot explain the increase in phosphatase activity in the pmu3Δ strain, but increased activity does correlate with an increase in PMU2 transcript levels. These results may suggest there may be feedback in the regulation of these genes, although we have not explored this further in this study.
Figure 4.—
CgPMU2 is strongly regulated by phosphate starvation conditions and the transcription factor Pho4. Quantitative real-time PCR was performed on each PMU gene and normalized to the expression level of ACT1. Each primer set was verified to amplify only the indicated gene by examining qPCR of deletion strains (Figure S3). CgPMU1 and CgPMU2 appear regulated by CgPho4, but CgPMU1 to a much lesser extent. The CgPMU3/CgACT1 ratio is ∼0.003 and does not change in response to phosphate condition or CgPHO4 deletion.
What makes CgPmu2 unique?
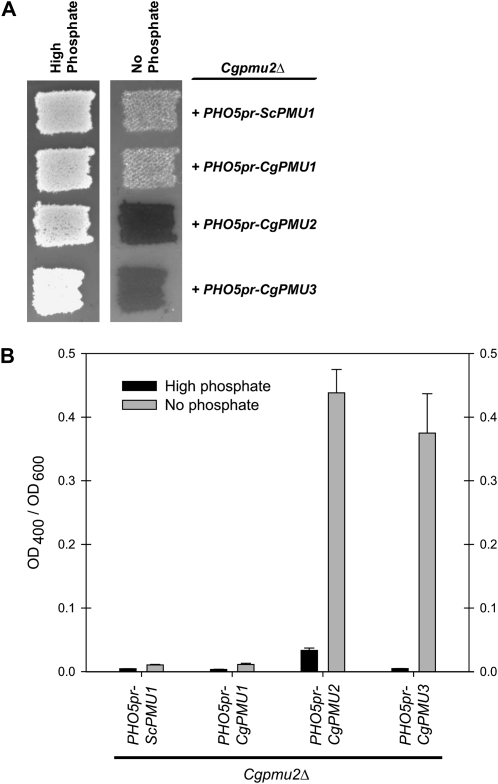
Because all three paralogs are ∼75% identical to one another, we asked whether any of the paralogs were capable of conferring inducible phosphatase activity when regulated by a phosphate starvation-inducible promoter (ScPHO5). If CgPMU1 or CgPMU3 were capable of conferring inducible phosphatase activity, then it would suggest that any paralog was capable of cleaving 1-NP like CgPmu2, but that it simply is not highly induced by phosphate starvation. When CgPMU3 is placed under control of the ScPHO5 promoter, complementation of the Cgpmu2Δ strain was observed on the basis of the semiquantitative plate assay, suggesting that both the CgPMU2 and CgPMU3 ORFs have activity against 1-NP (Figure 5A). Additionally, this is confirmed when we measure PNPP hydrolysis (Figure 5B). CgPMU1 and ScPMU1 have a lower degree of similarity to CgPMU2 and do not have significant phosphatase activity. We confirmed that the ScPHO5 promoter induced the expression of the PMU genes by performing qPCR on reverse-transcribed RNA from cells containing the appropriate plasmids (Figure S4). Furthermore, we confirmed that CgPMU2 and CgPMU3 encode significant 1-NP and PNPP phosphatase activity by placing these genes under the control of another phosphate starvation regulated promoter—CgPMU2 (Figure S5).
Figure 5.—
The CgPMU2 and CgPMU3 ORFs are able to hydrolyze 1-NP or PNPP efficiently. All four ORFs were placed under the control of the phosphate starvation-regulated ScPHO5 promoter and transformed into Cgpmu2Δ strain. Expression of each ORF was verified by RT–qPCR (Figure S4). Phosphatase activity is restored when CgPMU2 and CgPMU3 are expressed as judged by hydrolysis of 1-NP (A) or PNPP (B). Additionally, these ORFs were placed under the control of the CgPMU2 promoter and exhibited similar results (Figure S5). Multiple isolates were subjected to the phosphatase plate assay and these are representative results.
To determine the in vitro enzymatic differences between the three homologs, we purified from bacteria the three proteins of interest (Pmu1, Pmu2, and Pmu3) tagged with an N-terminal His10 tag by immobilized metal affinity chromatography. We determined that all three proteins had detectable phosphatase activity against PNPP (Figure 6), did not require Mg2+ or Ca2+ for activity (data not shown), and had maximal activity at a pH <4 (Figure S6), similar to ScPho5. Additionally, we characterized phosphatase activity against different organic phosphate compounds. We hypothesized that CgPMU1, because it is most similar to ScPMU1, would have significant activity against trehalose-6-phosphate and glycerol phosphate and that CgPmu2 might have new broader range substrate specificity.
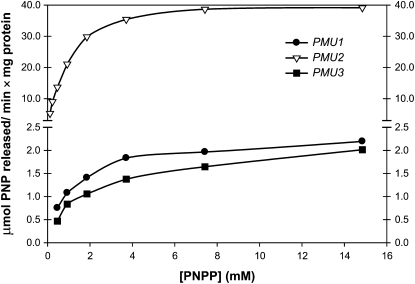
Figure 6.—
Substrate vs. velocity curve with PNPP. Equal amounts of purified enzyme were incubated with various concentrations of PNPP and the amount of PNP formed was monitored at OD400. All three exhibit Michaelis–Menten kinetics, and protein from a mock purification exhibited a velocity of ∼0.2 mmol PNP released/min × mg protein regardless of substrate concentration (data not shown).
To dissect specificity, we determined the activity of all three proteins against varying concentrations of PNPP (Figure 6). All three exhibited Michaelis–Menten kinetics against PNPP and a KM of ∼1 mm; however, CgPmu2 had greater than ∼27 times the Vmax of the other two homologs (Table 2 and Figure 6). In contrast, when we measured the KM and Vmax of the three enzymes using glycerol-1-phosphate and glycerol-2-phosphate, we observed that CgPmu2 had a lower Vmax than the other two paralogs (although CgPmu2 has a higher affinity for glycerol-1-phosphate than the other two paralogs). Furthermore, only CgPmu2 was capable of hydrolyzing phosphate from inosine-5′-monophosphate (Table 2). We conclude from these data that, whereas all three have phosphatase activity against a variety of substrates, CgPmu2 has evolved to have a novel broad-range phosphatase activity relative to the two other paralogs. This process is similar to the paralogous drug efflux pumps in C. albicans, where CaCDR2 is under positive selective pressure and the ancestral copy, CaCDR1, is under purifying selection (Holmes et al. 2006).
TABLE 2.
Activity of His10-tagged purified proteins (μmol phosphate released/min × mg protein) against various phosphate-containing compounds measured in millimolar concentrations
| Pmu1 |
Pmu2 |
Pmu3 |
||||
|---|---|---|---|---|---|---|
| Substrate | Km | Vmax | Km | Vmax | Km | Vmax |
| PNPP | 1.7 ± 0.83 | 2.6 ± 0.39 | 0.81 ± 0.03 | 46 ± 6.1 | 1.7 ± 0.2 | 2.2 ± 0.16 |
| Glycerol-1-phosphate | 24 ± 0.03 | 29 ± 2.2 | 2.2 ± 0.64 | 11 ± 1.0 | 98 ± 30 | 42 ± 8.0 |
| Glycerol-2-phosphate | 23 ± 3.3 | 29 ± 5.7 | 25 ± 11 | 14 ± 3.3 | ND | ND |
| IMP | ND | ND | 6.4 ± 3.4 | 11 ± 0.87 | ND | ND |
| Trehalose-6-phosphate | 0.26 ± 0.001 | 0.29 ± 0.006 | ND | ND | 1.1 ± 0.38 | 0.24 ± 0.08 |
ND, no activity detected.
Caution should be used in the interpretation of these in vitro results. When we overexpress CgPMU3 in yeast, we observe dramatic 1-NP and PNPP hydrolysis, demonstrating that CgPMU3 encodes a broad-range phosphatase, but when purified from bacteria, His10–Pmu3 does not have a high Vmax like His10–Pmu2. These in vivo data suggest that the only reason that CgPMU3 is not the primary acid phosphatase is that it is not highly expressed during phosphate starvation. Because the bacterially purified version of His10–Pmu3 has higher activity against glycerol-1-phosphate and trehelose-6-phosphate than His10–Pmu1 or His10–Pmu2, we believe that His10–Pmu3 is partially functional, but that our assays do not completely reflect its in vivo activity.
Evolutionary requirements for phosphatase activity:
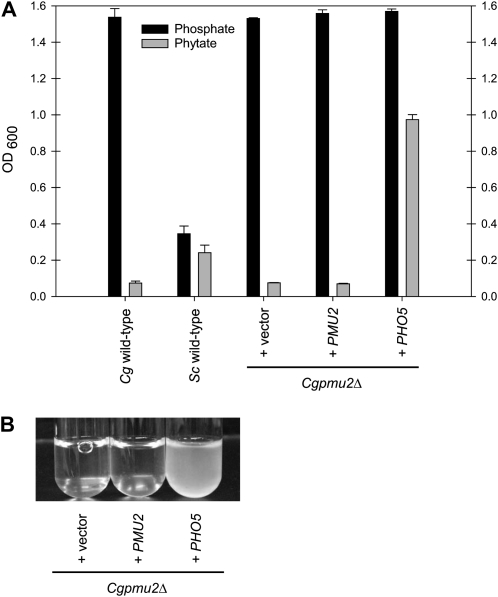
In the environment of S. cerevisiae, phytic acid (inositol hexakisphosphate) is likely a substantial source of phosphate as phytic acid is relatively abundant in plant matter (Lott et al. 2000). However, release of phosphate from this compound requires a specialized phosphatase, and ScPho5 has this phytase activity (Olstorpe et al. 2009) and we wanted to determine whether the Pmu paralogs possessed phytase activity. Purified proteins did not exhibit detectable phytase activity in our assay conditions; therefore, we examined phytase activity from phosphate-starved S. cerevisiae and C. glabrata whole cells. Phytic acid is cleaved slowly, but detectably, by S. cerevisiae (28 fmol phosphate released/cell/hr ± 3.0, n = 2) and much slower by C. glabrata (0.43 fmol phosphate released/cell/hr ± 0.34, n = 2). We hypothesized that C. glabrata would be unable to grow in medium with phytic acid as the only phosphate source. Because C. glabrata grows on mammalian tissue, which is unlikely to be rich in phytic acid (Grases et al. 2001), we expected there was no selective pressure to possess phytase activity. C. glabrata does not grow well in medium with phytic acid as the sole phosphate source; however, when C. glabrata contains ScPHO5, the cells are now able to grow (Figure 7, A and B).
Figure 7.—
ScPHO5 encodes a phytase that allows C. glabrata to grow with phytic acid as a sole phosphate source. (A) Strains were inoculated at an OD600 of 0.001 and grown for 24 hr in SD + 1.2 mm phosphate or 60 hr in SD + 200 μm phytic acid and monitored by measuring OD600. The strains at these times points were not dividing rapidly. C. glabrata consistently grows to a higher OD600 than S. cerevisiae. Deletion of ScPHO5 has a modest effect on growth in phytic acid (data not shown) because there are other phytases in the S. cerevisiae genome (Olstorpe et al. 2009). (B) A photograph of strains from A, but inoculated at a density of 0.0001 in SD + 200 μm phytic acid and grown for 4 days at 30°.
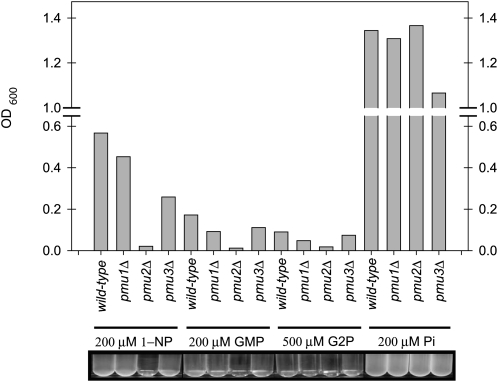
Loss of ScPHO5 is understandable in the context of not requiring phytase activity, but the gain of phosphatase activity through CgPMU2 suggests that there must have been selective pressure for C. glabrata to acquire or maintain secreted phosphatase activity. To determine whether C. glabrata requires phosphatase activity, we grew C. glabrata in media containing 1-NP, guanosine monophosphate, or glycerol-2-phosphate as the sole source of phosphate (Figure 8). Because phosphatase activity is required to release inorganic phosphate from these substrates, we hypothesized that C. glabrata growth should depend on CgPMU2 when these substrates are the only phosphate provided. CgPmu2 is required for growth on these organic phosphates as deletion of CgPMU2 lowers the ability of cells to grow, utilizing these substrates (Figure 8). However, these strains grow well with similar amounts of inorganic phosphate. It seems reasonable that it is advantageous for C. glabrata to be able to access inorganic phosphate from an organic compound, although the precise identity of that compound is unknown.
Figure 8.—
CgPMU2 is required for the growth of C. glabrata in media with organic phosphate as the sole phosphate source. Strains were inoculated at OD600 of 0.001 and grown for 20 hr in SD + 0.2 mm phosphate or 72 hr in SD + organic phosphate. GMP, guanosine monophosphate; G2P, glycerol-2-phosphate; Pi, inorganic phosphate. Strains grew much slower in organic phosphate sources and 500 μm G2P was required for measurable growth. This is one representative experiment, but was reproducible.
DISCUSSION
We have isolated the gene encoding the major phosphate starvation-inducible secreted acid phosphatase in C. glabrata. This gene, CgPMU2, is a part of a three gene family of phosphomutase domain-containing genes. CgPMU2 is the only member of this gene family that is regulated by the phosphate starvation-regulated transcription factor CgPho4. Furthermore, it is the only gene that has a high Vmax for hydrolysis of phosphate from p-nitrophenylphosphate and the only gene that substantially hydrolyzes inosine-5′-monophosphate. CgPmu2 is the functional analog of ScPho5; however, unlike ScPho5, it is unable to liberate phosphate from phytic acid.
Our data allow for a hypothetical reconstruction of events that dictated some niche specificity—the ability of S. cerevisiae to grow in phytic acid-rich environments and the inability of C. glabrata to grow in these environments. The ancestral Pmu gene product likely bound and possibly cleaved phosphate from intracellular phosphate-containing compounds, such as glycerol phosphate or trehalose phosphate. After speciation, CgPmu1 acquired a signal peptide, and a small-scale duplication event generated two copies of the PMU gene. This allowed for drift and sequence divergence of one copy of the gene, with CgPmu1 maintaining its ancestral function. The divergent copy then experienced a second duplication event generating CgPMU2 and CgPMU3. Both acquired the ability to hydrolyze 1-NP and PNPP but only CgPMU2 acquired regulation by phosphate starvation. CgPmu2 also acquired the ability to hydrolyze at least one additional organic phosphate molecule (inosine-5′-monophosphate).
There is no genomic sequence similar to ScPHO5 in the C. glabrata genome, but most Ascomycetes contain PHO5 homologs, suggesting that in most niches there is selective pressure to maintain a secreted acid phosphatase. C. glabrata also likely experienced selective pressure for secreted acid phosphatase but did not experience selective pressure to encode a phytase. Growth on mammalian tissue may have allowed for CgPMU2 to replace ScPHO5. Interestingly, only Aspergillus nidulans appears to have both duplicated PMU1 and lost PHO5, and future studies could explore whether a similar process has occurred in this species (Wapinski et al. 2007).
Evolution through natural selection suggests that unnecessary genes are usually lost, and there are excellent examples of this process, such as the loss of the genes encoding galactose catabolism when there is no galactose present over an evolutionary timescale (Butler et al. 2004; Hittinger et al. 2004). This “use it or lose it” process is likely what we are observing through the loss of ScPHO5. However, our data suggest a corollary to this process. In evolution, necessity can exert a strong selective pressure. If the C. glabrata genome encoded Pmu proteins with weak phosphatase activity and, at some point, no ScPHO5 homolog was present, there would be a strong selective pressure on these Pmu proteins for the acquisition of broad-range specificity for organic phosphate compounds in the environment to provide essential inorganic phosphate for the cell. We cannot determine when ScPHO5 was lost in speciation, but because it was replaced with the functional analog CgPMU2 in C. glabrata, we can hypothesize that neofunctionalization coupled with gene loss has acted on the phosphate signal transduction pathway. The coupling of gene loss and neofunctionalization could also reinforce speciation by isolating closely related species—i.e., allowing only one of the species to grow in phytic acid-rich environments. Similar small scale processes with other signal transduction pathways may be a key process in speciation.
Acknowledgments
We thank members of the Wykoff laboratory and Todd Jackman, Louise Russo, and Erin O'Shea for comments on this work. We thank Juliette Power for helping to generate plasmids used in this study. We also thank Brendan Cormack for C. glabrata strains. We also appreciate comments from anonymous reviewers that enhanced this work. This work was funded by the Department of Biology, the College of Arts and Sciences, and the Center for Undergraduate Research and Fellowships at Villanova University and by a grant from the National Science Foundation (RUI-MCB-0747799).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.120824/DC1.
References
- Barbaric, S., M. Munsterkotter, C. Goding and W. Horz, 1998. Cooperative Pho2-Pho4 interactions at the PHO5 promoter are critical for binding of Pho4 to UASp1 and for efficient transactivation by Pho4 at UASp2. Mol. Cell. Biol. 18 2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswanger, S., and W. Stephan, 2008. Evidence that strong positive selection drives neofunctionalization in the tandemly duplicated polyhomeotic genes in Drosophila. Proc. Natl. Acad. Sci. USA 105 5447–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen, J. D., H. Nielsen, G. von Heijne and S. Brunak, 2004. Improved prediction of signal peptides: signalP 3.0. J. Mol. Biol. 340 783–795. [DOI] [PubMed] [Google Scholar]
- Butler, G., C. Kenny, A. Fagan, C. Kurischko, C. Gaillardin et al., 2004. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc. Natl. Acad. Sci. USA 101 1632–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, K. P., and K. H. Wolfe, 2006. Visualizing syntenic relationships among the hemiascomycetes with the Yeast Gene Order Browser. Nucleic Acids Res. 34 D452–D455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P., T. Toribara and H. Warner, 1956. Microdetermination of phosphorus. Anal. Chem. 28 1756–1758. [Google Scholar]
- Conant, G. C., and K. H. Wolfe, 2008. Turning a hobby into a job: how duplicated genes find new functions. Nat. Rev. Genet. 9 938–950. [DOI] [PubMed] [Google Scholar]
- Cormack, B. P., and S. Falkow, 1999. Efficient homologous and illegitimate recombination in the opportunistic yeast pathogen Candida glabrata. Genetics 151 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue, R., I. Castano, A. De Las Penas, M. Zupancic, V. Lockatell et al., 2005. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science 308 866–870. [DOI] [PubMed] [Google Scholar]
- Elliott, B., R. S. Haltiwanger and B. Futcher, 1996. Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics 144 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferminan, E., and A. Dominguez, 1997. The KIPHO5 gene encoding a repressible acid phosphatase in the yeast Kluyveromyces lactis: cloning, sequencing and transcriptional analysis of the gene, and purification and properties of the enzyme. Microbiology 143(Pt 8): 2615–2625. [DOI] [PubMed] [Google Scholar]
- Grases, F., B. M. Simonet, R. M. Prieto and J. G. March, 2001. Phytate levels in diverse rat tissues: influence of dietary phytate. Br. J. Nutr. 86 225–231. [DOI] [PubMed] [Google Scholar]
- He, X., and J. Zhang, 2005. Rapid subfunctionalization accompanied by prolonged and substantial neofunctionalization in duplicate gene evolution. Genetics 169 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges, P., B. V. Driessche, L. Tafforeau, J. Vandenhaute and A. M. Carr, 2005. Three novel antibiotic marker cassettes for gene disruption and marker switching in Schizosaccharomyces pombe. Yeast 22 1013–1019. [DOI] [PubMed] [Google Scholar]
- Hittinger, C. T., A. Rokas and S. B. Carroll, 2004. Parallel inactivation of multiple GAL pathway genes and ecological diversification in yeasts. Proc. Natl. Acad. Sci. USA 101 14144–14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, A. R., S. Tsao, S. W. Ong, E. Lamping, K. Niimi et al., 2006. Heterozygosity and functional allelic variation in the Candida albicans efflux pump genes CDR1 and CDR2. Mol. Microbiol. 62 170–186. [DOI] [PubMed] [Google Scholar]
- Huang, S., and E. K. O'Shea, 2005. A systematic high-throughput screen of a yeast deletion collection for mutants defective in PHO5 regulation. Genetics 169 1859–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin, C. L., and D. D. Wykoff, 2009. Candida glabrata PHO4 is necessary and sufficient for Pho2-independent transcription of phosphate starvation genes. Genetics 182 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 953–961. [DOI] [PubMed] [Google Scholar]
- Lott, J. N. A., I. Ockenden, V. Raboy and G. D. Batten, 2000. Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci. Res. 10 11–33. [Google Scholar]
- O'Neill, E. M., A. Kaffman, E. R. Jolly and E. K. O'Shea, 1996. Regulation of PHO4 nuclear localization by the PHO80–PHO85 cyclin-CDK complex. Science 271 209–212. [DOI] [PubMed] [Google Scholar]
- Olstorpe, M., J. Schnürer and V. Passoth, 2009. Screening of yeast strains for phytase activity. FEMS Yeast Res. 9 478–488. [DOI] [PubMed] [Google Scholar]
- Rebora, K., B. Laloo and B. Daignan-Fornier, 2005. Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: a central role for a small molecule. Genetics 170 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Lopez, V., M. Lynch, C. Schmitt, R. Cook and J. D. Sobel, 1990. Torulopsis glabrata vaginitis: clinical aspects and susceptibility to antifungal agents. Obstet. Gynecol. 76 651–655. [PubMed] [Google Scholar]
- Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk and J. Bille, 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43 2753–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, K. R., R. L. Smith and E. K. O'Shea, 1994. Phosphate-regulated inactivation of the kinase PHO80–PHO85 by the CDK inhibitor PHO81. Science 266 122–126. [DOI] [PubMed] [Google Scholar]
- Springer, M., D. D. Wykoff, N. Miller and E. K. O'Shea, 2003. Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol. 1 E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting, C. T., S. C. Tsaur, S. Sun, W. E. Browne, Y. C. Chen et al., 2004. Gene duplication and speciation in Drosophila: evidence from the Odysseus locus. Proc. Natl. Acad. Sci. USA 101 12232–12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski, I., A. Pfeffer, N. Friedman and A. Regev, 2007. Natural history and evolutionary principles of gene duplication in fungi. Nature 449 54–61. [DOI] [PubMed] [Google Scholar]
- Wykoff, D. D., and E. K. O'Shea, 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff, D. D., A. H. Rizvi, J. M. Raser, B. Margolin and E. K. O'Shea, 2007. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol. Cell 27 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., 2003. Evolution by gene duplication: an update. Trends Ecol. Evol. 18 292–298. [Google Scholar]