Abstract
The Saccharomyces cerevisiae nuclear membrane is part of a complex nuclear envelope environment also containing chromatin, integral and peripheral membrane proteins, and large structures such as nuclear pore complexes (NPCs) and the spindle pole body. To study how properties of the nuclear membrane affect nuclear envelope processes, we altered the nuclear membrane by deleting the SPO7 gene. We found that spo7Δ cells were sickened by the mutation of genes coding for spindle pole body components and that spo7Δ was synthetically lethal with mutations in the SUN domain gene MPS3. Mps3p is required for spindle pole body duplication and for a variety of other nuclear envelope processes. In spo7Δ cells, the spindle pole body defect of mps3 mutants was exacerbated, suggesting that nuclear membrane composition affects spindle pole body function. The synthetic lethality between spo7Δ and mps3 mutants was suppressed by deletion of specific nucleoporin genes. In fact, these gene deletions bypassed the requirement for Mps3p entirely, suggesting that under certain conditions spindle pole body duplication can occur via an Mps3p-independent pathway. These data point to an antagonistic relationship between nuclear pore complexes and the spindle pole body. We propose a model whereby nuclear pore complexes either compete with the spindle pole body for insertion into the nuclear membrane or affect spindle pole body duplication by altering the nuclear envelope environment.
THE nuclear envelope is composed of distinct outer and inner nuclear membranes. The outer nuclear membrane is continuous with the endoplasmic reticulum. The inner nuclear membrane is associated with a unique set of proteins, some of which mediate interactions between the nuclear envelope and chromatin (reviewed in Zhao et al. 2009). Nuclear pore complexes traverse both membranes and allow transport of proteins and solutes between the cytoplasm and the nucleus. The inner and outer nuclear membranes fuse in the region surrounding each nuclear pore complex.
In animal cells, the nuclear envelope disassembles as cells enter mitosis and reassembles upon mitotic exit. Nuclear envelope breakdown allows the association of chromosomes with spindle microtubules, which are nucleated from centrosomes that reside in the cytoplasm. In contrast, certain types of fungi, such as the budding yeast Saccharomyces cerevisiae, undergo closed mitosis, where the nuclear envelope remains intact throughout the entire cell cycle. Closed mitosis is possible because the yeast centrosome-equivalent, the spindle pole body (SPB), is embedded in the nuclear envelope, allowing the SPB to nucleate both cytoplasmic and nuclear microtubules.
SPB duplication requires a mechanism for inserting the new SPB into the nuclear envelope (reviewed in Jaspersen and Winey 2004). The new SPB begins to form in late G1/early S phase as satellite material deposited on the cytoplasmic face of an electron-dense region of the nuclear envelope, called the half-bridge. The satellite material matures into a duplication plaque, which is then inserted into the nuclear membrane and becomes the daughter SPB. Many genes are known to be required for SPB duplication, and this process has been carefully examined cytologically (Rose and Fink 1987; Winey et al. 1991, 1993; Spang et al. 1995; Bullitt et al. 1997; Adams and Kilmartin 1999; Elliott et al. 1999; Schramm et al. 2000; Jaspersen et al. 2002; Nishikawa et al. 2003; Araki et al. 2006). However, the exact mechanisms by which SPB duplication and insertion occur remain a mystery.
Equally unclear is how nuclear pore complexes are inserted into an intact nuclear envelope (reviewed in Hetzer and Wente 2009). For both the SPB and nuclear pore complexes, the inner and outer nuclear membranes must fuse to allow insertion into the nuclear envelope. Yeast and vertebrate nuclear pore complexes each have four pore membrane (POM) nucleoporins containing transmembrane domains. Other nucleoporins have motifs with potential for bending membranes or sensing membrane curvature. Thus, certain nuclear pore complex components may have the ability to alter the nuclear membrane or stabilize particular membrane conformations (Devos et al. 2004, 2006; Alber et al. 2007; Drin et al. 2007). It is interesting to note that, in S. cerevisiae, nuclear pore complexes are enriched in the vicinity of the SPB (Heath et al. 1995; Winey et al. 1997; Adams and Kilmartin 1999), but the significance of this phenomenon is not known. The SPB and nuclear pore complexes share at least two common components, the integral membrane protein Ndc1p and the small calcium-binding protein Cdc31p (Chial et al. 1998; Fischer et al. 2004). Ndc1p is thought to play a role in insertion of both SPBs and nuclear pore complexes into the nuclear membrane.
SUN domain proteins are a conserved family of inner nuclear membrane proteins that interact with specific outer nuclear membrane proteins to form a physical bridge across the nuclear envelope (reviewed in Hiraoka and Dernburg 2009; Razafsky and Hodzic 2009). One of the components of the S. cerevisiae SPB is the SUN domain protein Mps3p. The N terminus of Mps3p is in the nucleoplasm, while the C terminus, containing the SUN domain, is found in the space between the inner and outer nuclear membranes. In addition to the SPB, Mps3p localizes to multiple foci at the nuclear periphery, and these two pools of Mps3p have distinct functions (Jaspersen et al. 2002, 2006; Nishikawa et al. 2003). At the SPB, Mps3p is required for half-bridge formation and early steps of SPB duplication, and cells compromised for Mps3p function accumulate in mitosis with a single SPB and a monopolar spindle (Jaspersen et al. 2002; Nishikawa et al. 2003). At the nuclear periphery, Mps3p is involved in tethering telomeres to the nuclear envelope in mitosis and meiosis, sequestering DNA double-strand breaks away from recombination factors, and associating with soluble chromatin proteins (Antoniacci et al. 2004, 2007; Bupp et al. 2007; Conrad et al. 2007, 2008; Oza et al. 2009; Schober et al. 2009).
While many structural features of the yeast nucleus have been identified, little is known about how the physical properties of the nuclear membrane contribute to processes that occur at the nuclear envelope. As noted above, resident proteins of the nuclear envelope may affect nuclear membrane properties. In addition, the nuclear membrane is affected by altering lipid biosynthesis, for example, by inactivating the phosphatidic acid (PA) phosphohydrolase Pah1p or by inactivating the phosphates complex, made of Spo7p and Nem1p, which activates Pah1p. In the absence of Spo7p, Nem1p, or Pah1p, cells exhibit nuclear envelope extensions and extensive ER membrane sheets, and they also have altered membrane lipid composition, including a decrease in phosphatidylcholine and an increase in PA, phosphatidylethanolamine, and phosphatidylinositol (Siniossoglou et al. 1998; Santos-Rosa et al. 2005; Campbell et al. 2006; Han et al. 2006). These three proteins are unique among phospholipid biosynthesis proteins in their ability to affect nuclear morphology upon gene disruption (Han et al. 2008). A similar phenotype was seen upon overexpression of DGK1, which counteracts the activity of Pah1p by converting diacylglycerol to PA, leading to an increase in PA levels at the nuclear envelope (Han et al. 2008). Consistent with a conserved role for Pah1p in regulating nuclear envelope processes, deletion of either NEM1 or SPO7 is synthetically lethal with deletions of certain nucleoporin genes (Siniossoglou et al. 1998), and inactivation of the PAH1 homolog in Caenorhabditis elegans, LPIN-1, results in defects in nuclear envelope disassembly and reassembly (Golden et al. 2009; Gorjanacz and Mattaj 2009).
To identify processes that are affected by altered nuclear membrane properties, we screened for pathways that are compromised in spo7Δ cells. We found that SPO7 inactivation strongly influences the SPB. By screening for proteins that could alleviate spo7Δ-induced SPB defects, we uncovered an unexpected inhibitory role for nucleoporins in SPB function, revealing that nuclear pore complexes, or components thereof, act antagonistically to the SPB in the nuclear envelope. Taken together, our findings indicate that the nuclear envelope environment is important for the function of protein complexes and biological processes occurring at the nuclear periphery.
MATERIALS AND METHODS
Yeast strains:
All yeast strains are derivatives of W303. The full genotype of each strain is described in Table 1. Strains used in supporting information are described in Table S1. The pURA3-MPS3 plasmid has been described previously (Jaspersen et al. 2006).
TABLE 1.
Yeast strains
| Strain name | Genotype |
|---|---|
| JCY565 | MATaura3-1 trp1-1 leu2-3,112 spo7Δ∷NAT can1-100 |
| KW228 | MATaura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 spo7Δ∷NAT mps3-1 can1-100 pURA3-MPS3 |
| KW548 | MATα leu2-3,112 trp1-1 ura3-1 spo7Δ∷NAT mps3-1 can1-100 pURA3-MPS3 |
| KW576 | MATaura3-1 trp1-1 mps3Δ∷HIS3MX6 leu2∷mps3-F592S-LEU2 spo7Δ∷NAT can1-100 pURA3-MPS3 |
| KW658 | MATaura3-1 trp1-1 mps3Δ∷HIS3MX6 leu2∷mps3-F592S-LEU2 can1-100 pURA3-MPS3 |
| KW660 | MATα leu2-3,112 trp1-1 ura3-1 spo7Δ∷NAT can1-100 |
| KW661 | MATα leu2-3,112 trp1-1 ura3-1 mps3-1 can1-100 pURA3-MPS3 |
| KW945 | MATaura3-1 leu2-3,112 trp1-1 mps3-1 nup157Δ∷KAN can1-100 pURA3-MPS3 |
| KW948 | MATaura3-1 leu2-3,112 trp1-1 nup157Δ∷KAN can1-100 pURA3-MPS3 |
| KW949 | MATaura3-1 leu2-3,112 trp1-1 mps3-1 can1-100 pURA3-MPS3 |
| KW951 | MATaura3-1 leu2-3,112 trp1-1 can1-100 pURA3-MPS3 |
| KW966 | MATaura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 pom152∷NAT can1-100 |
| KW967 | MATaura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 nup157Δ∷NAT can1-100 |
| KW969 | MATaspc42-11 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 nup157Δ∷KAN bar1∷hisG can1-100 |
| KW970 | MATamps2-1 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 nup157Δ∷KAN bar1∷hisG can1-100 |
| KW971 | MATamps2-1 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 pom152∷NAT bar1∷hisG can1-100 |
| KW972 | MATaspc29-3 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 nup157Δ∷KAN bar1∷hisG can1-100 |
| KW973 | MATaspc98-2 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 nup157Δ∷KAN bar1∷hisG can1-100 |
| KW983 | MATaspc42-11 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 pom152∷NAT bar1∷hisG can1-100 |
| KW990 | MATaura3-1 leu2-3,112 his3-11,15 mps3Δ∷HIS3MX pom152Δ∷NAT can1-100 |
| KW991 | MATaura3-1 leu2-3,112 his3-11,15 pom152Δ∷NAT can1-100 |
| KW992 | MATaura3-1 leu2-3,112 his3-11,15 can1-100 |
| KW993 | MATaura3-1 leu2-3,112 his3-11,15 lys2Δ mps3Δ∷HIS3MX nup157Δ∷NAT can1-100 |
| KW994 | MATaura3-1 leu2-3,112 his3-11,15 lys2Δ nup157Δ∷NAT can1-100 |
| KW995 | MATaura3-1 leu2-3,112 his3-11,15 lys2Δ can1-100 |
| KW1010 | MATakar1Δ17 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 bar1∷hisG nup157Δ∷NAT can1-100 pURA3-KAR1 |
| KW1012 | MATaura3-1 ade2-1 leu2-3,112 his3-11,15 ndc1Δ∷KAN ndc1-39-TRP1 nup157Δ∷NAT can1-100 |
| KW1013 | MATaura3-1 ade2-1 leu2-3,112 his3-11,15 ndc1Δ∷KAN ndc1-39-TRP1 can1-100 |
| OCF1533-1A | MATaura3-1 trp1-1 leu2-3,112 can1-100 |
| OCF1533-2C | MATα leu2-3,112 trp1-1 ura3-1 can1-100 |
| OCF1533-4B | MATaura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 can1-100 |
| SLJ001 | MATabar1∷hisG ura3-1 leu2-3,112 trp1-1 his3-11,15 ade2-1 can1-100 |
| SLJ715 | MATaspc42-11 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 bar1∷hisG can1-100 |
| SLJ717 | MATamps2-1 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 bar1∷hisG can1-100 |
| SLJ751 | MATaspc29-3 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 bar1∷hisG can1-100 |
| SLJ839 | MATaspc98-2 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 bar1∷hisG can1-100 |
| SLJ843 | MATakar1Δ17 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 bar1∷hisG can1-100 pURA3-KAR1 |
| SLJ910 | MATamps3-1 ura3-1 ade2-1 leu2-3,112 his3-11,15 trp1-1 mps3-1 can1-100 pURA3-MPS3 |
| SLJ2388 | MATanup60Δ∷HYGMX ura3-1 trp1-1 leu2-3,112 his3-11,15 ade2-1 ade3Δ can1-100 |
| SLJ2752 | MATanup42Δ∷KANMX mps3Δ∷NATMX ura3∷MPS3-GFP-URA3 htb2∷HTB2-mCherry-HYGMX trp1-1 leu2-3,112 his3-11,15 ade2-1 can1-100 |
| SLJ3654 | MATanup188Δ∷KANMX ura3-1 trp1-1 leu2-3,112 his3-11,15 ade2-1 can1-100 |
| SLJ4215 | MATα mps3-1 pom34Δ∷KANMX ura3-1 trp1-1 leu2-3,112 his3-11,15 ade2-1 can1-100 |
| SLJ4216 | MATamps3-1 pom152Δ∷HYGMX ura3-1 trp1-1 leu2-3,112 his3-11,15 ade2-1 can1-100 lys2Δ |
| SLJ4217 | MATapom34Δ∷KANMX ura3-1 trp1-1 leu2-3,112 his3-11,15 ade2-1 can1-100 |
| SLJ4218 | MATapom152Δ∷HYGMX ura3-1 trp1-1 leu2-3,112 his3-11,15 ade2-1 can1-100 lys2Δ |
| SLJ4221 | MATα mps3-1 nup60Δ∷HYGMX ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 |
| SLJ4222 | MATamps3-1 nup42Δ∷KANMX ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 lys2Δ |
| SLJ4223 | MATamps3-1 nup188Δ∷KANMX ura3-1 leu2-3,112 his3-11,15 can1-10 |
| SLJ4233 | MATamlp2Δ∷HIS5MX mlp1Δ∷KANMX MPS3-HA3-NATMX bar1∷hisG ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 |
| SLJ4234 | MATα mlp2Δ∷HIS5MX mlp1Δ∷KANMX mps3-1 bar1∷hisG ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 lys2Δ |
| SLJ4236 | MATanup84Δ∷KANMX MPS3-GFP-NATMX bar1∷hisG ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 lys2Δ |
| SLJ4237 | MATanup84Δ∷KANMX mps3-1 bar1∷hisG ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 lysΔ |
| SLJ4239 | MATα nup133Δ∷NATMX MPS3-GFP-KANMX bar1∷hisG ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 |
| SLJ4240 | MATα nup133Δ∷NATMX mps3-1 bar1∷hisG ura3-1 trp1-1 leu2-3,112 his3-11,15 can1-100 |
| SLJ4259 | MATapom152Δ∷HYGB mps3Δ∷NATMX ade2-1 ade3Δ100 his3-11,15 leu2-3,112 trp1-1 ura3-1 lys2 can1-100 |
| SLJ4260 | MATapom152Δ∷HYGB ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 lys2 can1-100 |
| SLJ4336 | MATα ade2-1 ade3Δ100 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 |
| SLJ4338 | MATα pom152Δ∷HYGB ade2-1 ade3Δ100 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 |
| SLJ4340 | MATα pom152Δ∷HYGB mps3Δ∷NATMX ade2-1 ade3Δ100 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100 |
Where indicated, mps3 mutant strains were maintained with pURA3-MPS3 until immediately prior to the experiment. To select for cells that spontaneously lost the plasmid, strains were struck on plates containing 5-fluoroorotic acid (5-FOA). Gene disruptions were performed according to Longtine et al. (1998) and Goldstein and McCusker (1999). Yeast transformation, sporulation, and tetrad dissection were done using standard protocols. For strain requests, contact O.C.-F. (for strains starting with KW, OCF, or JCY) or S.L.J. (for strains starting with SLJ).
Media and growth conditions:
Cells were grown in either rich media (YPD: 1% yeast extract, 2% peptone, 2% glucose) or synthetic media (SC: 0.17% yeast nitrogen base, 0.5% ammonium sulfate, 2% glucose, and amino acid mix lacking the appropriate amino acid or uracil), as indicated. 5-FOA was purchased from US Biologicals (Swampscott, MA). All serial dilutions were 10-fold. To determine cell cycle distribution of asynchronous cells, cell cultures in mid-log phase were temperature shifted as indicated and then fixed with 70% ethanol for 1 hr at room temperature. After sonication and DAPI staining (Vector Laboratories, Burlingame, CA), cells were examined using a Nikon Eclipse E800 fluorescent microscope. All samples were blinded prior to scoring. Cytological methods used for supporting information material are in File S1.
High-copy suppressor screen:
mps3-1 spo7Δ strains maintained with pURA3-MPS3 were transformed with a LEU2-marked 2μ library (American Type Culture Collection, Manassas, VA) and replica-plated to 5-FOA-containing plates. Surviving cells were retested by streaking on 5-FOA-containing plates. The suppressing 2μ plasmids were isolated and retransformed into new strains to confirm suppression. The gene responsible for suppression was then identified by transposon mutagenesis using the GPS-M mutagenesis system (New England Biolabs, Ipswich, MA). Briefly, a Tn7-based transposon was integrated randomly into the plasmid in vitro to disrupt function of individual genes. For NUP157 and NIC96, suppression by truncated forms of each gene was then confirmed by PCR-amplifying full-length and truncated genes with primers containing BamHI and SalI restriction sites, followed by cloning into expression vectors by standard methods of cloning and ligation. The absence of PCR errors was confirmed by DNA sequencing. Expression vectors were purchased from the American Type Culture Collection.
Flow cytometry:
Cells used for flow cytometry in Figure 4 were grown to mid-log phase, fixed with 70% ethanol for 1 hr at room temperature, treated with RNAse (Roche, Basel, Switzerland) and proteinase K (Roche), and then sonicated and resuspended in sytox buffer (180 mm Tris, 180 mm NaCl, 70 mm MgCl2, pH 7.5) containing 1:2500 Sytox Green (Invitrogen, Carlsbad, California). Flow cytometry was then performed on a BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ), using CellQuest Pro software (BD Biosciences). Cells used for flow cytometry in Figures 5 and 7 were treated as above, except they were grown to late log phase. After sonication, these cells were resuspended in a 1:1000 dilution of propidium iodide (Sigma-Aldrich, St. Louis), run on a CyAn ADP Analyzer (Beckman Coulter, Brea, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Figure 4.—
Deletion of NUP157 rescues the SPB defect of mps3-1 cells. (A) Wild-type (KW951), nup157Δ (KW948), mps3-1 (KW949), and nup157 mps3-1 cells (KW945), all initially maintained with the pURA3-MPS3 plasmid, were assayed for growth at 30° and 37° by serial dilution on control (SC) or 5-FOA-containing media. (B) DNA analysis by flow cytometry of cells described above and treated with 5-FOA to select for cells that have lost the pURA3-MPS3 plasmid and then grown to mid-log phase at 30° and fixed with ethanol. In each case, 20 individual cultures of cells with the indicated genotypes were tested, and representative examples are shown. (C) Cells described above were treated with 5-FOA to select for cells that had lost the pURA3-MPS3 plasmid, were grown to mid-log phase, and were then shifted to the indicated temperature for 3 hr. The percentage of total cells arrested just prior to anaphase as large-budded cells with a single nucleus is graphed. Light gray: growth at 30°. Dark gray: following a 3-hr shift to 37°. For each sample, 400 cells were counted over four independent experiments. Error bars indicate standard deviation. (D) Cells treated as in C were stained with antibodies to the SPB and microtubules as described in Figure 2. The graph illustrates the percentage of large-budded cells that contain monopolar spindles. For each sample, >100 cells were counted in each of two independent experiments. Error bars indicate standard deviation. The asterisk denotes statistical significance by Pierson's χ2 test (P-value < 0.0001).
Figure 5.—
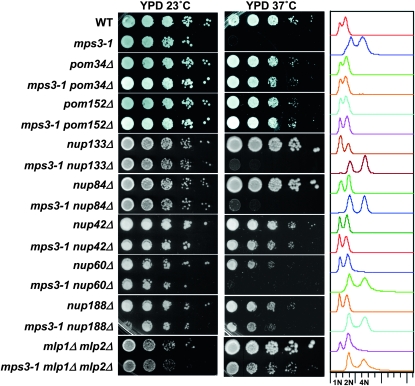
Specific nucleoporin gene deletions rescue mps3-1 phenotypes. Strains of the indicated genotypes were plated by serial dilution at 23° and 37°. Samples of each strain were grown at 23°, fixed with ethanol, and analyzed by flow cytometry (right); 1N, 2N, and 4N DNA peaks are indicated below. The strains used were SLJ001, SLJ910, SLJ4217, SLJ4215, SLJ4218, SLJ4216, SLJ4239, SLJ4240, SLJ4236, SLJ4237, SLJ2752, SLJ4222, SLJ2388, SLJ4221, SLJ3654, SLJ4223, SLJ4233, and SLJ4234.
Figure 7.—
mps3Δ strains are viable in the absence of Nup157p or Pom152p. (A and B) Strains of the indicated genotypes were plated by serial dilution at 30°. The following strains were included: (A) KW995 (wild type), KW994 (nup157Δ), and KW993 (mps3Δ nup157Δ). (B) KW992 (wild type), KW991 (pom152Δ), and KW990 (mps3Δ pom152Δ). (C) Asynchronous cells of the strains described in B were grown to mid-log phase, fixed with ethanol, and then examined for cell cycle distribution. Differences between wild type and the mps3Δ pom152Δ double mutant are not statistically significant (P > 0.05; for each sample, n = 300 over three independent experiments). Error bars indicate standard deviation. (D) Wild-type cells (SLJ4336), pom152Δ cells (SLJ4338), and the mps3Δ pom152Δ double mutants (SLJ4340) were grown to mid-log phase, fixed with ethanol, and analyzed by flow cytometry. (E) The same strains were grown to mid-log phase and processed for indirect immunofluorescence as described in Figure 2. Images show microtubules (green: anti-α-tubulin), SPBs (red: anti-Tub4p), and DNA (blue: DAPI). Arrows indicate short bipolar spindles, and arrowheads mark long bipolar spindles.
Immunofluorescence:
Cells were fixed for 45 min to 1 hr in 4% paraformaldehyde and then processed for indirect immunofluorescence microscopy as previously described (Rose et al. 1990). A 1:500 dilution of anti-Tub4p (Jaspersen et al. 2002) and a 1:50 or 1:500 dilution of the YOL1/34 anti-α-tubulin (Abcam, Cambridge, MA) antibody were used along with the appropriate secondary antibodies. DAPI was used to visualize the DNA.
Transmission electron microscopy:
Yeast cells were enriched in G1 using 1 μg/ml α-factor mating pheromone or left untreated and then frozen on the Leica EM-Pact (Wetzlar, Germany) at ∼2050 bar, transferred under liquid nitrogen into 2% osmium tetroxide/0.1% uranyl acetate/acetone, and transferred to the Leica AFS. The freeze substitution protocol was as follows: −90° for 16 hr, raised 4°/hr for 7 hr, −60° for 19 hr, raised 4°/hr for 10 hr, and −20° for 20 hr. Samples were then removed from the AFS, placed in the refrigerator for 4 hr, and then allowed to incubate at room temperature for 1 hr. Samples went through three changes of acetone over 1 hr and were removed from the planchettes. They were embedded in acetone/Epon mixtures to final 100% Epon over several days in a stepwise procedure as described (McDonald 1999). Sixty-nanometer serial thin sections were cut on a Leica UC6, stained with uranyl acetate and Sato's lead, and imaged on a FEI Technai Spirit (Hillsboro, OR).
RESULTS
Deletion of SPO7 affects SPB function:
Inactivation of Pah1p, or its activators Nem1p and Spo7p, leads to alterations in the nuclear and endoplasmic reticulum membrane (Siniossoglou et al. 1998; Santos-Rosa et al. 2005; Han et al. 2006). To search for processes that are affected by changing properties of the nuclear membrane, we screened for genetic interactions between spo7Δ and mutations in genes encoding nuclear envelope proteins. This screen was based on the idea that if the nuclear envelope environment affects certain nuclear periphery processes, then altering this environment by inactivating Spo7p might exacerbate the phenotype of mutants that cause mild defects in these processes.
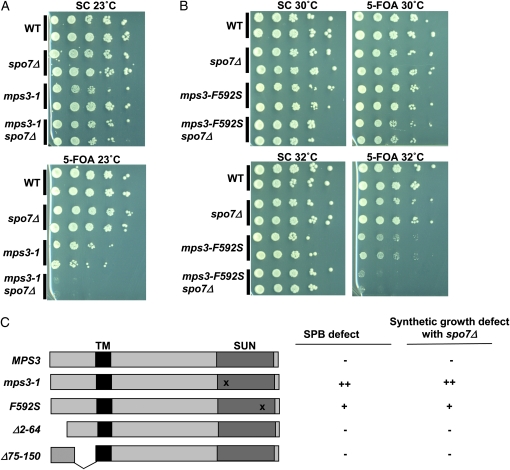
A gene of interest was MPS3, needed for SPB duplication and other processes at the nuclear periphery (Jaspersen et al. 2002, 2006; Nishikawa et al. 2003; Bupp et al. 2007; Oza et al. 2009; Schober et al. 2009). To test whether altering the nuclear membrane affects Mps3p-related processes, we combined spo7Δ with mps3-1, an allele that carries a point mutation in the SUN domain and is defective in SPB duplication at the nonpermissive temperature (Jaspersen et al. 2002). Even at the permissive temperature (23°), haploid mps3-1 cells rapidly diploidize (Jaspersen et al. 2002), suggesting that the protein expressed from this allele has reduced activity. Strains carrying mps3-1, spo7Δ, or both mutations were generated in the presence of a centromeric plasmid coding for MPS3 and URA3 (pURA3-MPS3). In this and subsequent experiments, growth in the absence of the plasmid was tested by selecting for cells that have lost the plasmid using 5-FOA, which is toxic to cells expressing URA3. While single mutants were viable on synthetic complete control media (SC) or 5-FOA, double mutants containing both the mps3-1 and spo7Δ alleles were unable to grow on 5-FOA at any temperature tested (Figure 1A; data not shown). These results suggest that the weakened activity provided by the mps3-1 allele is further compromised in the absence of Spo7p.
Figure 1.—
spo7Δ has genetic interactions with mps3 alleles specifically defective at SPB duplication. (A) Wild-type (OCF1533-2C), spo7Δ (KW660), mps3-1 cells containing pURA3-MPS3 (KW661), and mps3-1 spo7Δ cells containing pURA3-MPS3 (KW548) were plated by serial dilution on control media (SC) or plates containing 5-FOA at 23°. The presence of 5-FOA allows only cells lacking the URA3 gene to survive. (B) Wild-type (OCF1533-1A), spo7Δ (JCY565), mps3-F592S cells containing pURA3-MPS3 (KW658), and mps3-F592S spo7Δ cells containing pURA3-MPS3 (KW576) were plated by serial dilution on control media (SC) or plates containing 5-FOA at the indicated temperatures. (C) Summary of mps3 mutant alleles, their effects on SPB duplication, and their effects on cell growth in the absence of Spo7p. The Mps3p transmembrane domain (TM) and SUN domain are indicated. The “x” marks the approximate location of point mutations within the SUN domain. ++, +, and − denote a “strong defect,” “moderate defect,” and “no defect,” respectively. Data for SPB defects are from Jaspersen et al.(2002, 2006) and Bupp et al. (2007). Data for genetic interactions with spo7Δ are from Figure 1, A and B, and Figure S1.
While the mutation in mps3-1 is known to affect SPB duplication, formally it could also disrupt other functions of Mps3p at the nuclear periphery. To determine which of Mps3p's roles is compromised by spo7Δ, we tested existing mps3 alleles known to differentially influence Mps3p function. Alleles tested included the SUN domain mutant mps3-F592S and two N-terminal deletions, mps3Δ2-64 and mps3Δ75-150, which were previously shown to be proficient in the Mps3p SPB function but defective in its other roles at the nuclear envelope (Jaspersen et al. 2006; Bupp et al. 2007; Conrad et al. 2007). mps3-F592S spo7Δ double mutants could be recovered in the absence of pURA3-MPS3, indicating that these alleles are not synthetically lethal under standard growth conditions (Figure 1B). However, the combination of these two mutations led to lethality at elevated temperatures, as the double mutant failed to grow at 32°, a temperature at which spo7Δ cells grow well and mps3-F592S cells are sick but viable (Figure 1B). In contrast, spo7Δ showed no genetic interactions with either mps3Δ2-64 or mps3Δ75-150 at any temperature tested (Figure S1; data not shown). Thus, the extent of genetic interaction between the various mps3 alleles and spo7Δ correlated with the severity of the SPB defect of that mps3 allele (Figure 1C; Jaspersen et al. 2002, 2006; Bupp et al. 2007), suggesting that altering nuclear membrane properties by deleting the SPO7 gene further compromises mps3 mutants by exacerbating their SPB defect.
If the absence of Spo7p leads to a defect that compromises SPB function, one prediction is that the spo7Δ allele would have genetic interactions with mutations in other SPB components. To examine this, we tested a panel of SPB mutants coding for proteins from different parts of the SPB structure or required for different stages of SPB duplication (reviewed in Jaspersen and Winey 2004). Ndc1p and Mps2p are integral membrane proteins that localize to the periphery of the SPB, Kar1p is a transmembrane component of the half-bridge, and Spc42p and Spc29p are nonmembrane SPB proteins that form the central plaque. All five proteins are encoded by essential genes, and thus genetic interactions with spo7Δ were tested using conditional mutants. Temperature-dependent synthetic sickness was observed between spo7Δ and ndc1-39, kar1Δ17, spc29-3, and spc42-11 (Table 2; Figure S2). In addition, we observed genetic interactions between spo7Δ and mps2-381, which is thought to disrupt the physical interaction between Mps2p and Mps3p (Jaspersen et al. 2006), but not between spo7Δ and mps2-1, which instead affects a separate function of Mps2p in SPB insertion (Winey et al. 1991) (Table 2; Figure S2; data not shown). Taken together, the genetic interactions between spo7Δ and select mutations in SPB subunits indicate that, under certain conditions, Spo7p activity is needed for SPB-associated processes. This suggests that SPB function may be affected by properties of the nuclear membrane, such as lipid composition.
TABLE 2.
Genetic interactions between spo7Δ and SPB mutant alleles
| SPB mutant allele | Genetic interaction with spo7Δa |
|---|---|
| mps2-381 | + |
| mps2-1 | − |
| kar1Δ17 | + |
| ndc1-39 | + |
| spc29-3 | + |
| spc42-11 | + |
Double mutants were generated and scored for growth: “−” indicates no growth defect in the double mutant compared to the single mutants at any temperature tested; “+” indicates synthetic sickness evident by a growth defect in the double mutant compared to the single mutants.
Deletion of SPO7 exacerbates the SPB defect of mps3 mutants:
The genetic interactions presented above suggest that deletion of SPO7 adversely affects the activity of Mps3p in promoting SPB duplication. It was formally possible that spo7Δ cells had a SPB duplication defect of their own that was additive with the defect of mps3 alleles. However, using several methodologies at a range of temperatures, we did not detect any spindle or SPB abnormalities in spo7Δ cells (Figure S3; data not shown). Rather, it was likely that spo7Δ created a condition that further weakened the ability of mutated mps3 gene products to promote SPB duplication.
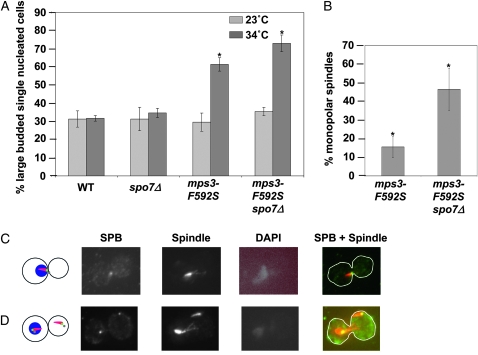
Failure in SPB duplication results in the accumulation of cells prior to anaphase (large-budded cells with a single nucleus) because of their inability to assemble a bipolar spindle. Indeed, at the nonpermissive temperature (37°), 90% of mps3-F592S cells accumulated as large-budded cells with a single nucleus, and nearly 60% of these cells had a monopolar spindle (Jaspersen et al. 2006). To examine the effect of spo7Δ on an mps3 mutant strain, a semipermissive temperature was necessary at which only a partial defect in SPB duplication would occur. To this end, we utilized the mps3-F592S allele because at semipermissive temperatures the growth defect of the single mps3-F592S mutant was notably less severe than that of the mps3-F592S spo7Δ double mutant (Figure 1B). Wild-type, spo7Δ, mps3-F592S, and mps3-F592S spo7Δ double-mutant cells were grown to mid-log phase at 23° and then shifted to 34° for 6 hr. Fixed cells were stained with DAPI and examined by microscopy to determine the cell cycle distribution before and after the temperature shift. At 23°, all four cultures had ∼30% large-budded cells with a single nucleus (Figure 2A, light gray bars). The temperature shift to 34° had no effect on the cell cycle distribution of wild-type or spo7Δ cells, but it doubled the percentage of large-budded, single-nucleated mps3-F592S cells. mps3-F592S spo7Δ double mutants had a small but reproducible and statistically significant increase over mps3-F592S cells in the fraction of cells that were large budded with a single nucleus (P < 0.0001) (Figure 2A, dark gray bars).
Figure 2.—
The SPB defect of mps3 mutants is exacerbated in the absence of Spo7p. (A) Wild-type (OCF1533-1A), spo7Δ (JCY565), mps3-F592S (KW658), and mps3-F592S spo7Δ cells (KW576) were grown to mid-log phase at 23° and then shifted to the indicated temperature for 6 hr. The percentage of cells just prior to anaphase (large-budded cells with a single nucleus) is graphed. Strains containing the mps3-F592S allele were maintained with the pURA3-MPS3 plasmid, which was removed using 5-FOA immediately prior to the experiment (see materials and methods). A total of 300 cells (wild type and spo7Δ) or 500 cells (mps3-F592S and mps3-F592S spo7Δ) were counted over three or five independent experiments. Error bars indicate standard deviation. (B) mps3-F592S (KW658; n = 183 over three independent experiments) and mps3-F592S spo7Δ cells (KW576; n = 218 over three independent experiments) were treated as in A and then subjected to indirect immunofluorescence using antibodies against Tub4p to visualize SPBs and against α-tubulin to visualize microtubules. The percentage of large-budded cells containing monopolar spindles is graphed. Error bars indicate standard deviation. The asterisk denotes statistical significance by Pearson's χ2 test (P-value < 0.0001). (C and D) Representative examples of large-budded cells stained with antibodies to Tub4p (SPB) or α-tubulin (spindle) and treated with DAPI. In the merged image, the SPB is shown in green and the microtubules in red. (C) Example of a large-budded cell with a monopolar spindle. (D) Example of a large-budded cell with duplicated, microtubule-associated SPBs in both mother and daughter cells, but only a single DAPI mass.
This result is consistent with an exacerbation of the mps3-F592S SPB duplication defect upon SPO7 deletion. To test this directly, we determined the fraction of monopolar spindles in large-budded, single-nucleated mps3-F592S and mps3-F592S spo7Δ cells. If deleting SPO7 indeed exacerbated the SPB defect of mps3-F592S, then the fraction of mitotic cells accumulating with monopolar spindles should be greater in the double mutant than in the mps3-F592S mutant alone. The SPB and spindle were visualized using antibodies against Tub4p (the yeast γ-tubulin homolog) and α-tubulin, respectively. After a 6 hr temperature shift to the semipermissive temperature, monopolar spindles were evident in >45% of the large-budded cells in the double mutant, but in only ∼15% of the large-budded cells in the single mps3-F592S mutant (Figure 2, B and C). Thus, spo7Δ exacerbated the SPB duplication defect of mps3-F592S.
Interestingly, at the semipermissive temperature, the mps3-F592S mutants with two SPBs fell into three classes. While many contained a conventional either short or long bipolar spindle, a sizable population (51%) had duplicated and separated SPBs, each with independent microtubule arrays, but only a single, unsegregated DNA mass associated with only one of the SPBs (Figure 2D). This phenotype was also observed in mps3-1 cells grown at the nonpermissive temperature (data not shown). In the majority of these cells, nuclear microtubules were limited to the SPB associated with the DNA mass. The second microtubule array was often smaller and was found outside the nuclear envelope upon staining with mAB414 antibodies that recognize nuclear pore complex antigens (Aris and Blobel 1989; data not shown). While this phenotype has never been reported for mps3 mutants, it is common among SPB mutants that fail to properly insert the newly made SPB into the nuclear envelope (Winey et al. 1991, 1993; Schramm et al. 2000; Araki et al. 2006). This suggests that Mps3p may also be involved in late stages of SPB duplication, such as SPB insertion.
A high-copy suppressor screen reveals a link between nucleoporins and Mps3p function:
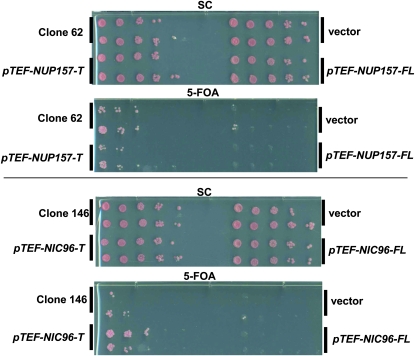
To uncover the biological basis for the genetic interaction between spo7Δ and mps3 mutants, we carried out a high-copy plasmid suppressor screen, aiming to unveil proteins that upon overexpression could suppress the lethality of spo7Δ mps3 double mutants. As expected, the screen recovered high-copy plasmids containing SPO7, MPS3, and PAH1 as strong suppressors (data not shown). We also recovered two additional suppressor plasmids, called clones 62 and 146, which encoded truncated forms of the large nucleoporins Nup157p and Nic96p, respectively. Both proteins are thought to play structural roles in the nuclear pore complex (Aitchison et al. 1995b; Zabel et al. 1996; Kosova et al. 2000). Interestingly, both suppressor clones were missing the 3′ ends of their respective nucleoporin genes. Clone 62 coded for the first 1204 amino acids of Nup157p (of 1391 amino acids), whereas clone 146 coded for the first 670 amino acids of Nic96p (of 839 amino acids). We named these suppressor alleles NUP157-T and NIC96-T, respectively. To compare full-length and truncated forms of each gene without contribution from overlapping open reading frames on the original plasmids, full-length and truncated forms of each gene were subcloned into a centromere-based plasmid behind the TEF promoter. In this context, NUP157-T suppressed as well as the original suppressor plasmid (clone 62), and NIC96-T suppressed better than clone 146 (Figure 3). In contrast, full-length forms of either gene had no suppressor activity (Figure 3). Further experimentation revealed that each truncated gene rescued the temperature sensitivity of the mps3-1 mutant but not the phenotypes associated with SPO7 deletion (Figure S4 and Figure S5). These results suggest that both NUP157-T and NIC96-T suppress synthetic lethality between mps3-1 and spo7Δ by fixing a defect associated with mps3-1.
Figure 3.—
Identification of high-copy suppressors of synthetic lethality in spo7Δ mps3 mutants. spo7Δ mps3-1 cells maintained with the pURA3-MPS3 plasmid (KW228) were transformed with the indicated suppressor plasmids (clones 62 and 146), an empty vector, and constructs containing full-length (-FL) or truncated forms (-T) of NUP157 and NIC96 cloned behind the TEF promoter. Each construct was tested by serial dilution for the ability to restore growth at 23° on plates containing 5-FOA or control (SC) media.
Deletion of NUP157 rescues the SPB defect of the mps3-1 allele:
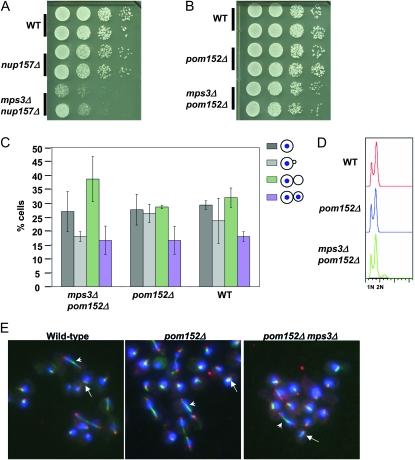
Because only truncated forms of NUP157 and NIC96 suppressed the temperature sensitivity of mps3-1, we speculated that the truncated genes might act as antimorphic alleles. For example, a nonfunctional truncated nucleoporin still might replace the full-length protein in the nuclear pore complex, effectively causing a loss-of-function phenotype. If this were the case, then suppressor activity might be phenocopied by the complete deletion of these genes. Because NIC96 is an essential gene (and thus cannot be deleted), but NUP157 is not, we focused on the deletion of NUP157. mps3-1 and mps3-1 nup157Δ strains, each containing an MPS3 plasmid, were assayed for the ability to grow without the plasmid at 37°. While strains containing the mps3-1 mutation alone were unable to grow without the plasmid at 37°, deletion of NUP157 restored viability under these conditions (Figure 4A). Thus, NUP157-T, and most likely NIC96-T, act as suppressors by causing loss of Nup157p and Nic96p function, respectively.
Because the essential function of Mps3p is its role in SPB duplication, we investigated whether NUP157 deletion improved SPB function in mps3-1 cells. As a consequence of SPB malfunction, haploid mps3-1 cells become diploid even at the permissive temperature immediately following loss of an MPS3-containing plasmid or immediately upon germination after meiosis (Jaspersen et al. 2002). To determine whether nup157Δ suppresses the diploidization phenotype of mps3-1 mutants, we analyzed the ploidy of wild-type, mps3-1, nup157Δ, and mps3-1 nup157Δ strains. Each strain was initially haploid due to the presence of a wild-type copy of MPS3 carried on the pURA3-MPS3 plasmid. After selection for cells that have lost the plasmid, independent cultures were grown to mid-log phase and examined by flow cytometry to determine their DNA content. As expected, mps3-1 mutant strains always had double the DNA content compared to a wild-type strain (n = 20; for a representative example, see Figure 4B). Strikingly, we found that the DNA content of mps3-1 nup157Δ double mutants (n = 20) was similar to wild-type cells, containing peaks representing 1N and 2N DNA content (Figure 4B). Thus, deleting NUP157 suppressed the diploidization phenotype of mps3-1 mutants, consistent with improved SPB function.
We also found that the mitotic delay of mps3-1 cells, caused by the inability to duplicate SPBs, was completely eliminated by deletion of NUP157 (Figure 4C). To confirm that this was due to restoration of SPB duplication, we used immunofluorescence to examine SPB and spindle morphology in large-budded cells of each genotype, as described in Figure 2B. While wild-type and nup157Δ cells had only a very small proportion of large-budded cells with monopolar spindles, mps3-1 mutants exhibited ∼45% monopolar spindles (Figure 4D). Deletion of NUP157 in mps3-1 cells reduced this number to wild-type levels (Figure 4D). Taken together, these results suggest that the SPB defects of mps3-1 cells are rescued by NUP157 deletion.
Specific nucleoporin deletions suppress the growth defects of mps3 mutants:
It was previously reported that deletion of genes coding for the POM nucleoporins Pom152p or Pom34p can rescue defects seen in certain mutants that disrupt SPB insertion, including ndc1, bbp1, and mps2 mutants (Chial et al. 1998; Sezen et al. 2009). To test whether POM gene deletions could also relieve defects associated with MPS3 mutation, POM152 and POM34 were individually deleted in haploid strains containing either wild-type MPS3 or mps3-1 alleles. Strains were then assayed for growth on rich media at 23° and 37°. Deletion of either POM gene restored growth at 37° to mps3-1 mutants and also rescued the genome diploidization phenotype (Figure 5). Thus, loss of POM nucleoporins is at least as effective as NUP157 deletion in alleviating the SPB defect of mps3 mutants (also see below).
To expand on the relationship between MPS3 and nucleoporin genes, we tested a panel of additional nucleoporin deletions to determine their effectiveness at rescuing mps3-1 phenotypes. Double mutants containing mps3-1 and individual deletion alleles of several nonessential nucleoporin genes were created and analyzed for ploidy and temperature sensitivity. Of the additional genes tested, only nup42Δ both rescued the diploidization phenotype and restored growth at elevated temperatures to the mps3-1 mutant (Table 3; Figure 5). This was somewhat surprising as Nup42p is a peripheral nucleoporin, rather than a structural nucleoporin like the other suppressors identified to this point (Strahm et al. 1999). nup188Δ acted as a partial suppressor, rescuing temperature sensitivity but not the diploidization phenotype, as did simultaneous deletion of MLP1 and MLP2 (Table 3; Figure 5). All other nucleoporin deletions tested, including nup84Δ, nup60Δ, nup133Δ, and mlp1Δ, and mlp2Δ individually, failed to suppress either temperature sensitivity or diploidization of mps3-1 (Table 3). Thus, while suppression of the SPB defect of mps3-1 cells is not an exclusive property of nup157Δ, it is specific to particular nucleoporin deletions. Moreover, while there is considerable overlap between the nucleoporin deletions that suppress mps2Δ and mps3-1, the spectrum of suppressing mutants is not identical: nup188Δ could partially suppress mps3-1 but not mps2Δ (Sezen et al. 2009), and nup157Δ suppressed mps3-1 but not mps2-1 (see below).
TABLE 3.
Suppression of mps3-1 phenotypes by nucleoporin gene deletions
| Full suppressiona | Incomplete suppressionb | No suppressionc |
|---|---|---|
| nup157Δ | nup188Δ | nup84Δ |
| pom152Δ | mlp1Δ mlp2Δ | nup60Δ |
| pom34Δ | nup133Δ | |
| nup42Δ | mlp1Δ | |
| mlp2Δ |
Suppression of both temperature sensitivity and ploidy phenotypes.
Suppression of temperature sensitivity but not ploidy phenotypes.
No suppression of either temperature sensitivity or ploidy phenotypes.
nup157Δ and pom152Δ rescue distinct SPB mutants:
The results above establish a genetic relationship between MPS3 and genes encoding subunits of the nuclear pore complex. To extend this analysis, we examined the spectrum of SPB mutants that could be suppressed by nucleoporin deletions, initially focusing on nup157Δ. NUP157 was deleted in cells containing one of several conditional mutant alleles that affect different structural parts of the SPB or different stages of SPB duplication (mps2-1, spc42-11, spc98-2, kar1Δ17, ndc1-39, and spc29-3). Growth of single SPB mutants and double mutants with nup157Δ was compared at permissive and restrictive temperatures. The temperature sensitivity of the spc42-11 allele was partially suppressed by the NUP157 deletion, which was evident by slight growth at 37° (Figure 6A). In contrast, nup157Δ did not rescue the temperature sensitivity of spc29-3, spc98-2, kar1Δ17, ndc1-39, or mps2-1 mutants (Figure 6A). This suggests that, while suppression by nup157Δ is not specific to mps3 mutants, it is limited to a small subset of SPB genes that likely collaborate in a common function.
Figure 6.—
The effects of nup157Δ and pom152Δ on various SPB mutant alleles. Strains of the indicated genotypes were plated on rich media (YPD) by serial dilution at 23° and 37°. The following strains were used: (A) OCF1533-4B, KW967, SLJ715, KW969, SLJ839, KW973, SLJ751, KW972, SLJ717, KW970, SLJ843, KW1010, KW1013, and KW1012. For SLJ843 and KW1010, 5-FOA was used immediately prior to the experiment to select for cells that have lost the pURA3-KAR1 plasmid. (B) The following strains were used: KW966, KW983, and KW971.
Since nup157Δ, pom152Δ, and pom34Δ equivalently suppress the temperature sensitivity of mps3-1 (Figure 5), we asked whether deletion of POM genes would also rescue temperature sensitivity of the spc42-11 allele. To test this, POM152 and POM34 were individually deleted in haploid strains containing spc42-11 or mps2-1 and assayed for growth on rich media at 23° and 37°. The mps2-1 allele was used as a control because POM deletions are known to rescue this mutant (Sezen et al. 2009). Neither POM gene deletion rescued the temperature sensitivity of the spc42-11 allele (Figure 6B; data not shown). Furthermore, while deletion of either POM152 or POM34 rescued the mps2-1 mutation (Figure 6B; data not shown), the NUP157 deletion did not (Figure 6A). Taken together, these data indicate that nucleoporin gene deletions have differential effects on the requirement for individual SPB subunits.
Deletion of NUP157 or POM152 renders MPS3 nonessential:
Loss of Nup157p or POM nucleoporins could affect Mps3p directly, for example, by changing the localization, stability, or conformation of the mps3-1 protein product; or it could act more generally to modulate SPB function such that Mps3p function is no longer required. To distinguish between these two possibilities, we made diploid strains heterozygous for mps3Δ and for either nup157Δ or pom152Δ. After meiosis and sporulation, progeny were inspected for segregation of the mps3Δ allele. As expected, no mps3Δ cells were recovered in otherwise wild-type backgrounds. Strikingly, we found that mps3Δ nup157Δ double mutants were viable, although compromised for growth compared to wild type and nup157Δ controls (Figure 7A).Furthermore, mps3Δ pom152Δ progeny were not only viable, but also exhibited robust growth similar to wild type and pom152Δ controls (Table 4; Figure 7B). Asynchronous mps3Δ pom152Δ cells had a cell cycle distribution similar to wild type (Figure 7C) and a haploid DNA profile by flow cytometry (Figure 7D). Thus, in the absence of either Nup157p or Pom152p, MPS3 is no longer an essential gene.
TABLE 4.
Suppression of SPB deletions with pom152Δ
| SPB deletion | Suppression by pom152Δa |
|---|---|
| mps3Δ | + |
| mps2Δ | + |
| mps1Δ | − |
| nbp1Δ | − |
| ndc1Δ | − |
| kar1Δ | − |
| nud1Δ | − |
| spc42Δ | − |
| spc98Δ | − |
Double mutants were generated in the presence of a URA3-marked plasmid containing a wild-type copyof the SPB gene and scored for growth on 5-FOA: “+” indicates robust growth similar to wild type strains; “−” indicates no growth on 5-FOA.
If deletion of other nucleoporin genes suppressed mps3-1 by a common mechanism shared with nup157Δ and pom152Δ, then they also might be expected to suppress the mps3Δ allele. Interestingly, despite their ability to rescue the mps3-1 allele (Figure 5), neither nup42Δ nor nup188Δ were able to restore growth to mps3Δ (data not shown). Thus, while different nucleoporin deletions can rescue SPB mutations, our results suggest that they do so in distinct ways. Those that rescue the mps3Δ allele alleviate the need for any Mps3p protein product, suggesting that under these conditions the process of SPB duplication occurs by a noncanonical pathway, completely independently of Mps3p. In contrast, those that can suppress mps3-1 but not mps3Δ may rescue the SPB defect by affecting the compromised Mps3p protein so that SPB duplication can still occur via the canonical pathway (i.e., Mps3-dependent), or they may activate the noncanonical pathway but to insufficient levels.
mps3Δ pom152Δ cells have intact spindles and SPBs:
How do the nucleoporin deletion mutations bypass the requirement for Mps3p? One possibility is that these bypass suppressors act by alleviating any need for the SPB, perhaps by allowing formation of acentrosomal spindles. While acentrosomal spindles have never been reported in S. cerevisiae, they are found in oocytes, plants, and some mutant animals lacking centrosomes (Walczak and Heald 2008). If this were the mechanism of suppression, then nup157Δ and pom152Δ would be expected to also restore viability to deletion alleles of other essential SPB genes. To test this, double mutants were made containing both pom152Δ and individual SPB deletions covered by a wild-type copy of the SPB gene on a URA3-marked plasmid and then tested for growth on plates containing 5-FOA. While POM152 deletion suppressed the mps2Δ allele, as reported previously (Sezen et al. 2009), it did not restore growth to kar1Δ, spc98Δ, nbp1Δ, spc42Δ, nud1Δ, mps1Δ, or ndc1Δ (Table 4; Figure S6; data not shown). This suggests that pom152Δ cells still require an intact SPB for spindle formation; however, this SPB requires neither Mps3p nor Mps2p.
To examine the SPB and spindle in mps3Δ pom152Δ cells directly, we performed immunofluoresence analysis of asynchronous cells using antibodies against Tub4p and α-tubulin to visualize SPBs and microtubules, respectively. This analysis revealed that mps3Δ pom152Δ mutants form bipolar spindles (Figure 7E). Quantitative measurement of spindle length revealed that average length and range of spindle lengths in mps3Δ pom152Δ cells were similar to those of wild-type and pom152Δ cells (Figure S7). However, compared to the controls, mps3Δ pom152Δ cells had a paucity of cells containing intermediate length spindles (Figure S7). This suggests that, while mps3Δ pom152Δ cells are capable of SPB duplication, bipolar spindle formation, and spindle elongation, their spindles are not identical to those of wild-type cells.
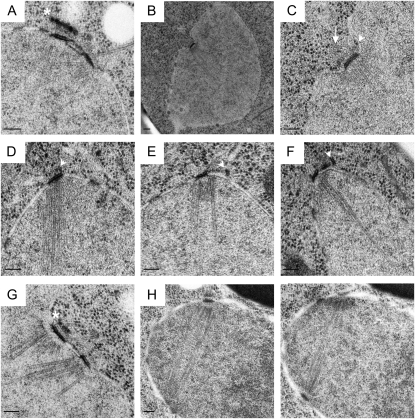
To examine the structure of the SPB in cells lacking Mps3p, pom152Δ and pom152Δ mps3Δ cells were examined by electron microscopy (EM). Complete serial sections through the nuclei of 10 pom152Δ and 25 pom152Δ mps3Δ mutants demonstrated that the overall architecture of the SPB, including its size, laminar structure, microtubule-organizing ability, and location in the nuclear envelope, was very similar between the two strains, with two notable exceptions (Figure 8). First, the density of the bridge or half-bridge was decreased in pom152Δ mps3Δ compared to pom152Δ. This was particularly notable on the nuclear side of the half-bridge (Figure 8, E–G); in some cases, the cytoplasmic region lost its association with the nuclear envelope (Figure 8D). Despite this difference, pom152Δ mps3Δ mutants still appear to undergo SPB duplication in much the same way as wild-type cells. We were able to observe all of the known intermediates in the canonical SPB duplication pathway in cells lacking Mps3, including a satellite (Figure 8E) and duplication-plaque structure (Figure 8F) at the distal tip of the half-bridge as well as duplicated side-by-side SPBs connected by a complete bridge (Figure 8G). Thus, the Mps3p-independent mechanism of SPB duplication is largely similar to the conventional Mps3p-dependent pathway at least at the cytological level. The second major difference that we frequently observed was that pom152Δ mps3Δ SPBs, but not pom152Δ SPBs, were often present on nuclear invaginations (8 of 25 SPBs; Figure 8, B and C) even though the overall shape of nuclei was round. Given that microtubule nucleation from the inner and outer plaque and the half-bridge appears normal in pom152Δ mps3Δ mutants and there are no obvious changes in nuclear morphology, we assume that this invagination does not interfere with SPB function. We suspect that it may be due to a nonessential role of Mps3p in chromosome segregation or perhaps in affecting the rigidity of the nuclear membrane surrounding the SPB. Whatever the defect, pom152Δ mps3Δ mutants are able to form bipolar spindles (Figure 8H), indicating that under certain conditions, Mps3p is not essential for SPB duplication and spindle formation.
Figure 8.—
Electron microscopy analysis of SPBs in cells lacking Mps3p. (A) EM image from pom152Δ cells (SLJ4260) of duplicated side-by-side SPBs connected by a bridge. Asterisk indicates vesicle structures in close proximity to the SPB. (B–H) EM images from pom152Δ mps3Δ mutant cells (SLJ4259). (B and C) Low magnification (B) and high magnification (C) image of SPB present on a nuclear invagination. An arrowhead points to electron-dense material resembling a satellite, and an arrow marks a cytoplasmic microtubule emanating from the half-bridge region of the SPB. (D) An unduplicated SPB with a half-bridge that appears to have lost association with the nuclear envelope (arrowhead). (E and F) SPBs that contain electron-dense material resembling a satellite (E, arrowhead) or duplication plaque (F, arrowhead) at the distal tip of the half-bridge. (G) Duplicated side-by-side SPBs connected by a bridge. Asterisk indicates vesicle structures located in close proximity to the SPB. (H) Serial section images showing a bipolar spindle. The first SPB is apparent in H (left) while the second SPB is apparent in H (right). Bars, 100 nm.
DISCUSSION
The function of Mps3p in SPB duplication is sensitive to the nuclear envelope environment:
In this study, we aimed to identify nuclear structures or processes that are affected by alteration of nuclear membrane properties. We found that the function of Mps3p was greatly affected by inactivation of Spo7p (Figure 1, A and B). Mps3p plays dual roles at the nuclear envelope, acting in both SPB duplication and as a molecular tether that physically links nucleoplasmic factors (such as telomeres) to the nuclear periphery. Three lines of evidence suggest that the synthetic lethality between mps3-1 and spo7Δ occurs because Spo7p affects the function of Mps3p at the SPB, rather than at the nuclear periphery. First, the genetic interactions between spo7Δ and mps3 are seen with mps3 alleles that are defective in SPB duplication, and not with mps3 alleles that are defective in telomere tethering (Figure 1). Second, spo7Δ has genetic interactions with mutant alleles of other SPB genes (Table 2; Figure S2). Finally, we saw an exacerbation of the SPB duplication defect in mps3-F592S mutants upon SPO7 deletion (Figure 2). Work from the Rose lab has identified genetic interactions between kar1Δ17 and deletion of the gene coding for Nem1p, Spo7p's partner in the phosphatase complex (Khalfan 2001). Like Mps3p, Kar1p is a transmembrane component of the SPB, and both have been implicated in Cdc31p recruitment early in the SPB duplication pathway. Expression of the dominant CDC31-16 allele rescued both temperature sensitivity of kar1Δ17 and its synthetic lethality with nem1Δ, suggesting that the genetic interaction between kar1Δ17 and nem1Δ stems from an SPB defect (Khalfan 2001), consistent with our findings here. Taken together, these data suggest that the Spo7p/Nem1p complex, possibly through its role in determining membrane composition, affects SPB processes in general and Mps3p function in particular.
How could spo7Δ affect SPB function?
The only known function of Spo7p is to regulate lipid biosynthesis through Pah1p, and inactivation of Pah1 leads to alteration in membrane composition. Thus, our data are consistent with the possibility that Mps3p is sensitive to changes in its membrane environment that occur upon SPO7 deletion. This is further supported by our observation that the genetic interaction between spo7Δ and mps3-1 can be suppressed by altering another nuclear envelope component, namely the nuclear pore complex (Figure 3). Both the SPB and the nuclear pore complexes are large structures embedded in the inner and outer nuclear membranes. Data from several groups suggest that physical properties of the nuclear membrane can affect the process of inserting nuclear pore complexes. For example, specific alleles of the ACC1 gene, affecting the limiting step in very-long-chain fatty acid synthesis, alter the structure of the nuclear envelope and inhibit nuclear pore complex assembly (Schneiter et al. 1996). Nuclear pore complex abnormalities are also seen upon deletion of the APQ12 gene, and these defects are remedied upon treatment with benzyl alcohol, which increases membrane fluidity (Scarcelli et al. 2007). More recently, it was shown that brr6-1 mutants with an abnormal accumulation of certain lipids also display defects in nuclear pore complex assembly (Hodge et al. 2010). Moreover, affecting membrane fluidity or rigidity by benzyl alcohol or palmitic acid, respectively, interferes with the normal distribution of the nucleoporin Gle2p (Izawa et al. 2004; Scarcelli et al. 2007). Finally, reticulon proteins required for membrane curvature have been implicated in NPC biogenesis in yeast and vertebrate cells (Dawson et al. 2009). Interestingly, spo7Δ cells also display enhanced sensitivity to mutation of nucleoporin genes (Siniossoglou et al. 1998). In light of these results, we interpret the genetic interactions between spo7Δ and SPB genes to indicate that changes in the nuclear membrane composition affect SPB duplication and insertion into the nuclear envelope.
SPB duplication defects in mps3 cells are suppressed by altering nuclear pore complex composition:
A high-copy suppressor screen revealed that deletion or mutation of certain nucleoporin genes suppresses the SPB duplication defect of mps3-1 (Figures 3–5). This raises the possibility that nuclear pore complexes may cause an additional type of nuclear envelope alteration that affects SPB function. Several of the nucleoporin genes that upon deletion suppress mps3 mutants code for proteins implicated in nuclear pore complex insertion or assembly, including Nup157p, Nic96p, Nup188p, Pom152p, and Pom34p (Nehrbass et al. 1996; Zabel et al. 1996; Gomez-Ospina et al. 2000; Madrid et al. 2006; Miao et al. 2006; Dawson et al. 2009; Flemming et al. 2009; Makio et al. 2009; Onischenko et al. 2009). Since many of these genes are not essential for viability, while nuclear pore complexes are essential, these gene deletions are unlikely to suppress the mps3-1 defect by eliminating nuclear pore complexes or drastically reducing their number. Indeed, many of these genes interact with other partially redundant nucleoporins also involved in nuclear pore complex insertion and biogenesis (e.g., NUP157 and NUP170). Furthermore, other labs have documented no obvious change in the steady-state number of nuclear pore complexes in the absence of either Nup157p or Pom152p (Madrid et al. 2006; Makio et al. 2009).
Thus, we propose two possible models for how nucleoporin gene deletions alter the nuclear pore complex to allow compromised SPBs to more easily duplicate. First, altered nuclear pore complexes lacking specific nucleoporins may affect the physical properties of the nuclear membrane in a way that creates a favorable condition for SPB duplication, allowing a compromised SPB duplication machinery to function more effectively. Consistent with this possibility is the observation that the strongest suppression of SPB mutants is mediated by deletion of the POM nucleoporins, which physically contact the nuclear membrane and are thus good candidates for proteins that can affect membrane properties. However, not all nucleoporins with predicted membrane-interaction domains are capable of suppression; for example, nup133Δ has no suppressor activity, despite encoding a protein with an ALPS domain (Drin et al. 2007). Conversely, not all suppressing nucleoporin deletions are membrane-associated proteins. For example, Nup42p has not been shown to be involved in either NPC assembly or insertion, and yet nup42Δ is a strong suppressor of mps3-1. It is, however, possible that deletion of NUP42 may influence NPC structure such that its interactions with the surrounding nuclear membrane are altered.
Several groups have reported altered nuclear membrane structure upon deletion or mutation of certain nuclear pore complex components. For example, mutants compromised for NUP116 (Wente and Blobel 1993); NUP145 (Wente and Blobel 1994), NUP120 (Aitchison et al. 1995a; Heath et al. 1995), NUP1 (Bogerd et al. 1994; DeHoratius and Silver 1996), NUP133 (Pemberton et al. 1995), NUP85 (Goldstein et al. 1996), NUP84 (Siniossoglou et al. 1996), or the combination of NUP170 and POM152 (Aitchison et al. 1995b) all have nuclear envelope structural abnormalities. It is possible that these previously described structural changes are related to those that facilitate SPB duplication in the absence of certain nucleoporins.
An alternative hypothesis for the mechanism of suppression is that the SPB and nuclear pore complexes compete for a limiting factor and that the SPB relies even more heavily on this putative factor when certain SPB proteins are weakened by mutation. Elimination of certain nucleoporins may reduce the affinity of the nuclear pore complex for this putative factor, thus favoring its association with the SPB instead. Indeed, the two complexes do have several features in common. First, several reports indicate that nuclear pore complexes may be preferentially inserted into the nuclear envelope in the vicinity of the SPB (Heath et al. 1995; Winey et al. 1997; Adams and Kilmartin 1999). Second, they share common components: Ndc1p is a functional part of both complexes, and Cdc31p and Mlp2p have each been reported to co-purify with both SPB and nuclear pore complex components (Chial et al. 1998; Fischer et al. 2004; Niepel et al. 2005). There may also be other shared components that have yet to be discovered. These shared features between the SPB and nuclear pore complexes raise the intriguing possibility that the two complexes may be inserted by common mechanisms or may rely on common membrane-remodeling machinery.
Competition for a limiting insertion factor is also consistent with the tendency of SPB mutants to diploidize. The propensity of SPB mutants to diploidize might be due to cell cycle progression in the absence of SPB duplication. But why do these cells remain as stable diploids rather than continue to increase in ploidy? When cells diploidize, not only their genetic content but also their cell and nuclear volumes increase 2-fold, possibly reflecting a doubling in protein synthesis capacity (Galitski et al. 1999; Jorgensen et al. 2007; Neumann and Nurse 2007). However, the surface area of the nucleus increases by only 1.6-fold. Thus, the relative concentration of this putative limiting factor in the nuclear membrane may be higher in diploids than in haploids, allowing cells with compromised SPBs to survive only as diploid cells.
An alternative SPB duplication pathway in cells lacking both SPB components and specific nucleoporins:
Remarkably, pom152Δ and nup157Δ were able to suppress the lethality associated with mps3Δ. Prior to this study, Mps3p was thought to be essential for SPB duplication. This observation is reminiscent of the finding that certain mutations in nuclear pore complex genes, including pom152Δ but not nup157Δ, can suppress the lethality associated with the deletion of several SPB genes, including mps2Δ, which are required for insertion of the daughter SPB into the nuclear membrane following SPB duplication. Our findings suggest that SPB duplication can occur through a noncanonical pathway that does not involve Mps3p and that this pathway resembles Mps3p-dependent SPB duplication in that the same structural intermediates were observed. Interestingly, the essential function of the other integral membrane component of the half-bridge, Kar1p, in SPB duplication can also be bypassed, not by deletion of the same nucleoporins that rescue mps3Δ, but by a dominant allele of CDC31 or by mutation of the small ubiquitin-like gene DSK2 (Vallen et al. 1994; Biggins et al. 1996). Although the structure of the SPB in cells lacking KAR1 was not determined, the fact that the two structural components of the half-bridge can be eliminated is consistent with the notion that the SPB may be able to duplicate by more than one mechanism and that there may be an intrinsic plasticity built into this organelle. In addition, the requirement for certain SPB proteins, particularly integral membrane components, might be highly dependent on proteins and other factors present in the nuclear membrane.
There is not a strict correlation between the SPB deletions that are rescued by nup157Δ and those rescued by POM nucleoporin deletions. The spc42-11 allele is rescued only by nup157Δ, whereas mps2-1 is rescued only by POM deletions (Figure 6). Similarly, only a subset of the nucleoporin deletions that rescue the mps3-1 allele restore viability to mps3Δ. Further studies are needed to determine the exact mechanism by which the different nucleoporin deletions suppress each SPB allele and to elucidate the mechanism by which SPBs can duplicate in the complete absence of Mps3p. However, regardless of the precise mechanism, our data show that Mps3p is required only for SPB duplication in the presence of intact nuclear pore complexes and that an alternative SPB duplication pathway exists under conditions where nucleoporin deletions have altered the nuclear envelope environment.
Acknowledgments
We thank Mark Winey for strains and helpful discussions, Joseph Campbell for advice and technical support, and Kathryn Wagner for assistance with flow cytometry. We are grateful to Rhonda Trimble for assistance with EM and to Neal Freedman for help with statistical analysis. We thank Mark Rose for sharing unpublished data and for insightful suggestions on immunofluorescence data. We also thank Kevin O'Connell, Leslie Barbour, Micah Webster, Daphna Joseph-Strauss, and William Glassford for discussion and comments on the manuscript. K.L.W. is funded by a National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) intramural Nancy Nossal fellowship award, O.C.F. is funded by an intramural NIDDK grant, and S.L.J. is supported by funds from the Stowers Institute for Medical Research.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.119149/DC1.
References
- Adams, I. R., and J. V. Kilmartin, 1999. Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae. J. Cell Biol. 145 809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison, J. D., G. Blobel and M. P. Rout, 1995a. Nup120p: a yeast nucleoporin required for NPC distribution and mRNA transport. J. Cell Biol. 131 1659–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitchison, J. D., M. P. Rout, M. Marelli, G. Blobel and R. W. Wozniak, 1995b. Two novel related yeast nucleoporins Nup170p and Nup157p: complementation with the vertebrate homologue Nup155p and functional interactions with the yeast nuclear pore-membrane protein Pom152p. J. Cell Biol. 131 1133–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber, F., S. Dokudovskaya, L. M. Veenhoff, W. Zhang, J. Kipper et al., 2007. The molecular architecture of the nuclear pore complex. Nature 450 695–701. [DOI] [PubMed] [Google Scholar]
- Antoniacci, L. M., M. A. Kenna, P. Uetz, S. Fields and R. V. Skibbens, 2004. The spindle pole body assembly component mps3p/nep98p functions in sister chromatid cohesion. J. Biol. Chem. 279 49542–49550. [DOI] [PubMed] [Google Scholar]
- Antoniacci, L. M., M. A. Kenna and R. V. Skibbens, 2007. The nuclear envelope and spindle pole body-associated Mps3 protein bind telomere regulators and function in telomere clustering. Cell Cycle 6 75–79. [DOI] [PubMed] [Google Scholar]
- Araki, Y., C. K. Lau, H. Maekawa, S. L. Jaspersen, T. H. Giddings, Jr. et al., 2006. The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol. Biol. Cell 17 1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aris, J. P., and G. Blobel, 1989. Yeast nuclear envelope proteins cross react with an antibody against mammalian pore complex proteins. J. Cell Biol. 108 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins, S., I. Ivanovska and M. D. Rose, 1996. Yeast ubiquitin-like genes are involved in duplication of the microtubule organizing center. J. Cell Biol. 133 1331–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd, A. M., J. A. Hoffman, D. C. Amberg, G. R. Fink and L. I. Davis, 1994. nup1 mutants exhibit pleiotropic defects in nuclear pore complex function. J. Cell Biol. 127 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt, E., M. P. Rout, J. V. Kilmartin and C. W. Akey, 1997. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell 89 1077–1086. [DOI] [PubMed] [Google Scholar]
- Bupp, J. M., A. E. Martin, E. S. Stensrud and S. L. Jaspersen, 2007. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J. Cell Biol. 179 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. L., A. Lorenz, K. L. Witkin, T. Hays, J. Loidl et al., 2006. Yeast nuclear envelope subdomains with distinct abilities to resist membrane expansion. Mol. Biol. Cell. 17 1768–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chial, H. J., M. P. Rout, T. H. Giddings and M. Winey, 1998. Saccharomyces cerevisiae Ndc1p is a shared component of nuclear pore complexes and spindle pole bodies. J. Cell Biol. 143 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, M. N., C. Y. Lee, J. L. Wilkerson and M. E. Dresser, 2007. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 104 8863–8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, M. N., C. Y. Lee, G. Chao, M. Shinohara, H. Kosaka et al., 2008. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 133 1175–1187. [DOI] [PubMed] [Google Scholar]
- Dawson, T. R., M. D. Lazarus, M. W. Hetzer and S. R. Wente, 2009. ER membrane-bending proteins are necessary for de novo nuclear pore formation. J. Cell Biol. 184 659–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoratius, C., and P. A. Silver, 1996. Nuclear transport defects and nuclear envelope alterations are associated with mutation of the Saccharomyces cerevisiae NPL4 gene. Mol. Biol. Cell 7 1835–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos, D., S. Dokudovskaya, F. Alber, R. Williams, B. T. Chait et al., 2004. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2 e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos, D., S. Dokudovskaya, R. Williams, F. Alber, N. Eswar et al., 2006. Simple fold composition and modular architecture of the nuclear pore complex. Proc. Natl. Acad. Sci. USA 103 2172–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin, G., J. F. Casella, R. Gautier, T. Boehmer, T. U. Schwartz et al., 2007. A general amphipathic alpha-helical motif for sensing membrane curvature. Nat. Struct. Mol. Biol. 14 138–146. [DOI] [PubMed] [Google Scholar]
- Elliott, S., M. Knop, G. Schlenstedt and E. Schiebel, 1999. Spc29p is a component of the Spc110p subcomplex and is essential for spindle pole body duplication. Proc. Natl. Acad. Sci. USA 96 6205–6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, T., S. Rodriguez-Navarro, G. Pereira, A. Racz, E. Schiebel et al., 2004. Yeast centrin Cdc31 is linked to the nuclear mRNA export machinery. Nat. Cell Biol. 6 840–848. [DOI] [PubMed] [Google Scholar]
- Flemming, D., P. Sarges, P. Stelter, A. Hellwig, B. Bottcher et al., 2009. Two structurally distinct domains of the nucleoporin Nup170 cooperate to tether a subset of nucleoporins to nuclear pores. J. Cell Biol. 185 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galitski, T., A. J. Saldanha, C. A. Styles, E. S. Lander and G. R. Fink, 1999. Ploidy regulation of gene expression. Science 285 251–254. [DOI] [PubMed] [Google Scholar]
- Golden, A., J. Liu and O. Cohen-Fix, 2009. Inactivation of the C. elegans lipin homolog leads to ER disorganization and to defects in the breakdown and reassembly of the nuclear envelope. J. Cell Sci. 122 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, A. L., and J. H. McCusker, 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15 1541–1553. [DOI] [PubMed] [Google Scholar]
- Goldstein, A. L., C. A. Snay, C. V. Heath and C. N. Cole, 1996. Pleiotropic nuclear defects associated with a conditional allele of the novel nucleoporin Rat9p/Nup85p. Mol. Biol. Cell 7 917–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ospina, N., G. Morgan, T. H. Giddings, Jr., B. Kosova, E. Hurt et al., 2000. Yeast nuclear pore complex assembly defects determined by nuclear envelope reconstruction. J. Struct. Biol. 132 1–5. [DOI] [PubMed] [Google Scholar]
- Gorjanacz, M., and I. W. Mattaj, 2009. Lipin is required for efficient breakdown of the nuclear envelope in Caenorhabditis elegans. J. Cell Sci. 122 1963–1969. [DOI] [PubMed] [Google Scholar]
- Han, G. S., W. I. Wu and G. M. Carman, 2006. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, G. S., L. O'Hara, G. M. Carman and S. Siniossoglou, 2008. An unconventional diacylglycerol kinase that regulates phospholipid synthesis and nuclear membrane growth. J. Biol. Chem. 283 20433–20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath, C. V., C. S. Copeland, D. C. Amberg, V. Del Priore, M. Snyder et al., 1995. Nuclear pore complex clustering and nuclear accumulation of poly(A)+ RNA associated with mutation of the Saccharomyces cerevisiae RAT2/NUP120 gene. J. Cell Biol. 131 1677–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer, M. W., and S. R. Wente, 2009. Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev. Cell 17 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka, Y., and A. F. Dernburg, 2009. The SUN rises on meiotic chromosome dynamics. Dev. Cell 17 598–605. [DOI] [PubMed] [Google Scholar]
- Hodge, C. A., V. Choudhary, M. J. Wolyniak, J. J. Scarcelli, R. Schneiter et al., 2010. Integral membrane proteins Brr6 and Apq12 link assembly of the nuclear pore complex to lipid homeostasis in the endoplasmic reticulum. J. Cell Sci. 123 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa, S., R. Takemura and Y. Inoue, 2004. Gle2p is essential to induce adaptation of the export of bulk poly(A)+ mRNA to heat shock in Saccharomyces cerevisiae. J. Biol. Chem. 279 35469–35478. [DOI] [PubMed] [Google Scholar]
- Jaspersen, S. L., and M. Winey, 2004. The budding yeast spindle pole body: structure, duplication, and function. Annu. Rev. Cell Dev. Biol. 20 1–28. [DOI] [PubMed] [Google Scholar]
- Jaspersen, S. L., T. H. Giddings, Jr. and M. Winey, 2002. Mps3p is a novel component of the yeast spindle pole body that interacts with the yeast centrin homologue Cdc31p. J. Cell Biol. 159 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen, S. L., A. E. Martin, G. Glazko, T. H. Giddings, Jr., G. Morgan et al., 2006. The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J. Cell Biol. 174 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, P., N. P. Edgington, B. L. Schneider, I. Rupes, M. Tyers et al., 2007. The size of the nucleus increases as yeast cells grow. Mol. Biol. Cell 18 3523–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalfan, W. A., 2001. Genetic interactions between a spindle pole body (SPB) gene KAR1, and the PKC1 MAP kinase cascade and nuclear envelope proteins. Ph.D. Thesis, Princeton University Press, Princeton, NJ.
- Kosova, B., N. Pante, C. Rollenhagen, A. Podtelejnikov, M. Mann et al., 2000. Mlp2p, a component of nuclear pore attached intranuclear filaments, associates with nic96p. J. Biol. Chem. 275 343–350. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, III, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 953–961. [DOI] [PubMed] [Google Scholar]
- Madrid, A. S., J. Mancuso, W. Z. Cande and K. Weis, 2006. The role of the integral membrane nucleoporins Ndc1p and Pom152p in nuclear pore complex assembly and function. J. Cell Biol. 173 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makio, T., L. H. Stanton, C. C. Lin, D. S. Goldfarb, K. Weis et al., 2009. The nucleoporins Nup170p and Nup157p are essential for nuclear pore complex assembly. J. Cell Biol. 185 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, K., 1999. High-pressure freezing for preservation of high resolution fine structure and antigenicity for immunolabeling. Methods Mol. Biol. 117 77–97. [DOI] [PubMed] [Google Scholar]
- Miao, M., K. J. Ryan and S. R. Wente, 2006. The integral membrane protein Pom34p functionally links nucleoporin subcomplexes. Genetics 172 1441–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehrbass, U., M. P. Rout, S. Maguire, G. Blobel and R. W. Wozniak, 1996. The yeast nucleoporin Nup188p interacts genetically and physically with the core structures of the nuclear pore complex. J. Cell Biol. 133 1153–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, F. R., and P. Nurse, 2007. Nuclear size control in fission yeast. J. Cell Biol. 179 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepel, M., C. Strambio-de-Castillia, J. Fasolo, B. T. Chait and M. P. Rout, 2005. The nuclear pore complex-associated protein, Mlp2p, binds to the yeast spindle pole body and promotes its efficient assembly. J. Cell Biol. 170 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, S., Y. Terazawa, T. Nakayama, A. Hirata, T. Makio et al., 2003. Nep98p is a component of the yeast spindle pole body and essential for nuclear division and fusion. J. Biol. Chem. 278 9938–9943. [DOI] [PubMed] [Google Scholar]
- Onischenko, E., L. H. Stanton, A. S. Madrid, T. Kieselbach and K. Weis, 2009. Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance. J. Cell Biol. 185 475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza, P., S. L. Jaspersen, A. Miele, J. Dekker and C. L. Peterson, 2009. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev. 23 912–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton, L. F., M. P. Rout and G. Blobel, 1995. Disruption of the nucleoporin gene NUP133 results in clustering of nuclear pore complexes. Proc. Natl. Acad. Sci. USA 92 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky, D., and D. Hodzic, 2009. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J. Cell Biol. 186 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, M. D., and G. R. Fink, 1987. KAR1, a gene required for function of both intranuclear and extranuclear microtubules in yeast. Cell 48 1047–1060. [DOI] [PubMed] [Google Scholar]
- Rose, M. D., F. Winston and P. Heither, 1990. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Santos-Rosa, H., J. Leung, N. Grimsey, S. Peak-Chew and S. Siniossoglou, 2005. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli, J. J., C. A. Hodge and C. N. Cole, 2007. The yeast integral membrane protein Apq12 potentially links membrane dynamics to assembly of nuclear pore complexes. J. Cell Biol. 178 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiter, R., M. Hitomi, A. S. Ivessa, E. V. Fasch, S. D. Kohlwein et al., 1996. A yeast acetyl coenzyme A carboxylase mutant links very-long-chain fatty acid synthesis to the structure and function of the nuclear membrane-pore complex. Mol. Cell. Biol. 16 7161–7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober, H., H. Ferreira, V. Kalck, L. R. Gehlen and S. M. Gasser, 2009. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 23 928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm, C., S. Elliott, A. Shevchenko and E. Schiebel, 2000. The Bbp1p-Mps2p complex connects the SPB to the nuclear envelope and is essential for SPB duplication. EMBO J. 19 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezen, B., M. Seedorf and E. Schiebel, 2009. The SESA network links duplication of the yeast centrosome with the protein translation machinery. Genes Dev. 23 1559–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou, S., C. Wimmer, M. Rieger, V. Doye, H. Tekotte et al., 1996. A novel complex of nucleoporins, which includes Sec13p and a Sec13p homolog, is essential for normal nuclear pores. Cell 84 265–275. [DOI] [PubMed] [Google Scholar]
- Siniossoglou, S., H. Santos-Rosa, J. Rappsilber, M. Mann and E. Hurt, 1998. A novel complex of membrane proteins required for formation of a spherical nucleus. EMBO J. 17 6449–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang, A., I. Courtney, K. Grein, M. Matzner and E. Schiebel, 1995. The Cdc31p-binding protein Kar1p is a component of the half bridge of the yeast spindle pole body. J. Cell Biol. 128 863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahm, Y., B. Fahrenkrog, D. Zenklusen, E. Rychner, J. Kantor et al., 1999. The RNA export factor Gle1p is located on the cytoplasmic fibrils of the NPC and physically interacts with the FG-nucleoporin Rip1p, the DEAD-box protein Rat8p/Dbp5p and a new protein Ymr 255p. EMBO J. 18 5761–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen, E. A., W. Ho, M. Winey and M. D. Rose, 1994. Genetic interactions between CDC31 and KAR1, two genes required for duplication of the microtubule organizing center in Saccharomyces cerevisiae. Genetics 137 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C. E., and R. Heald, 2008. Mechanisms of mitotic spindle assembly and function. Int. Rev. Cytol. 265 111–158. [DOI] [PubMed] [Google Scholar]
- Wente, S. R., and G. Blobel, 1993. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J. Cell Biol. 123 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente, S. R., and G. Blobel, 1994. NUP145 encodes a novel yeast glycine-leucine-phenylalanine-glycine (GLFG) nucleoporin required for nuclear envelope structure. J. Cell Biol. 125 955–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey, M., L. Goetsch, P. Baum and B. Byers, 1991. MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J. Cell Biol. 114 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey, M., M. A. Hoyt, C. Chan, L. Goetsch, D. Botstein et al., 1993. NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J. Cell Biol. 122 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey, M., D. Yarar, T. H. Giddings, Jr. and D. N. Mastronarde, 1997. Nuclear pore complex number and distribution throughout the Saccharomyces cerevisiae cell cycle by three-dimensional reconstruction from electron micrographs of nuclear envelopes. Mol. Biol. Cell 8 2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel, U., V. Doye, H. Tekotte, R. Wepf, P. Grandi et al., 1996. Nic96p is required for nuclear pore formation and functionally interacts with a novel nucleoporin, Nup188p. J. Cell Biol. 133 1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R., M. S. Bodnar and D. L. Spector, 2009. Nuclear neighborhoods and gene expression. Curr. Opin. Genet. Dev. 19 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]