Abstract
Microtubule-associated protein tau gene transfer to the substantia nigra of rats using the adeno-associated virus (AAV) vector previously led to neuropathology and neurodegeneration in young rats. In this study, we compared equal tau gene transfer in either 3 or 20 month old rats, in order to test the hypothesis that late middle-aged rats are more susceptible to neurodegeneration. Two intervals and two vector doses of the tau vector probed for age-related differences in the initial sensitivity to low level tau expression. Gene transfer efficiency was similar for both ages, but the tau vector caused more dopaminergic cell loss and a greater behavioral deficit in aged rats at specific doses and time points. Tau gene transfer caused microgliosis relative to the control vector, and to a greater extent in aged rats. The maximal microglial response ocurred at 2 weeks preceeding the peak dopaminergic cell loss by 8 weeks. The cellular and behavioral outcomes were more severe in the aged rats, validating the model for studies of age-related diseases.
Keywords: aging, adeno-associated virus, gene transfer, neurodegenerative diseases, microglia, microtubule-associated protein tau, progressive supranuclear palsy, substantia nigra
1. Introduction
Neurodegenerative diseases typically have onset with advanced age. For example, incidence rates of Alzheimer’s disease (AD), Parkinson’s disease (PD), and progressive supranuclear palsy (PSP) are low below age 50, then rise dramatically (Bower et al., 1997; Brookmeyer and Gray, 1998; von Campenhausen et al., 2005; Van Den Eeden et al., 2003). This study evaluated a gene vector based animal model of neurodegenerative disease in terms of the age relationship that occurs in humans, whether an experimental neurodegenerative disease state could mimic the greater prevalence in the aged. From two studies (Cass et al., 2002; Marshall et al., 1983), it appears that the dose-response curve of 6-hydroxydopamine (6-OHDA) is shifted to the left in aged rats, with both low and high doses reducing indices of the nigrostriatal dopamine system in aged rats whereas only the high dose did so in young rats. In mice, aged subjects are more susceptible to MPTP dopaminergic lesioning (Ali et al., 1993; Date et al., 1990; Ohashi et al., 2006; Ricuarte et al., 1987; Sugama et al., 2003). The purpose of the study was to test the hypothesis that aged rats would also be more susceptible to gene transfer model of dopaminergic neurodegeneration using an adeno-associated virus (AAV) vector for the microtubule-associated protein tau, or alternatively whether the vector induced disease state is irrelevant to aging.
Vector models of neurodegenerative diseases offer a rapid screen of disease processes and in the cases of gene transfer to rat nigrostriatal system, a well defined neuron population of 10,000 dopamine neurons for a precise readout index of lesioning or protective effects. We expressed disease related genes in the rat substantia nigra (SN) and observed lesioning effects from about 50% cell loss with alpha-synuclein and up to 95% loss with microtubule-associated protein tau with high doses of adeno-associated virus (AAV) vectors (Klein et al., 2002; Klein et al., 2008). The rationale for expressing tau in the rat SN stemmed from the tau neurofibrillary pathology found there in humans with AD, PSP, corticobasal degeneration (CBD), and frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), and the prominent neuronal loss in the SN in the latter three diseases (DiMaria et al., 2000; Mirra et al., 1999; Poorkaj et al., 2002; Schneider et al., 2002; Wakabayashi et al., 1994). We hypothesized that the vector model is relevant to human neurodegenerative diseases in terms of agedness (Bower et al., 1997; Brookmeyer and Gray, 1998; von Campenhausen et al., 2005; Van Den Eeden et al., 2003), that the aged are more susceptible to tau induced damage, and that this could be mimicked by a vector method in rats. An advantage of the vector approach is the ability to control expression onset, which we did in young or aged rats. In order to detect potentially small differences in disease susceptibility, we used lower level tau expression than in previous studies (Klein et al., 2008). To address at which stages there might be an age difference, we used two intervals and doses to attempt to range from early partial disease to a more penetrant disease. We predicted that low gene vector dose would reveal an aging effect, like low dose 6-OHDA (Cass et al., 2002; Marshall et al., 1983). Monitoring microglial staining addressed whether tau expression causes microgliosis as occurs in PSP (Ishizawa et al., 2000), whether the microglial response was altered in aged tissues as occurs in PD models (Kanaan et al., 2008; Sugama et al., 2003), and whether the timing of microgliosis matched effects on dopamine neurons.
2. Methods
2.1. DNAs and AAVs
An expression cassette flanked with AAV2 terminal repeats was cross-packaged into recombinant AAV9 (Klein et al., 2008). The hybrid cytomegalovirus/chicken β-actin promoter was used to drive expression. We made AAV9 vectors for either human wild-type tau including exons 2, 3 and 10 (four repeat microtubule-binding domains, 4R2N) or control green fluorescent protein (GFP) transgenes. Human embryonic kidney 293-T cells were co-transfected with either the tau or GFP transgene plasmid along with two packaging plasmids needed to make AAV9 (Klein et al., 2008). The cell lysate was applied to a discontinuous gradient of iodixanol (OptiPrep, Greiner Bio-One, Longwood, FL) and centrifuged (350,000 g for 1 hr). The AAV was then removed, diluted 2-fold with lactated Ringer’s solution (Baxter, Deerfield, IL), and then washed and concentrated by Millipore (Billerica, MA) Biomax 100 Ultrafree-15 units. The final stocks were sterilized by Millipore Millex-GV syringe filters into low adhesion tubes (USA Scientific, Ocala, FL). Vectors were aliquoted and stored frozen. Encapsidated genome copies were titered by dot-blot. The titer of the AAV9 tau was 1.2 × 1013 vector genomes (vg)/ml and the AAV9 GFP was 1.0 × 1013 vg/ml. Equal dose comparisons were made by normalizing titers with the diluent, lactated Ringer’s solution.
2.2. Animals and stereotaxic injections
Male Sprague-Dawley rats, either 3 or 20 months old (from Harlan, Indianapolis, IN), were anesthetized with a cocktail of 3 ml xylazine (20 mg/ml, from Butler, Columbus, OH), 3 ml ketamine (100 mg/ml, from Fort Dodge Animal Health, Fort Dodge, IA), and 1 ml acepromazine (10 mg/ml, from Boerhinger Ingelheim, St. Joseph, MO) administered intramuscularly at a dose of 1 ml/kg. Viral stocks were injected through a 27 gauge cannula connected via 26 gauge internal diameter polyethylene tubing to a 10 µl Hamilton syringe mounted to a microinjection pump (CMA/Microdialysis, North Chelmsford, MA) at a rate of 0.2 µl/min with 3 µl of vector/diluent injected. The stereotaxic injection coordinates for the SN were 5.3 P, 2.1 L, 7.6 V for 3 month old rats (Paxinos & Watson, 1988). In pilot experiments, we determined optimal coordinates for the SN in aged (18 month old) rats: 6.2 P, 2.1 L, 7.8 V. The needle remained in place at the injection site for 1 additional min before the cannula was removed slowly (over 2 min). The skin was sutured, and the animal was placed on a heating pad until it began to recover from the surgery, before being returned to its individual cage. All animal procedures followed protocols approved by our institutional ACUC committee as well as the NIH Guide for Care and Use of Laboratory Animals.
2.3. Vector dosing, age groups, and intervals
The purpose of the study was to compare rats at 3 or 20 months of age for indices of the nigrostriatal dopamine system after equivalent tau gene transfer. While 20 month old rats are not considered fully aged (Coleman, 2004), the period of late middle age is appropriate because the frequency of neurodegenerative diseases begins to rise in the 50s and increases in late middle age (Bower et al., 1997; Brookmeyer and Gray, 1998; Van Den Eeden et al., 2003; von Campenhausen et al., 2005). It is also important to use at least 3 ages (Coleman et al., 2004). Our study only used 2 ages, although we ran a number of doses/time points. While our histological readout was taken at 2 intervals (2 and 8 wk after gene transfer), the behavioral output tracked the rats at 4 intervals (4 d, 2, 4, 8 wk). Two tau vector doses, 2 and 6 × 109 vg, were chosen based on our previous results with 3 × 109 vg of AAV9 GFP or tau vectors (Klein et al., 2008), to evaluate young and aged during either low or high level tau expression. Intervals and vector doses were chosen to probe for age-related differences either during initial disease onset with low level expression or a more late-phase, complete disease state. Postmortem studies of the number of dopamine neurons, density of nigrostriatal axons, and glial markers were conducted at 2 and 8 weeks. We could analyze aging effects in relation to vector dose or interval for behavior, TH neurons, and TH axon density.
2.4. Western blot
The SN was dissected and frozen on dry ice. The samples were put in buffer (1% Nonidet-P40/0.5% sodium deoxycholate/0.1% SDS/PBS) with protease inhibitors (Halt protease inhibitor cocktail kit from Pierce, Rockford, IL) and the soluble fraction was prepared by Dounce homogenization and centrifugation. Protein content was determined by Bradford assay reagents (Bio-Rad, Hercules, CA) and subjected to 12% SDS/polyacrylamide gels (Bio-Rad), with the gel loaded with equal protein in each lane (∼30 µg protein). The primary antibodies for the immunoblot was GFP (Chemicon, Temecula, CA; 1:1000) and glyceraldehyde-3-phosphate dehydrogenase (Ambion, Austin, TX; 1:1000). The secondary antibody was from Santa Cruz (Santa Cruz, CA, 1:10000) and the ECL reagent from Amersham/GE Healthcare (Buckinghamshire, UK).
2.5. Immunostaining
Primary antibodies for immunostaining included: TH (Pel-Freez, Rogers, AR, 1:2000) for marking dopamine neurons, E-1 for detecting human specific tau (1:2000, provided by Leonard Petrucelli, Mayo Clinic Jacksonville, FL), CP13 for tau hyperphosphorylated at serine 202 (1:1000, provided by P. Davies, Albert Einstein College of Medicine, Bronx, NY), Ab39 (1:1000, provided by S.H. Yen, Mayo Clinic Jacksonville) to detect mature neurofibrillary pathology, glial fibrillary acidic protein (Chemicon, 1:1000) for astroglia, and CDllb (Chemicon, 1:1000) for microglia. Anesthetized animals were perfused with phosphate-buffered saline (PBS), followed by cold 4% paraformaldehyde in PBS. The brain was removed and immersed in fixative overnight at 4°C. Brains were equilibrated in a cryoprotectant solution of 30% sucrose/PBS at 4°C. Coronal sections (50 µm thick) were cut on a sliding microtome with a freezing stage. Primary antibody incubations on free-floating sections were overnight at 4°C on a shaking platform. For immunoperoxidase staining (TH , CP13, Ab39), endogenous peroxidase activity was quenched with 0.1% H2O2/PBS for 10 min. The sections were washed in PBS and incubated for 5 min in 0.3% Triton X-100/PBS, and washed before applying primary antibody. Biotinylated secondary antibodies for peroxidase staining from DAKO Cytomation (Carpinteria, CA; 1:2000) were incubated for 1 hr at room temperature. The sections were washed with PBS and labeled with horseradish peroxidase-conjugated Extravidin (Sigma, St. Louis, MO; 1:2000) for 30 min at room temperature. The chromogen was diaminobenzidine (0.67 mg; Sigma) in 0.3% H202, 80 mM sodium acetate buffer containing 8 mM imidazole and 2% NiSO4. After mounting on slides, the sections were dehydrated in a series of alcohol and xylene and coverslipped with Eukitt (Electron Microscopy Sciences, Hatfield, PA). For immunofluorescence (GFAP & CD11b), sections were incubated in primary antibody overnight, washed and incubated with Cy3-conjugated secondary antibodies (Jackson ImmunoResearch, 1:300) for 2 hr, followed by DAPI counterstaining (1 µg/ml), washes and coverslipping with glycerol/gelatin (Sigma).
2.6. Stereological estimates
The number of SN pars compacta neurons expressing TH immunoreactivity was estimated by unbiased stereology using the MicroBrightfield Inc. system. Eight sections evenly spaced throughout the SN pars compacta structure were analyzed for each probe. Optical dissectors were 50 × 50 × 16 µm cubes spaced in a systematic random manner 150 × 150 µm apart and offset 2 µm from the section surface. The fractionator sampling was optimized to yield about 150 counted cells per animal, for Gundersen error coefficients <0.10 (West et al., 1993).
2.7. Striatal TH analysis
Five sections evenly spaced through the striatum were processed for TH immunohistochemistry. The specific TH staining in the striatum was quantified using the Scion (Frederick, MD) imaging program. The striata were traced and then measured for optical density of staining (pixels). The ratio of the injected side relative to the contralateral uninjected side was calculated for each section. The value for each animal was an average from five sections.
2.8. Rotational behavior
Animals were challenged with d-amphetamine (free base, 2 mg/kg in saline, i.m., Sigma). The amphetamine was injected 20 min before placing the animals in an automated rotometer system from San Diego Instruments (San Diego, CA) for 10 min. Pilot studies determined that locomotor activity peaks by 20 min after injection and that a 10 min measurement is sufficient to determine whether a side-to-side rotational bias is present.
2.9. Statistics
Data are expressed as mean ± SEM. Statistical tests included ANOVAs, repeated measures ANOVAs, Bonferroni’s multiple comparisons, or two-tailed t-tests as indicated.
3. Results
3.1. Western blot testing for age-related change in gene transfer efficiency
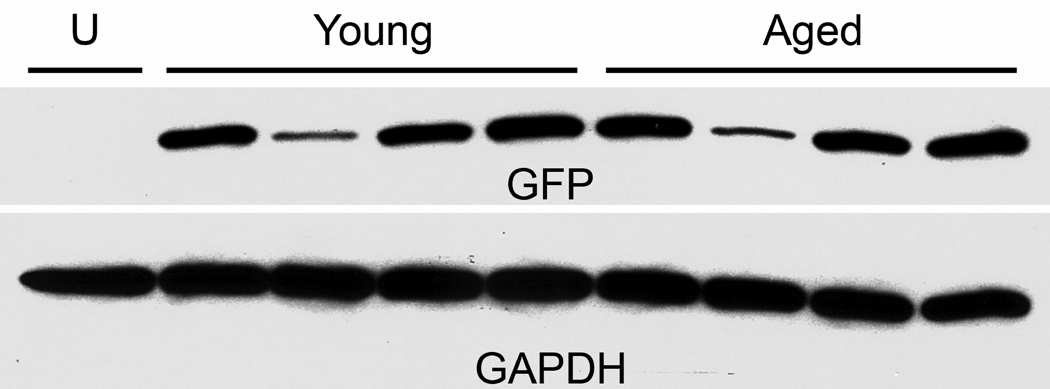
First we viewed gene transfer in young and aged subjects to ensure that aging differences in the tau disease model would not be attributable to gene transfer efficiencies. We used an AAV9 GFP vector to avoid tau-induced neurodegeneration. There were 4 rats per age group that received equal dose injections of 6 × 109 vg, with a 2 wk interval before evaluating levels of GFP in the SN. While there was variability within each age group, the average level per age group was similar (Fig. 1). The t-test of optical density measurements for the 2 age groups was not significant. There was no change in AAV9 GFP gene transfer efficiency as a result of aging, similar to results with AAV2 GFP vectors in an aging study by Wu et al., 2004. The variability, with one low expressing subject in each group, could be due to error in stereotaxic injection or dissection of the tissue, or variability of the gene transfer itself.
Fig. 1.
Equal AAV9 GFP gene transfer in the substantia nigra (SN) of young and aged rats. With 4 subjects per age group, there was no average difference in GFP levels (31 kDa band) in dissected SN. The housekeeping gene product glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 38 kDa band) is shown from the same blot. U, uninjected rat. Vector dose, 6 × 109 vg; 2 week interval.
3.2. Expression of GFP or tau on brain sections
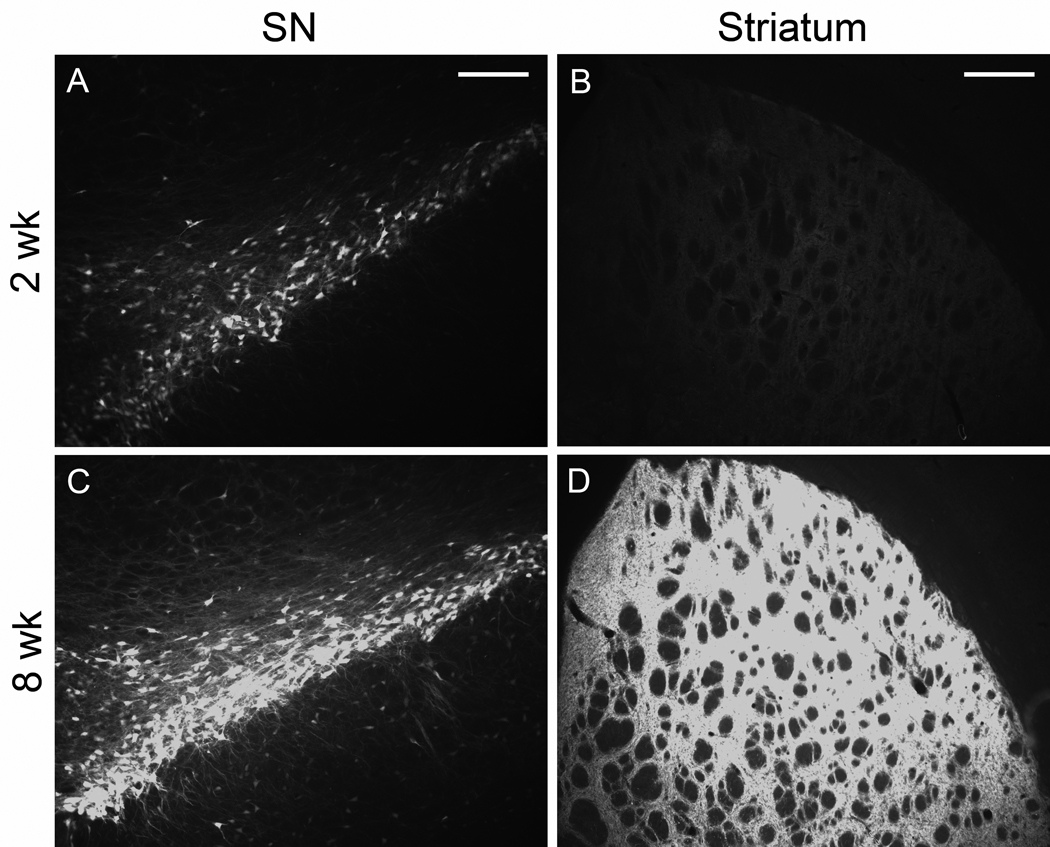
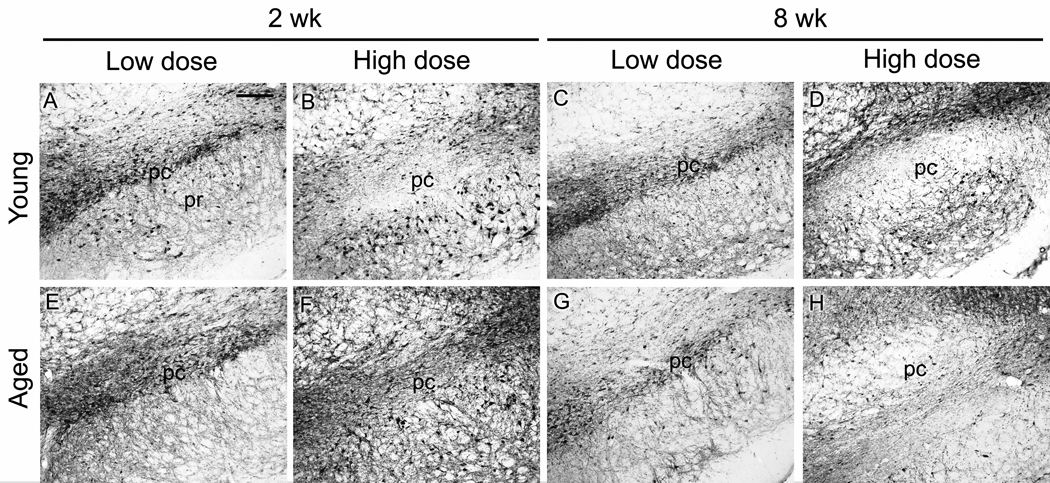
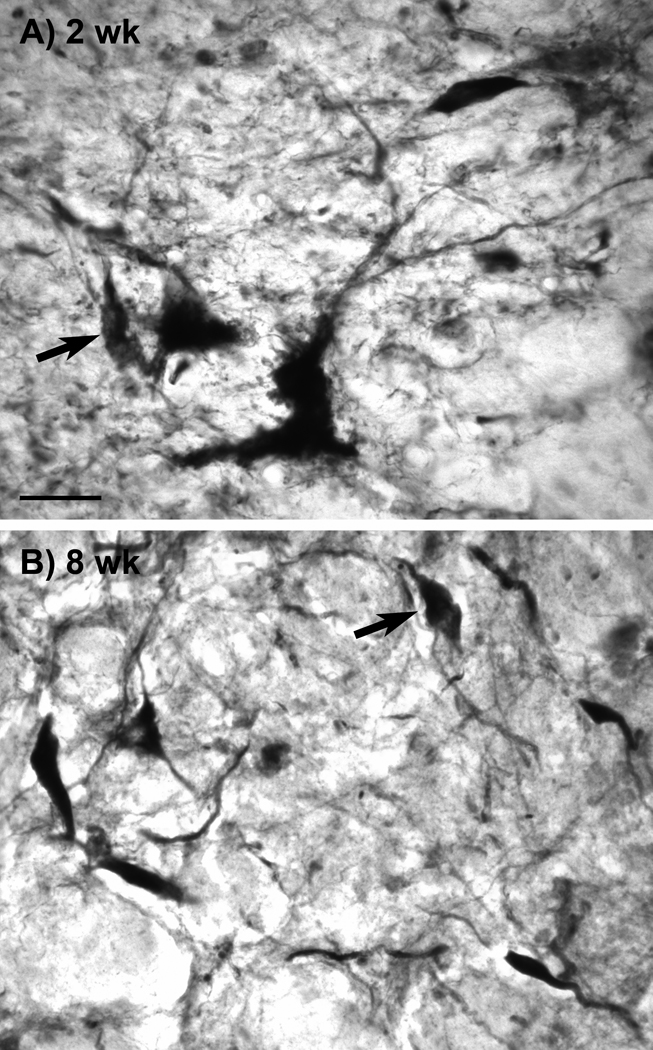
AAV9 GFP gene transfer in the nigrostriatal pathway of aged rats was efficient (Fig. 2), similar to what we observed in young rats (Klein et al., 2008). GFP levels increased dramatically in the SN and the striatum between 2 and 8 weeks when viewed with equal camera conditions (Fig. 2), which supported our goal of achieving submaximal, low to mid level tau expression at 2 weeks. To monitor tau vector expression, we used a human tau specific antibody, E-1 (Crowe et al., 1991), and an antibody for pathologically hyperphosphorylated tau (CP13; Jicha et al., 1999). Both antibodies labeled tissue associated with tau vector injections, but not in control vector injected tissue nor in surrounding non-transduced tissue. Staining with E-1 was consistently more robust than with CP13, and the CP13 reflected a consistent fraction of the total vector derived tau in both young and aged rats (not shown), but either antibody was sufficient to track the tau transgene expression pattern. CP13 labeled cells in the SN pars compacta, the SN pars reticulata, and along the needle track in the midbrain (Fig. 3). The best examples of CP13 staining in the pars compacta occurred at 2 wk and low dose at both ages. The pars compacta became blank for CP13 at the high dose and at 8 wk (Fig. 3D, H). We observed the pattern of loss of transgene expressing cells after tau vector injections in the pars compacta previously using a human specific tau antibody to mark transgene expression (Klein et al.; 2005; 2008), although tau expressing cells are preserved better in the pars reticulata more ventrally, previously and in the current study, e.g., Fig. 3B, D, F. The apparently selective loss of expression in the compacta relative to reticulata could have relevance for diseases that selectively affect the pars compacta such as PD. The tau transgene expression was also detected with the antibody Ab39 (Fig. 4) (Yen et al., 1985). Ab39, and a similar antibody, Ab69, are specific for mature tau neurofibrillary pathology and do not recognize somatodendritic pre-tangles. Ab39 or Ab69 are essentially alternatives to silver staining which produces similar detection of neurofibrillary tangles (Dennis Dickson, personal communication). For example, Ab69 has been used sucessfully to differentiate tangle-bearing neurons from tangle-free neurons in AD (Callahan and Coleman, 1995; Callahan et al., 2002). The Ab39 staining was a substantially smaller fraction relative to the E-1 or CP13 pattern. The cells harboring Ab39 immunoreactivity were a small subset of the transgene expressing cells. Ab39 was detected in all the tau vector groups, but not in non-transduced surround tissue. We considered that age differences in neurodegeneration could be directly due to enhanced tau pathology, or on the other hand, if tau aggregates were somehow a protective response, young rats could be more capable of making markers of tau pathology. However, both the CP13 and Ab39 staining appeared to be comparable at both ages and in all conditions. Ab39 labeled flame-shaped neurons as early as 2 wk (Fig. 4).
Fig. 2.
Increasing AAV9 GFP expression in the nigrostriatal pathway of aged rats over time. A) GFP fluorescence 2 weeks after injection of AAV9 GFP to the SN at a dose of 6 × 109 vg. B) GFP in striatum at 2 weeks. C) Substantia nigra, 8 weeks. D) Striatum, 8 weeks. Equal camera settings in A, C; B, D. Bars, A, C = 200 µm, B, D = 400µm
Fig. 3.
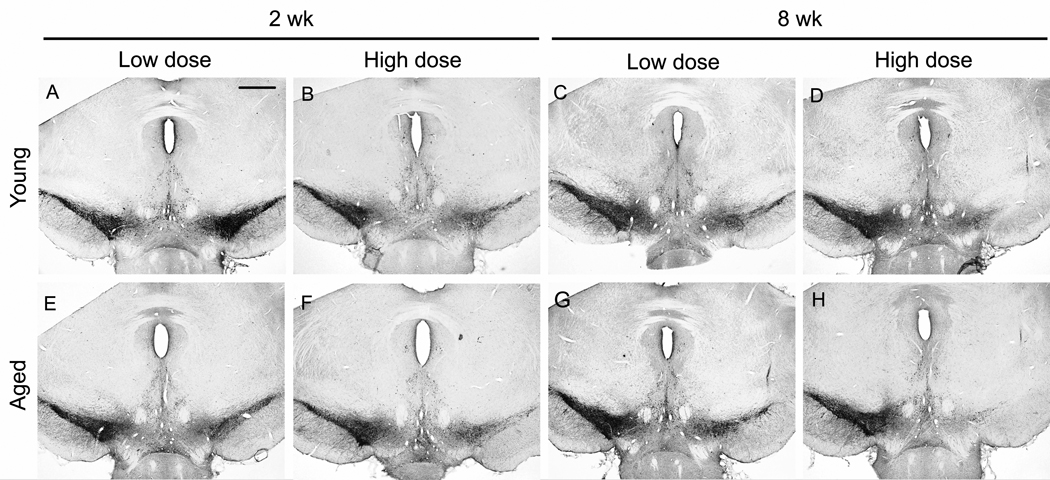
Pathologically hyperphosphorylated human tau transgene expression in young and aged rats with 2 vector doses and 2 intervals. A) Antibody CP13 immunoreactivity in the substantia nigra (SN) at the low AAV9 tau vector dose (2 × 109 vg) at 2 weeks post-injection. pc, pars compacta; pr, pars reticulata. A-D) young rats; E-H) aged rats. A, B, E, F, 2 weeks; C, D, G, H, 8 weeks. A, C, E, G, low dose; B, D, F, H, high dose (6 × 109). In both young and aged rats, at high dose and long interval (D, H), the CP13 staining became void in the SN pars compacta, which is consistent with severe loss of dopaminergic neurons, while CP13 staining persisted in midbrain areas outside the pars compacta. The loss of CP13 in the pars compacta appeared to be both time and dose dependent. A-H, bar = 200 µm.
Fig. 4.
Neuropathological tau expression in young and aged rats. The Ab39 antibody recognizes tau conformations associated with mature neurofibrillary pathology. A) The short interval of 2 wk was sufficient to produce Ab39 immunoreactivity in dystrophic and flame-shaped (arrows) neurons in the tau vector injected areas. A similar pattern of Ab39 staining resulted in both young and aged rats under all the conditions tested, with the high vector dose producing more Ab39 expression than the low dose. A young rat is shown in A and an aged rat at the 8 wk interval is shown in B. A, B, bar = 26 µm.
3.3. TH immunoreactivity in SN and striatum
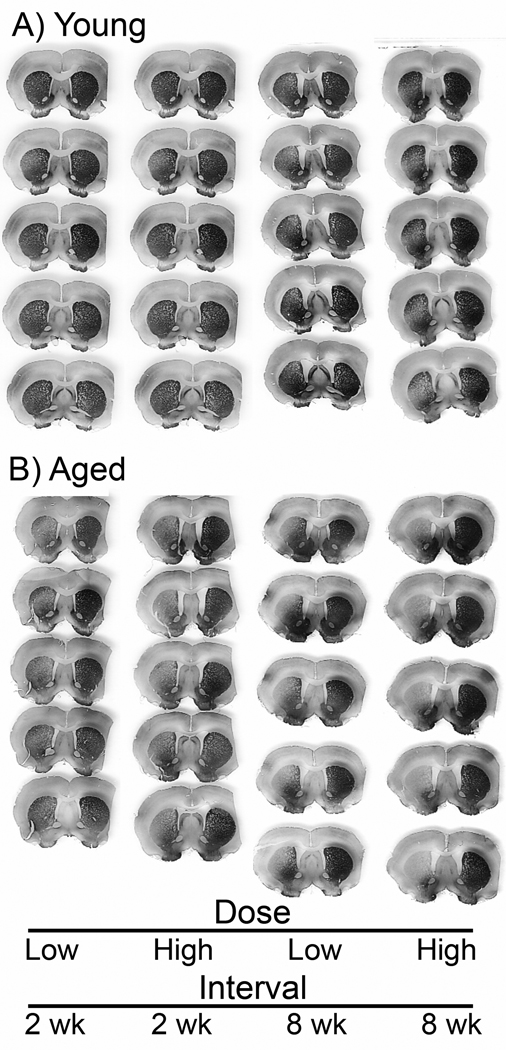
Tau vector gene transfer caused a loss of TH immunoreactivity in the SN and the striatum in young rats (Figs. 5, 6), as expected (Klein et al., 2008). The tau gene transfer had the same effect on TH immunoreactivity levels in aged rats (Fig. 5). For both ages, the loss of TH on the side of the tau vector relative to the contralateral uninjected side appeared greater with both increasing dose and interval in the SN (Fig. 5). However, both ages appeared similar in the TH cell loss response to tau expression, with the exception of the low dose and early interval where aged tissue did show an enhanced effect in the SN (Fig. 5A, E). This trend on sections was borne out by stereology (Fig. 7). Matching with the SN, tau vectors reduced TH immunoreactivity levels on the side of the tau vector injections in the striatum (Fig. 6), and greater effects on TH were associated with the high dose and long interval, at both ages. Again matching with the SN pattern, effects appeared similar at both ages. However, any trend of an aging effect in the striatum was most apparent at the high dose and long interval (Fig. 6).
Fig. 5.
Tyrosine hydroxylase (TH) immunoreactivity in the substantia nigra. Each panel shows a midbrain section with a tau vector injection on the right side and the uninjected side on the left. A-D) young rats; E-H) aged rats. A, B, E, F, 2 weeks; C, D, G, H, 8 weeks. A, C, E, G, low dose; B, D, F, H, high dose. After equal AAV9 tau vector doses, TH was overall similar in young and aged rats, with more TH loss at the high dose and the long interval. There was a visual trend for greater loss of TH in aged rats at the low dose and short interval (A, E). A-H, bar = 200 µm.
Fig. 6.
Tyrosine hydroxylase (TH) immunoreactivity in the striatum. Five serial sections from front to back in the striatum from an individual rat in each group. The right side of each section had a tau vector injection in the substantia nigra and the left side is uninjected. After equal AAV9 tau vector doses, there was some indication of more loss of TH staining in aged tissues relative to young under some conditions, for example rats at high dose and 8 weeks.
Fig. 7.
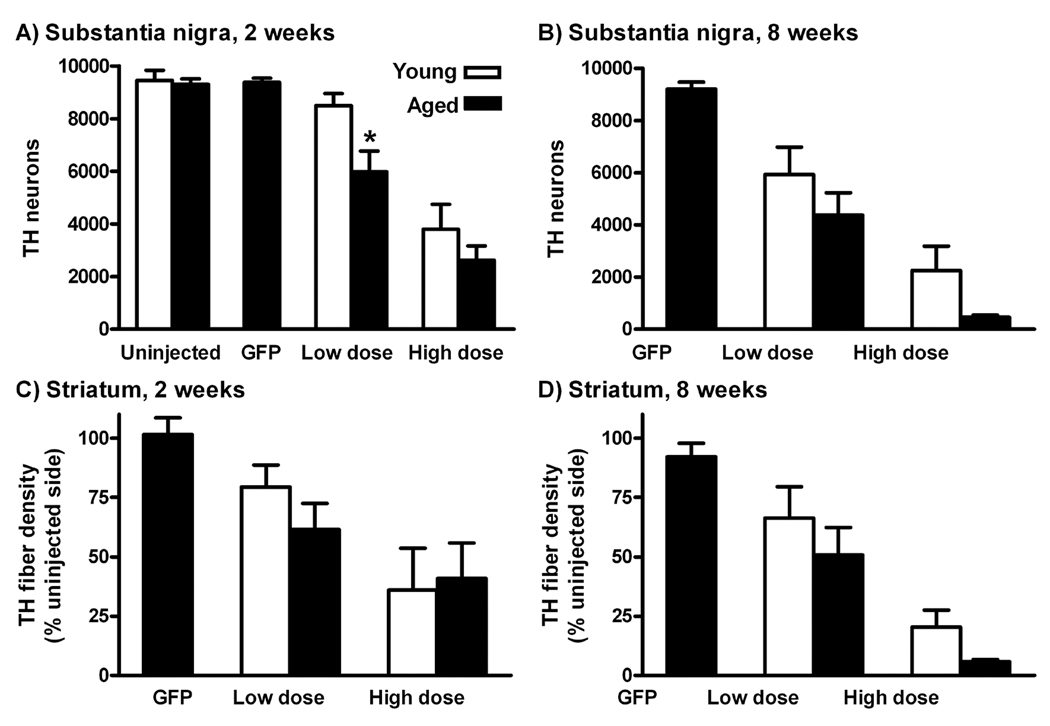
Quantification of tyrosine hydroxylase (TH) immunoreactivity after AAV9 tau gene transfer. A, B) Stereological estimates of TH neuron profiles in the substantia nigra (SN) pars compacta either 2 or 4 weeks after gene transfer. Control, uninjected young and aged rats are shown in A. There was a significant aging effect on the number of remaining TH neurons in age-by-vector dose ANOVAs both at 2 weeks (P < 0.02) and 8 weeks (P < 0.05), see Results. *, aged vs. young difference at low dose, 2 weeks (P < 0.05, Bonferroni post-test). C, D) Optical density measurements of TH immunoreactivity in the striatum (% of contralateral uninjected side) did not yield significant age differences, although there was a trend for greater loss in aged rats at the high dose, 8 weeks, similar to the SN results.
Looking at AAV9 GFP vector gene transfer to aged rats, both the number of TH neurons and the striatal fiber density were not altered relative to uninjected tissues (uninjected rats and contralateral uninjected side) at the high dose (Fig. 7). In contrast, the tau vector at two doses and two intervals lowered remaining dopamine neurons at both ages, as observed previously in young rats (Klein et al., 2008). We ran age-by-vector dose 2 way ANOVAs at either 2 wk or 8 wk. There was a significant effect of the animal’s age, as well as vector dose at 2 wk (effect of age P < 0.02, F1,17 = 6.76, effect of dose P <0.0001, F1,17 = 32.05) and 8 wk (effect of age P < 0.05 F1,16 = 4.58, effect of dose P < 0.0005, F1,16 = 23.42), with no interactions at either interval. However, in the Bonferroni post-tests, there was a difference at only one of the age comparison points, low dose at 2 wk (P < 0.05). The most robust age-related effect on TH neurons occurred with low level tau expression, at the early interval and low dose, consistent with accelerated early stage disease in the aged rats. We previously confirmed that tau-induced reduction of neurons positive for TH was truly cell loss and not loss of marker expression by staining for a neuronal marker and also pre-labeling neurons with GFP (Klein et al., 2008). It is therefore likely the fewer numbers of TH and CP13 transgene expressing neurons over time in this study is cell loss. Regarding the potential effect of mature tau pathology being causative for the TH cell loss, the small amount of Ab39 staining was not sufficient to account for the degree of cell loss. It is more likely that early stage pathology, the somatodendritic localization and pre-tangles, underlie tau induced neurodegeneration of dopamine neurons, based on the degree of staining for E-1 and CP13 and the degree of cell loss. The effect of early stage pathology is underscored by the age difference at the short interval and low dose.
Tau vector gene transfer erased TH fiber density in the striatum in young and aged rats (Fig. 6). The quantification of side-to-side ratios for each section demonstrated vector dose effects in the age-by-dose ANOVAs: 2 wk, P < 0.05, F1,17 = 5.36; 8 wk, P < 0.0005, F1,16 = 23.09. However, unlike the SN cell counts, age differences were not found, even though the averages for the groups stereology and fiber density were highly correlated (P < 0.0001, r = 0.98, N = 10 group averages in Fig. 7). It is possible the optical density measurements were more variable than the stereology and would require more rats. For example, the fiber density was 3.5-fold less in aged rats at the high vector dose and long interval, though not significant (P = 0.08, t-test). It is also possible that the subtle age-related decreases in cell survival do not cause changes in fiber density via compensation by remaining axons.
3.4. Microglia
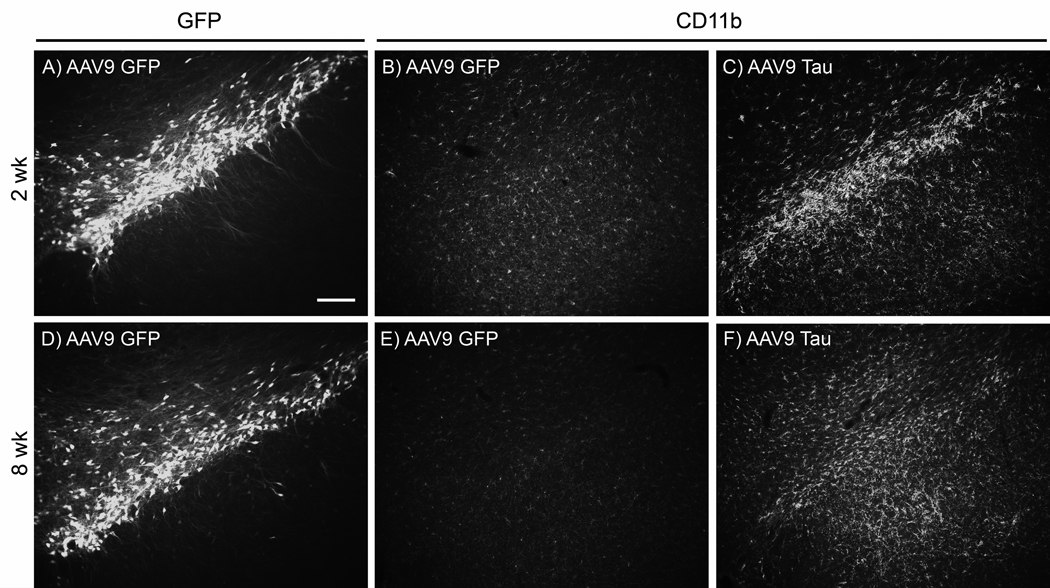
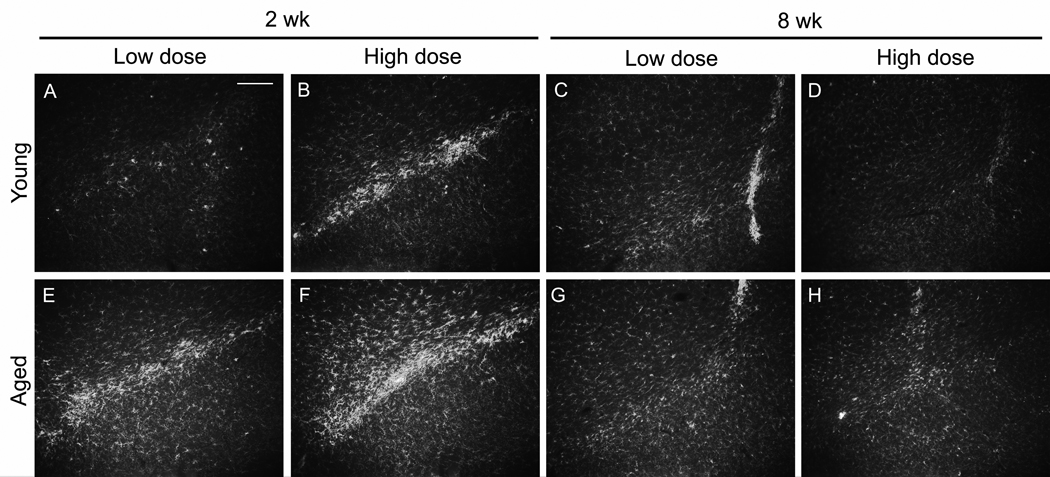
AAV9 GFP injections led to elevated microglial staining on the needle tracks, with little CD11b staining on sections not including the needle track at either 2 or 8 wks (Fig. 8). A time-course of CD11b staining of the needle track after AAV9 GFP injections in young rats showed little signal at 1 and 3 days after injection, an increase at 1 wk and another increase at 2 wk, and then less signal by 8 wk (not shown). The time-course was consistent with results from Reimsnider et al., 2007 using AAV GFP vectors. While GFP transgene expression levels continued to rise between 2 and 8 wk in the aged rats (Fig. 2), microglia responses attenuated after 2 wk. After tau gene transfer, there was a prodigious and specific microglia response relative to the control GFP vector, with elevated CD11b along both the needle tracks and throughout the SN pars compacta (pars compacta shown in Fig. 8). A time-course in young rats showed the most staining at 2 wk after tau vector injections as with the GFP (not shown). The tau vector injections also caused antibody CD11b staining at 8 wk in young and old rats, although the microgliosis was more widespread throughout the SN at 2 wk (Fig. 9). In Fig. 8, needle tracks are shown at 8 wk, while at 2 wk, areas 200–300 µm away from the needle tracks are shown, areas that were back to the baseline microglia pattern by 8 wk. While we did not attempt to quantify microglia, at both vector doses at 2 wk, there was a trend for more gliosis after tau gene transfer in the aged tissues, and to a lesser extent at 8 wk (Fig. 9). The elevated microglia staining at 2 wk caused by the tau vector is a candidate causative factor for the age-related dopaminergic neurodegeneration because there was increased microgliosis in the aged group at 2 wk, low dose along with more TH cell loss at 2 wk, low dose. However, microgliosis peaked earlier than the loss of TH staining in the SN or striatum. We also stained for astroglia with an antibody for glial fibrillary acidic protein. Neither the differences between GFP and tau vectors nor the differences between young and aged tissues that we observed for microglia occurred with staining for astroglia (not shown).
Fig. 8.
Microgliosis after tau, but not GFP gene transfer in aged rats. A-C) 2 wk; D-F) 8 wk. The AAV9 GFP vector produced GFP in the SN pars compacta at both 2 and 8 wk (A, D). A longer camera exposure time in A than in D was consistent with less GFP expression at 2 wk as shown in Fig. 2. B, E) The same sections as in A and D stained with an antibody for the microglia marker CDllb. With AAV9 GFP, areas with efficient gene transfer in the substantia nigra pars compacta displayed nearly baseline levels of microglia in the pars compacta relative to non-transduced areas (not shown). C, F) Unlike the GFP transductions, tau gene transfer elevated microglia staining, particularly at 2 wk. Equal camera settings in for CD11b in B, C, E, F. A-F, bar = 160 µm.
Fig. 9.
Microglia marker immunoreactivity in the substantia nigra after tau gene transfer in young and aged rats. A-D) young rats; E-H) aged rats. A, B, E, F; 2 weeks; C, D, G, H, 8 weeks. A, C, E, G, low dose; B, D, F, H, high dose. After equal AAV9 tau vector doses in young and aged rats, there was a microglia response at 2 weeks, that largely subsided by 8 weeks. At 8 weeks, the microglia staining was limited to the needle tracks only, while at 2 weeks, there was CD11b spread throughout the substantia nigra. At 2 weeks, there appeared to be a dose effect in both young and aged (A, B; E, F), while the microglia response viewed greater in aged rats (A, E; B, F). Equal camera settings in A-H. A-H, bar = 200 µm.
3.5. Rotational behavior
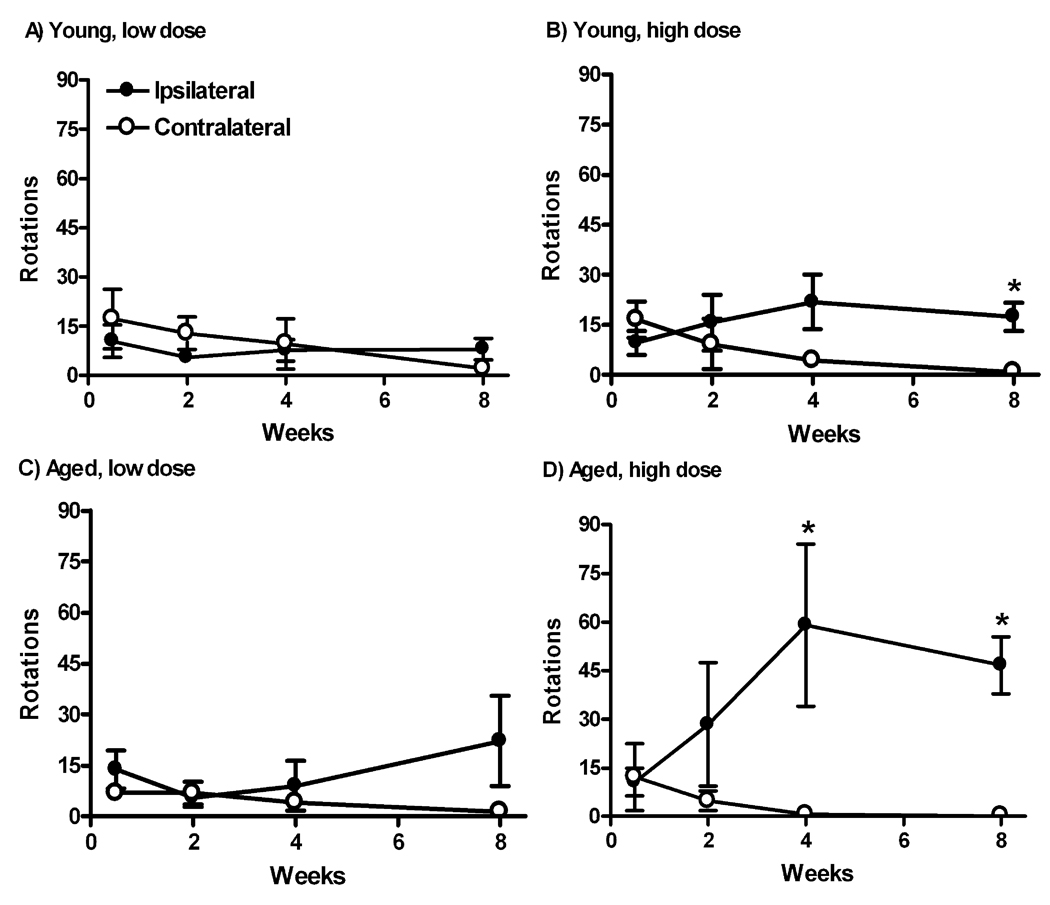
Across all the groups, the number of rotations in the 10 min sampling was small, consistent with partial or less than partial lesioning of the nigrostriatal pathway. For example, greater than 50% losses in dopaminergic markers are necessary for a rotational behavioral outcome (Hefti, 1980; Hudson et al., 1993). Each tau vector group was compared for ipsilateral vs. contralateral turning by 2-way time-by-direction repeated measures ANOVA (Fig. 10). An aged GFP vector group was also run in the same manner (not shown). Only one of the groups stood out with a clear trend for the vector treatment to drive ipsilateral turning consistent with a lesioning effect (Hefti, 1980; Hudson et al., 1993), the aged rats receiving high dose tau vector. The 2-way ANOVA for this group resulted in a directional effect (F1,24 = 6.84, P < 0.05) as well as significant time by direction interaction (F3,24 = 3.44, P < 0.05), indicative of a progressive lesion as expected. While young high dose tau group did not yield any significant effects by 2 way ANOVA, looking at 8 weeks only, there were more ipsialteral turns compared to contralateral turns (P < 0.01, t-test), consistent with a behavioral lesion effect by then. With the aged high dose group, there was a directional difference at both 4 wk (P < 0.05) and 8 wk (P < 0.001) when compared by t-tests. We made direct comparisons of net turns (ipsilateral minus contralateral) between the age groups at 8 weeks to address the central hypothesis, whether there was an aging difference for rotational behavior. Aged subjects had more net turns than young in the high dose groups at 8 wk (P < 0.02, t-test).
Fig. 10.
Amphetamine-stimulated rotational behavior after tau gene transfer in young and aged rats. A, B) Young rats. C, D) Aged rats. Low tau vector dose in A, C and high dose in B, D. Only the aged high dose group in D showed a directional turning bias over time by repeated measures 2-way ANOVA (P < 0.05, see Results). *, when looking at individual time points by t-tests, there was a directional difference at the high dose by 8 wk in the young group in B (P < 0.01), but at both 4 wk (P < 0.05) and 8 wk (P < 0.001) in the aged group in D. N = 5–6 per group.
4. Discussion
The ability to induce similar expression with an AAV9 vector in either young or aged naive animals has provided a unique way to examine the neurodegenerative prone environment of the aged brain. The aged rats were more sensitive to tau-induced degeneration of the nigrostriatal dopamine system. Aged subjects are therefore more susceptible to the rapid effects of dopaminergic neurotoxins (Ali et al., 1993; Cass et al., 2002; Date et al., 1990; Marshall et al., 1983; Ohashi et al., 2006; Sugama et al., 2003), as well as the more slowly progressing disease state caused by tau overexpression. The tau vector model in rats mimics age-related neurodegenerative diseases and can therefore be used to study age-related processes. Evidence for an age-related effect stemmed from age-by-vector dose ANOVAs at 2 wk and 8 wk, where there was a consistent age effect on numbers of TH neurons, although in the post-tests, only the low dose, short interval revealed a difference in the young vs. aged data points. The aging effect on loss of dopamine neurons occured at early onset, low tau expression levels. We infer from the data that early stage tau neuropathology, i.e., aberrant somatodendritic localization, could account for the neurodegeneration rather than mature neurofibrillary pathology which was detected in too few cells relative to those lost. Sensitivity to early stage pre-tangle tau pathology could potentially account for the age difference at the short interval and low dose.
While tau expression clearly reduced TH immunoreactivity in the SN and the striatum in a specific manner relative to a GFP control, the aging difference was subtle, underscored by the lack of an aging effect on TH fiber density in the striatum. There was a tendency for greater reductions of striatal TH staining in the aged rats, particularly at the high dose, long interval, though not statistically signficant. The mismatch in the two readouts of TH staining is puzzling considering the values were highly correlated. It could reflect a difference in sensitivity of the two methods or a functional difference such as axonal sprouting response to the tau-induced cell loss. Our functional readout, rotational behavior, confirmed differences in the severity of the tau pathology due to age. The behavior requires robust tau expression and major loss of TH neurons, so the lack of a behavioral effect in most of the groups was not surprising. The high dose tau vector resulted in a progressive rotational behavior phenotype in the aged rats but not the young rats. With the high dose tau vector, the aged rats turned more than the young rats when directly compared at 8 wk. The aging differences on cell loss occurred earlier and at a lower vector dose than the behavioral differences which required high dose, long interval. Amphetamine-induced rotational behavior is a threshold effect requiring over 50% cell loss (Hefti et al., 1980), not unlike PD where major degeneration of the nigrostriatal pathway preceeds motor symptoms. The subtle age-related differences were observed both for cell loss and behavior at stages where any lesion-like effect can begin to be detected, i.e. 10–20 % loss for TH stereology, and over 50% loss for behavior, illustrating the importance of the two dose, time-course strategy.
The tau vector model mimics PSP with respect to tau neuropathology and cell loss in the SN (Poorkaj et al., 2002), and with our findings here, also with respect to aging (Bower et al., 1997) and microgliosis (Ishizawa et al., 2000). The tau vector-induced microglia response was specific relative to a control vector, and the finding is consistent with microgliosis in tau transgenic mice (Yoshiyama et al., 2007). This was not surprising considering age-related differences in microgliosis in other types of dopaminergic lesioning models in mice (Sugama et al., 2003) and monkeys (Kanaan et al., 2008). While robust age-related differences were not striking on samples of TH staining, they were for microglia in a qualitative comparison. There was more TH cell loss in the aged rats at 2 wk (and low dose), at the time of peak microglia response, which could therefore be causative in the age-related neurodegeneration. Causation is also inferred from the earlier peak of microgliosis relative to loss of dopaminergic markers, consistent with results in tau transgenic mice where microgliosis preceeded synapse loss and the expression of neurofibrillary tangles (Yoshiyama et al., 2007). However, a potential caveat of a viral vector study is that immune response to the vector could be additive with the effect of the tau transgene. For example, intravenous injection of AAV into mice caused a transient upregulation of mRNAs for inflammatory cytokines in the liver (Zaiss et al., 2002), and control GFP AAV vectors do cause a small and transient degree of gliosis in the rat brain relative to vehicle sham injections (Reimsnider et al., 2007), plus the fact that GFP can achieve toxic levels at high vector doses (Klein et al., 2006). We used lower vector doses than previously and in this study, the high dose AAV9 GFP vector in aged rats did not induce microgliosis despite efficient gene transfer (Fig. 7), although we cannot rule out completely that the viral vector could have somehow primed the age-related gliosis in response to tau expression (Fig. 8).
The vector gene transfer model of neurofibrillary tangle diseases offers some unique advantages because equal transgene expression can be set on in normal young or aged rats. Other advantages of the model include the internal control of the non-transduced contralateral side after unilateral treatment and the facility for quantifying neuronal populations over time with stereology in rats. By switching on equal low level tau expression at different ages, we observed a difference in susceptibility to neurodegeneration. The vector approach for aging studies is a useful alternate to promoter-regulated expression in tau transgenic mice where tau expression can be induced and repressed at different ages, given that the currently available conditional tau model remains leaky in the repressed state (SantaCruz et al., 2005). We have noticed upregulation of mRNAs for microglia markers after tau gene transfer in unpublished microarray studies and wish to pursue the relationship of microglia to tau-induced dopaminergic neurodegeneration in young and aged subjects in our effort to shed light on mechanisms involved in tau diseases such as PSP, CBD, and FTDP-17.
Acknowledgements
Richard Zweig critiqued. National Institute of Neurological Disorders and Stroke R01 NS048450 and The Society for Progressive Supranuclear Palsy supported the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
There are no actual or potential conflicts of interest.
References
- Ali SF, David SH, Newport GD. Age-related susceptibility to MPTP-induced neurotoxicity in mice. Neurotox. 1993;14:29–34. [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology. 1997;49:1284–1288. doi: 10.1212/wnl.49.5.1284. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan LM, Coleman PD. Neurons bearing neurofibrillary tangles are responsible for selected synaptic deficits in Alzheimer's disease. Neurobiol Aging. 1995;16:311–314. doi: 10.1016/0197-4580(95)00035-d. [DOI] [PubMed] [Google Scholar]
- Callahan LM, Vaules WA, Coleman PD. Progressive reduction of synaptophysin message in single neurons in Alzheimer disease. J Neuropathol Exp Neurol. 2002;61:384–395. doi: 10.1093/jnen/61.5.384. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Bailey SL. Enhanced effects of 6-hydroxydopamine on evoked overflow of striatal dopamine in aged rats. Brain Res. 2002;938:29–37. doi: 10.1016/s0006-8993(02)02481-2. [DOI] [PubMed] [Google Scholar]
- Coleman PD. How old is old? Neurobiol. Aging. 2004;25:1. doi: 10.1016/j.neurobiolaging.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Coleman P, Finch C, Joseph J. The need for multiple time points in aging studies. Neurobiol. Aging. 2004;25:3–4. doi: 10.1016/j.neurobiolaging.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Crowe A, Ksiezak-Reding H, Liu WK, Dickson DW, Yen SH. The N terminal region of human tau is present in Alzheimer's disease protein A68 and is incorporated into paired helical filaments. Am. J. Pathol. 1991;139:1463–1470. [PMC free article] [PubMed] [Google Scholar]
- Date I, Felten DL, Felten SY. Long-term effect of MPTP in the mouse brain in relation to aging: neurochemical and immunocytochemical analysis. Brain Res. 1990;519:266–276. doi: 10.1016/0006-8993(90)90088-s. [DOI] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann. Neurol. 2000;47:374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195:123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Hudson JL, van Horne CG, Stromberg I, Brock S, Clayton J, Masserano J, Hoffer BJ, Gerhardt GA. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993;626:167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Lin WL, Tiseo P, Honer WG, Davies P, Dickson DW. A qualitative and quantitative study of grumose degeneration in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2000;59:513–524. doi: 10.1093/jnen/59.6.513. [DOI] [PubMed] [Google Scholar]
- Jicha GA, O'Donnell A, Weaver C, Angeletti R, Davies P. Hierarchical phosphorylation of recombinant tau by the paired-helical filament-associated protein kinase is dependent on cyclic AMP-dependent protein kinase. J. Neurochem. 1999;72:214–224. doi: 10.1046/j.1471-4159.1999.0720214.x. [DOI] [PubMed] [Google Scholar]
- Kanaan NM, Kordower JH, Collier TJ. Age and region-specific responses of microglia, but not astrocytes, suggest a role in selective vulnerability of dopamine neurons after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure in monkeys. Glia. 2008;56:1199–1214. doi: 10.1002/glia.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, King MA, Hamby M, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Human Gene Therapy. 2002;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol. Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Diaczynsky CG, Salvatore MF. Tau expression levels from various adeno-associated virus vector serotypes produce graded neurodegenerative disease states. Eur. J. Neurosci. 2008;27:1615–1625. doi: 10.1111/j.1460-9568.2008.06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, Drew MC, Neve KA. Recovery of function after mesotelencephalic dopaminergic injury in senescence. Brain Res. 1983;259:249–260. doi: 10.1016/0006-8993(83)91255-6. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA, Levey AI, Jones R, Green J, Shoffner JM, Wainer BH, Schmidt ML, Trojanowski JQ, Ghetti B. Tau pathology in a family with dementia and a P301L mutation in tau. J. Neuropathol. Exp. Neurol. 1999;58:335–345. doi: 10.1097/00005072-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Ohashi S, Mori A, Kurihara N, Mitsumoto Y, Nakai M. Age-related severity of dopaminergic neurodegeneration to MPTP neurotoxicity causes motor dysfunction in C57BL/6 mice. Neurosci. Lett. 2006;401:183–187. doi: 10.1016/j.neulet.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. fourth. San Diego: Academic Press; 1998. [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, Schellenberg GD. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann. Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N, Mandel RJ. Time course of transgene expression after intrastriatal psuedotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol. Ther. 2007;15:1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Irwin I, Forno LS, DeLanney LE, Langston E, Langston JW. Aging and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of dopaminergic neurons in the substantia nigra. Brain Res. 1987;403:43–51. doi: 10.1016/0006-8993(87)90120-x. [DOI] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Gilley DW, Kvarnberg DE, Mufson EJ, Bennett DA. Improved detection of substantia nigra pathology in Alzheimer’s disease. J. Histochem. Cytochem. 2002;50:99–106. doi: 10.1177/002215540205000111. [DOI] [PubMed] [Google Scholar]
- Sugama S, Yang L, Cho BP, DeGiorgio LA, Lorenzl S, Albers DS, Beal MF, Volpe BT, Joh TH. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,5-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s Disease: variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- von Campenhausen S, Bornschein B, Wick R, Bötzel K, Sampaio C, Poewe W, Oertel W, Siebert U, Berger K, Dodel R. Prevalence and incidence of Parkinson's disease in Europe. Eur. Neuropsychopharmacol. 2005;15:473–490. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta. Neuropathol. 1994;87:545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol. Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Wu K, Meyers CA, Guerra NK, King MA, Meyer EM. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol Ther. 2004;9:262–269. doi: 10.1016/j.ymthe.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Yen SH, Crowe A, Dickson DW. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 1. Identification of polypeptides. Am J Pathol. 1985;120:282–291. [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski TQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]