Abstract
Using a viral vector for mutant (P301L) tau, we studied the effects of gene transfer to the rat substantia nigra in terms of structural and functional properties of dopaminergic neurons. The mutant tau vector caused progressive loss of pars compacta dopaminergic neurons over time, reduced striatal dopamine content, and amphetamine-stimulated rotational behavior consistent with a specific lesion effect. In addition, structural studies demonstrated neurofibrillary tangles and neuritic pathology. Wild-type tau had similar effects on neuronal loss and rotational behavior. In contrast, mutant α-synuclein vectors did not induce rotational behavior, although α-synuclein filaments formed in nigrostriatal axons. Dopamine neuron function is affected by tau gene transfer and appears to be more susceptible to tau- rather than α-synuclein-related damage in this model. Both tau and α-synuclein are important for substantia nigra neurodegeneration models in rats, further indicating their potential as therapeutic targets for human diseases involving loss of dopamine neurons.
Keywords: Adeno-associated virus, α-Synuclein, Neurodegeneration, Neurofibrillary tangles, Substantia nigra, Tau
Deposits of the microtubule-associated protein tau in the form of neurofibrillary tangles (NFTs) are characteristic of many neurodegenerative diseases including Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), and in all of these diseases, NFTs are expressed in the substantia nigra (SN; Mirra et al., 1999; Poorkaj et al., 2002; Schneider et al., 2002; Wakabayashi et al., 1994). There is dramatic loss of pigmented SN neurons in PSP (Poorkaj et al., 2002) and FTDP-17 (Mirra et al., 1999) which may be causally related to toxic aggregation of tau. This study investigates the causal relationship of tau expression and SN dopamine neuron degeneration in an animal model.
Mutation in the tau gene causes FTDP-17 (Hutton et al., 1998), and particular variants are associated with increased risk for other parkinsonian disorders, including PSP (Baker et al., 1999) and CBD (Di Maria et al., 2000). Variants may also be a risk factor for Parkinson’s disease (PD; Healy et al., 2004; Martin et al., 2001). The P301L FTDP-17-related form of tau is particularly pathogenic as it exhibits accelerated filament formation in vitro (Nacharaju et al., 1999) and transgenic mice expressing P301L tau develop neurofibrillary tangles (NFTs; Lewis et al., 2000). While idiopathic PD is not associated with NFTs, tau has been demonstrated in a subpopulation of Lewy bodies (Ishizawa et al., 2003). Using a viral vector for P301L tau, we previously developed a rat model for NFT formation in the basal forebrain (Klein et al., 2004). Here we targeted wild type or P301L tau expression to the rat SN in order to study the causal relationship between neurofibrillary pathology and loss of dopamine neurons, as a number of tauopathies involve NFTs in and severe loss of pigmented neurons in the SN (Mirra et al., 1999; Poorkaj et al., 2002).
Viral vector gene transfer offers unique advantages for modeling, particularly for targeting brain regions and ages most associated with specific diseases. While α-synuclein (ASN) vector gene transfer models share features with PD such as loss of SN dopaminergic neurons in our previous study (Klein et al., 2002a) and two others (Kirik et al., 2002; Lo Bianco et al., 2002), motor dysfunction was not readily detected. To determine if the lack of behavioral effect was due to insufficient gene expression, we incorporated an enhancer element (Loeb et al., 1999) to boost ASN expression in this study. Because motor deficits are critical for a model of SN degeneration in order to test drugs and diets that could ameliorate symptoms, a goal of the present study was to generate a behaviorally significant gene transfer model. We used unilateral gene transfer to normal rats to study lateralized motor deficits and evaluate the functional significance of neuronal pathology.
Materials and methods
DNA and AAV constructs
The expression cassette was the same for all the DNA constructs expressed in the brain. It was flanked by the adeno-associated virus (AAV) serotype 2 terminal repeats, the only remaining sequence (and 4%) of the AAV-2 genome. The promoter/enhancer combination used to drive expression included the hybrid cytomegalovirus/chicken β-actin promoter (Niwa et al., 1991) and the 3′ enhancer woodchuck hepatitis virus post-transcriptional regulatory element (WPRE, Loeb et al., 1999) as described previously (Klein et al., 2002b). Plasmids for the reporter control green fluorescent protein (GFP) and the P301L form of human tau including exons 2, 3, and 10 (four repeat microtubule-binding domains, 4R2N) were described previously (Klein et al., 2002b, 2004).
Plasmids for human WT tau (4R2N), human ASN with A30P or A53T, and human glial cell line-derived neurotrophic factor (GDNF) were also constructed with the AAV-2 cytomegalovirus/chicken β-actin promoter-WPRE expression cassette. We previously showed that WPRE enhanced expression of GFP, nerve growth factor, or tau (Klein et al., 2002b,c, 2004); the same was true for ASN. Human embryonic kidney 293 cells were treated for 4 days with ASN AAV vectors either with or without WPRE. By comparing bands on Western blots with the Scion imaging program (Scion Corporation, Frederick, MD), levels of ASN were boosted 10-fold by the WPRE in titer-matched comparisons (data not shown).
The method for packaging plasmids in recombinant AAV-2 was previously described (Klein et al., 2002a). Human embryonic kidney 293 cells were transfected with an AAV terminal repeat-containing plasmid in an equimolar ratio with the AAV helper plasmid pDG (Grimm et al., 1998). The cell lysate was applied to a discontinuous gradient of iodixinol (OptiPrep, Greiner Bio-One, Longwood, FL) and centrifuged. The AAV was then removed and added to a heparin (Sigma, St. Louis, MO) affinity column, and the eluent was concentrated and washed using Millipore (Billerica, MA) Biomax 100 Ultrafree-15 units. AAV vector stocks were titered for physical particles, or copies of vector genomes, by dot-blotting against standard curves of known amounts of DNA using non-radioactive Psoralen-Biotin and BrightStar kits from Ambion (Austin, TX). Titers for the AAVs were normalized to 5 × 1012 particles per ml.
Animals and stereotaxic injections
Male Sprague–Dawley rats (3 months old, from Harlan, Indianapolis, IN) were anesthetized with a cocktail of 3 ml xylazine (20 mg/ml, from Butler, Columbus, OH), 3 ml ketamine (100 mg/ml, from Fort Dodge Animal Health, Fort Dodge, IA), and 1 ml acepromazine (10 mg/ml, from Boehringer Ingelheim, St. Joseph, MO) administered intramuscularly at a dose of 1 ml/kg. The stereotaxic injection coordinates for the SN were 5.4 mm bregma, 2.0 mm lateral, 7.6 mm ventral (Paxinos and Watson, 1998). Viral stocks were injected through a 27-gauge cannula connected via 26-gauge internal diameter polyethylene tubing to a 10-µl Hamilton syringe mounted to a microinjection pump (CMA/Microdialysis, North Chelmsford, MA). The pump delivered a total of 4 µl at a rate of 0.2 µl/min. The needle remained in place at the injection site for 1 additional minute before the cannula was removed slowly (over 2 min). The skin was sutured, and the animal was placed on a heating pad until it began to recover from the surgery, before being returned to their individual cages. Some animals were injected with 6-hydroxydopamine (6-OHDA, Sigma). The 6-OHDA solution was 4 mg/ml free base in 0.1% ascorbic acid/0.9% NaCl. 4 µl was injected into the striatum (1.2 A, 2.5 L, 5.0 V). The 6-OHDA lesioned rats were anesthetized with pentobarbital sodium (50 mg/kg, ip, Abbott, North Chicago, IL) and pretreated with methyl atropine nitrate (10 mg/kg, ip, Sigma) to aid post-operative feeding.
A total of 84 rats were used in the study with 3–16 rats/treatment group/interval. All animal care and procedures were in accordance with institutional IACUC and NIH guidelines.
Rotational behavior
Animals that had been unilaterally injected with AAV vectors or 6-OHDA were challenged with d-amphetamine (free base, 2 mg/kg in saline, im, Sigma) at several intervals after gene transfer. The amphetamine was injected 20 min before placing the animals in a Plexiglas cylinder (18-in. diameter by 12-in. tall) for 10 min. The number of completed circles either to the left or to the right was counted by an observer who did not know the experimental status of the animal. Turns were only counted if the animal moved continuously in one direction for the full 360°. One group of rats (WT tau) was tested using an automated rotometer system from San Diego Instruments (San Diego, CA) with the same conditions for drug challenge and trial time. The automated system resulted in greater numbers of rotations when the same animals were tested by both methods, although the WT tau group was tested only with the automated system. The number of rats used for behavioral assays was 3–16/group at intervals from 0 to 16 weeks after gene transfer.
Western blots
Standardized samples of brain tissue were dissected with the aid of a 1-mm brain block device and a 3-mm diameter biopsy puncher. Tissue samples were consistently ~15 mg and included either the entire SN or a portion of the dorsal striatum. The soluble fraction was prepared by Dounce homogenization and centrifugation. Samples were normalized for protein content by Bradford assay and subjected to 12% SDS/polyacrylamide gel electrophoresis. Antibodies for immunoblots included GFP (monoclonal from Chemicon, Temecula, CA), T14 (monoclonal antibody specific for human tau; Zymed, South San Francisco, CA), and LB509 (monoclonal antibody specific for human ASN; Zymed, South San Francisco, CA). Five rats/group were run for Western blots 16 weeks after gene transfer.
Immunohistochemistry and immunoelectron microscopy
Anesthetized animals were perfused with phosphate-buffered saline (PBS), followed by cold 4% paraformaldehyde in PBS. The brain was removed and immersed in fixative overnight at 4°C. For standard immunohistochemistry, the brain was equilibrated in a cryoprotectant solution of 30% sucrose/PBS at 4°C. Coronal sections (50 µm thick) were cut on a sliding microtome with a freezing stage. Antigen detection was conducted on free-floating sections by incubation in a blocking solution (2% goat serum/0.3% Triton X-100/PBS) for 1 h at room temperature, followed by primary antibody incubation overnight at 4°C on a shaking platform. Before blocking, endogenous peroxidase was quenched by incubation in 0.1% H2O2/PBS for 10 min. The sections were washed in PBS and incubated with biotinylated goat anti-rabbit or goat anti-mouse secondary antibody (DakoCytomation, Carpinteria, CA, 1:2000) for 1 h at room temperature. The sections were washed with PBS and labeled with horseradish peroxidase-conjugated Extravidin (Sigma, 1:2000) for 30 min at room temperature. The chromogen was diaminobenzidine (0.67 mg; Sigma) in 0.3% H2O2, 80 mM sodium acetate buffer containing 8 mM imidazole and 2% NiSO4.
Primary antibodies for immunostaining included: GFP (polyclonal from Molecular Probes, Eugene, OR, 1:5000); T-14 (1:2000); CP13 [monoclonal anti-phospho-tau; P. Davies, Albert Einstein College of Medicine, 1:200 (Jicha et al., 1999)]; Ab39 [monoclonal anti-NFT, S.H. Yen, Mayo Clinic Jacksonville, 1:500 (Yen et al., 1985)]; LB509 (1:5000); NACP-98 [polyclonal anti-ASN; 1:2000 (Gwin-Hardy et al., 2000)]; tyrosine hydroxylase [(TH) (polyclonal antibody, 1:2000; Chemicon)]. Some of the markers were visualized with immunofluorescence using tetramethyl-rhodamine isothiocyanate-conjugated or fluorescein isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA, 1:500).
For immunoelectron microscopy (immunoEM), animals were perfusion fixed and their brains were removed and immersion fixed overnight as above. The brains were then placed in PBS. The SN was dissected out and embedded in LR White as described (Lewis et al., 2000; Lin et al., 2003a,b). Antibodies for immunoEM included CP13, LB509, E1 [polyclonal antibody specific to human tau, S.H. Yen, Mayo Clinic Jacksonville (Crowe et al., 1991)], SMI31 [monoclonal antibody to high and mid-molecular weight phosphorylated neurofilament (NF-H and NF-M)], and SMI32 (monoclonal antibody to non-phosphorylated NF-H). SMI31 and SMI32 were purchased from Sternberger Monoclonals (Lutherville, MD). The number of rats processed for immunoEM was 2–3/group 16 weeks after gene transfer.
Stereological estimates
The number of SN pars compacta neurons expressing TH immunoreactivity was estimated by unbiased stereology using the MicroBrightfield Inc. (Williston, VT) system as previously described (Klein et al., 2002a). Seven sections evenly spaced throughout the SN pars compacta structure were analyzed for each probe. Optical dissectors were 50 × 50 × 16 µm cubes spaced in a systematic random manner 150 × 150 µm apart and offset 2 µm from the section surface. The fractionator sampling was optimized to yield about 150 counted cells per animal, for Gundersen error coefficients < 0.10. The number of rats used for stereology was 6–7/group measured either 3 or 16 weeks after gene transfer.
Concentrations of dopamine and DOPAC in striatal tissue
Consistent striatal samples were dissected as described above for Western blots. The striatal tissue punches were weighed and then frozen on dry ice. The frozen samples were homogenized using an ultrasonic probe in 0.1 M HClO4 containing 0.1 mM EDTA and N-methyl-dopamine (20 ng/ml) as an internal standard as described (Dunn, 1993). The samples were centrifuged to sediment the protein, and the supernatants were analyzed directly by HPLC. The columns were 250 × 4.4 mm Spherosorb 5 µm ODS-1 reverse-phase columns (Waters Corp., Milford, MA) maintained at 35°C by BAS LC22/23A column heaters (Bioanalytical Systems, West Lafayette, IN). The electrochemical cells were BAS TL12 with glassy carbon electrodes connected to BAS LC4B electrochemical detectors. The electrodes were maintained at potentials of approximately 0.78 and 0.9 V with respect to an Ag/AgCl reference electrode. The mobile phase was 0.1 M sodium phosphate (pH 3.0), 0.1 mM EDTA, 0.2–0.4 mM 1-octane sulfonic acid (Eastman Kodak, Rochester, NY), and 3–4% acetonitrile (v/v), filtered through a 0.45-µm filter, and pumped at 1.2 ml/min. Data were collected and integrated by a Waters Millennium system. Five P301L tau vector rats were analyzed 16 weeks after gene transfer.
Results
Effects of tau gene transfer on rotational behavior
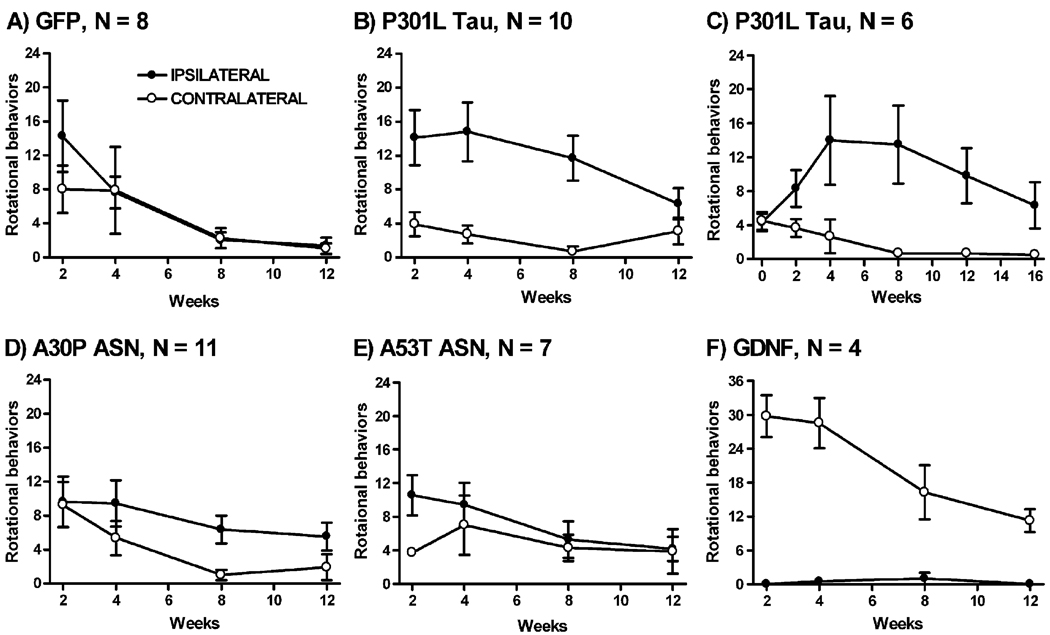
To determine if gene transfer could cause unilateral motor deficits, rats were monitored for amphetamine-stimulated rotational behavior up to 16 weeks after vector injections. The GFP control group showed no propensity to turn towards the injected (ipsilateral) side or the uninjected (contralateral) side between 2 and 12 weeks (Fig. 1A). The data were analyzed by two way repeated-measures ANOVA, which showed a main effect for trial interval (F(3,14) = 8.09, P = 0.0002). The animals completed less full circles over time. In the P301L tau group (Fig. 1B), there was a directional effect (F(1,18) = 12.20, P = 0.0026). There was also a main effect of trial interval in this group (F(3,18) = 4.47, P = 0.0071) as well as a significant interaction (F(3,18) = 4.22, P = 0.0094). A directional bias was not found at 12 weeks in this group, perhaps not detected as a result of the reduced overall turning over time for the rats in Figs. 1A and B or because of plasticity-related recovery. We did not expect to observe a directional bias as early as 2 weeks after gene transfer. To address if the data at 2 weeks truly reflected an effect of the P301L tau gene transfer and also to address the transient pattern of the turning bias at 12 weeks in Fig. 1B, we ran an additional separate group of six rats before tau gene transfer and up to 16 weeks after (Fig. 1C). This schedule showed no directional difference before gene transfer and then a persistent directional bias from 2 to 16 weeks. The data from the 0–16 week schedule with tau gene transfer in the separate group of 6 rats showed a directional effect (F(1,10) = 6.67, P = 0.03) and significant interaction (F(5,10) = 3.73, P = 0.006). In the A30P ASN group (Fig. 1D), there was a tendency for more ipsilateral turning, although this did not reach statistical significance. As in the GFP group, there was a main effect of interval (F(3,20) = 13.11, P < 0.0001) and no interaction. We also tested the A53T form of ASN because transgenic mice expressing this mutation have achieved the greatest degree of success in modeling PD (Giasson et al., 2002; Lee et al., 2002; Neumann et al., 2002). However, there were no directional or interval effects or interaction in the A53T ASN group (Fig. 1E). Despite the use of the WPRE enhancer and the A53T form, the two ASN-WPRE vectors did not result in motor deficits, similar to the earlier report of the A30P ASN vector without the WPRE (Klein et al., 2002a). Unilateral GDNF gene transfer to the rat SN has been shown to induce amphetamine-stimulated turning to the contralateral side (Kirik et al., 2000). We measured this phenomenon as a control for directional specificity after gene transfer (Fig. 1F). In the GDNF group, there was a directional effect (F(1,6) = 52.91, P = 0.0003). There was also a main effect of trial interval in this group (F(3,6) = 8.62, P = 0.0009) as well as a significant interaction (F(3,6) = 8.85, P = 0.0008). The total number of turns for the four sessions from 2 to 12 weeks post-injection is shown in Table 1. The data for P301L tau animals run under the same conditions in Figs. 1B and C are combined in Table 1. An additional 6-OHDA control group was included. The 6-OHDA induced ipsilateral turning as expected and with greater rates of turning (>6/min) than after gene transfer (Table 1). Data from sessions run at 16 weeks are shown in Table 1 and demonstrate persistent behavioral effects of P301L tau gene transfer (6 rats from Fig. 1C combined with 6 additional P301L rats), despite the attenuated effect at 12 weeks observed in Fig. 1B. We also ran six rats expressing the WT form of tau 2 weeks after gene transfer and observed similar turning as with the P301L tau vector at this interval (Table 1). In summary, ipsilateral turning was found in the P301L and WT tau groups and the 6-OHDA group, while contralateral turning was found in the GDNF group. Animals from the GFP, as well as the A30P and A53T ASN, groups did not show a directional bias.
Fig. 1.
Amphetamine-stimulated rotational behavior time-course. (A) Control GFP vector group 2–12 weeks after gene transfer. (B) P301L tau vector group 2– 12 weeks. (C) P301L tau vector group 0–16 weeks, separate group of rats from B. (D) A30P ASN vector group 2–12 weeks. (E) A53T ASN vector group 2–12 weeks. (F) GDNF vector group 2–12 weeks. The N values are shown on graphs. Repeated-measures ANOVA analyses described in Results. Directional differences found in the tau and GDNF vector groups (B, C, F; P < 0.01).
Table 1.
Amphetamine-stimulated circling
| Group | N | Interval (week) |
Sessions | Ipsilateral | Contralateral |
|---|---|---|---|---|---|
| GFP | 8 | 2–12 | 4 | 25.3 ± 5.2 | 19.1 ± 8.8 |
| Tau P301L | 16 | 2–12 | 4 | 46.5 ± 7.8* | 9.4 ± 2.4 |
| ASN A30P | 11 | 2–12 | 4 | 31.0 ± 8.6 | 17.6 ± 6.3 |
| ASN A53T | 7 | 2–12 | 4 | 29.4 ± 5.3 | 18.9 ± 7.2 |
| GDNF | 4 | 2–12 | 4 | 1.5 ± 1.2 | 85.8 ± 11.5* |
| 6-OHDA | 3 | 2, 4 | 2 | 128.3 ± 30.1** | 0.7 ± 0.7 |
| GFP | 3 | 16 | 1 | 8.7 ± 1.5 | 5.7 ± 2.2 |
| Tau P301L | 12 | 16 | 1 | 13.5 ± 4.4** | 2.8 ± 2.0 |
| ASN A30P | 6 | 16 | 1 | 6.3 ± 2.6 | 4.2 ± 1.5 |
| Tau WT | 6 | 2 | 1 | 24.8 ± 4.4** | 5.8 ± 2.2 |
Total number of 360° turns in 1, 2, or 4 × 10 min sessions. For the 2- to 12-week interval, there were 4 sessions run at 2, 4, 8, and 12 weeks after gene transfer. The data for tau P301L are pooled from rats run under the same conditions in Figs. 1B and C. For the 6-OHDA group, data are from sessions at 2 and 4 weeks after lesioning. The data for the WT tau group came from a different monitoring system, described in Materials and methods.
P < 0.0005.
P < 0.05, directional difference, t test.
Transgene expression on Western blots
We ran immunoblots for GFP, tau, or ASN to confirm transgene expression from the vectors at 4 months post-injection. GFP was only detected in midbrain and corpus striatum on the side of the GFP vector injections to the SN (Fig. 2A). Human-specific tau was consistently detected in the SN, but not the corpus striatum, of rats injected with the P301L tau vector (Fig. 2B). In contrast, human ASN expression was detected in both midbrain and striatum (Fig. 2C). Vector-derived tau expression did not build up in the anterograde projection axons in the corpus striatum to the extent that GFP or ASN did after injections of the respective vector into the SN.
Fig. 2.
Western blots of dissected substantia nigra (SN) and striatum (STR) showing GFP, tau, or ASN expression from the respective vector. (A) GFP immunoblot, after injecting 2 × 1010 particles of the GFP AAV unilaterally to the rat SN. Band ~31 kDa, as expected. Lane 1, uninjected SN; lane 2, injected SN; lane 3, STR uninjected side; lane 4, STR injected side. (B) Human tau immunoblot, after injecting 2 × 1010 particles of the human P301L tau AAV unilaterally to the SN. Band ~70 kDa, as expected for the longest form of tau. Lane 1, uninjected SN; lane 2, injected SN; lane 3, uninjected SN from a second rat; lane 4, injected SN from second rat. We did not detect human tau in samples from STR. (C) ASN immunoblot, after injecting 2 × 1010 particles of the A30P ASN-WPRE AAV unilaterally to the SN. Lane 1, uninjected SN; lane 2, injected SN; lane 3, STR uninjected side; lane 4, STR injected side. (A–C) 4 months after gene transfer. 60 µg protein loaded in each lane.
Tau gene transfer to the SN causes loss of pars compacta neurons
The injected areas were analyzed for the expression of immunoreactivity for GFP, tau, or ASN at 4 months post-injection. GFP fluorescence was detected in cell bodies in the SN and in neurites in the corpus striatum of animals injected with the GFP vector. Co-localization studies showed that the GFP fluorescence overlapped with TH immunofluorescent labeling in the majority of dopaminergic neurons in the SN pars compacta as we observed previously (Klein et al., 2002a). Co-localization studies with an antibody against parvalbumin, a marker for GABAergic neurons, demonstrated efficient expression of GFP in parvalbumin neurons of the SN pars reticulata as well (not shown). Immunostaining for GFP was more sensitive than monitoring its native fluorescence. GFP immunolabeling showed robust expression in the SN pars compacta in addition to the SN pars reticulata and along the needle track (Fig. 3A). The efficient expression of GFP did not alter TH immunoreactivity relative to the contralateral uninjected side (Fig. 3B).
Fig. 3.
Tau gene transfer results in the degeneration of the SN pars compacta. (A) GFP vector injections to the midbrain led to robust expression of GFP immunoreactivity in the SN pars compacta (SNc), the SN pars reticulata below (SNr), and above the SN along the needle track. (B) TH staining on the side of the GFP expression (left side of panel) was similar relative to the contralateral uninjected side. (C) Human-specific tau labeling after injection of the P301L human tau vector, showing characteristic blank area in the SN pars compacta. (D) Consistent with the human tau expression pattern, TH staining in the SN pars compacta was dramatically reduced after tau vector injections (left side of panel). (E) Human-specific ASN labeling after injection of the A30P human ASN vector. Unlike with tau gene transfer, there were many ASN-expressing neurons in the SN pars compacta in the ASN groups. (F) Reduced TH staining was associated with ASN vector injections (left side of panel) as we observed with a previous, less efficient version of the ASN vector (Klein et al., 2002a). The loss of staining with the P301L tau AAV was generally more intense and more consistent than observed with ASN vectors in this and the previous study. Staining controls in untransfected tissues were blank for GFP or human tau or ASN. (A–F) 4 months after gene transfer. Scale bars, A = 320 µm; B = 700 µm; C = 125 µm. C and E, and B, D, and F, are of the same magnification.
Immunoreactivity for human tau (T14 antibody) was detected in the SN after injections of tau containing AAV into the SN and was characterized by immunoreactivity in neuronal perikarya and processes, with a focal region devoid of neuronal labeling in the central region of the pars compacta (Fig. 3C), due to loss of neurons in this region (see below). Interestingly, the pars reticulata neurons appeared to continue to express tau immunoreactivity, even when tau-expressing neurons in the pars compacta were lost. This pattern was found even at 3 weeks post-injection, the earliest interval checked, and also with both WT tau and P301L tau vectors. Consistent with the tau expression pattern, TH immunostaining was reduced on the side of the tau vector injections (Fig. 3D).
Immunoreactivity for human ASN (LB509 antibody) in the SN pars compacta after injections of the A30P ASN vector (Fig. 3E) was similar to what we saw previously with the ASN vector lacking WPRE (Klein et al., 2002a). Human ASN was expressed strongly in the SN pars compacta relative to human tau expression in the tau vector groups, where the pars compacta had no immunoreactivity. The ASN gene transfer also produced loss of TH immunoreactivity in the pars compacta (Fig. 3F) as we observed previously (Klein et al., 2002a).
We evaluated the number of SN pars compacta dopaminergic neurons after tau gene transfer by unbiased stereology on sections immunostained for TH (Table 2). GFP gene transfer did not alter the number of dopaminergic neurons compared to uninjected tissues; however, the P301L vector did cause a significant loss of dopaminergic neurons relative to control GFP gene transfer (P < 0.001, one-way ANOVA/Bonferroni’s multiple comparison test) at 4 months post-injection. We tested both the P301L and WT tau vectors at a shorter 3-week interval and found a similar 26–28% reduction in dopaminergic neurons in both cases compared to uninjected tissues (P < 0.05 one-way ANOVA/Bonferroni’s multiple comparison test). For the P301L vector, cell loss increased between 3 weeks and 4 months, from 28% to 54% (P < 0.01 one-way ANOVA/Bonferroni’s multiple comparison test).
Table 2.
Substantia nigra pars compacta dopaminergic neurons
| Group | N | Interval | Dose (particles) | TH neurons |
|---|---|---|---|---|
| Uninjected | 6 | − | − | 10,379 ± 535 |
| GFP | 7 | 4 months | 2 × 1010 | 10,000 ± 355 |
| Tau P301L | 7 | 4 months | 2 × 1010 | 4576 ± 470* |
| Tau P301L | 7 | 3 weeks | 2 × 1010 | 7519 ± 597** |
| Tau WT | 6 | 3 weeks | 2 × 1010 | 7662 ± 195** |
Estimated by unbiased stereology and TH immunohistochemistry of sections.
P < 0.001 compared to GFP AAV.
P < 0.05 compared to uninjected; one-way ANOVA/Bonferroni’s multiple comparison test.
Dystrophic axons in SN after tau gene transfer
As an immunohistochemical marker for NFTs, we used Ab39 since this antibody detects fibrillary tau lesions, including intracellular and extracellular NFTs, but it does not detect earlier neuronal alterations referred to as pre-tangles (Dickson et al., 1992). As in previous studies (Klein et al., 2004), Ab39 immunoreactivity was found in NFTs in the P301L tau vector group, in a subset of neurons that showed immunoreactivity for the human tau antibody T14 (Fig. 4A). In addition to the NFTs, Ab39 stained dystrophic neuronal processes consistent with axonal spheroids in the SN of the P301L tau vector group (Fig. 4B). The axonal spheroids were 3–5 Am diameter and found most abundantly medial to the SN in both the P301L (Fig. 4B) and WT tau (Fig. 4C) groups. While there was more overall immunoreactivity with T14 than Ab39 on adjacent sections, Ab39 selectively labeled dystrophic axon spheroids. There was a tendency for fewer dystrophic axons at 16 weeks than at 3 weeks, although this was not formally assessed. The findings are comparable to studies of axonal pathology in the spinal cord in P301L transgenic mice (Zehr et al., 2004), suggesting that early tau pathology may be in neuronal processes with later shift of the fibrillary pathology to neuronal cell bodies.
Fig. 4.
Neurofibrillary pathology in SN neurons detected with antibody Ab39. (A) Neuronal cell bodies and processes in the lateral SN expressing Ab39 immunoreactivity, 4 months post-injection of the P301L tau vector. (B) Ab39 labeling was prevalent in axonal spheroids in the medially-projecting axons in the P301L tau vector group, 3 weeks post-injection. (C) Ab39 labeling and axonal spheroids also found in the wild-type tau vector group at 3 weeks. Ab39 labeling found only in tau vector groups. Scale bar = 32 µm. A–C, same magnification.
Corpus striatum in animals with SN injections
The corpus striatum was studied for expression of GFP, tau, or ASN to assess the degree of anterograde labeling in nigrostriatal fibers. In accord with their expression in the SN, GFP or human ASNs were detected in neuritic processes in the striatum, in the respective GFP or A30P ASN group, and only on the injected side (not shown). Some human tau-immunoreactive processes could be detected in the striata on the injected side in the P301L tau vector group, although these processes were detected less frequently than those labeled for GFP or ASN in the respective groups, consistent with our inability to detect human tau on Western blots of striatal samples. Expression of GFP reporter had no apparent effect on striatal TH immunoreactivity, although there was a tendency for reduced TH immunoreactivity associated with P301L tau expression, though we did not attempt to quantify striatal immunostaining. Some of the P301L tau-expressing rats were analyzed for striatal dopamine content 4 months after gene transfer, with the contralateral uninjected side used as a control. For the tau group, there was 9.41 ± 1.06 ng dopamine/mg tissue on the uninjected side and 6.79 ± 1.13 ng/mg on the injected side (N = 5, P < 0.01, paired t test), a 28% reduction relative to uninjected side. There was also a significant 30% increase in the DOPAC/dopamine ratio for the tau vector-injected side (0.134 ± 0.012) relative to the contralateral side (0.101 ± 0.010, N = 5, P < 0.05, paired t test).
Immunoelectron microscopy of tau or ASN in the substantia nigra
Similar to what we observed previously in the basal forebrain (Klein et al., 2004), the P301L tau vector led to the development of NFTs in the SN. Neurons with NFTs were characterized by eccentric nuclei (Fig. 5A) and cytoplasm filled with tau filaments that were straight and ~15 nm in diameter. The filaments formed highly organized and densely packed arrays in some neurons (Fig. 5B). They were immunoreactive for CP13, a monoclonal antibody to phospho-serine 202 of tau, (Figs. 5A and B) and for E1, a polyclonal antibody to an exon 1 sequence specific to human tau (not shown). In contrast to tau filaments, phosphorylated and non-phosphorylated neurofilaments were randomly oriented within SN neurons. They did not co-localize with tau filaments (not shown), but neurons with NFTs did show abnormal compartmentalization of neurofilament epitopes. Specifically, there was aberrant phosphorylated neurofilament in neuronal perikarya and non-phosphorylated neurofilaments in myelinated axons (not shown). Non-phosphorylated neurofilaments are usually compartmentalized to neuronal cell bodies and phosphorylated neurofilaments to axons (Sternberger and Sternberger, 1983).
Fig. 5.
Ultrastructural detection of tau or ASN filaments in the rat SN 4 months after gene transfer. (A) A SN neuron showing eccentric nucleus, pushed by densely packed filaments after P301L tau gene transfer. There is extensive deposition of 10-nm gold particles with immunoEM for hyperphosphorylated tau. (B) Higher magnification of straight ~15-nm tau filaments within the same neuron as A. (C) A myelinated axon in the substantia nigra containing ASN filaments. Filaments labeled with gold particles (10 nm) were found after A30P ASN gene transfer using the human-specific ASN antibody. The filaments did not co-label with antibodies for neurofilaments and were proximal to mitochondria (M). (D) Higher magnification of a field in C. CP13 (A and B) or LB509 (C and D) antibody labeling was not observed in controls. Scale bars, A = 1 µm; B = 180 nm; C = 300 nm; D = 60 nm.
At the electron microscopic level, NFTs were uncommon and this fits with the paucity of NFTs at the light microscopic level with Ab39 immunohistochemistry. In contrast, many neurons in the SN had tau immunoreactivity with human-specific and phospho-tau antibodies. These lesions were morphologically consistent with pretangles (Bancher et al., 1989) and had diffuse or granular cytoplasmic tau immunoreactivity, with occasional accentuation in the perinuclear region. At the ultrastructural level, the cytoplasm of such neurons had a meshwork of short tau-immunoreactive fibrils, but no aggregation or dense arrays of filaments.
While the WPRE-containing ASN vectors did not produce rotational behavior as we had hoped, they did produce lesions that contained filamentous ASN, which has not been previously reported in rats. The ASN-immunoreactive filaments were about 10 nm wide, as in human synucleinopathies (Lin et al., 2004) and in the spinal cord of ASN transgenic mice (Giasson et al., 2002), and they were most readily detected in myelinated axons in the SN (Figs. 5C and D). The ASN filaments did not co-label with antibodies to neurofilaments and were often in close proximity to mitochondria. Neuronal perikaryal inclusions similar to Lewy bodies were not detected at the light microscopic or ultrastructural level. The results for the A30P ASN (Figs. 5C and D) and A53T ASN vectors (not shown) were similar.
Discussion
This study shows that dopaminergic neurons in the rat SN are susceptible to tau-induced neurofibrillary degeneration and that this produces functional as well as structural consequences. This is the first example of vector-based disease modeling in the nigrostriatal system that involves a sufficient degree of motor dysfunction to be detected by amphetamine challenge. A clear and rapid behavioral effect induced by tau gene transfer should facilitate future studies of treatments that can either intensify or ameliorate motor deficits. The P301L tau vector caused turning behavior consistent with a specific lesion effect as early as 2 weeks after gene transfer, and it persisted for up to 16 weeks, the longest interval studied. In addition to successfully modeling dopamine-mediated motor dysfunction, the P301L tau gene transfer caused significant dopaminergic neuronal loss progressing from 3 weeks to 4 months, reduced striatal dopamine content and eventual NFT formation in SN neurons. The P301L tau gene transfer was initially associated with pretangles, as well as axonal dystrophy and abnormal distribution of neurofilaments, which are characteristic features in human neurodegenerative tauopathies (Lin et al., 2003b; Tsunoda et al., 2003). There was also decreased transgene expression in the corpus striatum after P301L tau gene transfer relative to the GFP control vector, which would be consistent with loss of nigrostriatal afferents. Because behavioral dysfunction occurred as early as 2 weeks after gene transfer while unequivocal examples of NFTs were rather uncommon at any interval, we hypothesize that mature NFTs are not required for tau-induced disruption of dopaminergic neurotransmission resulting in motor deficits.
The results with P301L tau gene transfer were remarkably consistent and reproducible, even though three separate batches of P301L tau vector were used throughout the course of this study. P301L tau has been used in a broad range of experimental model systems to study tau pathology. The most successful models of NFTs in rodents used this disease-related form of tau (Lewis et al., 2000) or a closely related mutation, P301S (Allen et al., 2002). In both of these models, transgenic mice develop profound motor dysfunction as early as 4 months of age, as well as NFTs and neuronal loss in the spinal cord and brain. The vector approach in normal rats was effective for targeting the SN and rapidly modulating motor behavior specific to the dopaminergic nigrostriatal system. The model is therefore relevant to several neurodegenerative diseases with tau pathology in the SN such as FTDP-17 (Mirra et al., 1999), PSP (Poorkaj et al., 2002), CBD (Wakabayashi et al., 1994), AD (Schneider et al., 2002), and some forms of PD (Duda et al., 2002).
Interestingly, the WT tau vector was also effective in producing a dopaminergic neurodegeneration model. We intended to demonstrate pathology specific for the mutant form of tau, but the WT tau gene transfer caused similar behavioral deficits, cell loss, and Ab39 immunoreactivity as early as 2–3 weeks. Clearly, more work is necessary to determine if P301L tau is more toxic or pathological than wild-type tau in this system, which will require dose titration to test for greater potency of the mutant. The use of WT tau is more relevant to developing models for the more common sporadic tauopathies, including PSP, CBD, and AD. It has been difficult to produce transgenic mice with functional deficits by using WT tau. While neuronal pathology in early tau transgenic mice had pretangles and no motor deficits (Götz et al., 2004), more recent models with transgenic mice expressing all six isoforms of tau have developed functionally significant neurofibrillary pathology (Andorfer et al., 2003). The vector-based method, with targeted and high expression of the transgene, is alternatively effective for studying WT tau pathogenesis with a single isoform.
The SN degeneration induced by the ASN-WPRE vectors did not appear to be enhanced relative to the ASN vector used previously lacking WPRE, because neither A30P nor A53T ASN gene transfer in this study resulted in motor dysfunction. It therefore appears that with respect to functional impairment in these short-term experiments, a substantial fraction of dopaminergic neurons in the SN can tolerate high levels of ASN, a conclusion also reached by others (Paterna and Bueler, 2002). Given recent genetic evidence that some forms of familial PD are associated with over-expression of ASN due to multiplication of the gene (Singleton et al., 2003) and that polymorphisms in the ASN gene that may lead to overexpression may also be a risk factor for PD (Farrer et al., 2001), the present results are hard to explain. It is possible that the acute nature of the model accounts for the paucity of functional repercussions and that longer intervals might reveal time-dependent degeneration. It should be noted that in familial PD, the expression level is related to the age of onset of the PD (Chartier-Harlin et al., 2004).
In this model system, SN dopaminergic neurons were more vulnerable to overexpression of tau than ASN. Although our attempts to model functionally significant ASN degeneration as assessed by rotational behavior were unsuccessful, the ASN-WPRE vectors did produce for the first time ASN filament formation in rats. Both A30P and A53T ASN vectors produced 10-nm filaments that could be labeled with the human-specific ASN antibody. The filaments were detected predominantly in axons. This pattern of neurodegeneration is reminiscent of findings in human synucleinopathies, where neuritic pathology precedes neuronal perikaryal inclusions (i.e., Lewy bodies) (Braak et al., 2001). These findings may help understand factors relevant to early neuronal degeneration in α-synucleinopathies. The ultrastructural coincidence of ASN filaments with mitochondria suggests a possible interaction between ASN filament formation and mitochondria.
There were two overall goals of the modeling studies: (1) to study the disease process and (2) to develop novel therapies. The present tau vector-induced neurodegeneration model will be useful for studying mechanisms involved in tau filament formation, dopaminergic neuronal death, and motor dysfunction. The ability to co-express gene products with the vector system permits screening gene targets that can either intensify tau pathology or block it. For example, chaperone co-factors such as Hsp70 can regulate tau processing (Dou et al., 2003; Petrucelli et al., 2004). If a specific gene product efficaciously blocks tau toxicity in rats, it could be pursued as a gene therapy for tauopathies, especially genetically determined tauopathies such as FTDP-17, because there are no treatments currently available for this fatal condition. Co-expression screening studies may help to identify specific underlying mechanisms of neurodegeneration caused by tau, which could then be targeted with small molecules.
Acknowledgments
We thank Dr. Adrian Dunn and Charles Dempsey for assistance with the HPLC measurements and Dr. Nicholas Goeders and Glenn Guerin for assistance with animal studies. This work was supported by The National Parkinson’s Foundation/Parkinson’s Disease Foundation, The Society for Progressive Supranuclear Palsy, and The Parkinson’s Disease Resource of NW Louisiana.
References
- Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003;86:582–890. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Tau and ubiquitin immunoreactivity at different stages of formation of Alzheimer neurofibrillary tangles. Prog. Clin. Biol. Res. 1989;317:837–848. [PubMed] [Google Scholar]
- Braak E, Sandmann-Keil D, Rub U, Gai WP, de Vos RA, Steur EN, Arai K, Braak H. Alpha-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol. (Berl) 2001;101:195–201. doi: 10.1007/s004010000247. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Crowe A, Ksiezak-Reding H, Liu WK, Dickson DW, Yen SH. The N terminal region of human tau is present in Alzheimer’s disease protein A68 and is incorporated into paired helical filaments. Am. J. Pathol. 1991;139:1463–1470. [PMC free article] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann. Neurol. 2000;47:374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Ksiezak-Reding H, Liu WK, Davies P, Crowe A, Yen SH. Immunocytochemistry of neurofibrillary tangles with antibodies to subregions of tau protein: identification of hidden and cleaved tau epitopes and a new phosphorylation site. Acta Neuropathol. (Berl) 1992;84:596–605. doi: 10.1007/BF00227736. [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. U. S. A. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ. Concurrence of α-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Neurochemical methods for evaluating cerebral biogenic amine responses to cytokines and their involvement in the central actions of interleukin-1. In: De Souza EB, editor. Methods in Neurosciences, Neurobiology of Cytokines. Part B. vol. 17. San Diego: Academic Press; 1993. pp. 209–222. [Google Scholar]
- Farrer M, Maraganore DM, Lockhart P, Singleton A, Lesnick TG, de Andrade M, West A, de Silva R, Hardy J, Hernandez D. Alpha-synuclein gene haplotypes are associated with Parkinson’s disease. Hum. Mol. Genet. 2001;10:1847–1851. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-syuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Götz J, Streffer JR, David D, Schild A, Hoerndli F, Pennanen L, Kurosinki P, Chen F. Transgenic models of Alzheimer’s disease and related disorders: histopathology, behavior and therapy. Mol. Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum. Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Gwin-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen S-H, Hardy J, Dickson DW. Distinctive neuropathology revealed by α-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- Healy DG, Abou-Sleiman PM, Lees AJ, Casas JP, Quinn N, Bhatia K, Hingorani AD, Wood NW. Tau gene and Parkinson’s disease: a case-control study and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2004;75:962–965. doi: 10.1136/jnnp.2003.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, Swieten JV, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein in Lewy bodies. J. Neuropathol. Exp. Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- Jicha GA, O’Donnell A, Weaver C, Angeletti R, Davies P. Hierarchical phosphorylation of recombinant tau by the paired-helical filament-associated protein kinase is dependent on cyclic AMP-dependent protein kinase. J. Neurochem. 1999;72:214–224. doi: 10.1046/j.1471-4159.1999.0720214.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J. Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum. Gene Ther. 2002a;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- Klein RL, Hamby ME, Gong Y, Hirko AC, Wang S, Hughes JA, King MA, Meyer EM. Dose and promoter effects of adeno-associated viral vector for GFP expression in the rat brain. Exp. Neurol. 2002b;176:66–74. doi: 10.1006/exnr.2002.7942. [DOI] [PubMed] [Google Scholar]
- Klein RL, Hamby ME, Sonntag CF, Millard WJ, King MA, Meyer EM. Measurements of vector-derived neurotrophic factor and green fluorescent protein levels in the brain. Methods. 2002c;28:286–292. doi: 10.1016/s1046-2023(02)00234-7. [DOI] [PubMed] [Google Scholar]
- Klein RL, Lin WL, Dickson DW, Lewis J, Hutton M, Duff K, Meyer EM, King MA. Rapid neurofibrillary tangle formation after localized gene transfer of mutated tau. Am. J. Pathol. 2004;164:347–353. doi: 10.1016/S0002-9440(10)63124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 to Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am. J. Pathol. 2003a;162:213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) tau. J. Neurocytol. 2003b;32:1091–1105. doi: 10.1023/B:NEUR.0000021904.61387.95. [DOI] [PubMed] [Google Scholar]
- Lin WL, DeLucia MW, Dickson DW. Alpha-synuclein immunoreactivity in neuronal nuclear inclusions and neurites in multiple system atrophy. Neurosci. Lett. 2004;354:99–102. doi: 10.1016/j.neulet.2003.09.075. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. Alpha-synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum. Gene Ther. 1999;10:2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH, Jr, Goetz CG, Small GW, Masterman D, Mastaglia F, Laing NG, Stajich JM, Ribble RC, Booze MW, Rogala A, Hauser MA, Zhang F, Gibson RA, Middleton LT, Roses AD, Haines JL, Scott BL, Pericak-Vance MA, Vance JM. Association of single-nucleotide polymorphisms of the tau gene with late-onset Parkinson disease. JAMA. 2001;286:2245–2250. doi: 10.1001/jama.286.18.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA, Levey AI, Jones R, Green J, Shoffner JM, Wainer BH, Schmidt ML, Trojanowski JQ, Ghetti B. Tau pathology in a family with dementia and a P301L mutation in tau. J. Neuropathol. Exp. Neurol. 1999;58:335–345. doi: 10.1097/00005072-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Nacharaju P, Lewis J, Easson C, Yen S, Hackett J, Hutton M, Yen SH. Accelerated filament formation from tau protein with specific FTDP-17 missense mutations. FEBS Lett. 1999;447:195–199. doi: 10.1016/s0014-5793(99)00294-x. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J. Clin. Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Bueler H. Recombinant adeno-associated virus vector design and gene expression in the mammalian brain. Methods. 2002;28:208–218. doi: 10.1016/s1046-2023(02)00225-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, McGowan E, Lewis J, Dillmann WH, Browne SE, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M. CHIP and Hsp70 regulate Tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, Schellenberg GD. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann. Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Gilley DW, Kvarnberg DE, Mufson EJ, Bennett DA. Improved detection of substantia nigra pathology in Alzheimer’s disease. J. Histochem. Cytochem. 2002;50:99–106. doi: 10.1177/002215540205000111. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad. Sci. U. S. A. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Kuang LQ, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. 1994;87:545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- Yen SH, Crowe A, Dickson DW. Monoclonal antibodies to Alzheimer neurofibrillary tangles: I. Identification of polypeptides. Am. J. Pathol. 1985;120:282–291. [PMC free article] [PubMed] [Google Scholar]
- Zehr C, Goodwin K, Lin WL, Dickson DW, McGowan E, Lewis J, Hutton M. Axonal degeneration in P301L tau transgenic mice. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Neurobiol. Aging. 2004;25 Suppl. 2:S243. [Google Scholar]