Abstract
Neurodegenerative diseases involving neurofibrillary tangle pathology are pernicious. By expressing the microtubule-associated protein tau, a major component of tangles, with a viral vector, we induce neuropathological sequelae in rats that are similar to those seen in human tauopathies. We tested several variants of the adeno-associated virus (AAV) vector for tau expression in the nigrostriatal system in order to develop models with graded onset and completeness. Whereas previous studies with AAV2 tau vectors produced partial lesions of the nigrostriatal system, AAV9 or AAV10 tau vectors were more robust. These vectors had formidable efficacy relative to 6-hydroxydopamine for dopamine loss in the striatum. Time-courses for tau transgene expression, dopamine loss and rotational behavior tracked the disease progression with the AAV9 tau vector. There was a nearly complete lesion over a delayed time-course relative to 6-hydroxydopamine, with a sequence of tau expression by 1 week, dopamine loss by 2 weeks and then behavior effect by 3–4 weeks. Relative to AAV2 or AAV8, tau expression from AAV9 or AAV10 peaked earlier and caused more dopamine loss. Varying vector efficiencies produced graded states of disease up to nearly complete. The disease models stemming from the AAV variants AAV9 or AAV10 may be useful for rapid drug screening, particularly for tau diseases that affect the nigrostriatal system, such as progressive supranuclear palsy.
Keywords: adeno-associated virus, gene transfer, neurodegenerative diseases, progressive supranuclear palsy, rats
Introduction
Several neurodegenerative diseases involve deposits of the micro-tubule-associated protein tau polymerized into neurofibrillary tangles known as tauopathies. Alzheimer’s disease pathology features both neurofibrillary tangles and amyloid plaques (Dickson, 1999), whereas primarily tau pathology occurs in progressive supranuclear palsy and frontotemporal lobar degeneration diseases-τ including Pick’s disease, frontotemporal dementia and parkinsonism linked to chromosome 17, and corticobasal degeneration (Cairns et al., 2007; Kumar-Singh & Van Broekhoven, 2007). The rationale for expressing tau in the rat substantia nigra (SN) in this study stems from the extensive neurofibrillary pathology there in humans with Alzheimer’s disease, progressive supranuclear palsy, frontotemporal dementia with parkinsonism linked to chromosome 17 and corticobasal degeneration, and the prominent neuronal loss in the SN in the latter three diseases (Mirra et al., 1999; Poorkaj et al., 2002; Schneider et al., 2002; Di Maria et al., 2000; Wakabayashi et al., 1994).
We compared several adeno-associated virus (AAV) serotype vectors for their efficacy in modeling tauopathy in rats. By expressing the microtubule-associated protein tau in rats with AAV2, we induced neurofibrillary tangle formation and mimicked features of human neurological diseases involving tangle or tau pathology (Klein et al., 2004). Tau vectors caused degeneration of the nigrostriatal pathway but the effects on cell loss and behavior were partial relative to the dopaminergic neurotoxin 6-hydroxydopamine (6-OHDA) (Klein et al., 2005). Recent studies by us and others have shown enhanced gene transfer in the brain with newer AAV vector variants relative to the originally most well-studied AAV2 (Cearley & Wolfe, 2006; Sondhi et al., 2007; Klein et al., 2008). We chose two serotype variants, AAV9 and AAV10, that showed enhanced expression for tau vectors in this study in order to compare strength of expression and rate of disease onset relative to previous vectors. 6-OHDA was a control to evaluate whether one of the tau vectors could produce similar neurodegeneration of the dopaminergic nigrostriatal system. We also ran a control vector for alpha-synuclein, a gene linked to Parkinson’s disease that was previously shown to damage the nigrostriatal system when overexpressed (Klein et al., 2002; Kirik et al., 2002; Yamada et al., 2004; St Martin et al., 2007; Gorbatyuk et al., 2008).
We previously reported on our partial disease model in rats using AAV2 or AAV8 tau vectors (Klein et al., 2005, 2006). In this study, we evaluated the time-courses of tau expression and dopamine (DA) depletion, using new AAV9 or AAV10 vectors for tau, and we concentrated on DA loss in the striatum because it provides a quantitative index of the nigrostriatal system. We tested whether the AAV9 tau gene vector-based disease was equally powerful, although over a slower time-course relative to rapid, complete 6-OHDA lesions of the nigrostriatal system, and if the tau effect was specific with regard to control vectors. Time-course studies compared the sequence of tau expression, DA loss and behavioral effect, and we also compared the onset and strength of tau expression from AAV2, AAV8, AAV9 or AAV10 tau vectors.
Materials and methods
DNAs and AAVs
An expression cassette flanked with AAV2 terminal repeats was packaged into recombinant AAV2 or cross-packaged into recombinant AAV8, AAV9 or AAV10 (AAV Rh 10) (Cearley & Wolfe, 2006; Sondhi et al., 2007; Klein et al., 2008). The promoter/enhancer combination used to drive expression included the hybrid cytomeg-alovirus/chicken β-actin promoter and the 3′ enhancer woodchuck hepatitis virus post-transcriptional regulatory element. Plasmids for the reporter control green fluorescent protein (GFP), the P301L form of human tau including exons 2, 3 and 10 (four repeat microtubule-binding domains, 4R2N) or human wild-type alpha-synuclein were the same as described previously (Klein et al., 2005).
Human embryonic kidney 293-T cells were cotransfected with one of the above transgene plasmids along with the respective packaging plasmid(s) for AAV2, AAV8, AAV9 or AAV10 (Klein et al., 2008). The cell lysate was applied to a discontinuous gradient of iodixinol (OptiPrep, Greiner Bio-One, Longwood, FL, USA) and centrifuged at 350 000 g for 1 h. The AAV was then removed, diluted twofold with lactated Ringer’s solution (Baxter, Deerfield, IL, USA) and then washed and concentrated by Biomax 100 Ultrafree-15 units (Millipore, Billerica, MA, USA). The final stocks were sterilized by Millex-GV syringe filters (Millipore) into low-adhesion tubes (USA Scientific, Ocala, FL, USA). Encapsidated genome copies were titered by dot-blot, which ranged from 1 × 1012 to 3 × 1013 vector genomes (vg)/mL. Vectors were aliquoted and stored frozen. Equal dose comparisons were made by normalizing titers with the diluent, lactated Ringer’s solution.
Animals and stereotaxic injections
Male Sprague-Dawley rats (3 months old, Harlan, Indianapolis, IN, USA) were anesthetized with a cocktail of 3 mL xylazine (20 mg/mL, Butler, Columbus, OH, USA), 3 mL ketamine (100 mg/mL, Fort Dodge Animal Health, Fort Dodge, IA, USA) and 1 mL acepromazine (10 mg/mL, Boerhinger Ingelheim, St Joseph, MO, USA) administered intramuscularly at a dose of 1 mL/kg. Viral stocks were injected through a 27 gauge cannula connected via 26 gauge internal diameter polyethylene tubing to a 10 µL Hamilton syringe mounted to a microinjection pump (CMA/Microdialysis, North Chelmsford, MA, USA) at a rate of 0.2 µL/min. The stereotaxic injection coordinates for the SN were 5.3 P, 2.1 L and 7.6 V with 3 µL injected (Paxinos & Watson, 1998). The needle remained in place at the injection site for an additional 1 min before the cannula was removed slowly (over 2 min). The skin was sutured and the animal was placed on a heating pad until it began to recover from the surgery, before being returned to its individual cage. Some rats were injected with 6-OHDA (Sigma, St Louis, MO, USA). The 6-OHDA solution was 4 mg/mL free base in 0.1% ascorbic acid/0.9% NaCl. This solution was injected into the medial forebrain bundle (2.5 µL at two sites; coordinates: 2.8 and 3.3 P, 1.5 L, 8.5 V). The 6-OHDA-lesioned rats were anesthetized with a combination of pentobarbital sodium (50 mg/kg, i.p., Abbott, North Chicago, IL, USA) and 0.2 mL/kg of the cocktail described above. All animal procedures followed protocols approved by the Animal Care and Use Committee at our institution and were in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Vector dosing, groups and intervals
We previously found that doses of 8 × 109–5 × 1010 vg of AAV8 GFP caused a dose-related 15–41% loss of tyrosine hydroxylase (TH)-immunoreactive neurons in the SN (Klein et al., 2006), so we chose a dose below 8 × 109 vg for all of the groups. AAV2 tau, AAV8 tau or AAV8 GFP vector groups were run at a dose of 6.4 × 109 vg. We found that even lower doses of the AAV9 or AAV10 tau vectors were sufficient for inducing robust loss of TH-immunoreactive cells in the SN in initial test rats, so AAV9 GFP or AAV10 tau groups were run at a dose of 3.0 × 109 vg. Despite the 2.1-fold lower dose in the AAV9/AAV10 relative to the AAV2/AAV8 vector groups, we made serotype comparisons and found even more pronounced effects with AAV9 or AAV10 tau vectors. Equal-dose comparisons were made between either the AAV8 tau or the AAV9 tau and its matching GFP vector. Time intervals for experiments were 1, 2 or 4 weeks after gene transfer with the number of rats indicated in the Results. We previously assessed TH cell loss in the SN with AAV2 or AAV8 tau vectors (Klein et al., 2006) and now, in addition, measured TH fiber density in the striatum and time-courses for striatal DA content and tau expression. For the AAV10 tau vector, a matching AAV10 GFP vector was not tested because we only addressed hypotheses relative to AAV10 tau compared with other tau serotype vectors or, in the case of stereological assessments, uninjected tissue. We included groups for 6-OHDA or AAV9 alpha-synuclein for some of the measures as reference controls. Animals included in neurochemical measurements were not run for behavior to avoid potential effects of the amphetamine.
Concentrations of DA and DOPAC in striatal tissue
Striatal samples were dissected in a consistent manner using a 1 mm brain block and a 3 mm diameter biopsy puncher. Tissue punches were weighed and then frozen on dry ice. The samples were homogenized using an ultrasonic probe in 0.1 m HClO4 containing 0.1 mm EDTA and N-methyl-dopamine (20 ng/mL) as an internal standard. The samples were centrifuged at 3 300 g for 5 min to sediment the protein and the supernatants were analysed directly by high-performance liquid chromatography. The columns were 250 × 4.4 mm Spherosorb 5 µm ODS-1 reverse-phase columns (Waters Corp., Milford, MA, USA) maintained at 35 °C by BAS LC22/23A column heaters (Bioanalytical Systems, West Lafayette, IN, USA). The electrochemical cells were BAS TL12 with glassy carbon electrodes connected to BAS LC4B electrochemical detectors. The electrodes were maintained at potentials of approximately 0.78 and 0.9 V with respect to an Ag/AgCl reference electrode. The mobile phase was 0.1 m sodium phosphate (pH 3.0), 0.1 mm EDTA, 0.2–0.4 mm 1-octane sulfonic acid (Eastman Kodak, Rochester, NY, USA) and 3–4% acetonitrile (v/v), filtered through a 0.45 µm filter and pumped at 1.2 mL/min. Data were collected and integrated by a Millennium system (Waters Corp.).
Western blots
The SN was dissected in a consistent manner with the aid of a brain block and biopsy puncher, and frozen on dry ice. The samples were put in buffer [1% Nonidet-P40/0.5% sodium deoxycholate/0.1% sodium dodecyl sulfate/phosphate-buffered saline (PBS)] with protease inhibitors (Halt protease inhibitor cocktail kit, Pierce, Rockford, IL, USA) and the soluble fraction was prepared by Dounce homogenization and centrifugation. Protein content was determined by Bradford assay reagents (Bio-Rad, Hercules, CA, USA) and subjected to 12% sodium dodecyl sulfate/polyacrylamide gels (Bio-Rad), with each gel loaded with equal protein in each lane (~50 µg protein depending on the gel). The primary antibodies for immunoblots were human tau T14 (1 : 1000, Zymed, South San Francisco, CA, USA) or glyceraldehyde-3-phosphate dehydrogenase (1 : 1000, Ambion, Austin, TX, USA). Secondary antibody and chemiluminescence reagents were from Amersham (Buckinghamshire, UK). In addition to loading equal proteins, glyceraldehyde-3-phosphate dehydrogenase immunoblots were run for normalization. The human tau bands were quantified with the ScionImage program (Scion, Frederick, MD, USA), expressing the tau band densities as a ratio to the housekeeping gene product glyceraldehyde-3-phosphate dehydrogenase. Each AAV serotype was analysed for tau expression at 1, 2 and 4 weeks, and the data processed for anovas to test for time dependence of expression. Other gels tested AAV serotypes 2, 8, 9 and 10 relative to each other at each interval. anova/Bonferroni’s multiple comparison test determined serotype differences.
Immunostaining
Anesthetized animals were perfused with PBS, followed by cold 4% paraformaldehyde in PBS. The brain was removed and immersed in fixative overnight at 4 °C. Brains were equilibrated in a cryoprotectant solution of 30% sucrose/PBS at 4 °C. Coronal sections (50 µm thick) were cut on a sliding microtome with a freezing stage. Antigen detection was conducted on free-floating sections. Primary antibody incubations were overnight at 4 °C on a shaking platform. For immunoperoxidase staining, endogenous peroxidase activity was quenched with 0.1% H2O2/PBS for 10 min. The sections were washed in PBS and incubated for 5 min in 0.3% Triton X-100/PBS, and washed before applying primary antibody. Primary antibodies for immunostaining included: TH polyclonal (1 : 2000, Pel-Freez, Rogers, AR, USA), human tau monoclonal T14 (1 : 2000, Zymed/Invitrogen, Carlsbad, CA, USA), human alpha-synuclein monoclonal (1 : 2000, Zymed/Invitrogen), GFP polyclonal (1 : 10 000, Molecular Probes/Invitrogen) and neuronal nuclei monoclonal (1 : 500, Chemicon, Temecula, CA, USA). Biotinylated secondary antibodies for peroxidase staining were from DAKO Cytomation (Carpinteria, CA, USA; 1 : 2000), incubated for 1 h at room temperature (22°C). The sections were washed with PBS and labeled with horseradish peroxidase-conjugated Extravidin (1 : 2000, Sigma) for 30 min at room temperature. The chromogen was diaminobenzidine (0.67 mg; Sigma) in 0.3% H2O2, 80 mm sodium acetate buffer containing 8 mm imidazole and 2% NiSO4. After mounting on slides, the sections were dehydrated in a series of alcohol and xylene, and coverslipped with Eukitt (Electron Microscopy Sciences, Hatfield, PA, USA). For immunofluorescence, sections were incubated in primary antibody overnight, washed and incubated with Cy3-conjugated secondary antibodies (1 : 300, Jackson ImmunoResearch) for 2 h, followed by DAPI counterstaining (1 µg/mL), washes and coverslipping with glycerol/gelatin (Sigma).
Stereological estimates
The number of SN pars compacta neurons expressing TH immunoreactivity was estimated by unbiased stereology using the Micro-Brightfield Inc. system. Eight sections evenly spaced throughout the SN pars compacta structure were analysed for each probe. Optical dissectors were 50 × 50 × 16 µm cubes spaced in a systematic random manner 150 × 150 µm apart and offset 2 µm from the section surface. The fractionator sampling was optimized to yield about 150 counted cells per animal, for Gundersen error coefficients <0.10 (King et al., 2002).
Striatal TH analysis
Five sections evenly spaced through the striatum were processed for TH immunohistochemistry. The specific TH staining in the striatum was quantified using the Scion imaging program. The striata were traced and then measured for optical density of staining (pixels). The ratio of the injected side relative to the contralateral uninjected side was calculated.
Rotational behavior
Animals were challenged with d-amphetamine (free base, 2 mg/kg in saline, i.m., Sigma). The amphetamine was injected 20 min before placing the animals in an automated rotometer system (San Diego Instruments, San Diego, CA, USA) for 10 min. Pilot studies determined that locomotor activity peaks by 20 min after injection and that a 10 min measurement is sufficient to determine whether a side-to-side rotational bias is present.
Statistics
Data are expressed as mean ± SEM. Statistical tests included anovas, repeated measures anovas, Bonferroni’s multiple comparisons or t-tests as indicated.
Results
Tau transgene expression on western blots
Western blot data unequivocally showed that tau expression from either AAV9 or AAV10 vectors reached higher levels at earlier intervals than either AAV2 or AAV8. Equal amounts of protein were loaded on each blot and, for quantification, the tau band at ~68 kDa was normalized to the band for glyceraldehyde-3-phosphate dehydrogenase at ~38 kDa on each blot. At 1 week (Fig. 1A), there was an effect of serotype (P < 0.002, F3,13 = 12.61) with significant differences in Bonferroni’s multiple comparison test for AAV2 vs. AAV9 (P < 0.01), AAV2 vs. AAV10 (P < 0.05), AAV8 vs. AAV9 (P < 0.01) or AAV8 vs. AAV10 (P < 0.05). At 2 weeks (Fig. 1B), there was an effect of serotype (P < 0.0001, F3,15 = 125.1) with significant differences in Bonferroni’s multiple comparison test for AAV2 vs. AAV9 (P < 0.001), AAV2 vs. AAV10 (P < 0.001), AAV8 vs. AAV9 (P < 0.001), AAV8 vs. AAV10 (P < 0.001) or AAV9 vs. AAV10 (P < 0.05). At 4 weeks (Fig. 1C), there was an effect of serotype (P < 0.0001, F3,19 = 25.52) with significant differences in Bonferroni’s multiple comparison test for AAV2 vs. AAV8 (P < 0.001), AAV2 vs. AAV9 (P < 0.001) or AAV2 vs. AAV10 (P < 0.001). Although it was clear that AAV9 or AAV10 produced relatively high levels of tau by 1 or 2 weeks, expression from AAV9 was even stronger than AAV10 at 2 weeks.
FIG. 1.
Comparative expression from AAV2, AAV8, AAV9 and AAV10 tau vectors at (A) 1 week, (B) 2 weeks and (C) 4 weeks. (D) Quantification of human tau expression, normalized to a constitutive gene product [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] expression (N) (*significant serotype differences, P < 0.05, anovas/Bonferroni’s multiple comparison tests, see Results for all comparisons). The dissected SN, the injected area, showed relatively strong and rapid expression from AAV9 or AAV10. AAV2 was weaker than either AAV9 or AAV10 at all intervals (P < 0.05). AAV8 expression was similar to AAV2 at 1 and 2 weeks but was greater than AAV2 by 4 weeks (P < 0.001), by which time AAV8 expression was similar to AAV9 or AAV10. Each sample is shown; equal proteins were loaded on gels. AAV2 or AAV8 was run at a vector dose of 6.4 × 109 vg, whereas AAV9 or AAV10 was run at a lower dose of 3 × 109 vg.
We also ran the 1, 2 and 4 week time-course for each serotype on individual gels in order to directly compare the onset timing with equal protein loading and normalization from the same gels with each serotype (not shown). For AAV2, there was a time-dependent increase in tau expression by anova (P < 0.005, F2,14 = 10.94) and significant difference in tau levels between 1 and 4 weeks (P < 0.05), and 2 and 4 weeks (P < 0.01) by Bonferroni’s multiple comparison test. Expression with AAV2 therefore continued to rise up to 4 weeks. For AAV8, similar to AAV2, there was a time-dependent increase in tau expression by anova (P < 0.0001, F2,14 = 67.55) and significant difference in tau levels between 1 and 4 weeks (P < 0.001), and 2 and 4 weeks (P < 0.001) by Bonferroni’s multiple comparison test. Expression with AAV8 therefore continued to rise up to 4 weeks, as with AAV2, and this was consistent with Fig. 1 where AAV8 expression rose at 4 weeks. For both AAV9 or AAV10, there was no time dependence between 1, 2 and 4 weeks.
Neurochemical data
The central goal of the study was to evaluate DA levels in the striatum after tau vector gene transfer. We also evaluated the DOPAC : DA ratio as an index of DA turnover. As for the raw levels in non-transduced tissues on one side of the brain, the non-transduced, uninjected side, from a group of six rats that received the AAV9 GFP vector 4 weeks earlier on the other side, contained 10.890 ± 0.440 ng/mg tissue DA and 0.114 ± 0.009 DOPAC : DA. The uninjected side in a group of six rats that received the AAV9 tau vector 4 weeks earlier contained 11.920 ± 1.074 ng/mg tissue DA and 0.110 ± 0.002 DOPAC : DA. There were no differences in the uninjected sides from these vector groups. The vector treatments were unilateral and the rest of the data are a ratio of treated : untreated side.
Complete medial forebrain bundle lesioning with 6-OHDA was used as a comparative control to the gene transfer models. At either 1 or 4 weeks after lesioning, DA was reduced by 98–100% relative to the uninjected side (Fig. 2A). The 6-OHDA also increased DOPAC : DA as expected (Hefti et al., 1985), being 8.3-fold greater on the injected side at 4 weeks. DA loss was similar at 1 and 4 weeks, although there was a time dependency for an increase in DOPAC : -DA between 1 and 4 weeks (P < 0.001, N = 3/interval).
Fig. 2.
Neurochemical data from rat striatum. (A) DA levels on the treated side relative to the untreated side of the brain, expressed as a ratio, were completely depleted by 6-OHDA at 1 and 4 weeks post-treatment. By 4 weeks, AAV8 tau reduced DA levels to a greater extent than AAV2 tau (P < 0.001, anova/Bonferroni’s multiple comparison test). AAV9 tau or AAV10 tau reduced DA more rapidly as each was different from AAV2 tau at 2 weeks (P < 0.001 for each) and both AAV9 and AAV10 tau produced greater loss than AAV8 tau at both 2 and 4 weeks (P < 0.001–0.05 for different comparisons). N = 3–11/data point. (B) The 4 week data from A, shown with control AAV8 or AAV9 GFP vectors. N-value indicated, as for C. (C) 6-OHDA drove up DA turnover as expected, as did AAV9 tau at 4 weeks (P < 0.001 compared with AAV9 GFP). *Difference between AAV tau and its cognate GFP vector (P < 0.001–0.01).
We compared AAV2, AAV8, AAV9 or AAV10 tau vectors at 1, 2 and 4 weeks for DA loss with 3–11 rats/serotype/interval (Fig. 2A). The two-way anova revealed significant effects of serotype (F3,52 = 23.92, P < 0.0001), time interval (F2,52 = 37.92, P < 0.0001) and interaction (F6,52 = 5.44, P < 0.0003). In Bonferroni post-tests, there was a difference between AAV2 and AAV8 at 4 weeks (P < 0.001). AAV2 was different from AAV9 or AAV10 at both 2 and 4 weeks (P < 0.001 for each test). AAV8 was different from AAV9 at 2 or 4 weeks (P < 0.001 for each) and different from AAV10 at 2 weeks (P < 0.001) or 4 weeks (P < 0.05), whereas there were no differences between AAV9 and AAV10. The tau vector serotypes therefore produced degrees of DA loss: AAV9 = AAV10 > AAV8 > AAV2. Whereas the 6-OHDA-induced DA loss was complete by 1 week, the progression was slower with AAV9 or AAV10 (1–2 weeks) or AAV8 (2–4 weeks). Control AAV8 or AAV9 GFP vectors were run at the 4 week interval (Fig. 2B). Interestingly, the AAV9 GFP resulted in a consistent 15% reduction in DA when compared with the intact uninjected side in a pairwise manner (P < 0.01, paired t-test). The small but significant loss of striatal DA with the AAV9 GFP in this study can be considered a toxic side-effect, although the AAV9 GFP did not affect the numbers of DA neurons in the SN (below).
The DOPAC : DA turnover data are in Fig. 2C, which shows a side-to-side ratio of the DOPAC : DA on the two sides. 6-OHDA drove up DA turnover. The AAV9 tau vector caused a 2.4-fold increase that was greater than the AAV9 GFP group, AAV2 tau, AAV8 tau or AAV10 tau (P < 0.001 for each, anova/Bonferroni’s multiple comparison test). The AAV10 tau group was also greater than the AAV2 tau (P < 0.01). The DA turnover was not elevated in the AAV8 tau group realtive to AAV8 GFP at 4 weeks. We investigated whether the elevated DA turnover in the AAV9 tau group was progressive over time. For the AAV9 tau, there was a significant effect of time on DOPAC : DA (F2,11 = 33.06, P < 0.0001) with differences between 1 and 2 weeks (P < 0.05), 1 and 4 weeks (P < 0.001), and 2 and 4 weeks (P < 0.01).
Expression of GFP or tau on brain sections and effects on TH neurons
The AAV9 GFP produced robust GFP expression targeted to the SN. GFP fluorescence in the SN pars compacta (Fig. 3A) overlapped with TH staining (Fig. 3B). The SN pars compacta is made up of mostly DA neurons (Oertel et al., 1982) and the GFP fluorescence and TH counterstaining colocalized in merged images. More evidence of efficient dopaminergic neuron transduction with AAV9 is the robust expression of GFP in striatal processes specifically on the injected side (Fig. 3C).
Fig. 3.
AAV9 GFP expression in the nigrostriatal pathway. (A) GFP fluorescence at 4 weeks after injection of AAV9 GFP to the SN at a dose of 3 × 109 vg. (B) The same section as A counterstained for TH showing good overlap with the GFP in A and the pars compacta DA neurons. (C) GFP was expressed unilaterally in corpus striatum after unilateral injections as in A. Scale bars: A and B, 132 µm; C, 528 µm.
Robust expression of GFP in the SN pars compacta from AAV9 GFP at 4 weeks is also shown in Fig. 4A. GFP expression in the SN pars reticulata neurons can also be seen in Fig. 4A, as observed previously with AAV injections to the SN (Klein et al., 2005). In stark contrast, human tau transgene expression from either AAV9 tau (Fig. 4B) or AAV10 tau (Fig. 4C) was mainly found in the pars reticulata but not the pars compacta.
Fig. 4.
Transgene expression and effects on DA neurons. Examples of transgene expression at 4 weeks after injections of (A) AAV9 GFP, (B) AAV9 tau, (C) AAV10 tau and (D) AAV9 alpha-synuclein. All of the vectors were injected at a dose of 3 × 109 vg. GFP fluorescence in the SN (A) or human alpha-synuclein immunoreactivity (D) was dense in the SN pars compacta, whereas with the tau vectors in B and C there was evidence of human tau staining in the pars reticulata but little or no remaining expression in the pars compacta. TH staining after unilateral injections of (E) AAV9 GFP, (F) AAV9 tau, (G) AAV10 tau and (H) AAV9 alpha-synuclein. The right side of the panel is the injected side for E–H. At this vector dose, TH was preserved in the GFP and alpha-synuclein groups in E and H, along with the transgene expression in the pars compacta above, whereas AAV9 or AAV10 tau vectors in F and G obliterated TH on the injected side. Scale bars: A–D, 90 µm; E–H, 360 µm.
Based on the highly efficient transduction with AAV9 tau, we tested an AAV9 vector for human wild-type alpha-synuclein to determine if the enhancement would lead to a more robust disease model than we observed previously with AAV2 alpha-synuclein (Klein et al., 2002, 2005). Similar to AAV9 GFP, and in contrast to the AAV9 or AAV10 tau vectors, AAV9 alpha-synuclein produced robust expression of human alpha-synuclein immunoreactivity in both the pars compacta and pars reticulata (Fig. 4D). The transgene expression in the pars compacta with either AAV9 GFP or AAV9 alpha-synuclein was preserved along with apparently full complements of SN pars compacta TH-immunoreactive neurons on the injected side relative to the uninjected sides, whereas AAV9 or AAV10 tau vectors erased TH immunoreactivity in the SN on the injected side (Fig. 4E–H).
Numbers of TH-immunoreactive neurons in the SN
We previously observed significant loss of TH-immunoreactive neurons in the SN with AAV2 or AAV8 tau vectors (Klein et al., 2005, 2006) and we did not repeat stereology studies with AAV2 or AAV8 vectors in this study. AAV2 and AAV8 tau vectors are used for the neurochemical and expression level and TH fiber density components. Here we found an 88% loss with AAV9 tau and a 94% loss with AAV10 tau relative to uninjected SN at 4 weeks (Fig. 5), consistent with the images in Fig. 4F and G. There was a group effect for TH cell numbers (F3,21 = 147.5, P < 0.0001) and specific differences between uninjected and either AAV9 or AAV10 tau (P < 0.001 for each, Bonferroni’s multiple comparison test), a difference between AAV9 tau and AAV9 GFP (P < 0.001) but not between uninjected and AAV9 GFP (N = 5–6/group). Although we did not attempt to quantify the percentage of the SN DA neurons transduced in this study, we assume that DA neuons that were lost had to have been transduced by the tau vectors, so the 88–94% loss probably reflects the transduction rate. In four rats injected with 3 × 109 vg AAV9 alpha-synuclein, the same dose as the AAV9 tau, there was no loss of DA neurons relative to uninjected controls (data not shown), confirming that tau is relatively more toxic as in Fig. 4.
Fig. 5.
Stereology estimates of SN DA neurons. AAV9 GFP vector did not alter numbers of DA neurons at 4 weeks relative to uninjected SN but AAV9 tau or AAV10 tau reduced DA neuron profiles by 88–94%. *P < 0.001, anova/Bonferroni’s multiple comparison test, N = 5–6/ group.
In order to determine whether the loss of TH in the SN was truly cell loss vs. down-regulation of the dopaminergic phenotype marker, we injected a mixture of AAV9 tau and AAV9 GFP on one side of the brain and a mixture of AAV9 GFP and lactated ringer’s solution on the other side. Both sides received 7.2 × 109 vg of AAV9 GFP and one side received 3.0 × 109 vg of AAV9 tau, whereas the other side received lactated ringer’s vehicle solution in an equal volume, and the interval was 4 weeks. Whereas AAV9 GFP/vehicle maintained robust GFP expression in the SN pars compacta and did not cause loss of neurons (Fig. 6A–C), tau coexpression obliterated both GFP and TH expression, and neuronal nuclei in the SN pars compacta (Fig. 6D–F), demonstrating neuronal cell loss. In viewing the SN pars reticulata in these rats, GFP expression was found on both sides (although Fig. 6 is not optimized for viewing GFP in this region), which suggests as in Fig. 4 that the killing of neurons by tau is specific, or at least selective, for those in SN pars compacta. These GFP results confirm those of tau expression in Fig. 4B and C, with loss of expressing cells in pars compacta but not pars reticulata.
Fig. 6.
AAV9 tau gene transfer obliterates GFP, TH and neuronal nuclei (NeuN) markers, demonstrating loss of DA neurons rather than solely loss of TH. A mixture of AAV9 GFP/lactated ringer’s solution on one side of the brain (A–C) or AAV9 GFP/AAV9 tau (D–F) was injected 4 weeks earlier. Without tau, there was GFP (A) and TH (B) expression and NeuN (C) present in the SN pars compacta on adjacent sections. With tau expression, there were fewer GFP- (D) and TH- (E) expressing neurons, and fewer neuronal nuclei (F) in the SN pars compacta on adjacent sections. Camera exposure times were equal for each side of the brain. Scale bar, 150 µm.
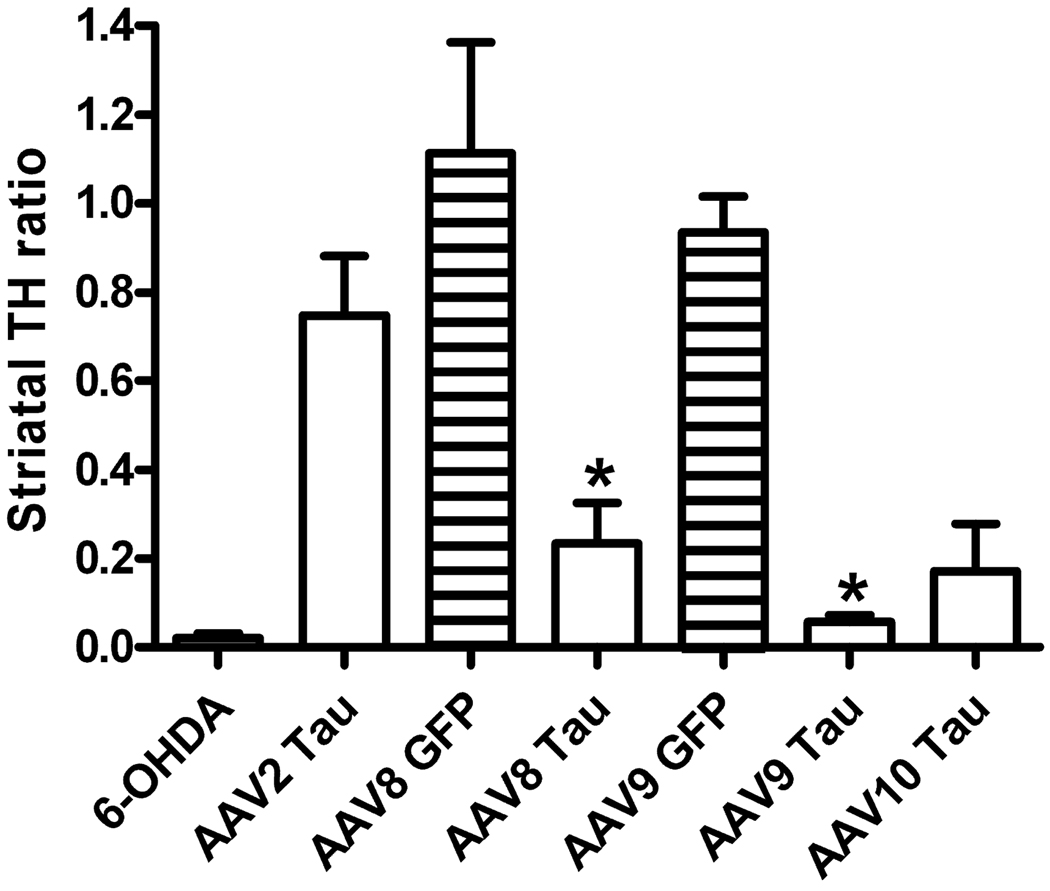
TH staining in striatum
Consistent with the loss of striatal DA and SN DA neurons in Figs 2 and 5, levels of TH in the striatum were also reduced at 4 weeks in the AAV8, AAV9 or AAV10 tau vector groups (Figs 7 and 8). There was a group effect comparing vectors at 4 weeks (F5,21 = 13.17, P < 0.0001) and, in the Bonferroni post-test, there were differences between AAV8 GFP and AAV8 tau (P < 0.01), AAV9 GFP and AAV9 tau (P < 0.001), and tau vector serotype differences between AAV2 and AAV9 (P < 0.01) or AAV2 and AAV10 (P < 0.05) with three to four rats/group.
Fig. 7.
TH fiber density in striatum. A series of five sections from one rat is shown for each vector group. The left hemisphere is the injected side, showing marked loss of striatal TH fiber density in the 6-OHDA, AAV8 tau, AAV9 tau or AAV10 tau vector groups.
Fig. 8.
Quantification of TH fiber density in striatum. The TH staining in striatum ipsilateral to the injection in the SN was measured as a ratio to the staining on the uninjected contralateral side. AAV8 (P < 0.01) or AAV9 (P < 0.001) tau caused loss of striatal TH immunoreactivity relative to its matching GFP vector at 4 weeks (*anova/Bonferroni’s multiple comparison test). The loss was greater with either AAV9 tau (P < 0.01) or AAV10 tau (P < 0.05) than AAV2 tau (N = 3–4/group).
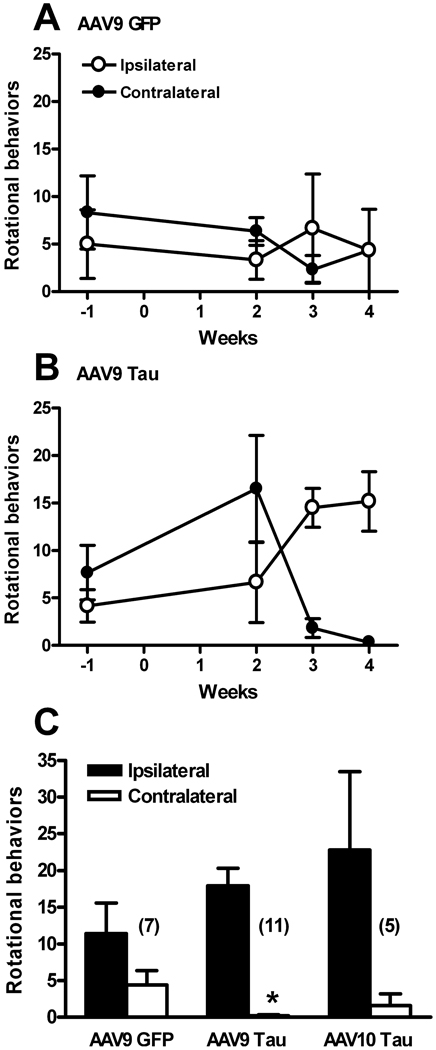
Rotational behavior
The purpose of the amphetamine challenge was to probe whether a side-to-side rotational bias was present within each unilateral treatment group. A large unilateral loss of nigrostriatal DA, 50% or more, leads to turning toward the side of DA depletion (Hefti et al., 1980). A time-course for the AAV9 GFP vector in three rats is shown in Fig. 9A and for the AAV9 tau vector in six rats in Fig. 9B. Repeated-measures anova showed no effect of time, turning direction or interaction in the GFP group. There was no effect of time or turning direction in the AAV9 tau either, although there was an interaction (F3,10 = 10.20, P < 0.0001), supporting the treatment affecting the turning direction in a time-dependent manner. The 4 week data in Fig. 9A and B were combined with other rats run at this interval only (Fig. 9C). At 4 weeks, there was no turning bias in the AAV9 GFP group (N = 7), although there was in the AAV9 tau group (P < 0.0001, t-test, N = 11). Despite 14-fold more ipsilateral than contralateral turns in the AAV10 tau group (N = 5), the difference was not significant. In a group of five rats lesioned with 6-OHDA and run for rotational behavior at 2 weeks post-lesion, there were 84.0 ± 13.4 ipsilateral rotations and no contralateral rotations. The rotational bias therefore occurred earlier in the case of 6-OHDA compared with the AAV9 tau, consistent with the time-course of DA loss (Fig. 2) and the behavioral effect was more pronounced with 6-OHDA.
Fig. 9.
Amphetamine-stimulated rotational behavior. (A) Rotational behavior at 1 week prior to and 2, 3 and 4 weeks after AAV9 GFP gene transfer (N = 3). (B) Rotational behavior before and after AAV9 tau gene transfer (N = 6). There was a signficiant interaction in the repeated-measures anova for turning direction over time in the AAV9 tau group (P < 0.0001). (C) 4 week data, N-value indicated. There was a significant turning bias in the AAV9 tau group (*P < 0.0001, t-test).
Discussion
Modeling tau diseases resulted in graded disease states with respect to the early expression levels and severity of striatal DA loss. More molecules of tau got expressed in the brain at 1 and 2 weeks from AAV9 or AAV10 compared with AAV2 or AAV8, presumably due to higher vector copy number in cells infected with AAV9 or AAV10 as the expression cassette was the same for each. To our knowledge, this marks the first example of AAV vector-based models approaching full efficacy for lesioning the nigrostriatal DA system. Earlier models with alpha-synuclein AAV produced partial lesions that were not behaviorally significant (Klein et al., 2002). It is clear that tau is more neurotoxic than alpha-synuclein, with matching doses of alpha-synuclein vector causing strong expression without affecting DA neurons, although we would expect that higher doses of the AAV9 alpha-synuclein, and longer intervals, would cause dopaminergic neurodegeneration (Kirik et al., 2002; Klein et al., 2002; Yamada et al., 2004; St Martin et al., 2007; Gorbatyuk et al., 2008). We previously observed that high doses of AAV8 GFP vector, above 1 × 1010 vg, led to obvious dopaminergic cell loss (Klein et al., 2006). We did not observe any trend towards dopaminergic cell loss with the AAV9 GFP in this study at a dose of 3 × 109 vg, so the tau-induced neurodegeneration was specific relative to GFP or alpha-synuclein expression. The AAV9 tau gene transfer clearly killed DA neurons (neuronal nuclei staining), rather than down-regulated their phenotypic marker (TH staining), and the degeneration was selective for SN pars compacta neurons, which are predominantly dopaminergic, rather than SN pars reticulata neurons, which are predominantly GABAergic (Oertel et al., 1982). The chemical neurotoxin 6-OHDA eliminated striatal DA more rapidly than the AAV9 tau gene transfer vector by 1 week but the AAV9 appeared equally robust by 4 weeks. At 4 weeks, we estimated 88% loss of TH neurons, 97% loss of striatal DA levels and 94% loss of TH immunoreactivity in the striatum with the AAV9 tau relative to non-transduced tissue. The specific tau vector disease has nearly full efficacy in terms of DA ablation, with a well-defined end-point, which could be important for screening therapeutics. Efficacious drugs in gene vector-based animal models such as this one will hopefully translate better in clinical trials than drugs developed in chemical lesion models (Parkinson Study Group PRECEPT Investigators, 2007).
Despite the robust losses in DA and dopaminergic markers, there was a paucity of rotational behavior with the AAV9 tau relative to 6-OHDA. We assayed rotational behavior as a confirmation that functional lesions were present, although the amount of turning relative to the loss of DA was mismatched, with the behavioral results indicating incomplete lesioning. Amphetamine-stimulated rotational behavior is a threshold effect rather than a graded one and there is poor correlation of turning over the entire range of lesioning (Hefti et al., 1980; Carman et al., 1991; Hudson et al., 1993; Lee et al., 1996). However, we cannot rule out inherent functional differences in the gene transfer vs. chemical neurotoxin models in terms of compensation, sprouting and state of quiescence in remaining cells, which could dampen the behavioral outcome.
Tau expression peaked earlier with AAV9 or AAV10 relative to AAV2 or AAV8, and the enhanced early expression was coupled with pronounced loss of DA in the striatum. The AAV8 appeared to catch up with AAV9 or AAV10 by 4 weeks at least partially in terms of tau expression and loss of DA and TH fiber density, whereas the AAV2-based tau disease was less robust throughout the study. The AAV8 tau led to more robust effects than AAV2 tau with respect to tau expression at 4 weeks and DA loss at 4 weeks, which is consistent with the greater loss of DA neurons that we previously observed with AAV8 tau relative to AAV2 tau (Klein et al., 2006). When directly comparing the AAV8 tau with AAV9 tau, there was greater tau expression at 1 and 2 weeks, greater DA loss at 2 and 4 weeks, and effects on DA turnover with the AAV9, but not AAV8, at 4 weeks. Effects with AAV9 tau were enhanced relative to AAV10 tau with respect to tau expression at 2 weeks and DA turnover at 4 weeks. The powerful disease induced by the AAV9 tau led to loss of DA and TH fiber density in the striatum, loss of TH cells in the SN and a motor behavior effect. Noteworthy was the sequence of tau expression by 1 week, loss of striatal DA by 2 weeks and the behavioral effect at 3–4 weeks with the AAV9 tau. Although we did not investigate the tau neurofibrillary pathology in this study as we have previously (Klein et al., 2004, 2005), we hypothesize that the rapid tau-specific killing of DA neurons does not require neurofibrillary tangle formation.
There are many transgenic mouse and other animal models for tau pathology (Lee et al., 2005). The tau AAV vector strategy is a useful complement for inducing tau disease in normal adult animals. AAV vector gene transfer is widely used in basic and clinical neuroscience, and is a complement to existing chemical and germ-line transgenic models for neurodegenerative diseases because it studies the pathological impact of a specific gene product in specific disease-related brain regions and in naive adults, who may not adapt as much as with germ-line models (Senut et al., 2000; Furler et al., 2001; Kirik et al., 2002; Klein et al., 2002; Yamada et al., 2004; Gong et al., 2006; St Martin et al., 2007). The vectors used in combination with amyloid-bearing transgenic mice study specific gene products that may alter the course of Alzheimer’s disease (Zhang et al., 2003; Klein et al., 2004; Hara et al., 2004; Iwata et al., 2004; Fukuchi et al., 2006; Levites et al., 2006). In searching for new therapies for neurodegenerative diseases, gene therapy is worth considering when there are inherited familial forms (Hutton et al., 1998; Mirra et al., 1999; Poorkaj et al., 2002) and when there are no effective drugs. AAV-based gene therapy clinical trials are underway for Parkinson’s disease, showing a good safety outcome (Kaplitt et al., 2007). However, the tragedy in an AAV clinical trial for rheumatoid arthritis in 2007 underscores the potential risk (Friedmann, 2007).
The different tau vectors caused graded levels of DA loss, which could platform for mimicking early pre-symptomatic and later, more debilitating stages of neurodegenerative diseases. The nearly complete effect over a 1 month time-course that is possible with AAV9 or AAV10 tau vectors will hopefully aid in studying the disease processes of progressive supranuclear palsy and other tauopathies. The neurodegenerative diseases characterized by neurofibrillary tangles are insidious, with limited therapeutic options, so new therapies should be explored. Disease modeling will hopefully generate leads for new therapies, including gene therapy. Although this study demonstrates that tau expression levels govern the disease outcome, the data do not shed light on tau’s neurodegenerative mechanism. Recent DNA microarray work demonstrates that an immune response is involved in the model (unpublished data), consistent with the microgliosis that is observed in progressive supranuclear palsy (Ishizawa et al., 2000) and in tau transgenic mice (Yoshiyama et al., 2007). The short time-course could be useful to pinpoint the sequelae.
Acknowledgements
Phillip Henning assisted and Richard Zweig critiqued. NIH/NINDS R01 NS048450 and The Society for Progressive Supranuclear Palsy supported the work.
Abbreviations
- AAV
adeno-associated virus
- DA
dopamine
- GFP
green fluorescent protein
- 6-OHDA
6-hydroxydopamine
- PBS
phosphate-buffered saline
- SN
substantia nigra
- TH
tyrosine hydroxylase
- vg
vector genomes
References
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. (Berl.) 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior. Brain Res. 1991;553:275–283. doi: 10.1016/0006-8993(91)90835-j. [DOI] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9 & rh10 in the mouse brain. Mol. Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Tau and synuclein and their role in neuropathology. Brain Pathol. 1999;9:657–661. doi: 10.1111/j.1750-3639.1999.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann. Neurol. 2000;47:374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Friedmann T. A new serious adverse event in a gene therapy study. Mol. Ther. 2007;15:1899–1900. doi: 10.1038/sj.mt.6300328. [DOI] [PubMed] [Google Scholar]
- Fukuchi K, Tahara K, Kim HD, Maxwell JA, Lewis TL, Accavitti-Loper MA, Kim H, Ponnazhagan S, Lalonde R. Anti-Abeta single-chain antibody delivery via adeno-associated virus for treatment of Alzheimer’s disease. Neurobiol. Dis. 2006;23:502–511. doi: 10.1016/j.nbd.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furler S, Paterna JC, Weibel M, Bueler H. Recombinant AAV vectors containing the foot and mouth disease virus 2A sequence confer efficient bicistronic gene expression in cultured cells and rat substantia nigra neurons. Gene Ther. 2001;8:864–873. doi: 10.1038/sj.gt.3301469. [DOI] [PubMed] [Google Scholar]
- Gong Y, Meyer EM, Meyers CA, Klein RL, King MA, Hughes JA. Memory related deficits following selective hippocampal expression of Swedish mutation amyloid precursor protein in the rat. Exp. Neurol. 2006;200:371–377. doi: 10.1016/j.expneurol.2006.02.136. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk O, Shoudong L, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc. Natl Acad. Sci. U.S.A. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Monsonego A, Yuasa K, Adachi K, Xiao X, Takeda S, Takahashi K, Weiner HL, Tabira T. Development of a safe oral Abeta vaccine using recombinant adeno-associated virus vector for Alzheimer’s disease. J. Alz. Dis. 2004;6:483–488. doi: 10.3233/jad-2004-6504. [DOI] [PubMed] [Google Scholar]
- Hefti F, Melamed E, Wurtman RJ. Partial lesions of the dopaminergic nigrostriatal system in rat brain: biochemical characterization. Brain Res. 1980;195:123–137. doi: 10.1016/0006-8993(80)90871-9. [DOI] [PubMed] [Google Scholar]
- Hefti F, Enz A, Melamed E. Partial lesions of the nigrostriatal pathway in the rat. Acceleration of transmitter synthesis and release of surviving dopaminergic neurones by drugs. Neuropharmacology. 1985;24:19–23. doi: 10.1016/0028-3908(85)90090-5. [DOI] [PubMed] [Google Scholar]
- Hudson JL, van Horne CG, Stromberg I, Brock S, Clayton J, Masserano J, Hoffer BJ, Gerhardt GA. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993;626:167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Lin WL, Tiseo P, Honer WG, Davies P, Dickson DW. A qualitative and quantitative study of grumose degeneration in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2000;59:513–524. doi: 10.1093/jnen/59.6.513. [DOI] [PubMed] [Google Scholar]
- Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, Gerard NP, Gerard C, Ozawa K, Saido TC. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J. Neurosci. 2004;24:991–998. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease. and open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- King MA, Scotty N, Klein RL, Meyer EM. Particle detection, number estimation, and feature measurement in gene transfer studies: optical fractionator stereology integrated with digital image processing and analysis. Methods. 2002;28:293–299. doi: 10.1016/s1046-2023(02)00235-9. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J. Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, King MA, Hamby ME, Meyer EM. Dopaminergic cell loss induced by human A30P alpha-synuclein gene transfer to the rat substantia nigra. Hum. Gene Ther. 2002;13:605–612. doi: 10.1089/10430340252837206. [DOI] [PubMed] [Google Scholar]
- Klein RL, Lin WL, Dickson DW, Lewis J, Hutton M, Duff K, Meyer EM, King MA. Rapid neurofibrillary tangle formation after localized gene transfer of mutated tau. Am. J. Pathol. 2004;164:347–353. doi: 10.1016/S0002-9440(10)63124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Lin WL, Dickson DW. Tau gene transfer, but not alpha-synuclein, induces both progressive dopamine neuron degeneration and rotational behavior in the rat. Neurobiol. Dis. 2005;20:64–73. doi: 10.1016/j.nbd.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol. Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV 8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol. Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Van Broekhoven C. Frontotemporal lobar degeneration: Current concepts in light of recent advances. Brain Pathol. 2007;17:104–113. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Sauer H, Björklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Lee VM, Kenyon TK, Trojanowski JQ. Transgenic animal models of tauopathies. Biochim. Biophys. Acta. 2005;1739:251–259. doi: 10.1016/j.bbadis.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Levites Y, Jansen K, Smithson LA, Dakin R, Holloway VM, Das P, Golde TE. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta40 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J. Neurosci. 2006;26:11 923–11 928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA, Levey AI, Jones R, Green J, Shoffner JM, Wainer BH, Schmidt ML, Trojanowski JQ, Ghetti B. Tau pathology in a family with dementia and a P301L mutation in tau. J. Neuropathol. Exp. Neurol. 1999;58:335–345. doi: 10.1097/00005072-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Oertel WH, Tappaz ML, Berod A, Mugnaini E. Two-color immunohistochemistry for dopamine and GABA neurons in rat substantia nigra and zona incerta. Brain Res. Bull. 1982;9:463–474. doi: 10.1016/0361-9230(82)90155-1. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edn. San Diego: Academic Press; 1998. [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, Schellenberg GD. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann. Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Gilley DW, Kvarnberg DE, Mufson EJ, Bennett DA. Improved detection of substantia nigra pathology in Alzheimer’s disease. J. Histochem. Cytochem. 2002;50:99–106. doi: 10.1177/002215540205000111. [DOI] [PubMed] [Google Scholar]
- Senut MC, Suhr ST, Kaspar B, Gage FH. Intraneuronal aggregate formation and cell death after viral expression of expanded polyglutamine tracts in the adult rat brain. J. Neurosci. 2000;20:219–229. doi: 10.1523/JNEUROSCI.20-01-00219.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi D, Hackett NR, Peterson DA, Stratton J, Baad M, Travis KM, Wilson JM, Crystal RG. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 Rhesus Macaque-derived adeno-associated virus vector. Mol. Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- St Martin JL, Klucken J, Outeiro TF, Nguyen P, Keller-McGandy C, Cantuti-Castelvetri I, Grammatopoulos TN, Standaert DG, Hyman BT, McLean PJ. Dopaminergic neuron loss and up-regulation of chaperone protein mRNA induced by targeted over-expression of alpha-synuclein in mouse substantia nigra. J. Neurochem. 2007;100:1449–1457. doi: 10.1111/j.1471-4159.2006.04310.x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. 1994;87:545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Over-expression of alpha synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alph-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J. Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski TQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H, Kong Q, Chen D, Ba D, He W. A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2003;14:365–379. doi: 10.1016/j.nbd.2003.07.005. [DOI] [PubMed] [Google Scholar]