Abstract
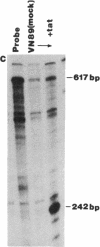
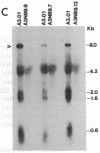
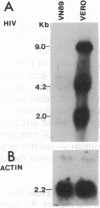
We have prepared stable cell lines, derived from Vero cells and A3.01 cells, that express a hybrid human alpha 2-interferon gene under control of the human immunodeficiency virus (HIV) long terminal repeat. These cells constitutively produced low levels (50-150 units/ml) of alpha 2-interferon. However, high levels of interferon (10(3) units/ml) could be induced upon trans-activation by the product of the tat gene (pIIIextatIII), and de novo infection by HIV resulted in a moderate increase (400 units/ml) in alpha 2-interferon synthesis. In contrast to the fully permissive HIV replication, in transfected Vero cells or infected A3.01 cells, the transcription and replication of HIV in Vero or A3.01 cells containing the HIV long terminal repeat--alpha 2-interferon hybrid gene (VN89 and A3N89 cells, respectively) was completely inhibited. These data suggest that virus-trans-activated alpha 2-interferon synthesis can be used as a selective inhibitor of HIV replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988 Sep 29;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Bednarik D. P., Mosca J. D., Raj N. B. Methylation as a modulator of expression of human immunodeficiency virus. J Virol. 1987 Apr;61(4):1253–1257. doi: 10.1128/jvi.61.4.1253-1257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benech P., Merlin G., Revel M., Chebath J. 3' end structure of the human (2'-5') oligo A synthetase gene: prediction of two distinct proteins with cell type-specific expression. Nucleic Acids Res. 1985 Feb 25;13(4):1267–1281. doi: 10.1093/nar/13.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilello J. A., Wivel N. A., Pitha P. M. Effect of interferon on the replication of mink cell focus-inducing virus in murine cells: synthesis, processing, assembly, and release of viral proteins. J Virol. 1982 Jul;43(1):213–222. doi: 10.1128/jvi.43.1.213-222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrom D. C., Fahey D., Kutny R., Korant B. D., Knight E., Jr Molecular characterization of the interferon-induced 15-kDa protein. Molecular cloning and nucleotide and amino acid sequence. J Biol Chem. 1986 Jul 5;261(19):8811–8816. [PubMed] [Google Scholar]
- Broder S. Pathogenic human retroviruses. N Engl J Med. 1988 Jan 28;318(4):243–245. doi: 10.1056/NEJM198801283180409. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Valentine F., Hojvat S., Allain J. P., Rubinstein P., Mirabile M., Czelusniak S., Leuther M., Baker L., Friedman-Kien A. E. Detection of HIV antigen and specific antibodies to HIV core and envelope proteins in sera of patients with HIV infection. Blood. 1987 Aug;70(2):575–578. [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Chen L., Mory Y., Zilberstein A., Revel M. Growth inhibition of human breast carcinoma and leukemia/lymphoma cell lines by recombinant interferon-beta 2. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8037–8041. doi: 10.1073/pnas.85.21.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. W., Morgan W. M., Hardy A. M., Jaffe H. W., Darrow W. W., Dowdle W. R. The epidemiology of AIDS: current status and future prospects. Science. 1985 Sep 27;229(4720):1352–1357. doi: 10.1126/science.2994217. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Ziemin S., Le Beau M. M., Pitha P., Smith S. D., Chilcote R. R., Rowley J. D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Folks T., Powell D. M., Lightfoote M. M., Benn S., Martin M. A., Fauci A. S. Induction of HTLV-III/LAV from a nonvirus-producing T-cell line: implications for latency. Science. 1986 Feb 7;231(4738):600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- Friedman A. D., Triezenberg S. J., McKnight S. L. Expression of a truncated viral trans-activator selectively impedes lytic infection by its cognate virus. Nature. 1988 Sep 29;335(6189):452–454. doi: 10.1038/335452a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Leung D. W., Dull T. J., Gross M., Lawn R. M., McCandliss R., Seeburg P. H., Ullrich A., Yelverton E., Gray P. W. The structure of eight distinct cloned human leukocyte interferon cDNAs. Nature. 1981 Mar 5;290(5801):20–26. doi: 10.1038/290020a0. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Lange J. M., Paul D. A., Dawson G. J. Antigenemia and antibody titers to core and envelope antigens in AIDS, AIDS-related complex, and subclinical human immunodeficiency virus infection. J Infect Dis. 1987 Mar;155(3):558–560. doi: 10.1093/infdis/155.3.558. [DOI] [PubMed] [Google Scholar]
- Higgins J. R., Pedersen N. C., Carlson J. R. Detection and differentiation by sandwich enzyme-linked immunosorbent assay of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus- and acquired immunodeficiency syndrome-associated retroviruslike clinical isolates. J Clin Microbiol. 1986 Sep;24(3):424–430. doi: 10.1128/jcm.24.3.424-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Hartshorn K. L., Rota T. R., Andrews C. A., Kaplan J. C., Schooley R. T., Hirsch M. S. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet. 1985 Mar 16;1(8429):602–604. doi: 10.1016/s0140-6736(85)92144-0. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. The (2'-5') oligoadenylate (pppA2'-5'A2'-5'A) synthetase and protein kinase(s) from interferon-treated cells. Eur J Biochem. 1979 Feb 1;93(3):515–526. doi: 10.1111/j.1432-1033.1979.tb12850.x. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Pitha P. M., Rowe W. P., Oxman M. N. Effect of interferon on exogenous, endogenous, and chroniv murine leukemia virus infection. Virology. 1976 Apr;70(2):324–338. doi: 10.1016/0042-6822(76)90275-0. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Hirsch M. S. Interferon and human immunodeficiency virus infection. Interferon. 1987;9:113–127. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Kellum M., Kelley K. A., Antrobus S., Pitha P. M. Differential regulation of interferon synthesis in lymphoblastoid cells. J Interferon Res. 1985 Summer;5(3):493–510. doi: 10.1089/jir.1985.5.493. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Till M. A., Ghetie V., Gregory T., Patzer E. J., Porter J. P., Uhr J. W., Capon D. J., Vitetta E. S. HIV-infected cells are killed by rCD4-ricin A chain. Science. 1988 Nov 25;242(4882):1166–1168. doi: 10.1126/science.2847316. [DOI] [PubMed] [Google Scholar]
- Vengris V. E., Stollar B. D., Pitha P. M. Interferon externalization by producing cell before induction of antiviral state. Virology. 1975 Jun;65(2):410–417. doi: 10.1016/0042-6822(75)90046-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Barré-Sinoussi F., Bolton V., Pedersen N. C., Gardner M. B. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986 Apr;6(2):143–152. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]