Abstract
Two recurring problems with stem/neural progenitor cell (NPC) transplantation therapies for spinal cord injury (SCI) are poor cell survival and uncontrolled cell differentiation. The current study evaluated the viability and differentiation of embryonic stem cell-derived neural progenitor cells (ESNPCs) transplanted within fibrin scaffolds containing growth factors (GFs) and a heparin-binding delivery system (HBDS) to enhance cell survival and direct differentiation into neurons. Mouse ESNPCs were generated from mouse embryonic stem cells (ESCs) using a 4−/4+ retinoic acid (RA) induction protocol that resulted in a population of cells that was 70% nestin positive NPCs. The ESNPCs were transplanted directly into a rat subacute dorsal hemisection lesion SCI model. ESNPCs were either encapsulated in a fibrin scaffold; encapsulated in fibrin containing the HBDS, neurotrophin-3 (NT-3) and platelet derived growth factor (PDGF-AA); or encapsulated in fibrin scaffolds with NT-3 and PDGF-AA without the HBDS. We report that the combination of GFs and fibrin scaffold (without HBDS) enhanced the total number of ESNPCs present in the treated spinal cords and increased the number of ESNPC-derived NeuN positive neurons 8 weeks after transplantation. All experimental groups treated with ESNPCs exhibited an increase in behavioral function 4 weeks after transplantation. In a subset of animals, the ESNPCs over-proliferated as evidenced by SSEA-1 positive/Ki67 positive ESCs found at 4 and 8 weeks. These results demonstrate the potential of tissue-engineered fibrin scaffolds to enhance the survival of NPCs and highlight the need to purify cell populations used in therapies for SCI.
Keywords: cell transplantation, regenerative medicine, biomaterials scaffold, growth factor
Introduction
Damage to the spinal cord causes the disruption of ascending and descending axonal tracts and the necrosis of cells around the site of injury. The overall result within the spinal cord is an environment that is intensely inhibitory to regeneration and functional recovery. Stem cells provide a potential source of cells to repopulate the damaged spinal cord and aid in functional recovery by replacing damaged circuits, increasing plasticity, and promoting the cell survival and the regeneration of host axons.
Neural progenitor cells (NPCs), a type of stem cell, are restricted to differentiate into one of the three major neural cell fates1. Several studies using NPCs transplanted into the injured spinal cord have consistently found that the survival of the transplanted cells is poor and differentiation is limited to a predominately glial fate2–4. One hypothesis for the tendency for glial differentiation following transplantation was given by Cao et al. who have found that the inflammatory environment in the spinal cord following injury inhibits differentiation of progenitors into a neuronal fate5. Poor survival of transplanted NPCs following SCI has also been attributed to the inflammatory environment of the injured spinal cord, which limits cell survival as well as integration with host tissue 4, 6, 7. Given the inhibitory nature of spinal cord lesions, additional factors are required for provide a more favorable environment for cell transplantation and nerve regeneration.
The adult spinal cord lesion lacks the molecular signals normally present during development that promote cell survival and differentiation. Nakamura et al. demonstrated that in a favorable environment (injured neonatal spinal cord) transplanted NPCs cells survive, integrate with host tissue, and differentiate into neurons and oligodendrocytes 7. Furthermore, they found that repopulation of the injured spinal cord with neurons and oligodendrocytes contributed significantly to plasticity and a return of lost function. Establishing a favorable environment in the injured adult spinal cord may promote survival, integration, and differentiation of transplanted stem cells into neurons leading to enhanced regeneration and functional recovery.
Research in our laboratory is focused on using tissue-engineered fibrin scaffolds to provide an environment in the adult spinal cord that is conducive to survival and differentiation of transplanted NPCs. To this end, the current study evaluated the effect of fibrin scaffolds on ESNPCs survival and differentiation following transplantation into a subacute model of SCI. ESNPCs were derived from mouse ESCs using a 4−/4+ RA induction protocol8. The ESNPCs were then transplanted as whole embryoid bodies (EBs) containing ~70% nestin positive ESNPCs into a subacute dorsal hemisection SCI as described previously9. The ESNPCs were encapsulated in fibrin scaffolds containing the heparin-binding delivery system (HBDS) that allowed for the controlled delivery of NT-3 and PDGF-AA10–14. The HBDS consists of a heparin-binding peptide, which is covalently crosslinked into fibrin scaffolds during polymerization by Factor XIIIa, heparin, which binds to the peptide, and a heparin-binding growth factor, which is sequestered by heparin within the scaffold10, 15. NT-3 and PDGF-AA were selected because in combination they have been found to enhance the survival of ESNPCs and increase the fraction of neurons obtained in vitro, both when in the culture medium and released from the HBDS11, 16. Previously, a short term (2 week) study evaluated fibrin scaffolds containing the ESNPCs and GFs in a subacute SCI model and found that the presence of NT-3 and PDGF delivered from the HBDS in the scaffold enhanced ESNPC survival and proliferation17. The current study evaluated the ability of tissue-engineered fibrin scaffolds to enhance the survival and neuronal differentiation of transplanted ESNPCs in a long term spinal cord injury model (4 and 8 weeks). Functional recovery of the experimental groups was evaluated weekly following transplantation to determine the effects of ESNPCs and growth factor delivery from the HBDS.
Experimental Section
Embryonic stem cell culture and embryoid body formation
CE3 mouse ESCs genetically engineered to express green fluorescent protein (GFP) under the β-actin promoter were obtained from D. Gottlieb and were cultured in T25 flasks (Fisher, Pittsburgh, PA, http://www.fishersci.com) coated with a 0.1% gelatin solution (Sigma, Saint Louis, MO, http://www.sigmaaldrich.com) in the presence of 1000 U/mL leukemia inhibitory factor (LIF; Chemicon, Temecula, CA, http://www.chemicon.com) and 10−4 M β-mercaptoethanol (BME; Invitrogen, Grand Island, NY, http://www.invitrogen.com) to maintain their undifferentiated state. These cells were grown in complete media consisting of Dubecco’s modified eagle media (DMEM) (Invitrogen) supplemented with 10% newborn calf serum (NBCS; Invitrogen), 10% fetal bovine serum (FBS, Invitrogen), and 0.3 M of each of the following nucleosides: adenosine, guanosine, cytosine, thymidine, and uridine (Sigma). ESCs were passaged at a ratio of 1:5 every two days.
Undifferentiated ES cells were induced to form EBs containing ESNPCs using the 4−/4+ RA treatment protocol 8. EBs were cultured in 100 mm Petri dishes (Fisher) coated with a 0.1% agar solution (MidSci, Saint Louis, MO, http://midsci.com/) in complete media in the absence of LIF and BME for 4 days. 500 nM RA (Sigma) was then added to the complete media for the final 4 days of culture. The media was changed every other day during this eight day process. The resulting EBs were 70% nestin positive NPCs 16.
Tissue-engineered fibrin scaffold preparation
All materials were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise noted. Fibrin scaffolds were made as described previously by mixing the following components: human plasminogen-free fibrinogen containing Factor XIII (10 mg/mL, Sigma), CaCl2 (5mM), and thrombin (12.5 NIH units/mL, Sigma) in Tris-buffered saline (TBS, 137 mM NaCl, 2.7 mM KCl, 33 mM Tris, pH 7.4)11. The bi-domain affinity peptide for the HBDS, denoted ATIII, was synthesized by standard solid phase Fmoc chemistry as described previously 10. In scaffolds containing the HBDS, ATIII peptide (0.25 mM) and heparin (6.25 μM, Sigma, sodium salt from porcine intestinal mucosa) were added to the fibrin polymerization mixture. In scaffolds containing growth factors, PDGF AA (20 ng, R&D Systems, Minneapolis, MN) and NT-3 (125 ng, Peprotech, Rock Hill NJ) were added to the fibrin polymerization mixture. In scaffolds containing ESNPCs, the EBs were added directly to the polymerization mixture prior to polymerization as described previously 17. Polymerized fibrin scaffolds (10 μL in volume) were formed by ejecting the polymerization mixture from a 20 μL pipette tip such that a sphere of the mixture formed on the tip of the pipette. The sphere was then allowed to polymerize on the pipette tip for 5 min prior to implantation. A second 20 μL polymerization solution devoid of EBs was then injected directly onto the sphere already in the injury site and allowed to polymerize in the lesion site.
In-vivo studies – dorsal hemisection subacute SCI and scaffold implantation
All experimental procedures on animals complied with the Guide for the Care and Use of Laboratory Animals and were performed under the supervision of the Division of Comparative Medicine at Washington University. Long-Evans female rats (250–275 g, Harlan, Indianapolis, IND) were anesthetized using 4% isoflurane gas (Vedco Inc., St Josephs, MO). The skin and muscle overlying the spinal column were incised and dissected away from the spinal column. Clamps were attached to the spinous processes and a rigid frame was used to immobilize the spinal column. A dorsal laminectomy was performed using fine rongeurs at level T-9 to expose the spinal cord. A lateral slit in the dura was made, and microdissection scissors mounted to a micromanipulator were lowered into the spinal cord 1.2 mm from the dorsal spinal cord surface. Using the microdissection scissors, a lateral incision was made to form a complete dorsal hemisection of the spinal cord. Finally the microdissection scissors were run through the incision to assure the hemisection was complete. The cord was then covered with a piece of artificial dura (generous gift of Synovis Surgical Innovations, St Paul MN), the muscles were sutured with degradable sutures, and the skin was stapled close. Two weeks following initial injury the lesion was re-exposed, and scar tissue was removed from the wound site of control and experimental groups in preparation of fibrin scaffold transplantation.
ESNPCs were transplanted as aggregated EBs (~10,000 ESNPCs in each EB). As described above, scar tissue was removed and either no treatment (control), a fibrin sphere alone (Fibrin), 10 EBs placed directly in the lesion site with no fibrin (10EB no Fibrin), a fibrin sphere containing 10 EBs (10EB + Fibrin), a fibrin sphere containing 10 EBs and containing the HBDS with 125ng NT-3 and 20ng PDGF-AA (10EB + DS + GF), or fibrin sphere containing 10 EBs with 125ng NT-3 and 20ng PDGF-AA with no HBDS (10EB + GF no DS) were placed in the injury site. The untreated control group received the same surgical removal of scar tissue but no treatment with fibrin scaffolds or cells. Table 1 shows a list of group numbers for each experimental group and relevant analysis.
Table 1.
Experimental Groups and Sample Size for Analysis
| Experimental Groups | Total “n” for study | Total “n” for Stereology | Total “n” for Differentiation Analysis | Total “n” for Behavioral analysis | ||||
|---|---|---|---|---|---|---|---|---|
| 4 week | 8 week | 4 week | 8 week | 4 week | 8 week | 1–4 week | 5–8 week | |
| 10EB no Fibrin | 8 | 10 | 6 | 7 | 6 | 7 | 12 | 7 |
| 10EB + Fibrin | 7 | 9 | 7 | 6 | 7 | 6 | 13 | 6 |
| 10EB + DS + GF | 9 | 10 | 8 | 5 | 8 | 5 | 13 | 5 |
| 10EB + GF no DS | 8 | 8 | 8 | 7 | 8 | 7 | 15 | 7 |
| Control | 6 | 8 | 14 | 8 | ||||
| Fibrin | 6 | 10 | 16 | 10 | ||||
| Includes rats with over-proliferation | √ | √ | ||||||
| Includes rats with no ESNPCs at end time point | √ | √ | √ | √ | √ | √ | √ | √ |
Animals were given cefazolin (15 mg/kg, twice a day) for five days following each surgical procedure as prophylactic against urinary tract infections. Bladders were expressed manually twice a day until they regained bladder control, approximately 5 to 6 days post initial injury. Animals with transplanted ESNPCs were immune suppressed with 10 mg/kg daily of cyclosporine (Novartis, East Hanover, New Jersey) because mouse ESCs were being transplanted into rats. The animals were euthanized at 4 and 8 weeks after transplantation using an overdose of Euthasol (Virbac, France). After transcardial perfusion using 4% paraformaldehyde (Sigma), spinal cords were dissected and post-fixed in 4% paraformaldehyde solution overnight. The cords were then cryoprotected in a 30% sucrose solution in phosphate buffered saline in preparation for frozen sections. The spinal cord was embedded in Tissue-Tek OCT compound Mounting Media (Sakura Finetek, Torence, CA) and cut into 20 μm sagittal sections using a cryostat.
Immunohistochemistry
Immunohistochemistry was used to analyze the differentiation of the transplanted ESNPCs and morphological aspects of the spinal cord from each experimental group. Sections were washed with phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton X-100 for 5 min. After washes in PBS, the sections were blocked with 10% bovine serum albumin (BSA, Sigma) and 2% normal goat serum (NGS, Sigma). Primary antibodies against glial fibrillary acidic protein (GFAP, rabbit polyclonal, recognizing astrocytes, 1:4, ImmunoStar, Hudson, WI), neuronal class III β-tubulin (Tuj1, mouse monoclonal, recognizing neurons, 1:200, Covance Research Products, Berkeley, CA), ED-1 (mouse monoclonal, recognizing macrophages/microglia, 1:100, Serotec, Oxford, UK), anti-oligodendrocyte marker O4 (O4, mouse monoclonal, recognizes oligodendrocytes, 1:200, Millipore http://www.millipore.com), anti-nestin (nestin, mouse monoclonal, recognizes NPCs, 1:200, Millipore), anti-neuronal nuclei (NeuN, mouse monoclonal, recognizes mature neuronal nuclei, 1:500, Millipore) and anti-SSEA-1 (SSEA-1, mouse monoclonal, recognizes mouse ESCs, 1:50, Millipore) were used to evaluate each section. Analysis of neuronal specific differentiation of transplanted ESNPCs was performed using the following primary antibodies; anti-serotonin (5HT, mouse monoclonal, recognizes serotonergic neurons, 1:100, Abcam Inc, Cambridge, MA), anti-tyrosine hydroxylase (TH, mouse monoclonal, recognizes dopaminergic neurons, 1:200 Millipore), anti-choline acetyltransferase (ChAT, mouse monoclonal, recognizes cholinergic neurons, 1:100, Millipore), anti-GAD67 (GAD, mouse monoclonal, 1:1000, recognizes GABAergic neurons, Millipore), and anti-vesicular glutamate transporter 2 (VGLUT2, mouse polyclonal, recognizes glutamatergic neurons, 1:100, Millipore). The primary antibody anti-Ki67 (rabbit monoclonal, recognizes proliferating cells, 1:100, Abcam) was used to identify transplanted cells that were still proliferating. Finally, appropriate secondary antibodies (Alexa Fluor 555, and 647 conjugated, 1:300 Invitrogen) diluted with 2% NGS were used and each section was stained with Hoechst nuclear stain (1:1000 Molecular Probes).
ESNPC proliferation
In some of the rats from all of the groups receiving ESNPCs, ESNPC over- proliferation was observed that overtook healthy tissue in the spinal cord. The animals were identified by distinct overgrowth of GFP positive ESNPCs that spanned from the dorsal to ventral surface of the spinal cord and a dramatic loss of behavioral function compared to controls. These animals were excluded from evaluations of transplant cell survival, differentiation and behavioral analysis.
Stereological analysis of transplanted cells
Sagittal sections of the frozen spinal cords were taken resulting in ~115 frozen serial sections. Every fifteenth section was then analyzed using stereological techniques resulting in ~7 sections from each animal being analyzed. Stereological cell counts of GFP+ cells to determine cell survival were performed on the fifteenth equidistant frozen section from each animal and counterstained with the nuclear dye Hoechst. To determine the number of GFP+ mature neurons present at the end of 4 and 8 weeks, stereological cell counts of GFP/NeuN double-positive cells was performed on the fifteenth equidistant frozen section from each animal. All stereological counts were performed using the Stereo Investigator Software using the optical fractionator function (Version 7, MBF Biosciences, Williston, VT), under 40× magnification and verified with a Gunderson coefficient of error less than 0.08. Animals with ESNPC over-proliferation were excluded from this analysis. Animals from each group in which ESNPCs were not seen in the spinal cord at the end of 4 or 8 weeks were included in the analysis as 0.
Differentiation analysis of transplanted ESNPCs
Sagittal sections of the frozen spinal cords were taken resulting in ~115 frozen serial sections. Every fifteenth section was then analyzed using stereological techniques resulting in ~7 sections from each animal being analyzed. The lesion area of every fifteenth serial spinal cord section was imaged at 40× magnification using an Olympus IX70 microscope (Olympus America, Melville, NY) and Magnafire Camera (Optronics, Goleta, CA). Imaging resulted in multiple 40× images per section that encompassed the entire lesion site, which were spliced together using Photoshop (Adobe, San Jose, CA) to yield a complete picture of the lesion. To ensure accurate quantification of GFP positive ESNPCs, control sections that did not receive cell transplants were imaged under the same conditions to account for auto fluorescence. The area of GFP positive pixels (representing ESNPCs), and the area of pixels positive for both GFP and one of the 5 other markers (Tuj1 (neurons), GFAP (astrocytes), O4 (oligodendrocytes), nestin (NPCs) or SSEA-1 (mouse ESCs)) was measured in each section using the binary image processing software Ia32 (Leco, St. Joseph, MI) as described previously 17. An average area across all the sections for each rat was then calculated to determine the average area of expression. An intensity threshold was set so that only pixels positive for the given marker were quantified within the lesion. This analysis was performed in the place of stereology because the nature of the tight organization of the transplanted cells and the markers used made delineation between adjacent cells difficult (Figure 1). Animals with ESNPC over-proliferation were excluded from this analysis. Animals from each group in which ESNPCs were not seen in the spinal cord at the end of 4 or 8 weeks were included in the analysis as 0.
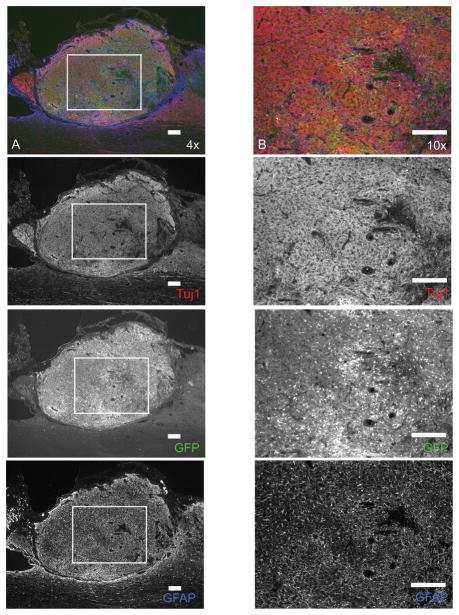
Figure 1.
Image of transplanted ESNPCs after 4 weeks stained with β-tubulin III (Tuj1) for neurons in red, GFP for transplanted ESNPCs in green, and GFAP for astrocytes in blue. The image shows the congested nature of ESNPC transplants that makes it difficult to analyze using stereology. The top image in column A and B is an overlain image of ESNPCs transplanted in the lesion site after 4 weeks stained with Tuj1 for neurons (red), GFP for ESNPCs (green), and GFAP for astrocytes (blue) (scale bar 200μm). The images in column A are 4× images of transplanted ESNPCs found in the lesion of a treated animal. The images in column B are 10× images of the boxed areas in the adjacent image from column A and highlight the congested nature of the transplants (scale bar 200 μm). Each subsequent image from the top of column A and B shows the individual staining of Tuj1 (neurons), GFP (ESNPCs), and GFAP (astrocytes).
Behavioral analysis
Hindlimb function was assessed weekly after injury using the Basso Beattie and Bresnahan (BBB) locomotor rating scale in an open field (2 × 4 feet) 18. For the BBB assessment, rats were observed for 4 min by a trained observer, and each hindlimb was scored individually from 0 (no observable movements) to 21 (normal gait). To complement the BBB, a grid walk test was utilized 19. For this test rats were observed while walking on a fixed grid of bars spaced 1 inch apart for 3 min. Footfalls, defined as missed steps resulting in the leg of the rat crossing the horizontal plane of the grid, were counted during the allotted time on the grid. The total number of footsteps taken on the grid was also recorded. Using both counts, the percentage of missed steps was determined and related to functional loss. Observers for each functional test were blind to the experimental groups being evaluated. Animals with ESNPC over-proliferation were excluded from the analysis of this functional testing.
Statistical analysis
All statistics were performed with fixed Analysis of Variance (ANOVA, planned comparison post-hoc test) using Statistica (StatSoft, Tulsa, OK). The statistical software automatically performs a Bonferroni correction for multiple comparisons. Significance was determined to be p < 0.05. Values are presented as mean ± SEM.
Results
Excessive proliferation of transplanted ESNPCs
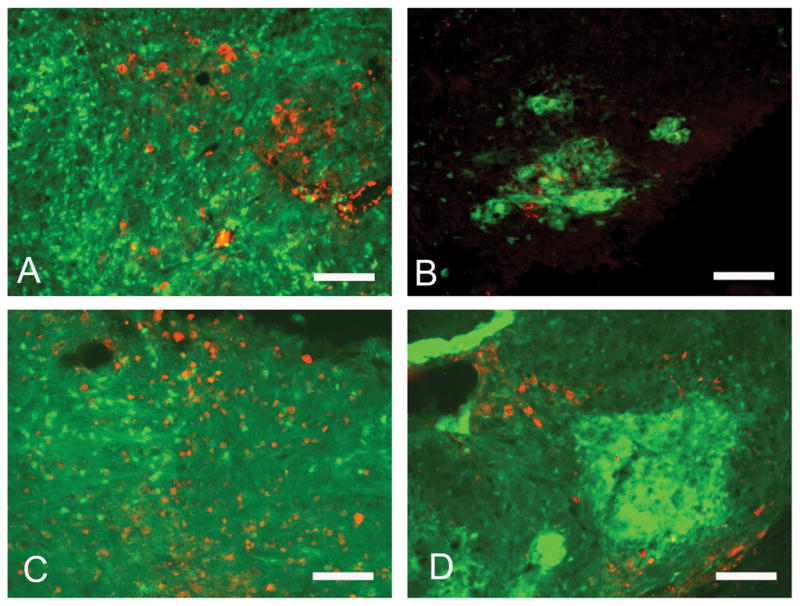
Excessive proliferation of transplanted ESNPCs was identified by growth of transplanted ESNPCs to an extent that the cells occupied a region that spanned the ventral to dorsal surfaces of a sagittal spinal cord section (Figure 2). Furthermore, the over-proliferation correlated with a dramatic loss of behavioral function compared to the control group. Table 2 shows the percentage of rats from each transplant groups that were identified to have ESNPCs over-proliferation. The highest percentage was found at 8 weeks in the 10EB + DS +GF group. Ki67 staining was performed to identify cells that continued to proliferate at 4 and 8 weeks. The majority of Ki67 staining colocalized with staining for SSEA-1, a mouse ESC marker (Figure 2B). The average area of SSEA-1 expression by ESNPCs at 2 weeks after transplantation (data from a previous study) correlates with the percentage of over-proliferation of ESNPCs after 8 weeks (Figure 3D, Table 2). The 10EB + DS + GF group showed the highest level of SSEA-1 expression at 2 weeks and the greatest percentage of animals with over-proliferation at 8 weeks. A comparison of GFP positive cells over the time course of this study and the prior 2 week study was performed to compare cell proliferation with respect to time between each experimental group (Figure 4A). Animals that exhibited over-proliferation as defined above were excluded from evaluations of transplant cell survival, differentiation and behavioral analysis, this left a small n (n=5) for some groups (Table 2), such as the 10EB + DS +GF group and thus this group is de-emphasized in the presentation of result below.
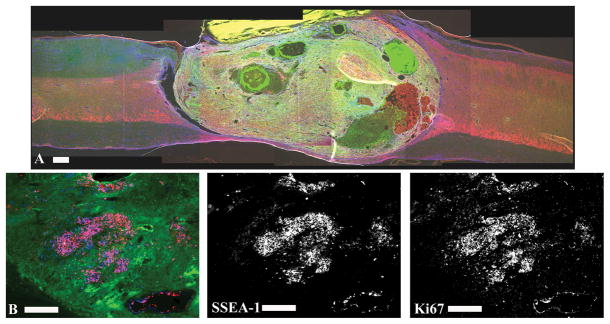
Figure 2.
Over-proliferation of transplanted ESNPCs. A subset of transplanted ESNPCs over-proliferated damaging uninjured spinal cord axons and cause a dramatic decrease in behavioral function. A) Over-proliferation was characterized by growth of ESNPCs that expanded from the ventral to the dorsal surface of the spinal cord. The image shows a sagittal section of a spinal cord with ESNPCs over-proliferation. The section was stained with Tuj1 (neurons, red), GFAP (astrocytes, blue) and GFP (ESNPCs, green). B) The proliferation marker Ki67 was used to visualize proliferating cells at 4 and 8 weeks after transplantation. The vast majority of Ki67 staining (blue) is co-localized with SSEA-1 (red) staining. The staining suggests that SSEA-1 positive mouse embryonic stem cells are contributing to the over-proliferation.
Table 2.
The occurrence of over-proliferation in transplanted groups
| A: 4 Weeks | |||
|---|---|---|---|
| Experimental Groups | Total n transplants | Total n with over- proliferation at end point | % with over- proliferation |
| 10EB no Fibrin | 8 | 2 | 29% |
| 10EB + Fibrin | 7 | 0 | 0% |
| 10EB + DS + GF | 9 | 1 | 11% |
| 10EB + GF no DS | 8 | 0 | 0% |
| Includes rats with over-proliferation | √ | √ | |
| Includes rats with no ESNPCs at end time point | √ | ||
| B. 8 Weeks | |||
|---|---|---|---|
| Experimental Groups | Total n transplants | Total n with over-proliferation at end point | % with over-proliferation |
| 10EB no Fibrin | 10 | 3 | 30% |
| 10EB + Fibrin | 9 | 3 | 30% |
| 10EB + DS + GF | 10 | 5 | 50% |
| 10EB + GF no DS | 8 | 1 | 13% |
| Includes rats with over-proliferation | √ | √ | |
| Includes rats with no ESNPCs at end time point | √ | ||
Figure 3.
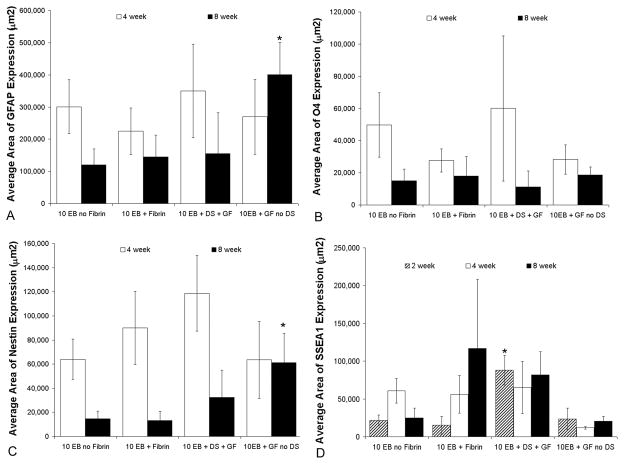
Effect of growth factors on differentiation of transplanted ESNPCs. A) The astrocyte marker GFAP was used to evaluate ESNPC differentiation into astrocytes. The average area of GFAP expression was not significantly different between experimental groups at 4 weeks. After 8 weeks, the average area of GFAP expression for ESNPCs embedded within fibrin scaffolds containing NT-3 and PDGF-AA without the HBDS (10EB + GF no DS, n = 8) was significantly greater from all other groups (Error bars represent SEM, * indicates p<0.05 vs all other groups at same time point). B) The oligodendrocyte marker O4 was analyzed to evaluate the differentiation of transplanted cells into oligodendrocytes. O4 was expressed at considerably lower levels compared to GFAP expression, and there were no significant difference between groups at either time point. C) Nestin expression was used to determine the ESNPCs that remain NPCs. The average area of nestin expression was not significantly different between all groups at 4 weeks. 8 weeks after transplantation the average area of nestin expression for ESNPCs embedded in fibrin scaffolds containing NT-3 and PDGF-AA with no HBDS (10EB + GF no DS, n = 8) was significantly greater than that for ESNPCs implanted directly into the lesion site and ESNPCs embedded in fibrin scaffolds alone (Error bars represent SEM, * indicates p < 0.05 vs. 10EB no Fibrin and 10EB + Fibrin at same time point). D) The mouse embryonic stem cells marker SSEA-1 was used to identify the transplanted cells that remained undifferentiated. The average area of SSEA-1 expression of transplanted cells after 2 weeks in ESNPCs transplanted in fibrin scaffolds with NT-3, PDGF-AA and the HBDS (10EB + DS + GF) was greater than all other groups (Error bars are SEM, * indicates p < 0.05 vs. all other groups).
Figure 4.
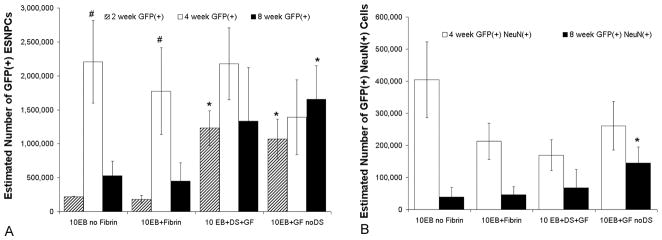
Stereological counts of GFP expressing ESNPCs 2, 4 and 8 weeks after transplantation and GFP expressing ESNPCs that also expressed the neuronal marker NeuN 4 and 8 weeks after transplantation. A) Count of GFP expressing ESNPCs was performed to analyze survival and proliferation of the transplants from each experimental group. There was significant increase in the estimated number of GFP positive ESNPCs between the 2 and 4 week time points in the 10EB no Fibrin and 10EB + Fibrin. No significant difference in the estimated number of GFP positive ESNPCs was observed between experimental groups at the 4 week time point. 8 weeks following transplantation, the ESNPCs transplanted in fibrin scaffolds containing NT-3 and PDGF-AA (10EB + GF no DS, n = 8) had a significantly greater number of ESNPCs. (Error bars represent SEM, # indicates p<0.05 versus same group at 2 weeks, * indicates p < 0.05 vs 10EB no Fibrin and 10EB + Fibrin at same time point) B) Count of GFP expressing ESNPCs that also expressed NeuN (marker for mature neurons), was performed to analyze neuronal differentiation. After 4 weeks, no significant difference in the estimated number of NeuN positive ESNPCs was found between experimental groups. The ESNPCs transplanted in fibrin scaffolds containing NT-3 and PDGF-AA with no HBDS (10EB + GF no DS) had a significantly higher count of NeuN positive ESNPCs when compared to ESNPCs transplanted alone (10EB no Fibrin). (Error bars represent SEM, * indicated p < 0.05 vs 10EB no Fibrin at same time point).
Evaluation of survival of ESNPCs and neuronal differentiation after transplantation
Transplanted ESNPCs were observed in subjects from all experimental groups at both the 4 and 8 week time points. The extent of survival depended on the method of transplantation and the presence of growth factors (Table 3). In the 10EB no fibrin control only 75% (6 of 8) of the treated animals were found to have any GFP positive ESNPCs present in their spinal cord at 4 weeks compared to 86% (6 of 7) of the 10EB + Fibrin group, 100% (9 of 9) of the 10EB + DS + GF group, and 100% (8 of 8) of 10EB + GF no DS group. Those percentages decreased after 8 weeks to 60% (6 of 10) in the 10EB no fibrin group, 67% (6 of 9) of the 10EB + Fibrin group, 80% (8 of 10) in the 10EB + DS + GF, and 88% (7 of 8) of the 10EB + GF no DS. These percentages include animals that had over-proliferation of transplanted ESNPCs.
Table 3.
Presence of ESNPCs in spinal cord after transplantation
| A: 4 Weeks | ||||
|---|---|---|---|---|
| Experimental Groups | Total n transplants | Total n ESNPCs at end point | Total n without ESNPCs at end point | % with ESNPCs at end point |
| 10EB no Fibrin | 8 | 6 | 2 | 75% |
| 10EB + Fibrin | 7 | 6 | 1 | 86% |
| 10EB + DS + GF | 9 | 9 | 0 | 100% |
| 10EB + GF no DS | 8 | 8 | 0 | 100% |
| Includes rats with over-proliferation | √ | √ | ||
| Includes rats with no ESNPCs at end time point | √ | |||
| B. 8 Weeks | ||||
|---|---|---|---|---|
| Experimental Groups | Total n transplants | Total n ESNPCs at end point | Total n without ESNPCs at end point | % with ESNPCs at end point |
| 10EB no Fibrin | 10 | 6 | 4 | 60% |
| 10EB + Fibrin | 9 | 6 | 3 | 67% |
| 10EB + DS + GF | 10 | 8 | 2 | 80% |
| 10EB + GF no DS | 8 | 7 | 1 | 88% |
| Includes rats with over-proliferation | √ | √ | ||
| Includes rats with no ESNPCs at end time point | √ | |||
The average number of transplanted ESNPCs remaining was quantified for each group at the 4 week and 8 week time point using stereological counts of GFP positive cells. Those animals that were observed to have an over-proliferation of transplanted ESNPCs were not included in this analysis. There was no significant difference between groups in the number of GFP positive cells after 4 weeks. There was however a significant difference in the number of GFP positive cells after 8 weeks when the ESNPCs were transplanted within fibrin scaffolds in the presence of NT-3 and PDGF-AA without DS (10EB GF no DS, 17 ± 5×105, n = 8) compared to the ESNPCs implanted into the lesion site alone (10EB no Fibrin, 5 ± 2×105, n = 7) and ESNPCs embedded in Fibrin with no growth factors (10EB + Fibrin, 4.5± 2.5×105, n = 6)(p < 0.05) (Figure 4A).
The groups receiving transplanted cells were also stained with the neuronal nuclei marker NeuN, which identifies mature neurons. The staining with NeuN revealed some of the ESNPCs differentiated into mature neurons. The number of ESNPC-derived neurons was quantified using stereological counts of the number of cells positive for NeuN and GFP (Figure 4B). After 4 weeks, there was no significant difference between experimental groups in the number of ESNPC-derived NeuN positive cells. At 8 weeks, the fibrin scaffolds containing ESNPCs, NT-3 and PDGF-AA without the DS (10EB + GF no DS, 1.5± 0.5×105, n = 8) had a higher number of ESNPC-derived NeuN positive neurons compared to transplantation of ESNPC directly into the lesion (p < 0.05 vs. 10EB no Fibrin, 0.3± 0.1×105, n = 7). The addition of GF promoted an increase in the number of ESNPC-derived NeuN positive neurons compared to transplantation of ESNPCs without GF (either alone or with fibrin but no growth factor) (Figure 4B).
Differentiation of transplanted ESNPCs
Due to the difficulty of acquiring accurate counts for the remaining neural markers using stereology, a method of automated image analysis was previously developed to quantify the expression of neural markers by the transplanted ESNPCs 17. The total area of pixels positive for both GFP and the marker of interest (Tuj1 (neurons), GFAP (astrocytes), O4 (oligodendrocytes), nestin (NPCs), or SSEA-1 (mouse ESCs)) was measured in every fifteenth section. The areas from the sections were then averaged to determine the average area of marker expression per rat. The average area of GFP expression for each experimental group was analyzed to correlate the differentiation analysis with the stereological data. No significant difference was observed in the average expression of GFP or Tuj1 (neurons) between experimental groups at 4 weeks (Figure 5), which is consistent with the stereological data of GFP positive ESNPCs and NeuN positive ESNPCs. Analysis of the other markers revealed no significant difference in the average area of expression of GFAP (astrocytes), O4 (oligodendrocytes), nestin (NPCs), or SSEA-1 (mouse ESCs) (Figure 3).
Figure 5.
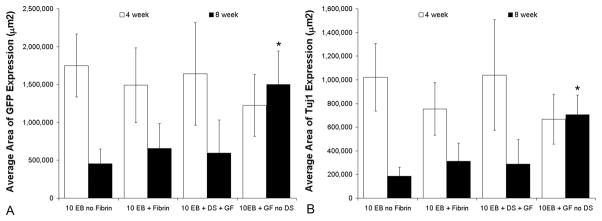
Average area of GFP and β-tubulin III (Tuj1) expression correlates with the stereological counts of GFP positive ESNPCs and NeuN ESNPCs. A) Average area of GFP expression. No significant difference in the average area of GFP expressing ESNPCs was found between experimental groups after 4 weeks. After 8 weeks, the average area of GFP expression was significantly greater when the ESNPCs were transplanted in fibrin scaffolds containing NT-3 and PDGF-AA with no HBDS (10EB + GF no DS, n = 8) when compared to ESNPCs transplanted alone and in fibrin scaffolds (10EB no Fibrin, n = 7 and 10EB + Fibrin, n = 7) (Error bars represent SEM, * indicates p < 0.05 vs. 10EB no Fibrin and 10EB + Fibrin at same time point). B) Average area of GFP and β-tubulin III co-expression. No significant difference in the average area of GFP and β-tubulin III co-expression was found between experimental groups after 4 weeks. After 8 weeks, the average area of GFP and Tuj1 co-expression was significantly greater when ESNPCs were transplanted in fibrin scaffolds containing NT-3, and PDGF-AA with no HBDS (10EB + GF no DS, n = 8) when compared to the 10EB no Fibrin. (Error bars represent SEM, * indicates p < 0.05 vs 10EB no Fibrin, n = 7 at same time point)
Differentiation analysis of GFP and the neuronal marker Tuj1 at 8 weeks was also consistent with the stereological analysis of GFP and NeuN expressing ESNPCs. The average area of GFP expression was significantly greater in the treatments containing ESNPCs transplanted with GFs without the HBDS (10EB + GF no DS, 15 ± 4×105 μm2, n = 8) when compared to treatments of ESNPCs placed directly in the lesion site (p < 0.05, 4.5 ± 2×105 μm2, n = 7) and the treatments of ESNPCs transplanted in fibrin scaffolds alone (p < 0.05, 6.5 ± 3×105 μm2, n = 7). The average area of Tuj1 expression was greater in the group with ESNPCs in fibrin scaffolds containing GFs without the HBDS (10EB + GF no DS, 7 ± 2×105 μm2, n = 8) compared to those treated with ESNPCs implanted directly into the lesion site (10EB no Fibrin, 2 ± 0.7×105 μm2, n = 7) (Figure 5B).
Two markers were used to evaluate the differentiation of ESNPCs into glia. The oligodendrocyte marker O4 was analyzed to evaluate the differentiation of transplanted cells into oligodendrocytes. O4 was expressed at considerably lower levels compared to the neuronal marker Tuj1, and there were no significant difference between groups at either time point. In contrast, considerable co-localization of the astrocytes marker GFAP and GFP was present in all groups containing transplanted ESNPCs. The average area of GFAP expression was not significantly different between experimental groups at the 4 weeks. After 8 weeks, the average area of GFAP expression from ESNPCs in fibrin with GFs without the HBDS (10EB + GF no DS, 4 ± 1×105 μm2, n = 8) was significantly greater than all other experimental groups (p<0.05) (Figure 3A). Finally the expression of the NPC marker nestin was used to identify those ESNPCs that did not differentiation into neuronal, astrocytes or oligodendrocytes. The average area of nestin expression was not significantly different between all of the experimental groups at 4 weeks (~0.8×105 μm2). At 8 weeks after transplantation, the average area of nestin expression in the group treated with ESNPCs in fibrin with GFs without HBDS (10EB + GF no DS, 0.6 ± 0.2×105 μm2, n = 8) was greater than the group with ESNPCs implanted directly into the lesion site and ESNPCs embedded in fibrin scaffolds alone (p < 0.05) (Figure 3C).
Expression of mature neuronal markers by transplanted ESNPCs
To determine if the transplanted ESNPCs expressed markers for different subclass of neurons, primary antibodies for dopaminergic (TH), serotonergic (5-HT), glutamatergic (VGLUT2), GABAergic (GAD67), and cholinergic (ChAT) neurons were used for immunohistochemistry at 4 and 8 weeks (Figure 6). After 4 weeks, the transplanted cells from all of the groups receiving ESNPCs expressed TH for dopaminergic neurons. The groups with ESNPCs transplanted in fibrin with GF (10EB + DS + GF and 10EB + GF no DS) contained cells that expressed ChAT at 4 and 8 weeks. After 8 weeks, transplanted cells from each group receiving ESNPCs expressed markers for dopaminergic neurons (TH) as well as the glutamatergic marker (VGLUT2). Cells transplanted in fibrin without GF (10EB + Fibrin) and transplanted directly in the lesion site (10EB no Fibrin) expressed a marker for serotonergic neurons (5-HT) 8 weeks after transplantation. These data are summarized in Table 4, and suggests that the transplanted ESNPC are not only surviving and expressing markers for the three different neural cell types, but that some of the cells are terminally differentiated and expressing markers for specific neuronal subtypes. The expression of the gabaminergic marker GAD-67 was not seen in the transplanted cells under any condition or time point.
Figure 6.
Expression of neuronal markers by transplanted ESNPCs (green) at 8 weeks after transplantation. A) The transplanted cells from all groups expressed TH for dopaminergic neurons (red). B) The groups with ESNPCs transplanted in fibrin scaffolds containing growth factors (10EB + DS + GF and 10EB + GF no DS) contained cells that expressed ChAT(red) for cholinergic neurons. C) Transplanted ESNPCs from each experimental group expressed markers for glutamatergic marker (VGLUT2, red). D) ESNPCs transplanted in fibrin scaffolds without growth factors (10EB no Fibrin and 10EB + Fibrin) expressed a marker for serotonergic neurons (5-HT, red). (Scale bar is 200μm)
Functional recovery of ESNPCs treated groups
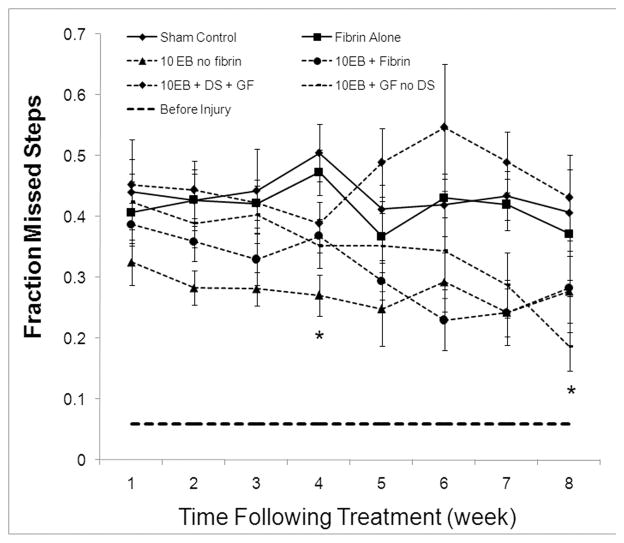
The ability of ESNPCs to promote functional recovery following SCI was evaluated using the BBB locomotor function and grid walk functional test 18, 19. Each experimental group showed spontaneous functional recovery over the time course of the study as evidenced by increases in BBB scores, however there were no significant difference between experimental groups using this assessment (data not shown). A significant difference between experimental groups was observed in the grid walk test (Figure 7). All groups receiving ESNPCs exhibited a significant decrease in the fraction of missed steps compared to the Control group (Control, n = 14, at 4 weeks) at 4 weeks after transplantation (p < 0.05). The significant difference compared to control seen at 4 weeks was not observed for the 10EB + DS + GF and 10EB + Fibrin group at 8 weeks (p > 0.05). The 10EB + GF no DS remained significantly different at 8 weeks (p < 0.05 vs. Control).
Figure 7.
Effect of controlled transplanted ESNPCs on functional recovery. Each experimental group was analyzed weekly following treatment for functional recovery using the grid walk test. A) Results of the grid walk analysis results for each group. The grid walk analysis measures the fraction of missed steps and increase in function is indicated by a decrease in the fraction of missed step. The groups receiving ESNPCs transplanted into the lesion site alone (10EB no Fibrin, n = 7) exhibited a significant decrease in fraction of missed steps compared to the Control (n = 6, p < 0.05) at 2 weeks following transplantation and remaining for the remainder of the study. All ESNPCs groups exhibited significant decrease in the fraction of missed step compared to the Control group 4 weeks after transplantation (p < 0.05, Control vs. 10EB no Fibrin, n = 14, 10EB + Fibrin, 10EB + DS + GF,, 10EB + GF no DS,). The difference compared to control receded for the 10EB + DS + GF and 10EB + Fibrin (0.28 ± .07, n = 7) groups at 8 weeks after transplantation. The 10EB + GF no DS remained significantly decreased at 8 weeks (Error bars represent SEM, * indicates p < 0.05 vs. Control).
Discussion
The injury environment following spinal cord injury is not conducive to the survival and differentiation of transplanted stem cells to treat SCI 2–4. In the current study we evaluated the ability of tissue-engineered fibrin scaffolds containing GFs to enhance the survival and affect the differentiation of ESNPCs transplanted in a subacute model of SCI. We found that transplantation within fibrin scaffolds enhanced the frequency with which transplanted ESNPCs were found within the spinal cord at the termination of the study (Table 3). The addition of growth factors to these scaffolds further increased the survival and proliferation of transplanted cells as seen in the increase in the number of GFP positive cells in the lesion site after 8 weeks. Furthermore, treatment with ESNPCs resulted in an increase in functional recovery at 4 weeks by a grid walk assessment when compared to controls not receiving treatment. However, several of the animals that received ESNPCs were found to have over-proliferating cells that damaged the native spinal cord. Undifferentiated mouse ESCs were identified as a potential source of over-proliferating cells, suggesting that cell purification prior to transplantation is essential for future studies.
In the development of cell transplantation strategies, it is important that the transplanted cells remain in the site of transplantation and survive. In this study, we observed that the method of transplantation affected the probability of transplanted cells being present within the spinal cord at 4 or 8 weeks after transplantation (Table 3). In animals where ESNPCs were embedded in fibrin scaffolds with GFs (with or without the HBDS), there was a higher probability that GFP+ cells were found in the cord. This probability decreased when growth factors were absent. The increase is likely the combined result of the fibrin scaffolds holding the transplanted EBs within the lesion site and the GFs promoting survival and/or proliferation. In the absence of the fibrin scaffold, it is possible that the transplanted cells were susceptible to becoming dislodged and being carried away from the lesion site by the cerebral spinal fluid. Others have shown that biomaterial scaffolds alone enhance the survival of transplanted cells 20, and fibrin scaffolds in particular have been shown to be a favorable environment for stem cell culture in vitro 21. Furthermore evidence from the literature suggests that the presence of GFs could have enhanced ESNPC survival and proliferation after engraftment within the spinal cord 6, 7, 17.
The addition of NT-3 and PDGF-AA (GF) to the tissue-engineered fibrin scaffolds containing ESNPCS was expected to increase the survival and proliferation compared to controls. In a previous 2 week study, it was found that the addition of NT-3 and PGDF-AA to fibrin scaffolds containing ESNPCS increased the proliferation of the transplanted cells17. It is possible that the HBDS combined with GF further increased this proliferation, due to the prolonged bioavailability of the GF, and thus increased the potential for overproliferation. Conversely in this study, there were no differences observed in the number of GFP positive ESNPCs between experimental groups at 4 weeks. The increases that were seen at 2 weeks in the previous study were likely negated at 4 weeks due to an increased proliferation rate of transplanted SSEA-1 positive cells in the 10EB no Fibrin and 10EB + Fibrin groups. As noted in the results, all groups with the exception of the 10EB + DS + GF group, expressed similar levels of SSEA-1/GFP expression at 2 weeks (Figure 3D). These levels likely translated to a similar total number of GFP positive cells in each group at 4 weeks. Interestingly, after 4 weeks only 3 of 32 animals from all experimental groups could be characterized as having over-proliferation based on the set criteria (cells spanning dorsal to ventral surface of the cord). Over-proliferation had likely begun in more animals, but at 4 weeks they had not yet met the set criteria to be removed from analysis and could explain the lack of differences seen between groups after 4 weeks. In contrast at 8 weeks, more animals could be characterized as exhibiting over-proliferation (12 of 37). The removal of the over-proliferating animals from each experimental group at the 8 weeks time point exposed differences between the experimental groups.
The number of GFP positive ESNPCs in the 10EB + GF no DS group was increased compared to the 10EB no Fibrin and 10EB + Fibrin group 8 weeks after transplantation. Additionally, the number of ESNPC-derived NeuN positive cells in the 10EB + GF no DS group was greater than 10EB no Fibrin group. Other studies have shown that the combination of NT-3 and PDGF-AA promotes NPC survival in models of CNS disease17, 22. PDGF-AA specifically has been found to promote NPC proliferation by acting as a mitogen in the early phase of stem cell differentiation to expand the pool of immature neurons23, 24. Soluble NT-3 promotes the differentiation of human ESCs into neurons in culture and treatment of NPCs with NT-3 results in the formation of bipolar neurons25, 26. The results at 8 weeks suggest that the addition of NT-3 and PDGF-AA to the fibrin scaffold without the use of the HBDS results in moderate proliferation and differentiation of transplanted ESNPCs into NeuN expressing neurons. However, these results must be taken under the consideration that over-proliferating ESNPCs were removed from the analysis.
Over-proliferation of ESNPCs was first observed 4 weeks after transplantation. The occurrence of ESNPCs over-proliferation was characterized by the presence of GFP positive cells spanning the ventral to dorsal surface of the spinal cord and was accompanied by a loss of behavioral function. The occurrence of over-proliferation is not surprising as others have found that transplantation of undifferentiated mouse ESCs can result in tumor formation27. The 10EB + DS + GF group, which contained the highest percentage of rats with over-proliferation at 8 weeks, was also found to have increased levels of SSEA-1 positive cells at 2 weeks compared to the other groups. SSEA-1 was consistently expressed in clusters of over-proliferating cells at 4 and 8 weeks and was the only marker observed to co-localize with the proliferation marker Ki67. Additionally, the 10EB+GF no DS group had the lowest occurrence of over-proliferation with only one animal from this experimental group displaying this characteristic and also exhibited the low levels of SSEA-1 staining at all time points (Figure 3D). While scaffolds help to promote cell survival at the lesion site, it is not clear whether they play a role in overproliferation (which varied between scaffold groups). Interestingly, the animals treated with transplanted ESNPCs that had not yet met the criteria for over-proliferation exhibited significant increases in functional recovery compared to a control group not receiving ESNPCs.
Functional recovery of the experimental groups was quantified using two functional assessments, the BBB and the grid walk test. The grid walk test, which requires forelimb-hindlimb coordination, reveals deficits that are not apparent during normal locomotion and thus it is more sensitive for recovery in this modest injury model 19. Differences from the control were seen in all of the ESNPC transplant groups at 4 weeks after transplantation. Unfortunately, as the time after transplantation extended to 8 weeks the functional recovery in the ESNPCs treated groups became inconsistent. These inconsistencies could be a result of the varying extents of ESNPC proliferation. A balance between too much cell proliferation, which adversely effects behavioral function and moderate cell proliferation, which may aide in functional recovery could cause these inconsistencies. It is possible that cyclosporine administration could have contributed to the improvements in functional recovery observed because it has been shown to improve BBB scores in modest contusion injuries28. However, cyclosporine has not been sufficient to promote functional regeneration in severe, complete transaction models29, 30. The mechanism for functional recovery exhibited after transplantation is unknown. However, the recovery is consistent with other studies that observe functional recovery after NPC transplantation into a SCI 6.
Conclusion
Transplanted NPCs can have a positive effect on functional recovery following spinal cord injury. However, previous studies have shown that survival is poor and differentiation of NPCs is limited to a glial fate following transplantation directly into the injured spinal cord. Biomaterial scaffolds have the potential to increase NPC survival following transplantation by shielding them from the harsh injury environment. In this study, we have demonstrated the ability of tissue-engineered fibrin scaffolds containing growth factors to augment the transplantation environment of the injured spinal cord in a way that enhanced the proliferation of ESNPC and in the 8 week 10EB+GF no DS group that increased the number of ESNPC-derived neurons. The study also found that transplantation of SSEA-1 positive mouse ESCs contributed to uncontrolled over-proliferation of transplanted cells. This finding suggests that removal of this population of cells may reduce the chance of ESNPCs over-proliferation. Finally we have demonstrated that if the transplanted ESNPCs can be sustained without inducing over-proliferation that they have the ability to increase functional recovery following SCI. Future studies will center on the transplanting pure populations of neural progenitors that are absent of SSEA-1 positive embryonic stem cells.
Acknowledgments
This study was supported by NIH RO1 NS051454. The authors thank Dan Hunter and Dr. Susan Mackinnon for the use of their cryostat and stereology equipment.
Footnotes
No benefit of any kind will be received either directly or indirectly by the authors.
References
- 1.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Chow SY, Moul J, Tobias CA, Himes BT, Liu Y, Obrocka M, Hodge L, Tessler A, Fischer I. Brain Res. 2000;874:87–106. doi: 10.1016/s0006-8993(00)02443-4. [DOI] [PubMed] [Google Scholar]
- 3.Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- 4.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 5.Cao QL, Howard RM, Dennison JB, Whittemore SR. Exp Neurol. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- 6.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura M, Okano H, Toyama Y, Dai HN, Finn TP, Bregman BS. J Neurosci Res. 2005;81:457–468. doi: 10.1002/jnr.20580. [DOI] [PubMed] [Google Scholar]
- 8.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 9.Johnson PJ, Parker SR, Sakiyama-Elbert SE. Journal of biomedical materials research. 92:152–163. doi: 10.1002/jbm.a.32343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakiyama-Elbert SE, Hubbell JA. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 11.Willerth SM, Rader A, Sakiyama-Elbert SE. Stem Cell Research. 2008;1:205–218. doi: 10.1016/j.scr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson PJ, Parker SR, Sakiyama-Elbert SE. Biotechnol Bioeng. 2009;104:1207–1214. doi: 10.1002/bit.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor SJ, Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. J Control Release. 2006;113:226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SJ, Sakiyama-Elbert SE. J Control Release. 2006;116:204–210. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. J Control Release. 2004;98:281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. Stem Cells. 2007;25:2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Cell Transplant. 2009 doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basso DM, Beattie MS, Bresnahan JC. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 19.Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- 20.Zahir T, Nomura H, Guo XD, Kim H, Tator C, Morshead C, Shoichet M. Cell Transplant. 2008;17:245–254. doi: 10.3727/096368908784153887. [DOI] [PubMed] [Google Scholar]
- 21.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fressinaud C. Glia. 2005;49:555–566. doi: 10.1002/glia.20136. [DOI] [PubMed] [Google Scholar]
- 23.Erlandsson A, Enarsson M, Forsberg-Nilsson K. J Neurosci. 2001;21:3483–3491. doi: 10.1523/JNEUROSCI.21-10-03483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandsson A, Brannvall K, Gustafsdottir S, Westermark B, Forsberg-Nilsson K. Cancer Res. 2006;66:8042–8048. doi: 10.1158/0008-5472.CAN-06-0900. [DOI] [PubMed] [Google Scholar]
- 25.Lachyankar MB, Condon PJ, Quesenberry PJ, Litofsky NS, Recht LD, Ross AH. Exp Neurol. 1997;144:350–360. doi: 10.1006/exnr.1997.6434. [DOI] [PubMed] [Google Scholar]
- 26.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Tissue Eng. 2005;11:506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 27.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Faseb J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 28.McMahon SS, Albermann S, Rooney GE, Moran C, Hynes J, Garcia Y, Dockery P, O’Brien T, Windebank AJ, Barry FP. J Anat. 2009;215:267–279. doi: 10.1111/j.1469-7580.2009.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibarra A, Hernandez E, Lomeli J, Pineda D, Buenrostro M, Martinon S, Garcia E, Flores N, Guizar-Sahagun G, Correa D, Madrazo I. Brain Res. 2007;1149:200–209. doi: 10.1016/j.brainres.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 30.Romero SE, Bravo G, Hong E, Rojas G, Ibarra A. Neurosci Lett. 2008;445:99–102. doi: 10.1016/j.neulet.2008.08.063. [DOI] [PubMed] [Google Scholar]