Abstract
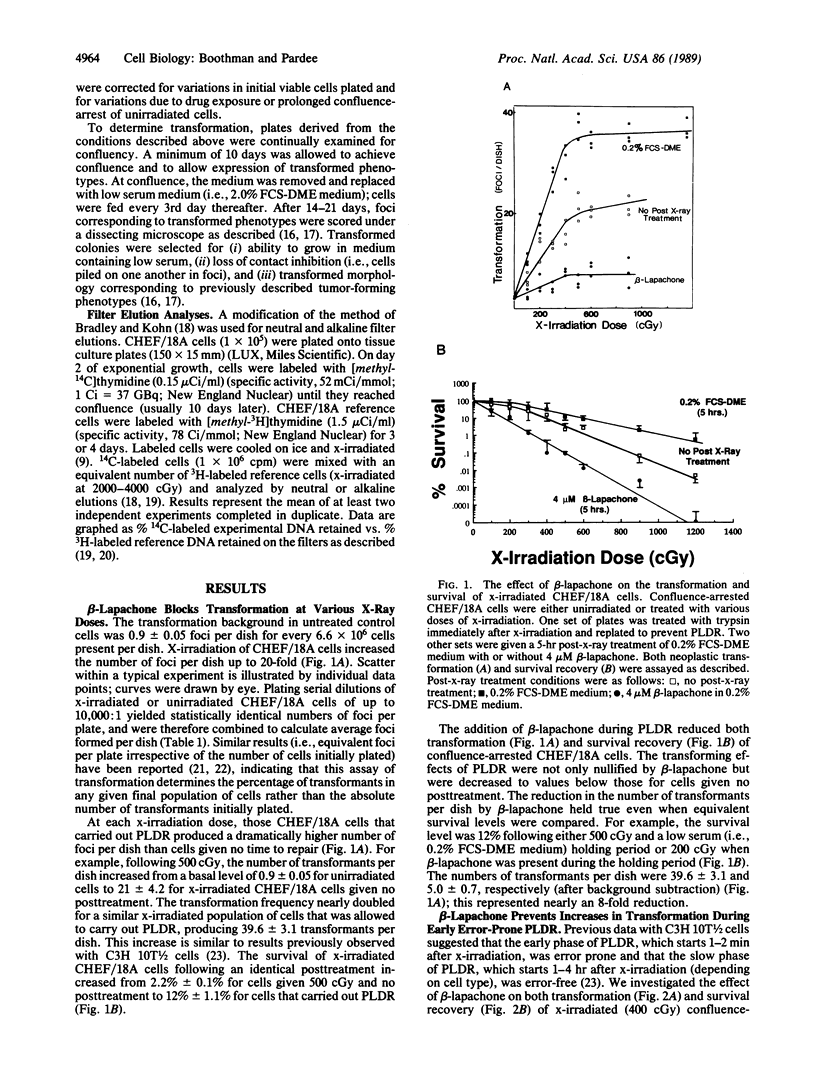
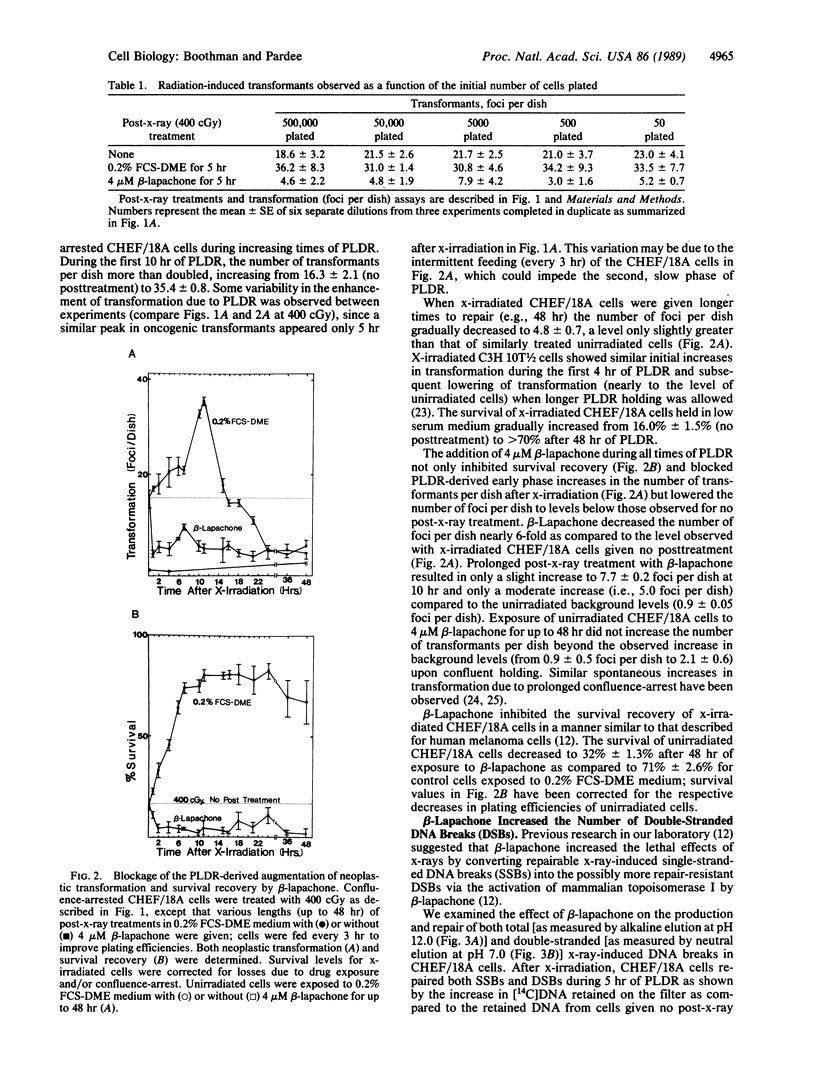
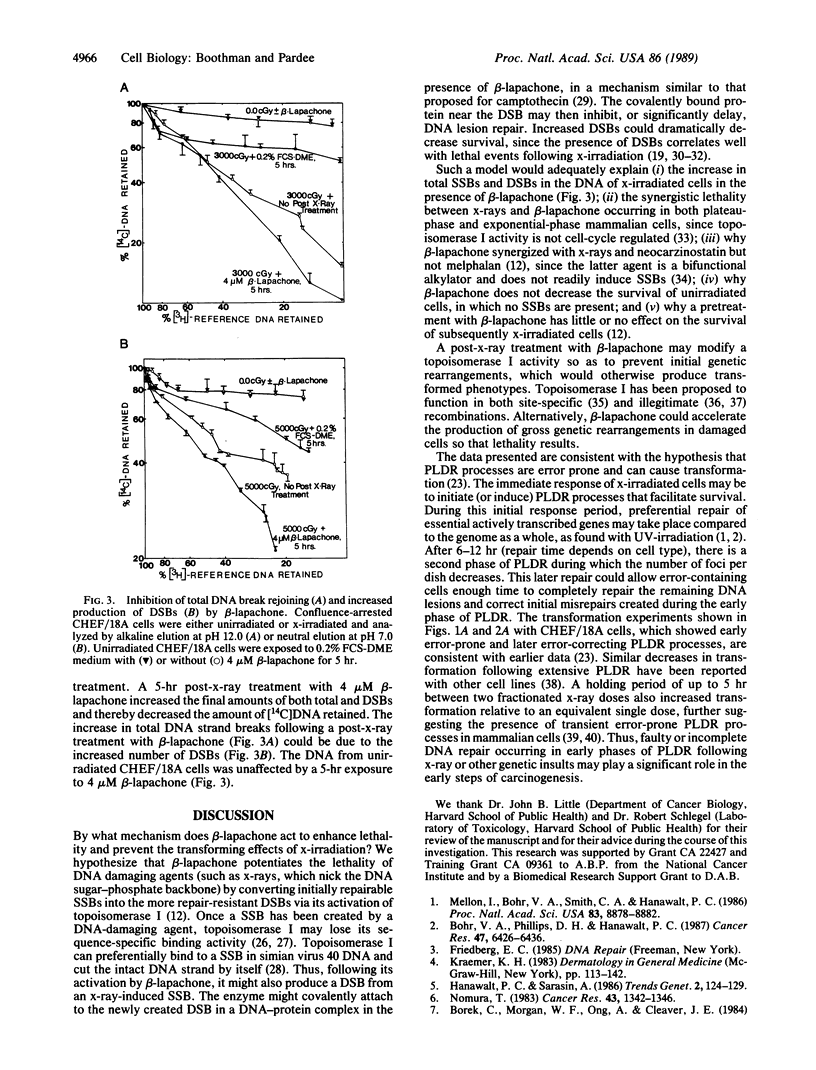
Beta-lapachone is a potent inhibitor of DNA repair in mammalian cells and activates topoisomerase I. We show that beta-lapachone can prevent the oncogenic transformation of CHEF/18A cells by ionizing radiation. Potentially lethal DNA damage repair (PLDR) occurs while x-irradiated cells are held in medium containing low serum prior to replating. PLDR processes permitted survival recovery but also drastically increased the number of foci per plate (i.e., transformation) of CHEF/18A cells. By blocking PLDR with beta-lapachone, both survival recovery and enhanced transformation were prevented. At equivalent survival levels, exposure of x-irradiated cells to beta-lapachone resulted in an 8-fold decrease in the number of foci per dish as compared to the number of transformants produced after PLDR. Early PLDR-derived increases in transformation may be the result of error-prone genetic rearrangements dependent on topoisomerase I, which are thereby prevented by beta-lapachone. Beta-lapachone exposure decreased the rejoining of DNA strand breaks and produced additional double-strand breaks in x-irradiated cells during PLDR. The activation of topoisomerase I by beta-lapachone may convert repairable single-strand DNA breaks into the more repair-resistant double-strand breaks, thereby preventing PLDR and neoplastic transformation. These results suggest a new direction for the development of anticarcinogenic agents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Boorstein R. J., Pardee A. B. Beta-lapachone greatly enhances MMS lethality to human fibroblasts. Biochem Biophys Res Commun. 1984 Feb 14;118(3):828–834. doi: 10.1016/0006-291x(84)91469-4. [DOI] [PubMed] [Google Scholar]
- Boothman D. A., Greer S., Pardee A. B. Potentiation of halogenated pyrimidine radiosensitizers in human carcinoma cells by beta-lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran- 5,6-dione), a novel DNA repair inhibitor. Cancer Res. 1987 Oct 15;47(20):5361–5366. [PubMed] [Google Scholar]
- Boothman D. A., Schlegel R., Pardee A. B. Anticarcinogenic potential of DNA-repair modulators. Mutat Res. 1988 Dec;202(2):393–411. doi: 10.1016/0027-5107(88)90201-1. [DOI] [PubMed] [Google Scholar]
- Boothman D. A., Trask D. K., Pardee A. B. Inhibition of potentially lethal DNA damage repair in human tumor cells by beta-lapachone, an activator of topoisomerase I. Cancer Res. 1989 Feb 1;49(3):605–612. [PubMed] [Google Scholar]
- Borek C. The induction and control of radiogenic transformation in vitro: cellular and molecular mechanisms. Pharmacol Ther. 1985;27(1):99–142. doi: 10.1016/0163-7258(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Bradley M. O., Kohn K. W. X-ray induced DNA double strand break production and repair in mammalian cells as measured by neutral filter elution. Nucleic Acids Res. 1979 Oct 10;7(3):793–804. doi: 10.1093/nar/7.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., McCoubrey W. K., Jr, Been M. D. DNA structural features that lead to strand breakage by eukaryotic type-I topoisomerase. Cold Spring Harb Symp Quant Biol. 1984;49:435–442. doi: 10.1101/sqb.1984.049.01.049. [DOI] [PubMed] [Google Scholar]
- Downes C. S., Johnson R. T. DNA topoisomerases and DNA repair. Bioessays. 1988 Jun;8(6):179–184. doi: 10.1002/bies.950080602. [DOI] [PubMed] [Google Scholar]
- Frankenberg D., Frankenberg-Schwager M., Blöcher D., Harbich R. Evidence for DNA double-strand breaks as the critical lesions in yeast cells irradiated with sparsely or densely ionizing radiation under oxic or anoxic conditions. Radiat Res. 1981 Dec;88(3):524–532. [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Induction of foci of morphologically transformed cells in synchronized populations of 10T1/2 cells by N-methyl-N'-nitro-N-nitrosoguanidine and the effect of spontaneous transformation on calculated transformation frequency. Cancer Res. 1988 Nov 1;48(21):5977–5983. [PubMed] [Google Scholar]
- Grisham J. W., Smith G. J., Lee L. W., Bentley K. S., Fatteh M. V. Spontaneous formation of foci of morphologically transformed cells in populations of C3H 10T1/2 (clone 8) cells. Cancer Res. 1988 Nov 1;48(21):5969–5976. [PubMed] [Google Scholar]
- Hansson J., Lewensohn R., Ringborg U., Nilsson B. Formation and removal of DNA cross-links induced by melphalan and nitrogen mustard in relation to drug-induced cytotoxicity in human melanoma cells. Cancer Res. 1987 May 15;47(10):2631–2637. [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Hsiang Y. H., Wu H. Y., Liu L. F. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988 Jun 1;48(11):3230–3235. [PubMed] [Google Scholar]
- Kakunaga T. Caffeine inhibits cell transformation by 4-nitroquinoline-1-oxide. Nature. 1975 Nov 20;258(5532):248–250. doi: 10.1038/258248a0. [DOI] [PubMed] [Google Scholar]
- Kelland L. R., Edwards S. M., Steel G. G. Induction and rejoining of DNA double-strand breaks in human cervix carcinoma cell lines of differing radiosensitivity. Radiat Res. 1988 Dec;116(3):526–538. [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. A. Nicking-closing activity associated with bacteriophage lambda int gene product. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3760–3764. doi: 10.1073/pnas.76.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin R. M., Sager R. Genetic analysis of tumorigenesis: V. Chromosomal analysis of tumorigenic and nontumorigenic diploid chinese hamster cell lines. Somatic Cell Genet. 1980 Jan;6(1):75–87. doi: 10.1007/BF01538697. [DOI] [PubMed] [Google Scholar]
- Kohen R., Szyf M., Chevion M. Quantitation of single- and double-strand DNA breaks in vitro and in vivo. Anal Biochem. 1986 May 1;154(2):485–491. doi: 10.1016/0003-2697(86)90019-9. [DOI] [PubMed] [Google Scholar]
- McCoubrey W. K., Jr, Champoux J. J. The role of single-strand breaks in the catenation reaction catalyzed by the rat type I topoisomerase. J Biol Chem. 1986 Apr 15;261(11):5130–5137. [PubMed] [Google Scholar]
- McGarrity G. J., Carson D. A. Adenosine phosphorylase-mediated nucleoside toxicity. Application towards the detection of mycoplasmal infection in mammalian cell cultures. Exp Cell Res. 1982 May;139(1):199–205. doi: 10.1016/0014-4827(82)90333-0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Hall E. J. X-ray dose fractionation and oncogenic transformations in cultured mouse embryo cells. Nature. 1978 Mar 2;272(5648):58–60. doi: 10.1038/272058a0. [DOI] [PubMed] [Google Scholar]
- Mordan L. J., Martner J. E., Bertram J. S. Quantitative neoplastic transformation of C3H/10T1/2 fibroblasts: dependence upon the size of the initiated cell colony at confluence. Cancer Res. 1983 Sep;43(9):4062–4067. [PubMed] [Google Scholar]
- Nomura T. Comparative inhibiting effects of methylxanthines on urethan-induced tumors, malformations, and presumed somatic mutations in mice. Cancer Res. 1983 Mar;43(3):1342–1346. [PubMed] [Google Scholar]
- Porter S. E., Champoux J. J. Mapping in vivo topoisomerase I sites on simian virus 40 DNA: asymmetric distribution of sites on replicating molecules. Mol Cell Biol. 1989 Feb;9(2):541–550. doi: 10.1128/mcb.9.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford I. R. Effects of hyperthermia on the repair of X-ray-induced DNA double strand breaks in mouse L cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 May;43(5):551–557. doi: 10.1080/09553008314550641. [DOI] [PubMed] [Google Scholar]
- Shishido K., Noguchi N., Ando T. Correlation of enzyme-induced cleavage sites on negatively superhelical DNA between prokaryotic topoisomerase I and S1 nuclease. Biochim Biophys Acta. 1983 May 20;740(1):108–117. doi: 10.1016/0167-4781(83)90127-6. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Sager R. Multistep origin of tumor-forming ability in Chinese hamster embryo fibroblast cells. Cancer Res. 1982 Feb;42(2):389–396. [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. Repair of potentially lethal radiation damage in mammalian cells is associated with enhancement of malignant transformation. Nature. 1975 Feb 13;253(5492):548–549. doi: 10.1038/253548a0. [DOI] [PubMed] [Google Scholar]
- vanAnkeren S. C., Murray D., Meyn R. E. Induction and rejoining of gamma-ray-induced DNA single- and double-strand breaks in Chinese hamster AA8 cells and in two radiosensitive clones. Radiat Res. 1988 Dec;116(3):511–525. [PubMed] [Google Scholar]


