Abstract
Endothermy and homeothermy are mammalian characteristics whose evolutionary origins are poorly understood. Given that fungal species rapidly lose their capacity for growth above ambient temperatures, we have proposed that mammalian endothermy enhances fitness by creating exclusionary thermal zones that protect against fungal disease. According to this view, the relative paucity of invasive fungal diseases in immunologically intact mammals relative to other infectious diseases would reflect an inability of most fungal species to establish themselves in a mammalian host. In this study, that hypothesis was tested by modeling the fitness increase with temperature versus its metabolic costs. We analyzed the tradeoff involved between the costs of the excess metabolic rates required to maintain a body temperature and the benefit gained by creating a thermal exclusion zone that protects against environmental microbes such as fungi. The result yields an optimum at 36.7°C, which closely approximates mammalian body temperatures. This calculation is consistent with and supportive of the notion that an intrinsic thermally based resistance against fungal diseases could have contributed to the success of mammals in the Tertiary relative to that of other vertebrates.
IMPORTANCE
Mammals are characterized by both maintaining and closely regulating high body temperatures, processes that are known as endothermy and homeothermy, respectively. The mammalian lifestyle is energy intensive and costly. The evolutionary mechanisms responsible for the emergence and success of these mammalian characteristics are not understood. This work suggests that high mammalian temperatures represent optima in the tradeoff between metabolic costs and the increased fitness that comes with resistance to fungal diseases.
Introduction
Endothermy and homeothermy are fundamental aspects of mammalian physiology whose evolutionary origin remains poorly understood. Although many explanations have been suggested for the origins of endothermy and homeothermy, none are fully satisfactory given their high metabolic costs (1, 2). Furthermore, the factors responsible for the mammalian set point remain unknown, posing the additional question of why mammals are so hot. Recently, the observation that fungal diseases are common in plants and insects but rare in mammals, combined with the thermal susceptibility of fungi, led to the proposal that mammalian endothermy and homeothermy create a thermal exclusionary zone that protects mammals against mycoses (3). Endothermy was also suggested to have provided a fitness advantage in the fungal bloom that followed the end of the Cretaceous such that it could have contributed to the success of mammals in the Tertiary (3, 4).
Assuming that a relationship exists between endothermy and reduced susceptibility to certain classes of microbes, we hypothesized a tradeoff relationship whereby the high costs of endothermy were mitigated by protection against infectious diseases. In other words, we posited that increases in body temperature would protect against microbes by creating a thermal exclusionary zone but that such increases would be increasingly costly with regard to metabolic rates as the host body temperature diverged from ambient temperatures. Given that there is robust information on fungal thermal tolerances (3), we decided to test this hypothesis by attempting to identify body temperatures that confer maximal fitness for certain metabolic rates.
To address this question, we propose a first-order model wherein a tradeoff exists between the excess metabolic rates required to maintain a body temperature, T, and the benefit gained by protection against deleterious microbes because of the creation of a thermal exclusion zone. Metabolism, the exchange of energy between the organism and its environment, as well as the transformation of that energy to material within an organism, is affected by two main factors, body mass, M, and body temperature, T. Due to the fractal nature of transport networks, that is, vessel architecture and branching (5, 6), over ontogeny, the resting metabolic rate, Brest, scales with body mass, m, as Brest , where B0 is a normalization constant for a given taxon. Also, the normalization coefficient, B0, exponentially increases with body temperature , where E0 is the average activation energy for the rate-limiting enzyme-catalyzed biochemical reactions of metabolism (ca. 0.65 eV), K is Boltzmann’s constant (8.62 × 10−5 eV/K), and T is body temperature (5, 7). The scaling relationship between resting metabolic rate and body mass, , has been predicted from allometric theories and supported by data on a diverse set of organisms, including mammals, birds, fish, and mollusks (8–11). As can be seen from the formulas above, body temperature affects the metabolic rate through its effects on rates of biochemical reaction kinetics according to Boltzmann’s factor, , where T is measured in kelvins (absolute temperature). The resting metabolic rate, , is proportional to the product of these two effects and again has been shown to be well approximated, within a biologically relevant temperature range (0°C to 40°C), as (5). The first part of our analysis examined the excess cost for an organism of body mass m to maintain a body temperature T (assuming no dependence of body mass on temperature).
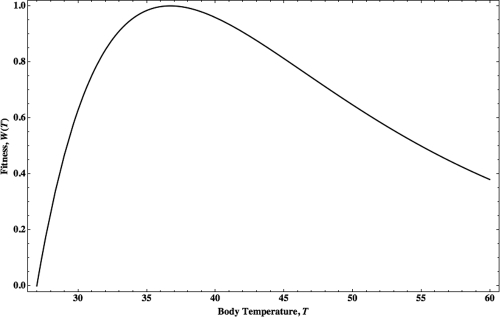
In the second part of our analysis, the benefit, noted here as , is calculated as the reduction in the number of fungal species capable of infecting a host; this number is reduced approximately by for every degree Celsius in the temperature range of 27°C to 40°C (3). The increased benefit of the successive elimination of fungal species can thus be expressed as , where F0 is a constant scaling factor. The quantity can represent the balance between cost and benefit; thus, can be viewed as the total fitness of an organism as a function of its body temperature. Within the biologically relevant temperature range, the proposed fitness measure reaches its maximum at approximately 37°C (Fig. 1). Note that in this formulation, the optimal body temperature, where attains its maximum value, does not depend on the organism’s body mass. Furthermore, the one parameter that is determined from biological observation is the reduction in the number of fungal species capable of infecting a host; thus, to determine our model’s dependence on this parameter, we calculated the optimal temperature over a wide range of possible reduction percentages, i.e., 4% to 8%. In this range, the optimal temperature was found to remain in a tight range of less than 2°C, from 37.7°C to 35.9°C, respectively, which is still within the biologically relevant range of mammalian body temperatures. The insensitivity of the model to its only parameter further strengthens our hypothesis.
FIG 1 .
Organism fitness as a function of body temperature. We normalized fitness, , to attain a maximum value of 1 and plotted body temperature in degrees Celsius over a range of 27°C to 60°C. Fitness reaches a maximum value at .
In summary, we present a minimal, parsimonious model to account for the cost of maintaining a high body temperature in mammalian organisms. A body temperature of 36.7°C maximizes fitness by restricting the growth of most fungal species relative to its metabolic cost. Our model suggests that no additional elaborations are required to explain the evolution of endothermy other than the tradeoff between protection against environmentally acquired microbial diseases and the cost of metabolism. Although we cannot rule out the possibility that this body temperature optimum arose by some remarkable coincidence, we think this highly unlikely because it emerges from considering two unrelated processes, fungal thermal tolerance and mammalian metabolic costs. Nonetheless, we acknowledge that similar temperature optima might emerge from other considerations. For example, the specific heat capacity of water has a minimum at 36°C, and if the efficiency of metabolic processes is related to heat capacity, then using this parameter as the optimality criterion may result in a similar range of solutions. Nevertheless, we note the internal consistency in the theme that fungal diseases are rare in immunologically intact mammals and the tradeoff between increased fitness and metabolic costs closely approximates mammalian body temperatures.
ACKNOWLEDGMENTS
Aviv Bergman is supported by 5P01AG027734-04 and 5R01AG028872-04. Arturo Casadevall is supported by AI33774-11, HL59842-07, AI33142-11, AI52733-02, and U54-AI057158-Lipkin.
Footnotes
Citation Bergman, A., and A. Casadevall. 2010. Mammalian endothermy optimally restricts fungi and metabolic costs. mBio 1(5):e00212-10. doi:10.1128/mBio.00212-10.
REFERENCES
- 1. Kemp T. S. 2008. The origin of mammalian endothermy: a paradigm for the evolution of complex biological structure. Zool. J. Linn. Soc. 147:473–488 [Google Scholar]
- 2. Ruben J. 1995. The evolution of endothermy in mammals and birds: from physiology to fossils. Annu. Rev. Physiol. 57:69–95 [DOI] [PubMed] [Google Scholar]
- 3. Robert V. A., Casadevall A. 2009. Vertebrate endothermy restricts most fungi as potential pathogens. J. Infect. Dis. 200:1623–1626 [DOI] [PubMed] [Google Scholar]
- 4. Casadevall A. 2005. Fungal virulence, vertebrate endothermy, and dinosaur extinction: is there a connection? Fungal Genet. Biol. 42:98–106 [DOI] [PubMed] [Google Scholar]
- 5. Gillooly J. F., Brown J. H., West G. B., Savage V. M., Charnov E. L. 2001. Effects of size and temperature on metabolic rate. Science 293:2248–2251 [DOI] [PubMed] [Google Scholar]
- 6. Savage V. M., Deeds E. J., Fontana W. 2008. Sizing up allometric scaling theory. PLoS Comput. Biol. 4:e1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85:1771–1789 [Google Scholar]
- 8. Brody S. 1964. Bioenergetics and growth. Hafner, New York, NY. [Google Scholar]
- 9. Moses M. E., Hou C., Woodruff W. H., West G. B., Nekola J. C., Zuo W., Brown J. H. 2008. Revisiting a model of ontogenetic growth: estimating model parameters from theory and data. Am. Nat. 171:632–645 [DOI] [PubMed] [Google Scholar]
- 10. Savage V. M., Gillooly J. F., Woodruff W. H., West G. B., Allen A. P., Enquist B. J., Brown J. H. 2004. The predominance of quarter-power scaling in biology. Funct. Ecol. 18:257–282 [Google Scholar]
- 11. West G. B., Brown J. H., Enquist B. J. 1997. A general model for the origin of allometric scaling laws in biology. Science 276:122–126 [DOI] [PubMed] [Google Scholar]