Abstract
Cholera remains a significant health threat across the globe. The pattern and magnitude of the seven global pandemics suggest that cholera outbreaks primarily originate in coastal regions and then spread inland through secondary means. Cholera bacteria show strong association with plankton abundance in coastal ecosystems. This review study investigates relationship(s) between cholera incidence and coastal processes and explores utility of using remote sensing data to track coastal plankton blooms, using chlorophyll as a surrogate variable for plankton abundance, and subsequent cholera outbreaks. Most studies over the last several decades have primarily focused on the microbiological and epidemiological understanding of cholera outbreaks. Accurate identification and mechanistic understanding of large scale climatic, geophysical and oceanic processes governing cholera-chlorophyll relationship is important for developing cholera prediction models. Development of a holistic understanding of these processes requires long and reliable chlorophyll dataset(s), which are beginning to be available through satellites. We have presented a schematic pathway and a modeling framework that relate cholera with various hydroclimatic and oceanic variables for understanding disease dynamics using latest advances in remote sensing. Satellite data, with its unprecedented spatial and temporal coverage, have potentials to monitor coastal processes and track cholera outbreaks in endemic regions.
Key Terms: bacteria, surface water hydrology, remote sensing, aquatic ecology, Cholera, SeaWiFS, chlorophyll, plankton
Introduction
Cholera, longest known water-borne epidemic disease in the history of mankind, was anecdotally reported as early as 400BC (Bishagratna, 1963). F. Pacini in 1854 was the first scientist to isolate Comma bacillius, now known as V. cholerae followed by a similar discovery by Robert Koch in 1884 (Bentivoglio and Pacini, 1995). John Snow, a British physician, was the first to discover that cholera spread through contaminated water. Ever since, cholera has been a subject of intense interest for a range of microbiological and epidemiological studies. The ongoing seventh pandemic of cholera, which started in 1960s, has been reported in over 50 countries and affected over 7 million people (Gleick, 2008). The disease remains a public health threat in many regions of the world, specifically in coastal areas of South Asia, Africa, and Latin America.
The causative agent of cholera, V. cholerae, is known to survive and multiply in favorable estuarine environments. Primary outbreaks of cholera over the last several decades in South Asia, Africa and South America have mostly originated in coastal areas (Gleick, 2008; Griffiths et al., 2006; Siddique et al., 1994; Huq and Colwell 1996). With the first correlative study relating cholera incidence and increased number of algae in water (Cockburn and Cassanos, 1960), several studies have postulated connection between initial cholera outbreak and oceanic plankton abundance (Siddique et al., 1994; Huq and Colwell 1996). Many of these microbiological and epidemiological studies have primarily focused on the annual and local scale variations of cholera with different ecological and climatic variables. Despite wide advances in the ecological and biological understanding of the cholera bacteria over the last half-century, our understanding of the influence of large scale climatic and geophysical processes on the global transmission of cholera remains limited.
Various environmental factors such as sunlight, precipitation, salinity, temperature, and nutrients are suggested for survival and growth of cholera bacteria in the aquatic environment (Singleton et al. 1982; Huq et al. 1984; Epstein, 1993). Cholera bacteria attach to zooplanktons by forming a thin pathogenic biofilm (Reidl and Klose, 2002). As copepods feed on phytoplankton, a high correlation is expected between the occurrence of copepods and phytoplankton bloom. Consequently, one may expect an observed abundance in phytoplankton to correspond to an increase in the number of cholera bacteria in the coastal waters. Recent advances in remote sensing may allow us to understand cholera outbreaks over large regions by observing chlorophyll concentration, as a surrogate measure of plankton abundance, from satellites.
Three empirical observations motivate the exploration of possible connections among the biology and ecology of the aquatic environment and large scale hydro-climatic processes: (a) almost all cholera outbreaks are originated near the coastal areas including reemergence of cholera in Latin America in 1991; (b) laboratory studies suggest a significant positive correlation between plankton abundance and pathogenic cholera bacteria; and (c) remote sensing provides unprecedented coverage of space-time measurements of chlorophyll variability in coastal regions around the world.
Marine plankton exhibits wide variability in time and space. Previous studies have primarily used in-situ plankton data with limited daily measurements and attempted to establish chlorophyll-cholera connections. However, day-to-day variations of chlorophyll over a range of spatial scales can be as large as an order of magnitudes (Uz and Yoder, 2004). Thus, analysis of in-situ measurements of chlorophyll may provide limited and somewhat incomplete understanding of the space-time variations of phytoplankton and consequently cholera dynamics. With availability of continuous measurement of satellite-estimated chlorophyll, we are now able to examine chlorophyll variation at various temporal scales (~daily, weekly, etc.) with a range of pixel resolutions (~ kilometers) over very large regions for last ten years. The objectives for this article are to (1) establish possible relationship(s) between cholera incidence and coastal processes and (2) explore the utility of using remote sensing data to track coastal plankton blooms and subsequent cholera outbreaks in vulnerable regions. In Section 2, we explore the coastal connections of cholera and present evidence that primary cholera incidence is usually reported in near coastal regions. Cholera incidence in this manuscript is defined as the percent of new cholera infected patients from a total pool of patients visiting the hospital for treatment in a given region. Similarly primary cholera outbreak refers to the start of the cholera disease in a given season or area. Section 3 examines the relationship of cholera dynamics with terrestrial hydrology and coastal ecology. Section 4 discusses use of remote sensing to measure chlorophyll and its potential to track cholera outbreaks. Section 5 integrates the understanding gained from previous sections with respect to coastal processes, terrestrial ecology and potential of using satellite observations to track cholera outbreaks over large regions across the globe. Section 6 provides a plausible modeling framework that relates cholera with various hydroclimatological and oceanic variables for understanding disease dynamics using latest advances in remote sensing.
Cholera and Coasts: A Geographical Overview
Cholera remains endemic in many countries of the developing world, mainly in coastal areas of South Asia and Africa (Colwell, 1996, Mouriño-Pérez, 1998) and has lately shown an unprecedented rise in infection and transmission in Africa (Griffith et al. 2006; Collins, 2003). The global awareness of cholera began in 1817 with the explosive epidemics breaking out in the lower Ganges River delta and spreading to the entire world in the form of pandemic (Sack et al, 2004). The seventh pandemic, still ongoing, started in Indonesia in 1961 and has already been reported in over 50 countries. Figure 1 shows the worldwide reach of the ongoing cholera pandemic. The global pattern and magnitude of the pandemics suggest that cholera outbreaks primarily originate in coastal environments, suggesting a link between changes in the near shore waters and outbreaks of the disease (e.g., Colwell and Huq, 2001; Mouriño-Pérez, 1998).
FIGURE 1.
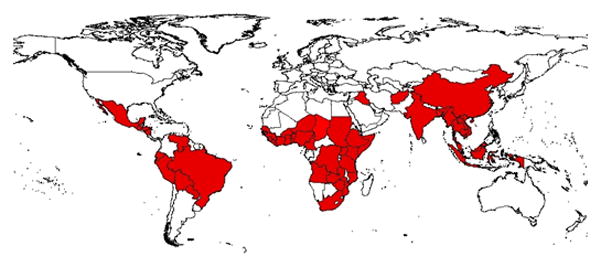
Countries affected by the Seventh Pandemic of Cholera (compiled from World Health Organization (WHO), Center for Disease Control and Prevention (CDC), and various news sources, Countries in the red shade have reported cholera outbreaks)
The coastal regions of South Asia, for example, have a long history of cholera incidence and are collectively considered the native homeland of the cholera disease since the early 19th century (Bouma and Pascal, 2001). Recent studies focusing on this region (Lipp et al. 2002, Pascual et al. 2002) suggest significant seasonal patterns in cholera incidence with a primary outbreak occurring in the coastal districts. The historic cholera mortality rates in this region show significant correlation between sea surface temperature (SST) and spring cholera deaths in the coastal districts (Bouma and Pascual, 2001). Although cholera cases have been reported in inland districts of the Indian Subcontinent, the regions of endemicity are most frequently found near coastlines (Lipp et al. 2002). Huq and Colwell (1996) presented three case studies to qualitatively explain plausible mechanisms of transmission of the disease from coastal regions to inland. In Sub-Saharan Africa, initial cholera outbreaks were concentrated along coastal regions before spreading to other parts of the continent. In 1991, over five hundred thousand people were affected by cholera in 20 Latin American countries, with over 5000 deaths. The initial outbreak of this explosive transmission of cholera was identified to be in a coastal village near Lima, Peru. Similarly, December 1992 cholera outbreak originated in coastal Bangladesh that affected over 47 thousand people and killed 846 (Siddique et al. 1994). A detailed cholera epidemiological study from Bangladesh (Sack et al. 2003) showed that areas closer to the coast such as Bakerganj and Matlab experienced recurrent spring cholera outbreaks. The primary outbreaks of cholera in most regions thus show a strong link with the coastal areas, implying a role of the near shore marine environment. Table 1 shows the origin of major cholera outbreaks of the current pandemic and the proximity of these locations from the nearest ocean coast, confirming the coastal links to primary cholera outbreaks. Similar role of coastal ecosystems, working as environmental reservoirs of V. cholerae, has been suggested for South Africa(Mendelsohn and Dawson, 2008; Bertuzzo et al. 2008) and Peru (Gil et al. 2004; Martinez-Urtaza et al. 2008).
Table 1.
Proximity to Coast for Major Seventh Pandemic Cholera Outbreaks
| Year | Country | Area/City | Affected Population | Distance to Coast(km) |
|---|---|---|---|---|
| 2006 | Angola | Luanda | 46,750 | 0 |
| 2005 | Senegal | Touba | > 31,000 | 200 |
| 2002 | Malawi | Lilongwe | 32,618 | 50~100 |
| 2000 | South Africa | Kwazulu- | 86,107 | 0 |
| 2000 | Madagascar | Antananarivo Natal | 15,173 | 0 |
| 1996 | Peru | Lima | 22,397 | 0 |
| 1992 | Bangladesh | Dhaka | > 30,000 | 150 |
| 1991 | Ecuador | Quito | 46284 | 0~200 |
| 1974 | Bangladesh | Dhaka | > 15,000 | 150 |
Cholera, Coastal Ecology, and Terrestrial Hydrology
A significant reservoir of V. cholerae is marine plankton, both phytoplankton and zooplankton (Colwell and Huq, 2001). Cholera bacteria attach themselves to the zooplankton, more specifically to crustacean copepods, to form a thin pathogenic biofilm, which provides protection from the external environment (Reidl and Klose, 2002). Phytoplankton serves as the primary food source for copepods and other zooplanktons, also releases nutrients into the water through disintegration. The bacteria then proliferate taking advantage of the nutrition conditions of the aquatic system (Lipp et al, 2002). Increase in phytoplankton has been associated with increased presence of copepods (Reidl and Klose, 2002). Phytoplankton and zooplankton, therefore, play vital role in facilitating the survival, growth, and transmission of V. cholerae in the natural aquatic environment (Lipp et al, 2002, Mouriño-Pérez, 1998). The role of sea surface temperature (SST) in creating and sustaining favorable environmental conditions for oceanic phytoplankton production is well documented (e.g., Timmermann and Jin, 2002; Legaard and Thomas, 2006; Garcia & Carr, 1999).
A closer look at cholera outbreaks and relevant oceanic and terrestrial variables, however, shows a lack of understanding of the seasonal and interannual variability and the processes governing cholera transmission. For example, cholera incidence data from Bangladesh shows bi-annual peaks while coastal phytoplankton primarily shows a single peak (Akanda et al, 2009; Jutla et al 2009 a,b). On the other hand, cholera incidence time series across most affected areas in Africa, such as Mozambique and Democratic Republic of Congo, show infection patterns with a single annual peak. Studies on historic mortality data and recent incidence data from Bangladesh show a coastal endemic pattern in the spring while a post-monsoon outbreak pattern in fall is usually observed further inland (Sack et al. 2003; Bouma and Pascual, 2001). However, other land locked regions such as inland districts of the Ganges-Brahmaputra-Meghna (GBM) basin and the East African lake region show epidemic cholera outbreaks in post-flood situation or after extreme precipitation events. In addition, global warming and an increasing number of natural disasters can contribute to an outbreak or occurrence of cholera in new places, or to the appearance of a new serotype of the causative agent (Koelle et al. 2005). For example, a new serogroup (O139 Bengal) caused epidemic cholera for the first time in history in 1992 in areas surrounding the Bay of Bengal (Siddique, 1994).
Cholera incidence data at the International Center for Diarrhoeal Disease Research in Bangladesh (ICDDR,B) is well documented and is perhaps one of the longest and most detailed cholera datasets in the world (Longini et al. 2002). The epidemic outbreaks in this region have been linked to a range of environmental and climate variables, such as, precipitation (Pascual et al. 2002; Hashizume et al. 2008), coastal phytoplankton abundance (Magny et al. 2008; Emch et al. 2008), floods (Koelle et al. 2005), peak river level (Schwartz et al. 2006), sea surface temperature (Cash et al. 2008; Lobitz et al. 2000), sea surface height (Lobitz et al. 2000), water temperature (Colwell 1996; Huq et al. 2005) and fecal contamination (Islam et al. 2006). Most recently, Akanda et al (2009) provided a preliminary explanation of the dual nature of the outbreaks through two distinctly different large scale hydroclimatic drivers. According to that study, intrusion of plankton and bacteria rich coastal water during the spring dry season is the primary mechanism of V. cholerae contamination of estuarine rivers and coastal cholera outbreaks; on the other hand, widespread monsoon flooding in the GBM Basin region and cross-contamination of water resources with bacteria already present in the ecosystem is primarily responsible for autumn outbreaks. Similar role of coastal rivers acting as conduits of cholera infection along the river have been proposed by Bertuzzo et al. (2008) for Southern Africa.
Cholera and Remote Sensing
Application of remote sensing to study cholera dynamics is an emerging research area with availability of longer datasets over the last decade (Harvell et al 2002). As mentioned in section 2, analysis of cholera incidence data from various regions of the world suggests transmission of cholera originate in coastal areas and then propagate to inland areas (Huq and Colwell, 1996). The causative agent of cholera outbreaks, V. cholerae, cannot be measured from space. However, the bacteria shows strong affinity with plankton blooms which can be estimated from satellites by measuring the green pigment (chlorophyll) present in plankton. Chlorophyll, a key biochemical component that gives plants its green color, is responsible for facilitating absorption of sunlight for photosynthetic purposes. Currently, satellite measured chlorophyll is the only effective way to monitor space-time variations of plankton abundance over large coastal areas.
Remote sensing of ocean color dates back to 1978 with successful launch of dedicated ocean satellite Coastal Zone Color Scanner (CZCS) on Nimbus7. CZCS was followed with Sea-viewing Wide Field-of-view Sensor (SeaWiFS) mission in 1997. Chlorophyll measured by SeaWiFS has been used in several studies ranging from detection of harmful algal blooms (Strumf et al., 2003; Tang et al., 2003), coastal pollution (Chen et al., 2007), oceanic processes (Tang et al., 2003; Yoder et al., 1987; Danling et al., 2002), land-ocean interaction (Lopez and Hidalgo, 2009; D’Sa and Miller, 2003; Jutla et al., 2009a) and marine fauna (Solanki et al., 2001; Polovina et al., 2003; Turley et al., 2000; Labiosa and Arrigo, 2003) . The SeaWiFS consists of eight channels at: 412, 443, 490, 510, 555, 670, 765, and 865 nm (nanometers: 1μm = 1,000 nm), each with bandwidths of 20 or 40 nm (O’Reilly et al., 2000). The orbital altitude of SeaWiFS is about 705 km (438 mi) with spatial resolution in the Local Area Coverage (LAC) of about 1.1 km (0.68 mi). The optimal resolution is 0.6 km at nadir. Currently, SeaWiFS data offer the longest available ocean color records for 10 years (1997 to till date) at various spatial (1.1km, 9km) and temporal scales (daily, monthly, annual). Current global SeaWiFS chlorophyll algorithm, OC4V4, is a fourth-order polynomial (Equation1) of the maximum band ratio of four bands (O’Reilly et al., 2000), and can be represented as:
| [1] |
where, chl is the chlorophyll in mg/m3 and Rrs() is the wavelength in nm.
Chlorophyll variations on a daily scale appear to be a random process with very limited memory (Sumich, 1999, Uz and Yoder, 2004). Our preliminary analysis reaffirms above findings and suggests that chlorophyll signatures for the coastal Bay of Bengal region resemble white noise for a range of pixel sizes (10–100km); the signal exhibits a lag one autocorrelation value of 0.20 with no apparent temporal structure (Jutla et al., 2009b). Chlorophyll variations on a daily scale, irrespective of spatial averaging, thus may not be useful for understanding chlorophyll-cholera relationships. On the other hand, chlorophyll variations on monthly scales show distinct seasonality in coastal Bay of Bengal with highest chlorophyll levels observed in September and lowest levels in February (Jutla et al 2009b). Figure 2 shows the ten year (1998–2007) climatological mean (2a), lowest (2b) and highest (2c) chlorophyll months in coastal Bay of Bengal. Figure 2 has been calculated using monthly SeaWiFS data for latitudes between 200 to 22.50N and longitudes between 860 to 930E. Chlorophyll levels are high along the coasts and decreases as we move away from the coast. Climatological mean chlorophyll within this domain is about 3 mg/m3 (Figure 2a), whereas the lowest and highest mean monthly chlorophyll values, 2.36 mg/m3 and 4.15 mg/m3, are observed during the months of February and September, respectively. Figures 2b and 2c shows the contrastingly different chlorophyll levels in these months.
FIGURE 2.
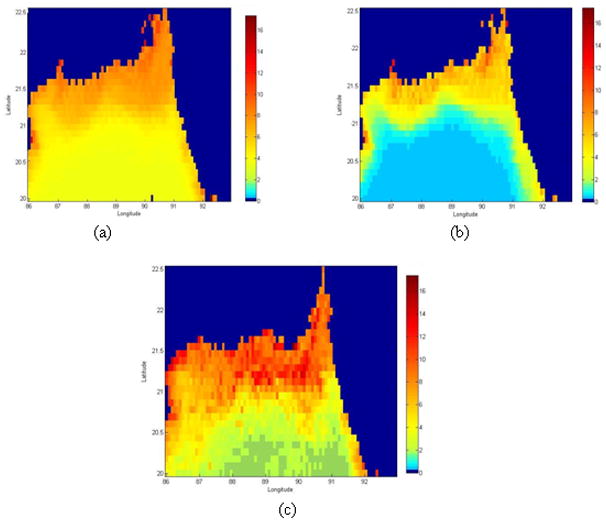
Space-time variations of chlorophyll (mg/m3) in the Bay of Bengal (a) Mean chlorophyll; (b) Lowest chlorophyll month (February) and (c) Highest chlorophyll month (September). This figure is based on climatology of 10 years of SeaWiFS data at 9km spatial resolution.
Remote sensing measurements of other relevant climate variables (e.g., sea surface temperature) may also help in understanding the possible controls on chlorophyll production in the coastal regions and its links to terrestrial hydrology. For example, using satellite measured chlorophyll from various ocean basins across the globe several recent studies suggest an inverse relationship between chlorophyll (and hence phytoplankton) and SST (e.g., Solanki et al., 2001, Uz and Yoder, 2004, Legaard and Thomas, 2006, Smyth et al. 2001). In the Bay of Bengal (BoB), however, a positive relationship between phytoplankton and SST is observed (Lobitz et al., 2000; Chaturvedi, 2005; Emch et al, 2008; Magny et al., 2008). Preliminary analyses, using SeaWiFS data, suggest that terrestrial nutrient transport through fresh water discharge from the Ganges and the Brahmaputra rivers is the dominant process affecting phytoplankton production in the coastal BoB region (Jutla et al. 2009a), which alters the usually observed inverse relationship between SST and chlorophyll. Akanda et al (2009) explain further role of regional freshwater discharge, where they associate dry and wet season discharge volumes with spring and autumn cholera outbreaks in Bangladesh, respectively.
Olsson (1996) was perhaps the first study to propose the potential of using satellite derived chlorophyll for studying cholera dynamics. Huq and Colwell (1996) suggested that remote sensing can be a helpful tool for tracking cholera outbreaks using ocean chlorophyll signatures. Lobitz et al (2000) used limited length SeaWiFS data (16 months) to stress the potential role of remotely sensed chlorophyll for understanding chlorophyll-cholera relationships. Since then there have been other studies that have qualitatively emphasized the use of remote sensing data for cholera (e.g., Colwell and Huq, 2001; Colwell et al., 2003; Koelle et al. 2005). There do not appear to be any quantitative analyses, however, that have used satellite based data to strengthen chlorophyll-cholera relationships. Recently, Magny et al (2008) developed a model for predicting cholera outbreaks based on several variables including coastal chlorophyll and other climatological data on a monthly time scale with approximately 100 km aggregated pixel scale. They concluded that there is approximately a month lag between plankton blooms in the Bay of Bengal and cholera incidence in Bangladesh. Magny et al (2008) also recommended that finer temporal and spatial scale chlorophyll data may be required for real-time tracking of cholera outbreaks. Emch et al., (2008) have used satellite chlorophyll measurements from two coastal regions in South Asia (Bangladesh and Vietnam) and reported a two month lag between plankton blooms and cholera outbreaks in Bangladesh. They have also suggested that chlorophyll may not be a useful variable for understanding the sporadic cholera in Vietnam. These studies have suggested the role of chlorophyll as a key variable to understand cholera dynamics; however, they have not elaborated on how the seasonal and interannual variability of cholera incidence are linked with the variations of other coastal processes and chlorophyll variations.
Colwell and Huq (1996) and Lipp et al (2002) proposed qualitative cholera infection and transmission pathways from coastal to inland regions. Here, with ten years of monthly SeaWiFS data, we quantitatively investigate how cholera incidence are associated with chlorophyll in the Bengal delta region. Figure 3 shows the climatological monthly mean for chlorophyll, river discharge and cholera incidence in the Bay of Bengal region. Monthly chlorophyll in the coastal region of BoB and river discharge from the Ganges-Brahmaputra rivers show high positive correlation (r = 0.75; p<0.05), thereby suggesting that nutrients carried by river discharge is influencing chlorophyll production. In this region, cholera incidence exhibit(s) biannual peaks, however, chlorophyll and river discharge show single annual peaks. Akanda et al (2009) provide a tentative explanation of the roles of river discharge and coastal plankton intrusion on the dual peak cholera incidence pattern seen in this region. Cholera outbreaks in spring (March-April-May) show strong negative correlation with dry season (February-March) river discharge (r = −0.65; p<0.05), i.e., bigger spring cholera peaks are typically seen in water scarce years (Akanda et al 2009). However, a new transmission environment emerges in autumn, when water abundance contributes to elevated cholera outbreaks, i.e., bigger autumn peaks are seen in high flood years. Figure 4 shows the monthly river discharge, phytoplankton and cholera incidence patterns in Mozambique, plotted in a fashion similar to Figure 3. A peak in marine plankton blooms is observed in the coastal areas off southern Mozambique and the capital city of Maputo, during August which follows increased outbreaks of cholera from November. Increase in river discharge in March actually leads to decrease in cholera incidence, a very similar phenomenon observed in Bay of Bengal (Akanda et al, 2009). Mendelsohn and Dawson (2008) also reported similar lags and mechanisms between chlorophyll peaks off South African coast and cholera outbreaks in the Kwazulu-Natal province. Figures 3 and 4 summarize the codependent relationships among chlorophyll, streamflow, and cholera outbreaks, and quantitatively reaffirm the potential use of remote sensing data for understanding cholera dynamics on large scales.
FIGURE 3.
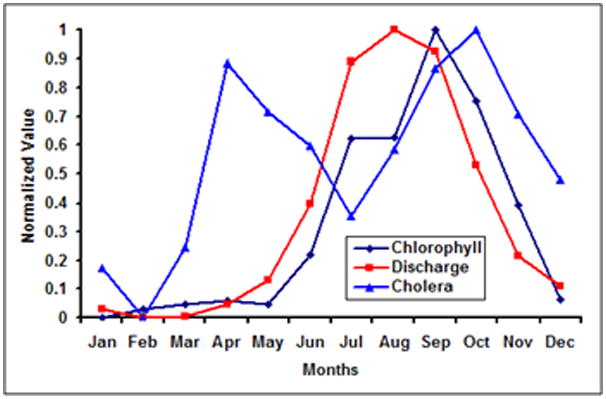
Cholera, River Discharge and Chlorophyll in Bay of Bengal. The climatology has been calculated using ten years of monthly (a) SeaWiFs data for chlorophyll, (b) incidence data of cholera incidence from ICDDR,B and (c) discharge data obtained from Bangladesh University of Engineering and Technology. The data has been normalized between 0 and 1.
FIGURE 4.
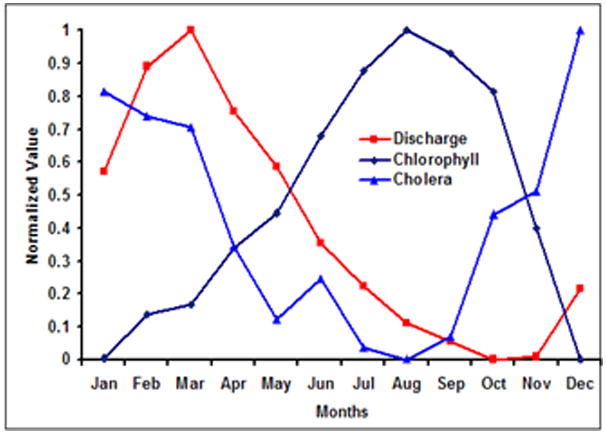
Cholera, River Discharge and Chlorophyll in Coastal Mozambique. The climatology has been calculated using ten years of monthly (a) SeaWiFs data for chlorophyll, (b) incidence data of cholera incidence from literature and (c) discharge data obtained from River Discharge Data (RIVDIS: www.rivdis.sr.unh.edu). The data has been normalized between 0 and 1.
Cholera, Coast, Terrestrial Hydrology and Remote Sensing
Cholera is perhaps the only endemic disease interfacing oceans and human health for several centuries. Cholera has a strong coastal connection; a number of studies have associated coastal ecosystem processes with cholera outbreaks. We have compiled a list of studies and reports to show that majority of primary cholera outbreaks usually occurs along coastal areas and then spread inland through secondary means (Figure 1; Table 1). However, much of this information remained qualitative because of the lack of data over coastal areas on a range of space-time scales. With the availability of over ten years of remotely sensed data to examine space-time variations of chlorophyll (and hence plankton abundance), we are able to explore possible relationships between plankton abundance and cholera dynamics. Our quantitative analysis, Figures 3 and 4, supported by qualitative understanding in literature suggests that initial cholera outbreaks primarily occur in regions close to the coast, and may be related with coastal plankton. For example, in the Bay of Bengal region of South Asia, plankton intrusion through coastal waters in the dry season leads to early cholera outbreaks (Jutla et al. 2009b). Similarly, in Mozambique, there is strong evidence that coastal chlorophyll intrusion leads to cholera outbreaks, a phenomenon similar to observed processes in the BoB region. These results suggest that it may be feasible to develop predictive models of cholera using large scale oceanic and hydroclimatic signatures using remotely sensed observations.
Chlorophyll production in the coastal areas with freshwater discharge may be controlled by the river discharge (Arker et al., 2005; Jutla et al., 2009a). In other regions, coastal chlorophyll production is driven by upwelling and shows inverse association with SST (Legaard and Thomas, 2006, Smyth et al. 2001). For example, in the Bay of Bengal, plankton blooms immediately follow the peak monsoon discharge volumes carrying terrestrial nutrients, whereas in Mozambique, chlorophyll peak does not follow the discharge peaks immediately. Given the high relative differences between the discharge volumes (628 km3/yr in the Bay of Bengal vs. 14 km3/yr in Mozambique; Dai and Trenberth, 2003), it is likely that the production of chlorophyll in coastal regions of Mozambique may be governed by oceanic processes rather than terrestrial discharge. Identification of the appropriate drivers for chlorophyll production is thus important for understanding controls over cholera dynamics.
Significant heterogeneity observed in space-time variability of coastal chlorophyll cannot be captured with sporadic in-situ observations. For example, chlorophyll data resembles white noise on daily time scales irrespective of spatial averaging (Uz and Yoder, 2004). Consequently, use of satellite remote sensing to track phytoplankton abundance through chlorophyll measurement is the most efficient and cost-effective way to develop a large scale understanding of the cholera-plankton relationship. With ten years of available data and ongoing measurements of reliable chlorophyll data from SeaWiFS, remote sensing has a great potential to be used in a systematic development of the understanding of the relationship between coastal ecology and cholera dynamics in various endemic regions across the world.
As it has been discussed above, cholera is endemic in many regions and is affected by coastal processes. We now attempt to identify ocean-corridors from where plankton intrusion may be possible to inland waters using 10 years of SeaWiFS data (Figure 5). In regions 2, 3, 5, 6, cholera remains endemic and these regions are also active river discharge regions. The Senegal, Congo, Ganges and Brahmaputra, and Changjiang Rivers in the regions 2, 3, 5, 6, respectively, discharge into the ocean in areas where there is a high possibility of plankton laden coastal water intrusion and subsequent contamination of inland water bodies. Region 7 drains the Amazon River with the largest freshwater discharge volumes, but cholera has not been reported to be endemic in that region. This can be explained as river discharge in Amazon remains high throughout the year (Dai and Trenberth, 2001) and there is negligible coastal intrusion in this estuary along with the fact that the population in the Amazonia region is much scarcer compared to regions 5 or 6. In regions 1 and 4, cholera outbreaks are sporadic. There are no major rivers or coastal deltas in the region and the possible cause of disease outbreaks may be contaminated food from the coast and human interaction.
FIGURE 5.
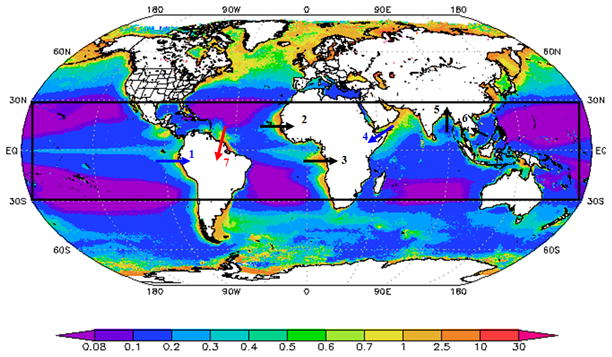
Possible ocean corridors for cholera outbreaks along tropical coastal regions (shown as the black box spanning between 30N to 30S) cholera outbreaks. The figure has been constructed using ten years (September 1997- March 2010) of monthly chlorophyll data. The global chlorophyll visualization were produced with the Giovanni online data system, developed and maintained by the NASA GES DISC.
This global overview of chlorophyll concentrations and possible vulnerable regions for cholera outbreaks is supported by other studies; Huq and Colwell (1996) presented an epidemiological global picture of cholera outbreaks in three continents (Africa, South Asia and South America) suggesting the role of coastal regions behind cholera outbreaks. This is perhaps one of the first attempts to quantify the chlorophyll-cholera relationships on a global scale using satellite remote sensing data. To summarize, section 6, we provide a schematic pathway integrating terrestrial hydrology, coastal ecology into existing microbiological framework for a holistic understanding of the cholera dynamics and associated large scale controls on cholera transmission using latest advances in remote sensing.
An Integrated Modeling Framework for Cholera Prediction
We propose a modeling framework to provide an adaptive understanding and prediction of cholera dynamics where “macro” (hydrological, ecological, climatic and coastal processes) and “micro” (microbiological, genetic, and human intestine scale processes) environmental conditions are integrated. . This distinction between “macro” and “micro” environmental controls on cholera dynamics is critical because the cholera bacterium can survive and proliferate in two distinctively different environments. We recognize the importance of micro-environmental (e. g.: microbiological, genetic, human intestines) understanding of cholera (Schoolnik and Yildiz 2000) to develop effective vaccines or treatment protocols. However, as V. cholerae exists naturally in aquatic habitats and there is strong evidence of new biotypes emerging, it is highly unlikely that cholera will be fully eradicated. Consequently, it is imperative that a broader perspective be taken to prevent cholera epidemics and minimize its impact by understanding the effects of macro-environmental conditions on cholera
One of the approaches to understand effects of macro environmental controls on cholera dynamics is by using the Susceptible-Infected-Recovered (SIR) based epidemiological models (e.g., Codeco, 2001; Joh et al., 2009). The basic idea of SIR models is to compute the theoretical number of people infected with a contagious illness over time and how the disease spread through a given population using various parameters. More details of SIR models can be found in Kermack and McKendrik (1972). Within the framework, we suggest a new class of SIR (Susceptible-Infected-Recovered) model, where macro-environmental factors inform traditional SIR model -will allow us to examine different facets of cholera dynamics. Our proposed model, Macro-SIR, will integrate macro-environmental and micro-environmental determinants of cholera occurrences and transmission. It will synthesize existing knowledge and new information from hydroclimatology, ecology, and remote sensing. Figure 6 provides a framework for the development and refinement of this modeling framework.
Figure 6.
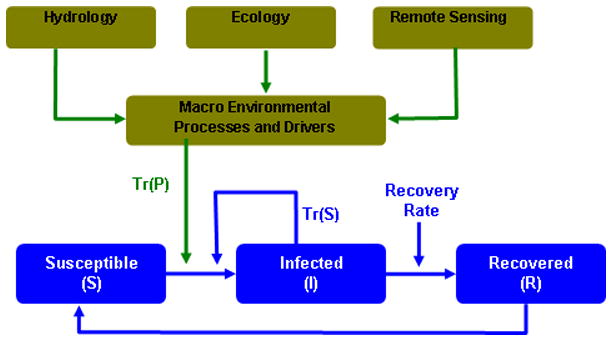
Plausible Flow Diagram for a Macro-SIR modeling framework
Cholera based SIR models usually start with the premise that cholera bacteria are transmitted via human to human interaction. Recently, role of indirect transmission via environmental reservoir has been introduced in SIR models (e.g., Codeco, 2001; Joh et al., 2009). Studies have highlighted the role of environmental conditions for creating seasonality in cholera (Koelle et al., 2005; Pascual et al., 2008) but did not elaborate on plausible physical mechanisms related to seasonality of outbreaks. Similarly, Bertuzzo et al. (2008; 2009) incorporated an SIR-type framework with a spatially distributed cholera transmission model, but the seasonality of transmission in that model was introduced with a-priori knowledge or assumption of the distribution of infections. Despite its ubiquitous nature and its importance in the timing of the outbreaks, the seasonality of cholera is not well understood (Fisman 2007). To our best knowledge, currently there are no models that can predict cholera outbreaks several months ahead.
Issues of seasonality and prediction lead time for cholera are particularly important for the endemic areas of the Bengal Delta where cholera exhibits two peaks per year. To examine the origin of such seasonal patterns, one may focus on relative roles of two routes of transmission – primary or environmental transmission, Tr (P), and secondary or person to person transmission, Tr (S), - for cholera (Miller et al., 1985). Most SIR models focus on the secondary transmission mechanisms, Tr(S), as shown in Figure 6. Few studies included, Tr (P), as an environmental reservoir (e.g., Codeco 2001; Jensen et al 2006). Traditional SIR models presume exponential decay for bacteria in the reservoir even though this phenomenon is not frequently observed (Joh et al., 2009). The Macro-SIR modeling framework may be used to evaluate the roles of macro- and micro-environmental drivers in creating and sustaining primary and secondary transmission mechanisms for cholera outbreaks and promoting epidemic and endemic cholera.
The dynamics of direct disease transmission in humans have been studied using variants of SIR models. In these models, primarily micro-environmental conditions are emphasized and basic reproductive ratio (defined as “the number of secondary cases caused by a small number of infected individuals” Joh et al., 2009) is used as a central concept (Joh et al., 2009; Dietz 1993). But, these models cannot create seasonality unless some of the model parameters are a-priori chosen to vary seasonally. Such is the case for a recent study by Pascual et al (2008) where a complex SIR type model is used with susceptible fraction and transmission rate as a-priori chosen seasonally varying parameters. In the absence of plausible physical mechanisms to explain this choice of seasonally varying parameters, predictive capabilities of these models remain uncertain. For example, Pascual et al (2008) reported only 7% improvement in predictive capability when effects of El-Nino are included in their model. In a related study, Koelle et al (2005) reported low frequency variations in transmission rate to be negatively correlated with rainfall in Northeast India (r = −0.797, p < 0.05, lag = 14 months). There are no plausible hydroclimatological explanations for such a lagged relationship between rainfall and cholera transmission.
Instead of a-priori choosing transmission mechanisms (primary or secondary) that create and sustain seasonality in cholera, one can use an adaptive modeling framework (Figure 6). In a Macro-SIR framework, pathogen dynamics and within human transmission dynamics may be explicitly coupled. It recognizes that seasonality of cholera may be dependent on geography and climate (e.g., dual peak in the Bengal delta and single peak in Mozambique) and transmission rates must be estimated based on regional macro-environmental drivers. Such a regionalized approach will allow one to accurately estimate transmission that will result in better prediction.
Development of a holistic understanding off cholera dynamics and its relationship with coastal processes requires long and reliable chlorophyll data over a range of space and time scales, which is beginning to be available through satellite observations. Remote sensing observations, with its wide spatial coverage and continuous measurement capabilities, will thus play an important role in monitoring coastal processes and tracking potential cholera outbreaks in vulnerable regions of the world. We hope this study will provide the rationale and motivation for future research in this direction to explore how coastal processes, terrestrial hydrology, large scale climatic controls and remote sensing can be integrated into a combined environmental and epidemiological modeling framework to enhance the existing knowledge base to develop global and regional scale cholera tracking and prediction models.
Acknowledgments
This research was supported, in part, by a Research Challenge Grant from the National Institutes of Health (1RC1TW008587-01) under the American Recovery and Reinvestment Act of 2009 and by a summer fellowship from Tufts Institute of the Environment, Tufts University, Medford, MA USA.
Contributor Information
Antarpreet S Jutla, WE REASoN (Water and Environmental Research, Education, and Actionable Solutions Network), Department of Civil and Environmental Engineering, Tufts University, Medford, MA 02155.
Ali S Akanda, WE REASoN, Department of Civil and Environmental Engineering, Tufts University, Medford, MA 02155.
Shafiqul Islam, Email: Shafiqul.Islam@tufts.edu, Civil and Environmental Engineering, School of Engineering, Water and Diplomacy, The Fletcher School of Law and Diplomacy, Bernard M. Gordon Senior Faculty Fellow in Engineering. 113 Anderson Hall, 200 College Avenue, Tufts University, Medford, MA 02155 Shafiqul Islam.
Literature Cited
- Acker JG, Harding LW, Leptoukh G, Zhu T, Shen S. Remotely-sensed Chl-a at the Chesapeake Bay Mouth is Correlated with Annual Freshwater Flow to Chesapeake Bay. Geophys Res Lett. 2005;32:L05601. doi: 10.1029/2004GL021852. [DOI] [Google Scholar]
- Akanda AS, Jutla AS, Islam S. Bimodal Cholera Transmission in Bengal Delta: A Hydroclimatological Explanation. Geophy Res Lett. 2009;36:L19401. doi: 10.1029/2009GL039312. [DOI] [Google Scholar]
- Bentivoglio M, Pacini P. Filippo Pacini: A Determined Observer. Brain Res Bull. 1995;38(2):161–165. doi: 10.1016/0361-9230(95)00083-q. [DOI] [PubMed] [Google Scholar]
- Bertuzzo E, Azaele S, Maritan A, Gatto M, Rodriguez-Iturbe I, Rinaldo A. On the Space-Time Evolution of a Cholera Epidemic. Water Resour Res. 2008;44:W01424. [Google Scholar]
- Bertuzzo E, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A. On spatially explicit models of cholera epidemics. J R Soc. 2009 doi: 10.1098/rsif.2009.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishagratna K. An English Translation of the Sushruta Samhita. Chowkhamba Sanskrit Series Office; Varanasi, India: 1963. pp. 352–356. [Google Scholar]
- Bouma MJ, Pascual M. Seasonal and Interannual Cycles of Endemic cholera in Bengal 1891–1940 in Relation to Climate and Geography. Hydrobiologia. 2001;460(1–3):1573–5117. [Google Scholar]
- Cash BA, Rodo X, Kinter JL. Links between Tropical Pacific SST and Cholera Incidence in Bangladesh: Role of the Eastern and Central Tropical Pacific. J Clim. 2008;21(18):4647–4663. [Google Scholar]
- Chaturvedi N. Variability of Chlorophyll Concentration in the Arabian Sea and Bay of Bengal as Observed from SeaWiFS Data from 1997–2000 and its Interrelationship with Sea Surface Temperature (SST) Derived from NOAA AVHRR. Int J Remote Sen. 2005;26:3695–3706. [Google Scholar]
- Chen C, Tang S, Pan Z, Zhan H, Larson M, Jönsson L. Remotely Sensed Assessment of Water Quality Levels in the Pearl River Estuary, China. Mar Poll Bull. 2007;54(8):1267–1272. doi: 10.1016/j.marpolbul.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Codeço CT. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infectious Diseases. 2001;1:1. doi: 10.1186/1471-2334-1-1. http://www.biomedcentral.com/1471-2334/1/1. [DOI] [PMC free article] [PubMed]
- Cockburn TA, Cassanos JG. Epidemiology of Endemic Cholera. Public Health Reports. 1960;75(9):791–803. [PMC free article] [PubMed] [Google Scholar]
- Collins AE. Vulnerability to Coastal Cholera Ecology. Soc Science & Med. 2003;57:1397–1407. doi: 10.1016/s0277-9536(02)00519-1. [DOI] [PubMed] [Google Scholar]
- Colwell RR, Huq A, Islam MS, Aziz KMA, Yunus M, Khan NH, Mahmud A, Sack RB, Nair GB, Chakraborty DA, Sack DA, Russek-Cohen E. Reduction of Cholera in Bangladeshi Villages by Simple Filtration. PNAS. 2003;100(3):1051–1055. doi: 10.1073/pnas.0237386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR. Global Climate and Infectious Disease: The Cholera Paradigm. Science. 1996;274:5295. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Colwell RR, Huq A. Marine Ecosystems and Cholera. Hydrobiologia. 2001;460:141–145. [Google Scholar]
- Dai A, Trenberth KE. Estimates of Freshwater Discharge from Continents: Latitudinal and Seasonal Variations. J Hydrometeorology. 2002;3:660–687. [Google Scholar]
- Danling T, Kester DR, Ni I, Kawamura H, Huasheng H. Upwelling in the Taiwan Strait during the Summer Monsoon Detected by Satellite and Shipboard Measurements. Remote Sens Environ. 2002;83(3):457–471. [Google Scholar]
- Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Meth Med Res. 1993;2:23–41. doi: 10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- D’Sa EJ, Miller RL. Bio-optical Properties in Waters Influenced by the Mississippi River During Low Flow Conditions. Remote Sens Environ. 2003;84(4):538–549. [Google Scholar]
- Emch M, Feldacker C, Yunus M, Streatfield PK, Thiem V, Canh D, Mohammad A. Local Environmental Predictors of Cholera in Bangladesh and Vietnam. Am J Trop Med Hyg. 2008;78:823–832. [PubMed] [Google Scholar]
- Epstein PR. Algal Blooms in the Spread and Persistence of Cholera. Biosystems. 1993;31:209–221. doi: 10.1016/0303-2647(93)90050-m. [DOI] [PubMed] [Google Scholar]
- Fisman DN. Seasonality of infectious diseases. Annual Review of Public Health. 2007;28:127–143. doi: 10.1146/annurev.publhealth.28.021406.144128. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Carr M. The Climatological Annual Cycle of Satellite Derived Phytoplankton Pigments in the Alboran Sea. Geophys Res Lett. 1999;26(19):2985–2988. [Google Scholar]
- Gil AI, V, Louis R, Rivera ING, Lipp E, Huq A, Lanata CF, Taylor DN, Russek-Cohen E, Choopun N, Sack RB, Colwell RR. Occurrence and Distribution of Vibrio cholerae in the Coastal Environment of Peru. Environ Microbiol. 2004;6(7):699–706. doi: 10.1111/j.1462-2920.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- Gleick P. The World’s Water 2008–2009: The Biennial Report on Freshwater Resources. Island Press; USA: 2008. [Google Scholar]
- Griffith DC, Kelly-Hope LA, Miller MA. Review of reported cholera outbreaks worldwide, 1995–2005. Am J Trop Med Hyg. 2006;75(5):973–977. [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296(5576):2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque A, Hayashi T, Sack D. The Effect of Rainfall on the Incidence of Cholera in Bangladesh. Epidemiology. 2008;19(1):103–110. doi: 10.1097/EDE.0b013e31815c09ea. [DOI] [PubMed] [Google Scholar]
- Huq A, Colwell RR. Vibrios in the Marine and Estuarine Environment: Tracking Vibrio cholerae. Ecosystem Health. 1996;2(3):198–214. [Google Scholar]
- Huq A, West PA, Small EB, Huq MI, Colwell RR. Influence of Water Temperature, Salinity, and ph on Survival and Growth of Toxigenic Vibrio cholerae Serovar O1 Associated With Live Copepods in Laboratory Microcosms. Appl Environ Microbiol. 1984;48(2):420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A, Morris JG, Khan MNH, Siddique AK, Yunus M, Albert MJ, Sack DA, Colwell RR. Critical Factors Influencing the Occurrence of Vibrio Cholerae in the Environment of Bangladesh. Appl Environ Microbiol. 2005;71(8):4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Brooks A, Kabir MS, Jahid IK, Islam MS, Goswami D, Nair GB, Larson C, Yukiko W, Luby S. Fecal Contamination of Drinking Water Sources of Dhaka City During the 2004 Peak Flow in Bangladesh. J Appl Microbiol. 2006:80–87. doi: 10.1111/j.1365-2672.2006.03234.x. [DOI] [PubMed] [Google Scholar]
- Jensen MA, Faruque SM, Mekalanos JJ, Levin BR. Modeling the role of bacteriophage in the control of cholera outbreaks. Proc Natl Acad Sci USA. 2006;103:4652–4657. doi: 10.1073/pnas.0600166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh RI, Wang H, Weiss H, Weitz JS. Dynamics of indirectly transmitted infectious diseases with immunological threshold. Bull of Mathematical Biol. 71(4):845–862. doi: 10.1007/s11538-008-9384-4. [DOI] [PubMed] [Google Scholar]
- Jutla AS, Akanda AS, Islam S. Relationship between Phytoplankton, Sea Surface Temperature and River Discharge in Bay of Bengal. Geophysical Research Abstracts; EGU2009–1091–2, EGU General Assembly 2009; Vienna, Austria. 2009a. [Google Scholar]
- Jutla AS, Akanda AS, Islam S. Satellites and Human Health: Potential for tracking cholera outbreaks; American Geophysical Union, Fall Meeting; San Francisco, USA. 2009b. [Google Scholar]
- Kermack WO, McKendrik AG. A contribution to the mathematical theory of epidemics. Proc R Soc Lond A. 1927;115:700–721. [Google Scholar]
- Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory Periods and Climate Forcing in Cholera Dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- Koelle K, Pascual M, Yunus Md. Pathogen adaptation to seasonal forcing and climate change. Proceedings of the Royal Society B: Biological Sciences. 2005;272(1566):971–977. doi: 10.1098/rspb.2004.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labiosa RG, Arrigo KR. The interplay between Upwelling and Deep Convective Mixing in Determining the Seasonal Phytoplankton Dynamics in the Gulf of Aqaba: Evidence from SeaWiFS and MODIS. Limnol Oceanogr. 2003;48(6):2355–2368. [Google Scholar]
- Legaard KL, Thomas AC. Spatial Patterns in Seasonal and Interannual Variability of Chlorophyll and Sea Surface Temperature in the California Current. J Geophys Res. 2006;111:C06032. doi: 10.1029/2005JC003282. [DOI] [Google Scholar]
- Lipp EK, Huq A, Colwell RR. Effects of Global Climate on Infectious Disease. Clin Microbiol Rev. 2002;15(4):757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobitz BM, Beck LR, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell RR. Climate and Infectious Disease: Use of Remote Sensing for Detection of Vibrio Cholerae by Indirect Measurement. PNAS. 2000;97(4):1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longini IM, Yunus M, Zaman K, Siddique AK, Sack BB. Epidemic and Endemic Cholera Trends over a 33-Year Period in Bangladesh. J Infectious Diseases. 2002;186:246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- Lopez BM, Hidalgo JZ. Seasonal and Interannual Variability of Cross-shelf Transports of Chlorophyll in the Gulf of Mexico. J Mar Sci. 2009;77(1–2):1–20. [Google Scholar]
- Magny G, Murtugudde R, Sapianob M, Nizam A, Brown C, Busalacchi A, Yunus M, Nair G, Gil A, Calkins J, Manna B, Rajendran K, Bhattacharya M, Huq A, Sack R, Colwell RR. Environmental Signatures Associated with Cholera Epidemics. PNAS. 2008;105:46. doi: 10.1073/pnas.0809654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Urtaza J, Huapaya B, Gavilan RG, Blanco-Abad V, Ansede-Bermejo J, Cadarso-Suarez C, Figueiras A, Trinanes J. Emergence of Asiatic Vibrio Diseases in South America in Phase With El Nino. Epidemiology. 2008;19(6):829–837. doi: 10.1097/EDE.0b013e3181883d43. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J, Dawson T. Climate and Cholera in KwaZulu-Natal, South Africa: The Role of Environmental Factors and Implications for Epidemic Preparedness. Int J Hyg Environ Health. 2008;211(1–2):156–162. doi: 10.1016/j.ijheh.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Feachem RG, Drasar BS. Cholera epidemiology in developed and developing countries: new thoughts on transmission, seasonality, and control. Lancet. 1985;1(8423):261–262. doi: 10.1016/s0140-6736(85)91036-0. [DOI] [PubMed] [Google Scholar]
- Mouriño-Pérez RR. Oceanography and the Seventh Global Cholera Pandemic. Epidemiology. 1998;9(3):355–357. doi: 10.1097/00001648-199805000-00024. [DOI] [PubMed] [Google Scholar]
- Olsson T. Malaria and cholera are traced by satellites. Lakartidningen. 1996;93(36):3037–40. [PubMed] [Google Scholar]
- O’Reilly JE, Maritorena S, Siegel D, O’Brien MC, Toole D, Mitchell BG, et al. Ocean color chlorophyll a algorithms for SeaWiFS, OC2, and OC4: Version 4. In: Hooker SB, Firestone ER, editors. SeaWiFS postlaunch technical report series, SeaWiFS postlaunch calibration and validation analyses, Part 3. Vol. 11. NASA/GSFC; 2000. pp. 9–23. [Google Scholar]
- Pascual M, Bouma MJ, Dobson AP. Cholera and Climate: Revisiting the Quantitative Evidence. Microbes and Infection. 2002;4(2) doi: 10.1016/S1286–4579(01)01533–7. [DOI] [PubMed] [Google Scholar]
- Pascual M, Chaves LF, Cash B, Rodó X, Yunus Md. Predicting endemic cholera: The role of climate variability and disease dynamics. Climate Research. 2005;36(2):131–140. [Google Scholar]
- Polovina JJ, Balaz GH, Howell EA, Parker DM, Seki MP, Dutton PH. Forage and Migration Habitat of Loggerhead (Caretta caretta) and Olive Ridley (Lepidochelys olivacea) Sea Turtles in the Central North Pacific Ocean. Fisheries Oceanogr. 2003;13(1):36–51. [Google Scholar]
- Reidl J, Klose KE. Vibrio Cholerae and Cholera: Out of the Water and into the Host. FEMS Microbiol Rev. 2002;26(2):125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Sack RB, Siddique AK, Huq A, Longini IM, Nair GB, Qadri F, Yunus M, Albert MJ, Sack DA, Colwell RR. A 4-Year Study of the Epidemiology of Vibrio Cholerae in Four Rural Areas of Bangladesh. J Infectious Diseases. 2003;187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363(9404):223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Harris JB, Khan AI, LaRocque RC, Sack DA, Malek MA, Faruque ASG, Ryan ET. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. American J of Trop Medicine and Hygiene. 2006;74(6):1067–1073. [PMC free article] [PubMed] [Google Scholar]
- Schoolnik GK, Yildiz FH. The complete genome sequence of Vibrio cholerae: a tale of two chromosomes and two lifestyles. Genome Biology. 2000;1(3):1016.1–1016.3. doi: 10.1186/gb-2000-1-3-reviews1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique AK, Zaman K, Akram K, Mutsuddy P. Emergence of a New Epidemic Strain of Vibrio cholerae in Bangladesh: An Epidemiological Study. Trop Geograph Med. 1994;46:147–150. [PubMed] [Google Scholar]
- Singleton FL, Attwell R, Jangi S, Colwell RR. Effects of Temperature and Salinity on Vibrio Cholerea Growth. App Environ Microbiol. 1982;44(5):1047–1058. doi: 10.1128/aem.44.5.1047-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth TJ, Miller PI, Groom SB, Lavender SJ. Remote Sensing of Sea Surface Temperature and Chlorophyll during Lagrangian Experiments at the Iberian Margin. Prog Oceanogr. 2001;51:269–281. [Google Scholar]
- Solanki HU, Dwivedi RM, Nayark SR. Synergistic Analysis of SeaWiFS Chlorophyll Concentration and NOAA-AVHRR SST Feature for Exploring Marine Living Resources. Int J Rem Sens. 2001;22:3877–3882. [Google Scholar]
- Stumpf RP, Culver ME, Tester PA, Tomlinson M, Kirkpatrick GJ, Pederson BA, Truby E, Ransibrahmanakul V, Soracco M. Monitoring Karenia Brevis Blooms in the Gulf of Mexico using Satellite Ocean Color Imagery and Other Data. Harmful Algae. 2003;2(2):147–160. [Google Scholar]
- Sumich JL. Introduction to the Biology of Marine Life. WCB McGraw Hill; New York, USA: 1999. [Google Scholar]
- Tang D, Kester DR, Ni I, Qi Y, Kawamura H. In Situ and Satellite Observations of A Harmful Algal Bloom And Water Condition at The Pearl River Estuary in Late Autumn 1998. Harmful Algae. 2003;2(2):89–99. [Google Scholar]
- Timmermann A, Jin FF. Phytoplankton influences on tropical climate. Geophy Res Lett. 2002;29(23):2104. [Google Scholar]
- Turley CM, Bianchi M, Christaki U. Relationship between Primary Producers and Bacteria in an Oligotrophic Sea - The Mediterranean And Biogeochemical Implications. Mar Eco-Prog Ser. 2000;193:11–18. [Google Scholar]
- Uz BM, Yoder JA. High Frequency and Mesoscale Variability in SeaWiFS Chlorophyll Inagery and its Relation to Other Remotely Sensed Oceanographic Variables. Deep-Sea Res -II. 2004;51:1001–1071. [Google Scholar]
- Worden AZ, Seidel M, Wick A, Malfatti F, Bartlett D, Azam F. Trophic Regulation of Vibrio Cholerae in Coastal Marine Waters. Environ Microbiol. 2006;8(1):21–29. doi: 10.1111/j.1462-2920.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Yoder JA, McClain CR, Blanton JO, Oey L. Spatial Scales in CZCS-chlorophyll Imagery of the Southeastern US Continental Shelf. Limnol Oceanogr. 1987;32(4):924–941. [Google Scholar]


