Abstract
Bacterial infection relies on the micro-organism's ability to orchestrate the host's cell signalling such that the immune response is not activated. Conversely, the host cell has dedicated signalling pathways for coping with intrusions by pathogens. The autophagy of foreign micro-organisms (known as xenophagy) has emerged as one of the most powerful of these pathways, although the triggering mode remains largely unknown. In the present paper, we discuss the role that certain post-translational modifications (primarily ubiquitination) may play in the activation of xenophagy and how some bacteria have evolved mechanisms to subvert or hijack this process. In particular, we address the role played by P62/SQSTM1 (sequestosome 1). Finally, we discuss how autophagy can be subverted to eliminate bacteria-induced danger signals.
Keywords: autophagy, bacteria, P62/sequestosome 1 (SQSTM1), pathogens, ubiquitin, xenophagy
Abbreviations: Atg, autophagy related protein; DUB, deubiquitinating enzyme; ERK, extracellular-signal-regulated kinase; GFP, green fluorescent protein; IL, interleukin; LC3, microtubule-associated protein 1 light chain 3; LIR, LC3-interacting region; LLO, listeriolysin O; LPS, lipopolysaccharide; MEF, mouse embryonic fibroblast; NBR1, neighbour of the BRCA1 gene 1; NDP52, nuclear dot protein 52 kDa; NEMO, NF-κB essential modulator; NF-κB, nuclear factor κB; NOD, nucleotide-binding oligomerization domain; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor; ROS, reactive oxygen species; SCV, Salmonella-containing vacuolar; SQSTM1, sequestosome 1; SUMO, small ubiquitin-related modifier; TBK-1, Tank-binding kinase-1; TRAF6, tumour-necrosis-factor-receptor-associated factor 6; Ub, ubiquitin; UBA, Ub-associated; YFP, yellow fluorescent protein
Introduction
For successful invasion of the host cell, pathogenic micro-organisms subvert intracellular signalling machineries in order to avoid triggering an immune response. This requires hijacking of molecular complexes involved in membrane traffic; the pathogen can then invade a host compartment within which it can hide from degradation pathways and replicate in safety. It has now been established that PAMPs (pathogen-associated molecular patterns) expressed by the micro-organisms are sensed by PAMP recognition receptors [PRRs (pattern recognition receptors)] (Kumar et al., 2009a) present at the host cell surface (such as the Toll-like receptors: Gay and Gangloff, 2007; O'Neill and Bowie, 2007; Kumar et al., 2009b) or inside the cytoplasm [such as the Nod (nucleotide-binding oligomerization domain)-like receptors (Franchi et al., 2009), which sense intracytoplasmic replicating bacteria]. These PRRs then activate signalling cascades that influence inflammatory responses and the fate of the infected cells (e.g. the activation of cell death pathways). The establishment of an intracellular niche requires the bacterium to escape lysosomal fusion of the vacuolar compartment. Autophagosomes are double-membrane structures involved in the cellular response to starvation and in the targeting of damaged cellular structures (e.g. mitochondria, misfolded protein aggregates) to the lysosomes. As such, autophagosomes have been implicated in the host cell's defence against bacterial invasion (Levine and Deretic, 2007; Deretic and Levine, 2009). Post-translational modifications of cell proteins (such as ubiquitination) may be involved in the regulation of both membrane trafficking (e.g. endosomal targeting through mono-ubiquitination) and protein degradation (e.g. proteosomal targeting of polyubiquitinated proteins) (Kerscher et al., 2006; Dikic et al., 2009; Hochstrasser, 2009). Recently, ubiquitination has also been implicated in the autophagy pathway (Kirkin et al., 2009b; Korolchuk et al., 2009b).
In the present paper, we discuss (i) emerging evidence for a link between post-translational modifications (such as ubiquitination) and the regulation of autophagy, (ii) the P62 protein's key role at the crossroads of several pathways and (iii) the importance of autophagy regulation in pathogen-induced downstream signalling. Finally, we suggest that the mechanisms involved are reminiscent of other processes triggered by membrane damage. Studying the initial steps in bacterial infection may help us to understand basic cell biology issues related to cell signalling responses to membrane stress.
Ubiquitination during bacterial invasion
Ub (ubiquitin) is a small, 76-amino acid protein which is highly conserved and widely expressed in all eukaryotic cells (Goldstein et al., 1975). Ubiquitin conjugation was first identified as a protein degradation system through proteasome targeting of polyubiquitinated proteins and is dependent on Ub's Lys48 residue (Ciechanover et al., 1978; Chau et al., 1989). Ubiquitination involves one or more covalent additions of Ub to the lysine residues of target proteins (i.e. mono- or multi-ubiquitination) or of Ub itself (i.e. polyubiquitination). Three classes of enzymes are required: Ub-activating E1, Ub-conjugating E2 and Ub ligase E3 (Kerscher et al., 2006; Dikic et al., 2009; Hochstrasser, 2009). However, distinct mechanisms have been revealed whereby E3 is not essential (Hoeller et al., 2007). The HECT (homologous to the E6-AP C-terminus)-domain E3 ligases function as monomers, whereas the RING E3 ligases can act as monomers (e.g. the Culin-Ring ligases) or multimers (e.g. c-Cbl and Parkin) (Dye and Schulman, 2007). Ubiquitination is reversible, due to the presence of DUBs (deubiquitinating enzymes) that can cleave Ub from modified proteins (Isaacson and Ploegh, 2009). As a result of a structure/function analysis of the linkage on different lysine residues (Lys29, Lys48 and Lys63), ubiquitination was found to be involved in signal transduction events (Kerscher et al., 2006) and membrane trafficking (Hicke and Dunn, 2003). In the latter case, mono-ubiquitination has been demonstrated to play an important role in endocytosis and sorting events (Hicke and Dunn, 2003; Raiborg and Stenmark, 2009).
Given (i) the importance of ubiquitination in cell physiology and (ii) the subversion of host pathways by invading bacteria, it is not surprising that a growing list of bacterial effectors has been shown to interfere with the host's ubiquitination system and thus to achieve successful infection (for a review, see Boyer and Lemichez, 2004; Angot et al., 2007; Munro et al., 2007; Rytkonen and Holden, 2007; Spallek et al., 2009). Several strategies have been developed by pathogenic bacteria. Some act directly on the ubiquitination pathway by mimicking host cell proteins [e.g. E3 ligases, DUBs and the SUMO (small ubiquitin-related modifier) protease], whereas others act indirectly by expressing or interfering with the host Ub pathway (e.g. Escherichia coli CNF1, Shigella OspG) (Spallek et al., 2009). Interestingly, bacterial effector function can be regulated by ubiquitination. Localization of the Salmonella type III secretion system effector SopB (an inositol polyphosphate phosphatase that modulates host cell phospholipids at the plasma membrane) has been shown to depend on its ubiquitination status (Knodler et al., 2009; Patel et al., 2009). SopB present at the cell surface modulates actin-mediated bacterial entry and the pro-survival Akt/PKB (protein kinase B) ubiquitination. Following multiple mono-ubiquitination (via a ligase that remains to be identified), SopB translocates to an internal SCV (Salmonella-containing vacuolar) compartment concomitantly with down-regulation of Akt and cell surface association of the actin cytoskeleton network. SopB ubiquitination was shown to be required for delivery and retention on SCVs and its binding depends on hydrophobic interactions. Bacteria replication in SCVs was found to depend on SopB ubiquitination. Various pathogenic bacteria hijack receptor ubiquitination in order to trigger their own uptake or the uptake of toxins into host cells. InlA-mediated internalization of Listeria monocytogenes is dependent on polyubiquitination of E-cadherin by the E3 ligase Hakai (Bonazzi et al., 2008), whereas InlB-mediated internalization involves Cbl E3 ligase-dependent mono-ubiquitination of the Met receptor (Veiga and Cossart, 2005). Cbl ligases also play a role in the endocytosis of other bacterial (e.g. Rickettsia conorii) or toxins (e.g. the Bacillus anthracis toxin) via the ubiquitination of mammalian receptors called Ku70 (Martinez et al., 2005) or tumour endothelial marker 8 respectively (Abrami, 2006). Ubiquitination of receptors localized within specialized domains (e.g. lipid rafts: Simons and Ikonen, 1997) mediates endocytosis for signalling cascade activation, as for the FcϵRI, for example (Lafont and Simons, 2001; Molfetta et al., 2009). These specialized domains have been implicated in the entry of a variety of pathogens (Lafont et al., 2004; Lafont and Van Der Goot, 2005; Pietiäinen et al., 2005; Zaas et al., 2005). Hence, one can legitimately expect to find bacteria exploiting the raft-associated receptor ubiquitination involved in endocytosis. We are convinced that an interdisciplinary approach combining cell microbiology and biophysics may help decipher these events. Innovative high-resolution imaging approaches photo-activated localization microscopy (Shroff et al., 2008), stochastic optical reconstruction microscopy (Rust et al., 2006) and atomic force microscopy (Yersin et al., 2007; Roduit et al., 2008, 2009) have been validated over recent years as investigating tools and should provide us with a better spatial understanding of the host cell's Ub-dependent processes which take place during host–pathogen interactions (Enninga and Rosenshine, 2009; Hoppe et al., 2009).
Sumoylation and host–pathogen interactions
Closely related post-translational modifications activated during pathogen infection (Stulemeijer and Joosten, 2008) involve covalent links to Ub-like proteins, such as the NEDD8 (neural-precursor-cell-expressed developmentally down-regulated 8) or SUMO-1 to -4. However, less is known about these biological functions than for Ub (Kerscher et al., 2006; Geiss-Friedlander and Melchior, 2007; Hochstrasser, 2009). Although the sequence homology between Ub and SUMO proteins is only approx. 18%, the two have the same structure and the respective enzyme reactions in ubiquitination and sumoylation are very similar. Sumoylation can also be reversed by SUMO proteases (Seeler and Dejean, 2001). A key difference between Ub and SUMO is the latter's inability to self-conjugate (Pickart, 1997). It has been assumed that sumoylation (in contrast to ubiquitination) is not a degradation signal (Geiss-Friedlander and Melchior, 2007), although this has been questioned recently (Geoffroy and Hay, 2009). However, there are some data to show that pathogens interfere with the host cell's sumoylation system in order to achieve successful infection. For example, it has been demonstrated that XopD (an effector expressed by Xanthomonas campestris) can act directly on the sumoylation pathway by mimicking host cell proteins such as SUMO protease (Hotson et al., 2003; Chosed et al., 2007; Kim et al., 2008a). This bacterial strategy inhibits the transcription of target genes involved in the activation of an innate immune defence reaction.
Surprisingly, Listeria infection leads to a decrease in overall sumoylation, which is dependent on LLO (listeriolysin O) activity (Ribet et al., 2010). LLO induces a decrease in the level of the E2 SUMO enzyme (Ubc9), which acts independently of (i) the calcium efflux triggered by LLO and (ii) p38 and ERK (extracellular-signal-regulated kinase) activities. Furthermore, LLO alone is able to alter the half-life of Ubc9. SMAD4, a transducer of TGF-β (transforming growth factor β) is stabilized by sumoylation (Lin et al., 2003; Kang et al., 2008). Both infection and LLO alone decrease SMAD4 levels; unsurprisingly, the TGF-β response is impaired in infected cells (Ribet et al., 2010). These in vitro observations were confirmed by the depressed Ubc9 level measured in the liver of Listeria-infected C57Bl/6J mice 48 and 72 h post-infection. This finding suggests that several pathways can be regulated in this way by pathogens. It is very likely that this phenomenon will be found in a broad range of infectious processes, as highlighted by the data on pore-forming toxins. Interestingly, the pore-forming toxins from Clostridium perfringens (perfringolysin O) and Streptococcus pneumoniae (pneumolysin O) act in much the same way (Ribet et al., 2010).
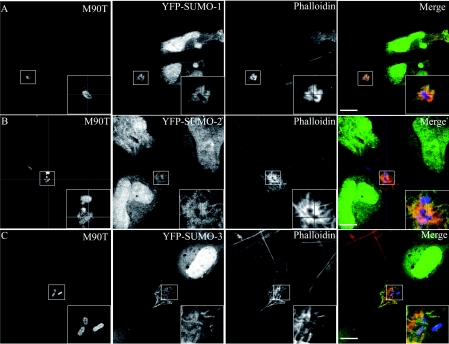
Indeed, our own data show that certain sumoylated proteins are recruited at the Shigella flexneri entry site in epithelial cells (Figure 1). Identification of the sumoylated protein involved requires further work, but should allow us to discover the bacterium's strategy for achieving successful infection via host–pathogen interactions.
Figure 1. Distribution of galectin-3 and SUMO during the early stages of Shigella infection of epithelial cells.
HeLa cells were transfected with YFP (yellow fluorescent protein)–SUMO-1 (A), YFP–SUMO-2 (B) or YFP–SUMO-3 (C) proteins and then infected with S. flexneri M90T for 30 min at 37°C. Infected cells were next fixed and processed for labelling. M90T bacteria and galectin-3 were visualized by immunostaining with specific primary antibodies and then a Marina-conjugated or an Alexa Fluor®555-conjugated secondary antibody respectively. Scale bar, 10 μm.
P62 stands at the crossroads of several signalling pathways
Apart from the proteasome route, misfolded proteins (polymerized through hydrophobic interaction of partially unfolded polypeptides and leading to the formation of cytoplasmic scattered structures, inclusion bodies and aggregosomes) can be targeted to degradation by macroautophagy (Kopito, 2000). Recently, cross-talk between the proteasomal and the macroautophagy pathways has been inferred by analysis of P62/SQSTM1 (sequestosome 1)–NBR1 (a neighbour of the BRCA1 gene 1) complexes (Kirkin et al., 2009b; Korolchuk et al., 2009b). P62/SQSTM1 (the 62 kDa sequestosome 1 protein, hereafter referred to as P62) plays a role in a number of cell functions, including signalling and protein turnover (Moscat et al., 2007; Seibenhener et al., 2007). P62 has been shown to bind ubiquitinated proteins [via its C-terminal UBA (Ub-associated) domain] and LC3 [microtubule-associated protein 1 light chain 3; via its LIR (LC3-interacting region)] (Bjorkoy et al., 2005; Komatsu et al., 2007; Pankiv et al., 2007; Ichimura et al., 2008b; Noda et al., 2008; Shvets et al., 2008). P62 targets protein aggregates to degradation via autophagy. After autophagy inhibition, P62 accumulates and leads to the impaired delivery of UPS (Ub-proteasome system) substrates to the proteasome (Korolchuk et al., 2009a). The P62-binding protein NBR1 possesses a functional LIR domain and is recruited to Ub-positive aggregates (Kirkin et al., 2009a). P62 and NBR1 can function independently, since NBR1-positive phagosomes can be degraded in P62-deficient cells; this explains why neither P62 nor NBR1 is required for the formation of all Ub aggregates. Interestingly, NBR1 depletion impaired the formation of puromycin-induced P62 bodies. Furthermore, P62-dependent aggregates were attenuated in LC3-deficient HeLa cells upon down-regulation of NBR1 by RNA silencing (Lamark et al., 2009). This strongly suggests that P62 and NBR1 act as cargo receptors for selective autophagosomal degradation of ubiquitinated targets. P62 is not only involved in the autophagy targeting of ubiquitinated protein aggregates but also plays a role in autophagy-mediated degradation of ubiquitinated-labelled peroxisomes (Kim et al., 2008b) and the autophagy modulation of tumorigenesis. Strikingly, the Ras/ERK-dependent survival and proliferation of cancer cells are observed in solid tumours in which cells experience hypoxia and up-regulation of autophagy (leading to P62 degradation) (Pursiheimo et al., 2009). P62 is indeed at the crossroads of many signalling pathways involved in controlling autophagy, tumorigenesis and the cell death/survival balance (Moscat and Diaz-Meco, 2009). Indeed, it was recently suggested that autophagy could act as a tumour suppressor gene by limiting P62 accumulation; this illustrates P62's complex role in the regulation of the cell death/survival balance. In tumour cells, allelic loss of the autophagy-essential beclin1 gene leads to P62 accumulation in response to oxidative stress; autophagy-incompetent Atg5−/− cells (Atg is autophagy related protein) overexpressing P62–EGFP (enhanced green fluorescent protein) were more prone to grow tumours than Atg5+/+ cells (Mathew et al., 2009). P62 accumulation in autophagy-defective cells leads to severe cellular perturbations, such as the production of ROS (reactive oxygen species) (which in turn increases mitochondrial damage), elevated ER (endoplasmic reticulum) chaperone levels and chromosomal instabilities. Similarly, at a later stage of cancer development, autophagy is induced concomitantly with an increase in hypoxic tumour regions. During prolonged MEF (mouse embryonic fibroblast) cell hypoxia, mitochondrial autophagy is one of the HIF-1 (hypoxia-induced factor-1)-mediated metabolic adaptations required to prevent increased ROS levels and cell death (Zhang et al., 2008). P62 accumulation is also reportedly associated with Ub-dependent activation of caspase 8 in hypoxia in carcinoma cells and it has been suggested that autophagy-induced clearance of P62 is involved in the survival pathway in these cells (Jin et al., 2009). Another P62-mediated cytoprotective effect could be linked to the autophagy-dependent clearing of inefficient mitochondria, as reported in Parkinson's disease (Geisler et al., 2010; Lee et al., 2010). It has been shown that mitochondria are targeted to mitophagy following ubiquitination by the Parkin ligase. Two independent studies have shown that mitophagy occurs via selective autophagy, which is dependent on a complex involving Atg8, Atg11 (involved in pexophagy and cytoplasm-to-vacuole targeting pathways) and specifically Atg32 (Okamoto et al., 2009; Kanki et al., 2009). Remarkably, Atg32 is induced during mitochondria growth, localizes and serves as a receptor recruiting mitochondria during starvation and, when overexpressed, induces mitophagy. In Atg32−/− cells, no mitochondrial alterations are observed. Atg8–Atg32 binding is dependent on Atg32's conserved WXXI motif. The motif is also present on P62 and also affects Atg8 binding (Pankiv et al., 2007; Ichimura et al., 2008a; Noda et al., 2008). These findings raise the question of whether or not this type of regulation occurs during the pathogen-dependent activation of autophagy.
P62's involvement in bacterial infection
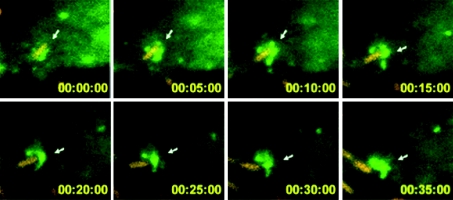
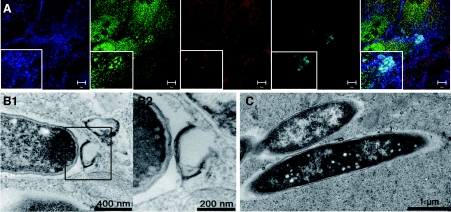
A growing body of evidence suggests that P62 is at a similar decision point during bacterial invasion, when the protein appears to orchestrate autophagy mostly as a protective response (i.e. xenophagy). Indeed, Listeria was found to be targeted to autophagy and lysosomal degradation following chloramphenicol-induced metabolic arrest (Rich et al., 2003). Targeting of Listeria during intercellular infection was, however, found to depend on the bacterium's phospholipases A and B; these activities are required for disruption of the double vacuolar membrane resulting from both donor and acceptor cells (Birmingham et al., 2007; Py et al., 2007). Furthermore, Listeria with low lysteriolysin-O activity is targeted to large, autophagosome-like compartments (Birmingham et al., 2008). Interestingly, ActA mutant bacteria were still able to disrupt the Listeria-containing vacuole but resided in the cytoplasm and were more frequently trapped by the autophagy system (Rich et al., 2003). Indeed, even though the recruitment of host proteins [such as the Arp2/3 complex (actin-related protein 2/3 complex), VASP (vasodilator-stimulated phosphoprotein) or actin] is prevented by ActA knockout, the bacteria become ubiquitinated and recruit P62 and LC3 (Yoshikawa et al., 2009). The level of GFP (green fluorescent protein)–LC3 recruitment on the ActA mutant was found to be dramatically lower in P62−/− MEFs (Yoshikawa et al., 2009). It has thus been suggested that the ActA and actin-binding protein scaffold around the bacteria acts as a shield against autophagy (Yoshikawa et al., 2009). Consistently, the diaminopimelic acid-type peptidoglycan of Listeria was shown to have autophagy-stimulating activity in Drosophila (Yano et al., 2008). During Salmonella typhimurium infection, approx. 25–35% of the invading bacteria reach the cytoplasm and are targeted to autophagy (Birmingham et al., 2006). These bacteria become ubiquitinated and recruit P62 1 h post infection in HeLa cells (Zheng et al., 2009). Mutations within the LIR or UBA domain interfere with P62-dependent autophagy and thus demonstrate P62's function as an adaptor protein (Zheng et al., 2009). However, in Atg5−/− MEFs, 20% of the bacteria are found in multilamellar organelles; this emphasizes the role of bacterial virulence factors (Zheng et al., 2009). In Atg5−/− MEFs, P62 can be recruited on to the bacteria, which shows that P62's association with S. typhimurium can occur independently of autophagy (Zheng et al., 2009). However, autophagy was still observed in P62 siRNA (small interfering RNA)-treated cells, which suggests the presence of an alternate autophagy-regulating pathway in intracellular S. typhimurium (Zheng et al., 2009). Interestingly, the adaptor NDP52 (nuclear dot protein 52 kDa) was found to bind Ub-coated S. typhimurium and recruit the TBK-1 (Tank-binding kinase-1) involved in type I IFNs (interferons) production. It has been suggested that TBK-1 controls the integrity of Salmonella-containing vacuoles by limiting the activity of the water channel aquaporin-1 (Radtke and O'Riordan, 2008; Thurston et al., 2009). NDP52 can also bind LC3 (Thurston et al., 2009). Knockdown of NDP52 was found to decrease the percentages of Ub-coated S. typhimurium delivered to autophagosomes in HeLa cells (Thurston et al., 2009). P62 was also found to be situated at the crossroads of several signalling pathways during infection with Shigella, a bacterium that replicates in the cytoplasm. Once the bacterium has escaped from its vacuolar compartment, the membrane remnants were found to be ubiquitinated (Figure 2 and Supplementary Movie 1 at http://www.biolcell.org/boc/102/boc1020621add.htm), accumulated P62 and were targeted to autophagosomes (Dupont et al., 2009). Inflammasome components [Ipaf (interleukin-1β-converting enzyme protease-activating factor), ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD, q.v.)) and NLRP3] and the TRAF6 (tumour-necrosis-factor-receptor-associated factor 6)/NEMO [NF-κB (nuclear factor κB) essential modulator] complex were also localized to these membranes, where TRAF6 (at least) was seen to be ubiquitinated (Dupont et al., 2009). In Atg5−/− MEFs, elevated ROS production and a lower mitochondrial potential (versus wild-type infected cells) suggested that necrosis signals were targeted to degradation, in order to protect cells from deleterious effects (Dupont et al., 2009). This is consistent with the observation that non-myeloid cells can die via necrosis upon Shigella infection (Carneiro et al., 2009). Pyroptosis is an inflammatory type of cell death, characterized by caspase 1 activation and osmotic-dependent cytolysis. The pyroptosis-inducer caspase 1 was also associated with these membranes and targeted to degradation via autophagy (Dupont et al., 2009), suggesting the presence of active, autophagy-dependent, protective mechanisms. Transient transfection with Atg4B C74A (which impairs with the maturation autophagosomes by inhibiting closure) (Fujita et al., 2008) and Atg5 knockdown were found to interfere with the NF-κB inflammatory response (Dupont et al., 2009). However, under certain conditions, the bacterium itself can also be targeted to autophagy (Ogawa et al., 2005). Deletion of the T3SS-secreted effector IcsB (which interacts with VirG/IcsA, involved in the actin comet-dependent motility of Shigella) has been shown to increase the autophagy of Shigella (Ogawa et al., 2005). Thus, both membrane remnants and bacteria can be included in autophagosomes (Figure 3). Innate immunity is activated following the detection of bacterial motifs by Nods, which then trigger the NF-κB response via the Rip2 adaptor (Inohara et al., 2000; Abbott et al., 2004). Interestingly, it has now been shown that in non-myeloid cells, Nod1 can recruit Atg16L1 independently of Rip2 – resulting in the autophagy targeting of Shigella (Travassos et al., 2010). In Nod1-deficient MEFs, a high proportion of ΔIcsB bacteria are found in the cytoplasm; this suggests that Nod-dependent autophagy restricts intracellular proliferation (Travassos et al., 2010). Surprisingly, the autophagy response is Rip2-insensitive, since Rip2-deficient cells and control cells had similar levels of autophagosome-containing bacteria (Travassos et al., 2010). Tagged versions of Nods and ATG16L1 were found to be associated with the plasma membrane and were recruited to the bacterial entry sites (Travassos et al., 2010). Polymorphisms in the ATG16L1 and NOD2 genes have been associated with the development of Crohn's disease (Hugot et al., 2001; Hampe et al., 2007; Rioux et al., 2007). Both Nod1 and Nod2 co-immunoprecipitate with ATG16L1 and a cytoplasmic variant (ΔN85-Atg16L1, lacking the first 85 amino acids). Shigella infection of HeLa cells transfected with Nod1 and ΔN85-Atg16L1 or with Nod2 and ΔN85-Atg16L1 leads to the recruitment of Nods and Atg16L1 around the bacteria at the entry foci (Travassos et al., 2010). In cells expressing the allelic forms of Nod2 or Atg16L1 associated with Crohn's disease, autophagy levels were found to be lower and the cell surface association of these proteins was impaired (Travassos et al., 2010). Atg16L1 did not localize to the plasma membrane upon expression of Nod2fs (the most prevalent polymorphism in Crohn's disease) (Travassos et al., 2010), suggesting that sequestration of Atg16L1 by Nod2fs in the cytoplasm interferes with the autophagosomal delivery of the bacteria. The important question of whether dysregulation of Nod2-Atg16L1-dependent autophagy is involved in the onset of Crohn's disease will certainly be investigated in the near future. Furthermore, the exact role of the Rip2-independent response in Crohn's disease remains to be evaluated, because Rip2 is required for the ubiquitination of NEMO (Abbott et al., 2004) and the Nod1-dependent activation of the NF-κB response induced by Shigella infection (Fukazawa et al., 2008; Carneiro et al., 2009). The role of ubiquitination in the Nod–Atg16L1 complex is also not clear at this stage, although the importance of the Ub-dependent NF-κB pathway in mediating the immune response to pathogen infection is well established (Skaug et al., 2009; Chiu et al., 2009).
Figure 2. Ub recruitment on Shigella vacuolar membrane remnants (from Ub movie).
Infection by DsRed-protein-expressing S. flexneri of HeLa cells transiently expressing the Ub–YFP protein. Time is indicated in h:min:s. The arrow points to membrane remnants.
Figure 3. Autophagy targeting of both the ΔIcsB S. flexneri mutant and its vacuolar membrane remnants.
(A) SKBR3 cells stably expressing the Gal-3 (galectin-3)–GFP (green) construct were infected with ΔIcsB S. flexneri for 30 min before paraformaldehyde fixation and labelling of cholesterol with filipin (blue). Sample was immunostained for Shigella (anti-LPS antibody, cyan) and poly-Ub (FK1 antibody). Scale bar 10 μm. (B, C) Ultrastructural analysis in transmission electron microscopy of HeLa cells infected for 30 min with the Shigella ΔIcsB strain. (B2) The inset of (B1) at a higher magnification.
Deretic and co-workers recently discovered a key role for P62 in myeloid cells (and possibly in other cell types) (Ponpuak et al., 2010): P62 is required if cytosolic proteins are to be targeted to lysosomes, where they are proteolytically processed into neo-antimicrobial peptides. This demonstrates that proteins involved in cell function may reveal cryptic antibacterial functions after autophagy-dependent conversion. This insight may lead to important breakthroughs in understanding the innate immune system's responses to microbial invasion.
Membrane damage and bacterial infection: how common is the associated cell signalling response?
The host uses PRRs to sense translocation of PAMPs into the cytoplasm; this ‘violation of the sanctity of the cytoplasm’ activates the cell signalling response (Lamkanfi and Dixit, 2009). The latter consists of pro-inflammatory signals involving mainly the NF-κB pathway and caspase 1-activating complexes termed inflammasomes (Lamkanfi and Dixit, 2009; Pedra et al., 2009). The inflammasomes' composition varies as a function of the activation models, which rely on a wide variety of pathogen-derived stimuli (Lamkanfi and Dixit, 2009; Pedra et al., 2009; Vance et al., 2009). Activation of the cryopyrin/NLRP3 inflammasome can occur following potassium efflux upon ATP binding to the purinergic P2X7 receptor, LPS (lipopolysaccharide) pre-treatment of the cells, cytoplasmic translocation of DNA, RNA or bacterial effectors via the type III, IV and VII secretions systems or exposure of the cell surface to pore-forming toxins [e.g. L. monocytogenes LLO, Aeromonas hydrophila aerolysin (leading to K+ efflux) and Staphylococcus aureus haemolysins]. In contrast, mycobacteria reportedly inhibit inflammasome activation (Master et al., 2008). It is not clear whether the cell interprets K+ efflux as a danger signal, which then triggers the inflammasome, since the efflux is not sufficient to activate cryoporin/NLRP3 and depends on the recruitment of a hemichannel (pannexin-1) which induces large pores (Pelegrin and Surprenant, 2006). The IPAF (ICE protease activating factor)/NLRC4 inflammasome is activated (possibly via a Birc1e/Naip5-dependent pathway) by cytosolic flagellins (e.g. from S. typhimurium, Legionella pneumophila, Pseudomonas aeruginosa etc.) and unknown type III-secreted effectors from S. flexneri. The flagellin can trigger the inflammasome, leading to IL-1(interleukin-1)/IL-18 release and pyroptosis. It is thus difficult to know whether the secretion system-induced membrane damage or the bacterial-derived material itself is responsible for the pyroptosis response (Bergsbaken et al., 2009). The NALP1b/NLRP1b inflammasome is activated by the protease activity of anthrax lethal factor (Boyden and Dietrich, 2006) and membrane damage does not appear to be the primary signal. In fact, cell membranes can sustain huge pores before repair mechanisms are triggered (Bischofberger et al., 2009). We have already discussed the LLO-dependent targeting of Listeria to autophagosomes. The Hla (alpha haemolysin) toxin secreted by S. aureus is essential for the recruitment of LC3 to the bacteria-containing autophagosome (Mestre et al., 2010). When the maturation of this compartment is blocked (probably via H+ efflux upon pore formation), membrane damage might induce a protective cellular response via the activation of autophagy. Hence, autophagy itself may sense membrane damage. Treatment of cells with the Vibrio cholerae pore-forming cytolysin leads to the recruitment of lipidated LC3 to the autophagosome (Gutierrez et al., 2007). Although VacA from Helicobater pylori induces autophagy, the VacA-dependent large vacuoles appear to differ from autophagosomes (Terebiznik et al., 2009). The induction of autophagy decreases the stability of VacA, which in turn affects large vacuole biogenesis. Autophagy may thus constitute a mechanism for limiting the toxin-mediated damage caused by excessive vacuolation (Terebiznik et al., 2009). Given that VacA can disrupt the degradative properties of the endocytosis pathway, the resulting membrane stress could be sufficient to trigger autophagy (Terebiznik et al., 2006). Alternatively, autophagy could be induced by VacA-dependent mitochondrial dysfunction (Yamasaki et al., 2006).
Cell death pathways include apoptosis-, necrosis- and pyroptosis-dependent mechanisms that may be associated with autophagy. Although apoptotic and autophagic processes are known to be silent, necrosis and pyroptosis induce an inflammatory response. Apoptosis and autophagy lead to the clearance of bacteria in apoptotic bodies and autolysosomes. The recent description of autophagy perturbations and correlated changes in infection-mediated necrosis and pyroptosis highlight a potential link between the autophagy pathway and the inflammatory cell death pathways during infection.
Conclusions
There is a growing body of evidence to suggest that post-translational modifications (e.g. ubiquitination, possibly sumoylation) regulate the intracellular traffic of pathogens. Certain micro-organisms are capable of subverting or hijacking these machineries and thus escaping the immune response. In the cell's defensive response, autophagy appears to be an alternative to the phagolysosome degradation route. It remains to be seen how the selection between these two routes is made. Nevertheless, autophagy now clearly appears to be yet another pathway targeted by invading pathogens. A number of key factors have been identified (such as the P62 protein), together with independent pathways similar to that involving phospholipase D, phosphatidic acid and diacylglycerol in Salmonella infection (Shahanazari et al. 2010). Another intriguing question concerns the identity of the donor compartment contributing to xenophagy. Pathogens come from the extracellular environment and are engulfed in a vacuolar membrane derived from a sub-compartment of the ER (Hayashi-Nishino et al., 2009) and/or the plasma membrane (Ravikumar et al., 2010). However, other sources have been identified: nuclear membranes during viral antigen presentation (English et al., 2009) and mitochondrial membranes during starvation (Hailey et al., 2010). It will thus take some time to decipher the exact contribution of each membrane compartment as a function of the autophagy pathway involved and the cell type. Another important question relates to the lipid composition of the membrane domain into which the vacuolar proteins involved in the pathway decision and the signalling cascade partition. The lipidomics of autophagosomes (especially in relation to pathogen invasion) is still a challenging field.
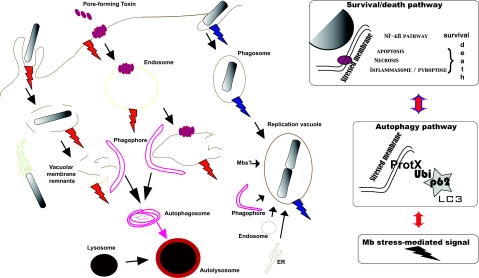
Another variable identified by these studies is the role of ubiquitination, sumoylation or autophagy as a danger signal and its effect on the cell's orchestration of the signalling response. According to Matzinger's ‘danger model’, PRRs have evolved to respond to damage more than to foreignness (Matzinger, 2002). During pathogen-dependent changes in membrane integrity (e.g. the action of pore-forming toxins, intracellular replicating bacteria, etc.), the accumulation of ubiquitinated proteins triggers specific signals (with the involvement of key adaptors like P62) and can modulate the stimulation of NOD-dependent trafficking (Travassos et al., 2010) and signalling (Franchi et al., 2009) pathways. Understandably, membrane stress can also re-route intracellular membrane to the fast-growing Atg8/LC3+ replication intravacuolar niche for bacteria which can subvert the autophagy pathway (Yersinia pestis and Yersinia pseudotuberculosis, for instance) (Pujol et al., 2009; Moreau et al., 2010) (Figure 4). This not only coordinates the fate of the pathogens (either targeting to a replication niche or destruction in autophagolysosomes) but can also control the survival/cell death pathway in the host (Carneiro et al., 2009; Dupont et al., 2009). The formation of signalling platforms on the damaged membranes is regulated both dynamically (in time over the course of infection and spatially along the membrane-trafficking pathway) and functionally (via the post-translational modification-dependent recruitment of signalling molecules). These platforms could act as danger signals and their targeting to autophagy might contribute to the regulation of the inflammatory and immune responses which occur during infection.
Figure 4. Autophagy and successful bacterial infection.
In contrast to xenophagy, autophagy can be activated during successful bacterial invasion, as illustrated herein by the S. flexneri and Y. pseudotuberculosis examples. During Shigella invasion, the signalling complexes associated with membrane remnants are targeted to autophagy. This may favour the silencing of danger signals and allow the bacteria to escape the immune response and thus replicate within the cytosol. Yersinia is able to replicate within an LC3-positive compartment in which maturation towards an acidic pH has been stopped. The host cell has to accommodate the growth of the Yersinia-containing vacuole as the bacteria replicate. This induces re-routing of intracellular membrane compartments. In both of these cases, the survival/death signalling pathway balance could be regulated by autophagy activation (left panel). Although it is known that P62 is involved in the regulation of these pathways during Shigella infection, this question remains open for Yersinia.
These hypotheses and working models make the field very attractive and stimulating. New excitements are certain to come that will pave the way for future investigations.
Online data
Glossary
- Autophagy
A ubiquitous process among eukaryotes in which cytosolic proteins and/or organelles are engulfed into double-membrane vesicles called autophagosomes, transported to, and degraded in lysosomes/vacuoles. This process is involved in recycling nutrients under starvation conditions as well as in the removal of old organelles and protein aggregates by degrading unwanted cytoplasmic constituents. Along with its metabolic function, autophagy functions as a cell-autonomous effector mechanism of innate immunity against invading bacteria and other pathogens.
- Apoptosis
A programmed cell death that occurs during the normal development of multicellular organisms and continues throughout adult life. Apoptotic cell death can be triggered by many different cellular stimuli, resulting in activation of apoptotic signalling pathways including caspases and mitochondria. The morphology associated with this phenomenon was characterized by condensation and fragmentation of nuclear chromatin, compaction of cytoplasmic organelles, dilatation of the endoplasmic reticulum, a decrease in cell volume and alterations to the plasma membrane resulting in the recognition and phagocytosis of apoptotic cells, thus preventing an inflammatory response. Apoptosis plays a complementary role to mitosis and cytokinesis in maintaining stable cell populations within tissues.
- Necrosis
The pathological process that occurs when cells are exposed to a serious physical or chemical insult. This is in contrast to apoptosis, which is a naturally occurring cause of cellular death. Necrosis is characterized by cellular swelling and disruption of the plasma membrane, and leads to a rapid release of the cellular cytoplasmic content into the cell environment. This release, in turn, results in massive inflammatory responses in the physiological environment around the dying cell.
- Pyroptosis
A pathway of cell death which is associated with caspase 1 activation, a protease that also activates the inflammatory cytokines IL-1 and IL-18, involved in host inflammatory responses. It is still controversial whether pyroptosis, which can be induced by bacterial or non-bacterial pathological stimuli, represents a cell death modality on its own or whether it constitutes a special case of apoptosis or necrosis.
Acknowledgements
We are grateful to Nicolas Barois (MICPaL Facility, CIIL and IFR142) for the EM data. We thank Kevin Moreau for a critical reading of the manuscript.
Funding
Work in the authors' laboratory is supported by grants from the French Ministry of Research and Technology (Chaire d'Excellence) and the French Research National Agency [MIIM05 and MIE09] to F.L.
References
*Articles of special interest
- Abbott D.W., Wilkins A., Asara J.M., Cantley L.C. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr. Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Abrami L. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J. Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot A., Vergunst A., Genin S., Peeters N. Exploitation of eukaryotic ubiquitin signaling pathways by effectors translocated by bacterial type III and type IV secretion systems. PLoS Pathog. 2007;3:e3. doi: 10.1371/journal.ppat.0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham C.L., Canadien V., Gouin E., Troy E.B., Yoshimori T., Cossart P., Higgins D.E., Brumell J.H. Listeria monocytogenes evades killing by autophagy during colonization of host cells. Autophagy. 2007;3:442–451. doi: 10.4161/auto.4450. [DOI] [PubMed] [Google Scholar]
- Birmingham C.L., Canadien V., Kaniuk N.A., Steinberg B.E., Higgins D.E., Brumell J.H. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature. 2008;451:350–354. doi: 10.1038/nature06479. [DOI] [PubMed] [Google Scholar]
- Birmingham C.L., Smith A.C., Bakowski M.A., Yoshimori T., Brumell J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006;281:11374–11383. doi: 10.1074/jbc.M509157200. [DOI] [PubMed] [Google Scholar]
- Bischofberger M., Gonzalez M.R., Van Der Goot F.G. Membrane injury by pore-forming proteins. Curr. Opin. Cell Biol. 2009;21:589–595. doi: 10.1016/j.ceb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M., Veiga E., Pizarro-Cerda J., Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell. Microbiol. 2008;10:2208–2222. doi: 10.1111/j.1462-5822.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- Boyden E.D., Dietrich W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- Boyer L., Lemichez E. Targeting of host-cell ubiquitin and ubiquitin-like pathways by bacterial factors. Nat. Rev. Microbiol. 2004;2:779–788. doi: 10.1038/nrmicro1005. [DOI] [PubMed] [Google Scholar]
- Carneiro L.A., Travassos L.H., Soares F., Tattoli I., Magalhaes J.G., Bozza M.T., Plotkowski M.C., Sansonetti P.J., Molkentin J.D., Philpott D.J., Girardin S.E. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe. 2009;5:123–136. doi: 10.1016/j.chom.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Chau V., Tobias J., Bachmair A., Marriott D., Ecker D., Gonda D., Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science. 1989;243:1576–1583. doi: 10.1126/science.2538923. [DOI] [PubMed] [Google Scholar]
- Chiu Y., Zhao M., Chen Z. Ubiquitin in NF-kappaB signaling. Chem. Rev. 2009;109:1549–1560. doi: 10.1021/cr800554j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosed R., Tomchick D.R., Brautigam C.A., Mukherjee S., Negi V.S., Machius M., Orth K. Structural analysis of Xanthomonas XopD provides insights into substrate specificity of ubiquitin-like protein proteases. J. Biol. Chem. 2007;282:6773–6782. doi: 10.1074/jbc.M608730200. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem. Biophys. Res. Commun. 1978;81:1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- Deretic V., Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I., Wakatsuki S., Walters K.J. Ubiquitin-binding domains – from structures to functions. Nat. Rev. Mol. Cell Biol. 2009;10:659–671. doi: 10.1038/nrm2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont N., Lacas-Gervais S., Bertout J., Paz I., Freche B., Van Nhieu G.T., Van Der Goot F.G., Sansonetti P.J., Lafont F. Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe. 2009;6:137–149. doi: 10.1016/j.chom.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Dye B., Schulman B. Structural mechanisms underlying posttranslational modification by ubiquitin-like proteins. Annu. Rev. Biophys. Biomol. Struct. 2007;36:131–150. doi: 10.1146/annurev.biophys.36.040306.132820. [DOI] [PubMed] [Google Scholar]
- English L., Chemali M., Duron J., Rondeau C., Laplante A., Gingras D., Alexander D., Leib D., Norbury C., Lippe R., Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J., Rosenshine I. Imaging the assembly, structure and activity of type III secretion systems. Cell. Microbiol. 2009;11:1462–1470. doi: 10.1111/j.1462-5822.2009.01360.x. [DOI] [PubMed] [Google Scholar]
- Franchi L., Warner N., Viani K., Nunez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N., Hayashi-Nishino M., Fukumoto H., Omori H., Yamamoto A., Noda T., Yoshimori T. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol. Biol. Cell. 2008;19:4651–4659. doi: 10.1091/mbc.E08-03-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa A., Alonso C., Kurachi K., Gupta S., Lesser C.F., McCormick B.A., Reinecker H.C. GEF-H1 mediated control of NOD1 dependent NF-kappaB activation by Shigella effectors. PLoS Pathog. 2008;4:e1000228. doi: 10.1371/journal.ppat.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N.J., Gangloff M. Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- Geisler S., Holmström K., Skujat D., Fiesel F., Rothfuss O., Kahle P., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Geiss-Friedlander R., Melchior F. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Geoffroy M., Hay R. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat. Rev. Mol. Cell Biol. 2009;10:564–568. doi: 10.1038/nrm2707. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D.H., Niall H.D., Boyse E.A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Natl. Acad. Sci. U.S.A. 1975;72:11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M.G., Saka H.A., Chinen I., Zoppino F.C., Yoshimori T., Bocco J.L., Colombo M.I. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1829–1834. doi: 10.1073/pnas.0601437104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey D., Rambold A., Satpute-Krishnan P., Mitra K., Sougrat R., Kim P., Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe J., Franke A., Rosenstiel P., Till A., Teuber M., Huse K., Albrecht M., Mayr G., De La Vega F.M., Briggs J., et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Hayashi-Nishino M., Fujita N., Noda T., Yamaguchi A., Yoshimori T., Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- Hicke L., Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458:422–429. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller D., Hecker C., Wagner S., Rogov V., Dötsch V., Dikic I. E3-independent monoubiquitination of ubiquitin-binding proteins. Mol. Cell. 2007;26:891–898. doi: 10.1016/j.molcel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Hoppe A.D., Seveau S., Swanson J.A. Live cell fluorescence microscopy to study microbial pathogenesis. Cell Microbiol. 2009;11:540–550. doi: 10.1111/j.1462-5822.2009.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotson A., Chosed R., Shu H., Orth K., Mudgett M.B. Xanthomonas type III effector XopD targets SUMO-conjugated proteins in planta. Mol. Microbiol. 2003;50:377–389. doi: 10.1046/j.1365-2958.2003.03730.x. [DOI] [PubMed] [Google Scholar]
- Hugot J.P., Chamaillard M., Zouali H., Lesage S., Cezard J.P., Belaiche J., Almer S., Tysk C., O'Morain C.A., Gassull M., et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Ichimura Y., Kominami E., Tanaka K., Komatsu M. Selective turnover of p62/A170/SQSTM1 by autophagy. Autophagy. 2008a;4:1063–1066. doi: 10.4161/auto.6826. [DOI] [PubMed] [Google Scholar]
- Ichimura Y., Kumanomidou T., Sou Y., Mizushima T., Ezaki J., Ueno T., Kominami E., Yamane T., Tanaka K., Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. J. Biol. Chem. 2008b;283:22847–22857. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- Inohara N., Koseki T., Lin J., del Peso L., Lucas P.C., Chen F.F., Ogura Y., Nunez G. An induced proximity model for NF-kappaB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 2000;275:27823–27831. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- Isaacson M., Ploegh H. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5:559–570. doi: 10.1016/j.chom.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Li Y., Pitti R., Lawrence D., Pham V., Lill J., Ashkenazi A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Kang J., Saunier E., Akhurst R., Derynck R. The type I TGF-beta receptor is covalently modified and regulated by sumoylation. Nat. Cell Biol. 2008;10:654–664. doi: 10.1038/ncb1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T., Wang K., Cao Y., Baba M., Klionsky D. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell. 2009;17:98–109. doi: 10.1016/j.devcel.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher O., Felberbaum R., Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Kim J.G., Taylor K.W., Hotson A., Keegan M., Schmelz E.A., Mudgett M.B. XopD SUMO protease affects host transcription, promotes pathogen growth, and delays symptom development in xanthomonas-infected tomato leaves. Plant Cell. 2008a;20:1915–1929. doi: 10.1105/tpc.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Hailey D., Mullen R., Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl. Acad. Sci. U.S.A. 2008b;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V., Lamark T., Sou Y.S., Bjorkoy G., Nunn J.L., Bruun J.A., Shvets E., McEwan D.G., Clausen T.H., Wild P., et al. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell. 2009a;33:505–516. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kirkin V., McEwan D., Novak I., Dikic I. A role for ubiquitin in selective autophagy. Mol. Cell. 2009b;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Knodler L., Winfree S., Drecktrah D., Ireland R., Steele-Mortimer O. Ubiquitination of the bacterial inositol phosphatase, SopB, regulates its biological activity at the plasma membrane. Cell Microbiol. 2009;11:1652–1670. doi: 10.1111/j.1462-5822.2009.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Waguri S., Koike M., Sou Y., Ueno T., Hara T., Mizushima N., Iwata J., Ezak I.J., Murata S., et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Kopito R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- Korolchuk V., Mansilla A., Menzies F., Rubinsztein D. Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol. Cell. 2009a;33:517–527. doi: 10.1016/j.molcel.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk V., Menzies F., Rubinsztein D. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2009b;584:1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. PRR pathogen recognition in the innate immune response. Biochem. J. 2009a;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009b;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Lafont F., Simons K. Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3180–3184. doi: 10.1073/pnas.051003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F., Van Der Goot F. Bacterial invasion via lipid rafts. Cell Microbiol. 2005;7:613–620. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- Lafont F., Abrami L., Van Der Goot F.G. Bacterial subversion of lipid rafts. Curr. Opin. Microbiol. 2004;7:4–10. doi: 10.1016/j.mib.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Lamark T., Kirkin V., Dikic I., Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle. 2009;8:1986–1990. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M., Dixit V.M. Inflammasomes: guardians of cytosolic sanctity. Immunol. Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- Lee J., Nagano Y., Taylor J., Lim K., Yao T. Diseasecausing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Liang M., Liang Y., Brunicardi F., Feng X. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J. Biol. Chem. 2003;278:31043–31048. doi: 10.1074/jbc.C300112200. [DOI] [PubMed] [Google Scholar]
- Martinez J., Seveau S., Veiga E., Matsuyama S., Cossart P. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell. 2005;123:1013–1023. doi: 10.1016/j.cell.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Master S., Rampini S., Davis A., Keller C., Ehlers S., Springer B., Timmins G., Sander P., Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224–232. doi: 10.1016/j.chom.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Karp C., Beaudoin B., Vuong N., Chen G., Chen H., Bray K., Reddy A., Bhanot G., Gelinas C., et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- Mestre M., Fader C., Sola C., Colombo M. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy. 2010;6:110–125. doi: 10.4161/auto.6.1.10698. [DOI] [PubMed] [Google Scholar]
- Molfetta R., Gasparrini F., Peruzzi G., Vian L., Piccoli M., Frati L., Santoni A., Paolini R. Lipid raft-dependent FcepsilonRI ubiquitination regulates receptor endocytosis through the action of ubiquitin binding adaptors. PLoS ONE. 2009;4:e5604. doi: 10.1371/journal.pone.0005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau K., Lacas-Gervais S., Fujita N., Sebbane F., Yoshimori T., Simonet M., Lafont F. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell Microbiol. 2010;12:1108–1123. doi: 10.1111/j.1462-5822.2010.01456.x. [DOI] [PubMed] [Google Scholar]
- Moscat J., Diaz-Meco M. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J., Diaz-Meco M., Wooten M. Signal integration and diversification through the p62 scaffold protein. Trends Biochem. Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Munro P., Flatau G., Lemichez E. Bacteria and the ubiquitin pathway. Curr. Opin. Microbiol. 2007;10:39–46. doi: 10.1016/j.mib.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Noda N., Kumeta H., Nakatogawa H., Satoo K., Adachi W., Ishii J., Fujioka Y., Ohsumi Y., Inagaki F. Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells. 2008;13:1211–1218. doi: 10.1111/j.1365-2443.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- O'Neill L.A., Bowie A.G. The family of five: TIRdomain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Yoshimori T., Suzuki T., Sagara H., Mizushima N., Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–731. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Kondo-Okamoto N., Ohsumi Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell. 2009;17:87–97. doi: 10.1016/j.devcel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Pankiv S., Clausen T.H., Lamark T., Brech A., Bruun J.A., Outzen H., Overvatn A., Bjorkoy G., Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Patel J.C., Hueffer K., Lam T.T., Galan J.E. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell. 2009;137:283–294. doi: 10.1016/j.cell.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedra J.H., Cassel S.L., Sutterwala F.S. Sensing pathogens and danger signals by the inflammasome. Curr. Opin. Immunol. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P., Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2×7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart C.M. Targeting of substrates to the 26S proteasome. FASEB J. 1997;11:1055–1066. doi: 10.1096/fasebj.11.13.9367341. [DOI] [PubMed] [Google Scholar]
- Pietiäinen V., Marjomäki V., Heino J., Hyypiä T. Viral entry, lipid rafts and caveosomes. Ann. Med. 2005;37:394–403. doi: 10.1080/07853890510011976. [DOI] [PubMed] [Google Scholar]
- Ponpuak M., Davis A., Roberts E., Delgado M., Dinkins C., Zhao Z., Virgin H.T., Kyei G., Johansen T., Vergne I., Deretic V. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C., Klein K., Romanov G., Palmer L., Cirota C., Zhao Z., Bliska J. Yersinia pestis can reside in autophagosomes and avoid xenophagy in murine macrophages by preventing vacuole acidification. Infect. Immun. 2009;77:2251–2261. doi: 10.1128/IAI.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiheimo J., Rantanen K., Heikkinen P., Johansen T., Jaakkola P. Hypoxia-activated autophagy accelerates degradation of SQSTM1/p62. Oncogene. 2009;28:334–344. doi: 10.1038/onc.2008.392. [DOI] [PubMed] [Google Scholar]
- Py B.F., Lipinski M.M., Yuan J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy. 2007;3:117–125. doi: 10.4161/auto.3618. [DOI] [PubMed] [Google Scholar]
- Radtke A.L., O'Riordan M.X. Homeostatic maintenance of pathogen-containing vacuoles requires TBK1-dependent regulation of aquaporin-1. Cell Microbiol. 2008;10:2197–2207. doi: 10.1111/j.1462-5822.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- Ravikumar B., Moreau K., Jahreiss L., Puri C., Rubinsztein D. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat. Cell Biol. 2010;12:747–757. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribet D., Hamon M., Gouin E., Nahori M., Impens F., Neyret-Kahn H., Gevaert K., Vandekerckhove J., Dejean A., Cossart P. Listeria monocytogenes impairs SUMOylation for efficient infection. Nature. 2010;464:1192–1195. doi: 10.1038/nature08963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich K.A., Burkett C., Webster P. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol. 2003;5:455–468. doi: 10.1046/j.1462-5822.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- Rioux J.D., Xavier R.J., Taylor K.D., Silverberg M.S., Goyette P., Huett A., Green T., Kuballa P., Barmada M.M., Datta L.W., et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roduit C., Sekatski S., Dietler G., Catsicas S., Lafont F., Kasas S. Stiffness tomography by atomic force microscopy. Biophys. J. 2009;97:674–677. doi: 10.1016/j.bpj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roduit C., Van Der Goot F.G., De Los Rios P., Yersin A., Steiner P., Dietler G., Catsicas S., Lafont F., Kasas S. Elastic membrane heterogeneity of living cells revealed by stiff nanoscale membrane domains. Biophys. J. 2008;94:1521–1532. doi: 10.1529/biophysj.107.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.J., Bates M., Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat. Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytkonen A., Holden D.W. Bacterial interference of ubiquitination and deubiquitination. Cell Host Microbe. 2007;1:13–22. doi: 10.1016/j.chom.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler J.S., Dejean A. SUMO: of branched proteins and nuclear bodies. Oncogene. 2001;20:7243–7249. doi: 10.1038/sj.onc.1204758. [DOI] [PubMed] [Google Scholar]
- Seibenhener M.L., Geetha T., Wooten M.W. Sequestosome 1/p62 – more than just a scaffold. FEBS Lett. 2007;581:175–179. doi: 10.1016/j.febslet.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahanazari S., Yen W.-L., Birmingham C.L., Shiu J., Namolavan A., Zheng Y.T., Nakayama K., Klionsky D., Brumell J.H. A diacylglyerol-dependent signaling pathway contributes to regulation of anti-bacterial autophagy. Cell Host Microbe. 2010;8:137–146. doi: 10.1016/j.chom.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff H., Galbraith C.G., Galbraith J.A., Betzig E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods. 2008;5:417–423. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvets E., Fass E., Scherz-Shouval R., Elazar Z. The N-terminus and Phe52 residue of LC3 recruit p62/SQSTM1 into autophagosomes. J. Cell Sci. 2008;121:2685–2695. doi: 10.1242/jcs.026005. [DOI] [PubMed] [Google Scholar]
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Skaug B., Jiang X., Chen Z. The role of ubiquitin in NF-kappaB regulatory pathways. Annu. Rev. Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- Spallek T., Robatzek S., Gohre V. How microbes utilize host ubiquitination. Cell Microbiol. 2009;11:1425–1434. doi: 10.1111/j.1462-5822.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- Stulemeijer I., Joosten M. Post-translational modification of host proteins in pathogen-triggered defence signalling in plants. Mol. Plant Pathol. 2008;9:545–560. doi: 10.1111/j.1364-3703.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terebiznik M.R., Raju D., Vazquez C.L., Torbricki K., Kulkarni R., Blanke S.R., Yoshimori T., Colombo M.I., Jones N.L. Effect of Helicobacter pylori's vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy. 2009;5:370–379. doi: 10.4161/auto.5.3.7663. [DOI] [PubMed] [Google Scholar]
- Terebiznik M.R., Vazquez C.L., Torbicki K., Banks D., Wang T., Hong W., Blanke S.R., Colombo M.I., Jones N.L. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect. Immun. 2006;74:6599–6614. doi: 10.1128/IAI.01085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston T.L., Ryzhakov G., Bloor S., von Muhlinen N., Randow F. The TBK1adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 2009;10:1215–1221. doi: 10.1038/ni.1800. [DOI] [PubMed] [Google Scholar]
- Travassos L.H., Carneiro L.A., Ramjeet M., Hussey S., Kim Y.G., Magalhaes J.G., Yuan L., Soares F., Chea E., Le Bourhis L., et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 2010;11:55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- Vance R.E., Isberg R.R., Portnoy D.A. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E., Cossart P. Listeria hijacks the clathrindependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Yamasaki E., Wada A., Kumatori A., Nakagawa I., Funao J., Nakayama M., Hisatsune J., Kimura M., Moss J., Hirayama T. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J. Biol. Chem. 2006;281:11250–11259. doi: 10.1074/jbc.M509404200. [DOI] [PubMed] [Google Scholar]
- Yano T., Mita S., Ohmori H., Oshima Y., Fujimoto Y., Ueda R., Takada H., Goldman W.E., Fukase K., Silverman N., et al. Autophagic control of listeria through intracellular innate immune recognition in Drosophila. Nat. Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yersin A., Hirling H., Kasas S., Roduit C., Kulangara K., Dietler G., Lafont F., Catsicas S., Steiner P. Elastic properties of the cell surface and trafficking of single AMPA receptors in living hippocampal neurons. Biophys. J. 2007;92:4482–4489. doi: 10.1529/biophysj.106.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y., Ogawa M., Hain T., Yoshida M., Fukumatsu M., Kim M., Mimuro H., Nakagawa I., Yanagawa T., Ishii T. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- Zaas D., Duncan M., Rae Wright J., Abraham S. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta. 2005;1746:305–313. doi: 10.1016/j.bbamcr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang H., Bosch-Marce M., Shimoda L., Tan Y., Baek J., Wesley J., Gonzalez F., Semenza G. Mitochondrial autophagy is a HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zheng Y.T., Shahnazari S., Brech A., Lamark T., Johansen T., Brumell J.H. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 2009;183:5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.