Abstract
The flowering plant genus Hypericum (Hypericaceae) contains the well-known medicinally valuable species Hypericum perforatum (common St. John’s wort). Species of Hypericum contain many bioactive constituents, including proanthocyanins, flavonoids, biflavonoids, xanthones, phenylpropanes and naphthodianthrones that are characterized by their relative hydrophilicity, as well as acylphloroglucinols and essential oil components that are more hydrophobic in nature. A concise review of the scientific literature pertaining to constituents of Hypericum essential oils and volatile fractions is presented.
Keywords: Hypericum, Hypericaceae, essential oil, antimicrobial, volatiles
The genus Hypericum contains, by the most recent count, 469 species that have been classified into 36 taxonomic sections [1,2]. Hypericum perforatum L. (Common St. John’s wort), the most thoroughly studied taxon of the genus, is well known for its historical and contemporary use as a medicinal plant, particularly for the treatment of mild to moderate depression [3]. Monographs for the crude drug (Hyperici herba), extracts of which are prepared from the aerial flowering portions of the plant, have been included in the European Pharmacopoeia (fully integrated as of Ph Eur 4) [4]. An infused oil of the flowers (Oleum hyperici), which is prepared by macerating fresh flowers in olive or sunflower oil and exposing the mixture to sunlight for two to three weeks, has a history of traditional use in Europe for treatment of burns and ulcers. The topical application is included in the monograph of the German Commission E and the Swiss Pharmacopoeia [5,6].
Oleum hyperici has a red color when either fresh (i.e. water-containing) flowers are extracted or heat is applied during the maceration process, although the naphthodianthrones (specifically hypericin and pseudohypericin) are not extracted into the oil. It has been proposed that a related emodin-derivative(s), specifically a degradation product of hypericin upon exposure to sunlight, is responsible for this coloration, but these have not yet been isolated [7-10]. The lipophilic phloroglucinol derivative, hyperforin, considered to be one of the primary bioactive (e.g. antibacterial, antidepressant) constituents of H. perforatum, along with a related compound, adhyperforin, are extracted into the oil [3,9,11-12].
Maisenbacher and Kovar [11] reported that these compounds degraded quickly in the presence of air, heat and light, and Isacchi et al. [12] demonstrated that when stored in the dark at −20°C, the hyperforin content had decreased by more than 60% and adhyperforin was no longer detectable after one year.
Primary production areas for H. perforatum in Europe include Germany, Italy and Romania, and the majority of the harvest goes toward the production of the crude drug [13]. A small fraction of the plant material is sold for the production of Oleum hyperici, which is also used as a carrier oil in aromatherapy, and for the distillation of the essential oil. The applications of the infused oil in aromatherapy and massage therapy mirror to some extent that of Hyperici herba, being used to treat anxiety, depression, bruises, mild wounds, rheumatic pain and sunburn [5,6,14]. Studies have shown that the infused oil demonstrates anti-inflammatory activity and speeds wound healing through the stimulation of epithelial tissue production when applied ex vivo, and exhibits gastroprotective effects when taken orally [10,15]. The steam-distilled essential oil, which has only recently become available on a broader scale on the market, is largely produced by small companies in Serbia, Croatia, Poland, Bulgaria, France, Canada and the United States [16]. Although evidence for the absorption of certain components of essential oils through the skin or lungs exists [14], clinical data for the efficacy of Hypericum essential oil applied topically in a pure form or diffused in a carrier oil are still lacking.
Hypericum species are generally classed as essential oil-poor plants (generally oil yield <1%, w/w) [17,18]. The content of essential oils in healthy H. perforatum plants is highest during the full-bloom stage versus pre-bloom or fruiting stage (0.35% versus 0.12 and 0.18%, respectively) [18-20]. Research has shown that essential oil extraction efficiency with liquid or supercritical CO2 (yield 1.00%, w/w) is higher than with steam distillation (0.06%, w/w). Hyperforin and derivatives can be extracted using liquid or supercritical CO2, but not with steam distillation [21,22].
Exudate-containing glands and canals are characteristic features of members of Hypericaceae, as well as of the related families Clusiaceae and Calophyllaceae (Guttiferae sensu lato), and are found on most organs of the plants [23,24]. In Hypericum L. (Hypericaceae), these glands appear as lines or dots, and are of two types: translucent (pale yellow to amber in color) and dark (red to nearly black), found variously on the stems, leaves, sepals, petals and the anther connective [1]. Anatomical and histochemical studies of H. perforatum have shown that the translucent glands are actually sub-epidermal cavities, lined with two layers of flattened, thin-walled secretory cells [25]. Dark glands, meanwhile, are specialized clusters of cells containing wax and the intensely red naphthodianthrone pigments hypericin and pseudohypericin [26-28]. The number and size of the dark glands in H. perforatum have been shown to correlate positively with naphthodianthrone content [29].
High hyperforin content (ca. 7 mg/g fresh tissue) has been measured in isolated translucent glands, but detected in only minor amounts (ca. 0.4 mg/g fresh tissue) in dark glands (possibly due to cross-contamination), and not detected at all in non-secretory tissue [28]. The isoprenoid moiety of hyperforin is produced via the same biosynthetic pathway leading to the monoterpenes, which are commonly major constituents of essential oils in Hypericum (e.g. α- and β-pinene, limonene). Evidence suggests that essential oil components and hyperforin (as well as derivatives) are biosynthesized within chloroplasts present in the thin cells surrounding the translucent glands and then secreted into the cavities [28,30,31].
Secretory canals in the floral and vegetative tissue in H. perforatum are of three types. Type A canals generally have a narrow diameter (although this can be variable in the pistil and root tissue) and are delimited by four or more polygonal cells. Type B and C canals are structurally similar to the translucent glands, but have different patterns of ontogeny [25]. Piovan et al. [32] identified hyperforin in sepal tissue containing Type B canals in H. elodes L., which is interesting in light of the shared ontogeny between this canal type and the translucent glands [25,28,32]. The distribution of translucent and dark glands; Type A, B and C canals; and hypericin and hyperforin in H. perforatum are summarized in Table 1. Anatomical and histochemical examinations of the translucent and dark glands have been performed in more than 20 other Hypericum species, including H. androsaemum, H. erectum, H. seniawinii and H. richeri [33-37].
Table 1.
Distribution of glands, canal types and particular chemical constituents in Hypericum perforatum.
| Secretory Structures | Sepals | Petals | Stamens | Pistil | Leaf | Stem | Root | Reference |
|---|---|---|---|---|---|---|---|---|
| Translucent Glands | + | + | − | − | + | − | − | 25, 28 |
| Dark Glands | + | + | + | − | + | + | 26-28 | |
| Type A canals | + | + | − | + | + | + | + | 25 |
| Type B canals | + | + | − | − | − | + | − | 25 |
| Type C canals | − | − | − | + | − | − | − | 25 |
| Chemical Constituents | ||||||||
| Hypericin/ Pseudohypericin1 |
+ | + | + | + | + | + | − | 1, 27 |
| Hyperforin2 | + | + | ? | + | + | ? | ? | 28, 38 |
Only detected in dark glands
Only detected in translucent glands
Methods undertaken to prepare samples prior to detection of either naphthodianthrones or acylphloroglucinols included excision of individual glands and/or pieces of tissue using razor blades [32], syringe tips [28], silica microcapillaries, and the technique of laser microdissection [38]. Once samples were excised from the respective tissue, they were either extracted in an organic solvent that was analyzed using HPLC-MS [28,32] or they were directly analyzed using laser desorption/ionization mass spectrometric (LDI-MS) imaging [38]. The solid phase micro extraction (SPME) technique, in which volatile and semi-volatile organic compounds are directly adsorbed on to a polymer coating on a thin fiber and subjected to GC-MS or HPLC-MS analysis, could potentially be used to examine the chemical content of stalked glands that occur in some species of Hypericum (e.g. on the margins of the sepals and/or petals), the glands on the anther connectives, or the glands on the outer surface of the ovaries.
Essential oil and volatile constituents that have been most frequently reported from Hypericum include the aliphatic hydrocarbons n-nonane and n-undecane; the monoterpenes α- and β-pinene; and the sesquiterpenes β-caryophyllene and caryophyllene oxide. The results of essential oil and volatile constituent analyses for representatives from 22 of the 36 taxonomic sections of Hypericum have been published, and a summary of major constituents is provided in Table 2 (except H. perforatum). A number of major components have been identified from these species that have a relatively limited occurrence among higher plants. Because some of these compounds may have a potential as food and/or beverage additives (flavoring agents), in cosmetics, or as aroma chemicals (e.g. see uses of β-caryophyllene in [96]), an interest in further research on targeted breeding programs for selected Hypericum species exists.
Table 2.
Reported major essential oil components in Hypericum (other than H. perforatum).
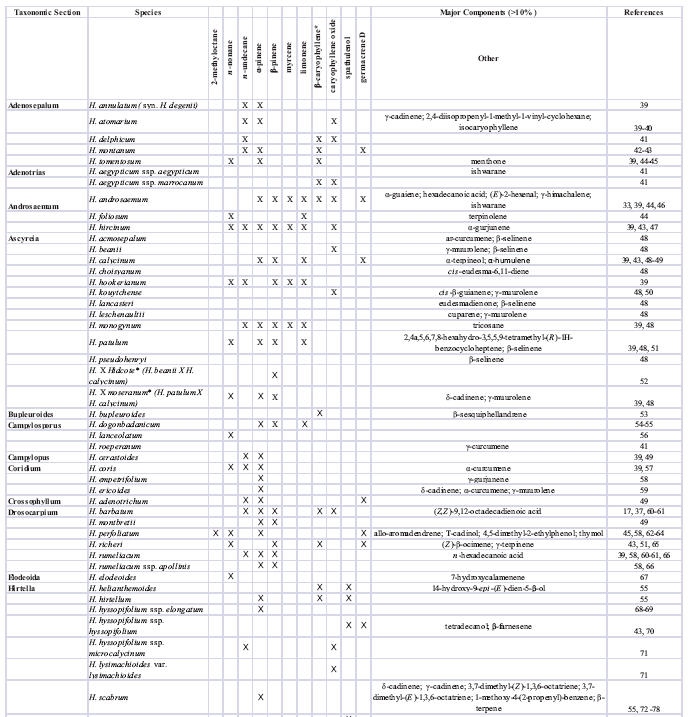
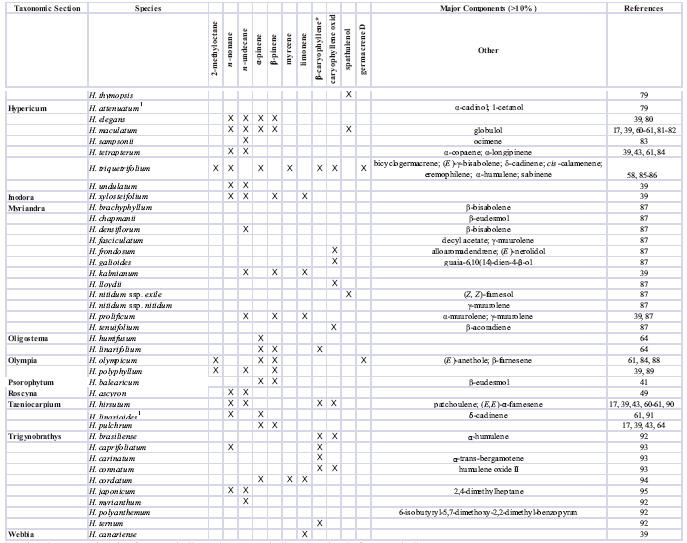
Variously reported as (E )-β-caryophyllene, (E )-caryophyllene, or simply β-caryophyllene
no components reported >10%; constituents >5% reported
Many studies of the essential oil content of H. perforatum, in which samples from a single population were taken, have been performed. The results of these studies are summarized in Table 3, and indicate the enormous variability inherent in the volatile chemistry of this species. Typical essential oil constituents for this species include the monoterpenes α- and β-pinene, limonene and myrcene; the sesquiterpenes β-caryophyllene and caryophyllene oxide; and hydrocarbons such as n-decane, C16- and C29 alkanes and C24-, C26-, and C28-alkanols [123]. Much variation between subspecies has also been documented, which is not surprising given the broad range of morphological variability encompassed by this species [124]. When the essential oils from flowers alone from two subspecies of H. perforatum collected in central Italy were directly compared, α-pinene and 2,6-dimethyloctane were identified as dominant in ssp. veronense, while β-caryophyllene and 2,6-dimethylheptane dominated in ssp. perforatum [52]. The oil yield from plants identified as H. perforatum ssp. perforatum growing in Serbia was markedly greater than from those identified as ssp. angustifolium, indicating that variability in both content and composition exists at this taxonomic level [120].
Table 3.
Reported major essential oil components in Hypericum perforatum.
| Collection Location | Major Components (>10%) |
References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β-pinene | α-pinene | 2-methyloctane | spathulenol | caryophyllene oxide | germacrene D | β-caryophyllene | Other | ||
| China | X | X | X | 102 | |||||
| China | hexadecanoic acid | 102 | |||||||
| China | X | X | 83, 104 | ||||||
| China* | X | 100 | |||||||
| China* | X | 101 | |||||||
| France | X | X | X | X | n -nonane; n -undecane; humulene | 39, 97-98 | |||
| France | X | X | 105 | ||||||
| France | X | X | X | 1-tetradecanol, β-funebrene, 1-dodecanol, and γ-muurolene | 106 | ||||
| France | X | X | 107 | ||||||
| France | X | X | X | n -decane | 108 | ||||
| Germany | X | X | 18 | ||||||
| Greece | X | X | X | X | 109 | ||||
| Greece | X | X | 84 | ||||||
| India | X | 99 | |||||||
| Iran | X | X | X | β-selinene; α-selinene | 110 | ||||
| Iran | X | X | hexadecanoic acid; n -tetradecanol; thymol | 111 | |||||
| Iran | X | X | α- and γ-eudesmol; bicyclogermacrene | 112 | |||||
| Iran | X | α-amorphene | 122 | ||||||
| Italy | X | X | X | 113 | |||||
| Italy | X | X | X | 114 | |||||
| Italy1 | X | 2,6-dimethylheptane | 52 | ||||||
| Italy1 | X | X | 43 | ||||||
| Italy2 | X | 2,6-dimethyloctane | 52 | ||||||
| Italy2 | X | X | 43 | ||||||
| Italy3 | X | X | X | 114 | |||||
| Lithuania | X | X | X | viridiflorol; tetradecanol |
115 | ||||
| Lithuania | X | X | X | 116 | |||||
| Portugal | X | X | β-selinene | 117 | |||||
| Portugal | X | X | X | 118 | |||||
| Serbia | X | X | X | α-bergamotene; (E )-anethole |
61 | ||||
| Serbia | X | β-farnesene | 121 | ||||||
| Serbia | X | X | 88 | ||||||
| Serbia* | X | X | (Z)-β-farnesene | 60 | |||||
| Serbia1 | X | bis-(2-ethyl)-1,2-benzene-dicarboxylic acid; n -eicosane; 1- tetradecanol; palmitic acid; 10-methyl-1-undecene |
120 | ||||||
| Serbia3 | X | 119 | |||||||
| Serbia3 | 10-methyl-1-undecene; 1-tetradecanol |
120 | |||||||
| Uzbekistan | X | 72 |
no components reported >10%; constituents >5% reported
Identified as H. perforatum ssp. perforatum
Identified as H. perforatum ssp. veronense
Identified as H. perforatum ssp. angustifolium
Numerous observations of intra- and interpopulation variability in H. perforatum oil content and composition for plants have been made. Because the plants for these studies were collected from the field rather than grown under controlled conditions, environmental factors are proposed to have contributed to this observed variability. For example, variation in oil content (0.04-1.93%) and dominant components was reported for plants from six different localities in Serbia. Terpenoid constituents, particularly sesquiterpenes, were described as primary constituents in the oil of the plants collected from mountainous regions, while long-chain waxes and fats were reported as dominating in plants from lowland areas [120]. Previous studies with other plant species have shown similar results, with concentrations of monoterpenoids and sesquiterpenoids increasing with increasing altitudes, hypothetically due to the role of these compounds in helping the plant deal with abiotic stress factors (e.g. UV radiation) [125]. An examination of H. perforatum plants growing in 10 defined habitat types in Lithuania allowed the identification of three distinct chemotypes, dominated respectively by β-caryophyllene, caryophyllene oxide and germacrene D [116,126]. A similar study performed in southeastern Poland identified significant differences among both the content and composition of essential oils from plants growing in 16 habitats, although two major constituents (2-methyloctane and α-terpineol) were produced by representatives of most populations [110]. It is not yet clear whether these reports indicate general trends for plants growing at particular altitudes or in particular habitats. Transplantation studies, in which plants from one altitude (or habitat) are moved to another altitude (or habitat) and later examined for their essential oil content, have not yet been conducted with Hypericum, but would be of value to test such hypotheses. Carefully controlled experiments examining the influence of genotypic background on the essential oil composition and yield in H. perforatum have not yet been made, but would be of significant interest.
In addition to the interspecific, inter- and intrapopulation variability observed, each organ selected from a particular plant for extraction may display a unique chemical profile. An examination of 11 accessions of H. perforatum leaves and flowers growing in a single population in Lithuania indicated that β-caryophyllene and caryophyllene oxide dominated in leaves, while spathulenol, tetradecanol and viridiflorol were dominant constituents of the flowers [115]. A similar study conducted on plants growing in northeastern Iran revealed α- and β-pinene and α- and β-selinene as the primary volatile constituents of the leaves and flowers, while germacrene D was predominant in the oil extracted from the stems and roots [110]. In an examination of H. androsaemum, a comparison of the volatile components present in translucent glands distributed just within the margin of the leaves (marginal glands) and glands distributed on the lamina (laminar glands) was made. The marginal glands contained β-caryophyllene and germacrene B as their dominant volatile components, while the laminar glands contained mainly β-pinene and limonene [33].
The antimicrobial and antioxidant properties of plant essential oils have been well-documented [127-130]. Lipophilic compounds, including terpenoid derivatives, have been shown to disrupt cellular membranes in bacteria and fungi, thus inhibiting cellular respiration and ionic transport [131,132]. Table 4 summarizes bioactivity data reported to date for essential oils and volatile fractions from Hypericum species. The antimicrobial activities of α- and β-pinene, as well as β-caryophyllene, have been well-documented and, as these compounds represent dominant components in the essential oils of many Hypericum species (see Tables 1 and 2), such effects are not unexpected. Further investigations with essential oils, volatile fractions and infused oils from Hypericum species would be of interest due to the ex vivo anti-inflammatory activity and in vivo gastroprotective effects that have been demonstrated with H. perforatum infused oils [10,15].
Table 4.
Bioactivity of essential oils and volatile fractions from Hypericum.
| Taxonomic Section | Species | Activity Reported | Test organism | Bioactivity* | Reference |
|---|---|---|---|---|---|
| Androsaemum | H. androsaemum | Nematicidal | Meloidogyne incognita | Essential oil showed preliminary activity: carvacrol (100% mortality), thymol (90% mortality), geraniol (74% mortality) |
133 |
| Ascyreia | H. calycinum | Antibacterial | Bacillus subtilis | 78 μg/mL (MIC) | 43 |
| H. kouytchense | Antibacterial | Bacillus coli | moderate GI | 50 | |
| Bacillus pyocyaneus | moderate GI | 50 | |||
| Antifungal | Candida albicans | moderate GI | 50 | ||
| Coridium | H. coris | Antibacterial | Saccharomyces cerevisiae | 100 μg/mL (CI) | 57 |
| Staphylococcus aureus | 100 μg/mL (CI) | 57 | |||
| Drosocarpium | H. barbatum | Antibacterial | Agrobacterium tumefaciens | 25 μg/mL (MIC) | 60 |
| Bacillus cereus | 6.25 μg/mL (MIC) | 60 | |||
| Escherichia coli | 25 μg/mL (MIC) | 60 | |||
| Micrococcus luteus | 6.25 μg/mL (MIC) | 60 | |||
| Proteus mirabilis | 25 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 50 μg/mL (MIC) | 60 | |||
| Salmonella eteritidis | 50 μg/mL (MIC) | 60 | |||
| Sarcina lutea | 6.25 μg/mL (MIC) | 60 | |||
| Staphylococcus aureus | 6.25 μg/mL (MIC) | 60 | |||
| Antifungal | Candida albicans | 25 μg/mL (MIC) | 60 | ||
|
H. richeri (published as H. alpinum) |
Antibacterial | Agrobacterium tumefaciens | 25 μg/mL (MIC) | 60 | |
| Bacillus cereus | 12.5 μg/mL (MIC) | 60 | |||
| Escherichia coli | 50 μg/mL (MIC) | 60 | |||
| Micrococcus luteus | 12.5 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 50 μg/mL (MIC) | 60 | |||
| Salmonella eteritidis | 50 μg/mL (MIC) | 60 | |||
| Sarcina lutea | 12.5 μg/mL (MIC) | 60 | |||
| Staphylococcus aureus | 12.5 μg/mL (MIC) | 60 | |||
| H. rumeliacum | Antibacterial | Agrobacterium tumefaciens | 25 μg/mL (MIC) | 60 | |
| Bacillus cereus | 12.5 μg/mL (MIC) | 60 | |||
| Candida albicans | 25 μg/mL (MIC) | 60 | |||
| Enterobacter cloacae | 17.2 mg/mL (MIC) (α- and/or β-pinene contributes to effect?) |
66 | |||
| Escherichia coli | 3.8 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Escherichia coli | 25 μg/mL (MIC) | 60 | |||
| Klebsiella pneumoniae | 9.3 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Micrococcus luteus | 12.5 μg/mL (MIC) | 60 | |||
| Proteus mirabilis | 50 μg/mL (MIC) | 60 | |||
| Pseudomonas aeruginosa | 7.4 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Pseudomonas aeruginosa | 25 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 25 μg/mL (MIC) | 62 | |||
| Salmonella enteritidis | 25 μg/mL (MIC) | 62 | |||
| Sarcina lutea | 6.25 μg/mL (MIC) | 62 | |||
| Staphylococcus aureus | 7.8 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Staphylococcus aureus | 6.25 μg/mL (MIC) | 62 | |||
| Staphylococcus epidermidis | 11.2 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Antifungal | Candida albicans | 6.3 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | ||
| Candida glabrata | 4.8 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Candida tropicalis | 5.3 mg/mL (MIC) (α-pinene contributes to effect?) |
66 | |||
| Heterophylla | H. heterophyllum | Antifungal | Rhizoctonia solani (AG-11) | 36% GI with oil (dose = 0.5 mg/μL); GI 85% and 79% by α-longipinene and caryophyllene oxide, respectiveLy (1 mg/mL) |
69 |
| Rhizoctonia solani (AG-4) | 18% GI with oil (dose = 0.5 mg/μL); GI 84% with caryophyllene oxide |
69 | |||
| Rhizoctonia solani (AG-9) | 12% GI (dose = 1 mg/μL); GI 71% and 59% by caryophyllene oxide and β- caryophyllene, respectively (1 mg/mL) |
69 | |||
| Fusarium acuminatum | 33% GI with oil (dose = 0.5 mg/μL); GI 64% with β-caryophyllene (1 mg/mL) |
69 | |||
| Hirtella |
H. hyssopifolium ssp. elongatum (syn: H. elongatum) |
Antibacterial | Bacillus subtilis | GIZ 8-10 mm by 8 μL | 68 |
| Enterococcus faecalis | GIZ 6-8 mm by 8 μL | 68 | |||
| Pseudomonas aeruginosa | GIZ 8-10 mm by 8 μL | 68 | |||
| Salmonella typhi | GIZ 8-10 mm by 8 μL | 68 | |||
| Staphylococcus aureus | GIZ 8-10 mm by 8 μL | 68 | |||
| Staphylococcus epidermidis | GIZ 8-10 mm by 8 μL | 68 | |||
| Candida albicans | GIZ 6-8 mm by 8 μL | 68 | |||
| Antifungal | Candida kefyr | GIZ 8-10 mm by 8 μL | 68 | ||
|
H. hyssopifolium ssp. elongatum |
Antifungal | Fusarium acuminatum | 39% GI with oil (dose = 1 mg/mL); 31% GI with α-terpineol (1 mg/mL) |
69 | |
| Rhizoctonia solani (AG-11) | 36% GI with oil (dose = 0.5 mg/μL); GI 79% and 77% by α-pinene and α- terpineol, respectively (1 mg/mL) |
69 | |||
| Rhizoctonia solani (AG-3) | 147% GS with oil (0.5 mg/mL); GI 71% with β-caryophyllene (1mg/mL); GI 53% with α-terpineol (1 mg/mL); GS 129% with α-pinene and p-cymene |
69 | |||
| Rhizoctonia solani (AG-4) | 11% GI with oil (dose = 0.5 mg/μL); GI 38% and 89% with α-phellandrene and α- terpineol, respectively (1 mg/mL) |
69 | |||
| Rhizoctonia solani (AG-5) | 30% GI (dose = 0.5 mg/mL); GI 57% and 87% with β-caryophyllene and α- terpineol, respectively |
69 | |||
| Rhizoctonia solani (AG-9) | 6% GI with oil (dose = 5 mg/mL); GI 65% and 71% with β-pinene and α-terpineol, respectively (1 mg/mL) |
69 | |||
| H. hyssopifolium * | Insecticidal | Tribolium confusum | 50% mortality (dose = 40 μL) | 134 | |
|
H. hyssopifolium ssp. hyssopifolium |
Antibacterial | Enterococcus hirae | moderate GI | 70 | |
| Escherichia coli | moderate GI | 70 | |||
| Saccharomyces cerevisiae | moderate GI | 70 | |||
| Saccharomyces aureus | moderate GI | 70 | |||
|
H. hyssopifolium ssp. microcalycinum |
Antibacterial | Bacillus brevis | 16 mm GIZ (60 μg/disc) | 71 | |
| Bacillus cereus | 10 mm GIZ (40 μg/disc) | 71 | |||
| Escherichia coli K12 | 14 mm GIZ (40 μg/disc) | 71 | |||
| Escherichia coli PBR 322 | 10 mm GIZ (40 μg/disc) | 71 | |||
| Escherichia coli PUC 9 | 8 mm GIZ (40 μg/disc) | 71 | |||
| Pseudomonas aeruginosa | 12 mm GIZ (40 μg/disc) | 71 | |||
| Staphylococcus aureus | 12 mm GIZ (40 μg/disc) | 71 | |||
| Streptococcus pyogenes | 14 mm GIZ (60 μg/disc) | 71 | |||
| Antifungal | Candida albicans | 12 mm GIZ (40 μg/disc) | 71 | ||
|
H. lysimachioides var. lysimachioides |
Antibacterial | Escherichia coli K12 | 12 mm GIZ (40 μg/disc) | 71 | |
| Escherichia coli PBR 322 | 16 mm GIZ (40 μg/disc) | 71 | |||
| Escherichia coli PUC 9 | 12 mm GIZ (40 μg/disc) | 71 | |||
| Pseudomonas aeruginosa | 12 mm GIZ (40 μg/disc) | 71 | |||
| Staphylococcus aureus | 12 mm GIZ (40 μg/disc) | 71 | |||
| Streptococcus pyogenes | 8 mm GIZ (40 μg/disc) | 71 | |||
| Antifungal | Candida albicans | 8 mm GIZ (40 μg/disc) | 71 | ||
| H. scabrum | Insecticidal | Sitophilus granarius | 73% mortality (dose = 10 μL) | 135 | |
| Ephestia kuehniella | 72% mortality (dose = 10 μL) | 135 | |||
| Antibacterial | Bacillus brevis | 10 mm GIZ (40 μg/disc) | 136 | ||
| Bacillus cereus | 14 mm GIZ (60 μg/disc) | 136 | |||
| Escherichia coli K12 | 18 mm GIZ (40 μg/disc) | 136 | |||
| Escherichia coli PBR 322 | 10 mm GIZ (40 μg/disc) | 136 | |||
| Escherichia coli PUC 9 | 14 mm GIZ (60 μg/disc) | 136 | |||
| Pseudomonas aeruginosa | 16 mm GIZ (40 μg/disc) | 136 | |||
| Staphylococcus aureus | 16 mm GIZ (40 μg/disc) | 136 | |||
| Streptococcus pyogenes | 10 mm GIZ (40 μg/disc) | 136 | |||
| Antifungal | Candida albicans | 18 mm GIZ (60 μg/disc) | 136 | ||
| H. scabroides | Antibacterial | Bacillus brevis | 12 mm GIZ (40 μg/disc) | 136 | |
| Bacillus cereus | 10 mm GIZ (60 μg/disc) | 136 | |||
| Escherichia coli K12 | 16 mm GIZ (40 μg/disc) | 136 | |||
| Escherichia coli PBR 322 | 16 mm GIZ (40 μg/disc) | 136 | |||
| Escherichia coli PUC 9 | 20 mm GIZ (40 μg/disc) | 136 | |||
| Pseudomonas aeruginosa | 14 mm GIZ (40 μg/disc) | 136 | |||
| Staphylococcus aureus | 10 mm GIZ (40 μg/disc) | 136 | |||
| Streptococcus pyogenes | 10 mm GIZ (40 μg/disc) | 136 | |||
| Antifungal | Candida albicans | 16 mm GIZ (40 μg/disc) | 136 | ||
| Hypericum | H. maculatum | Antibacterial | Agrobacterium tumefaciens | 25 μg/mL (MIC) | 60 |
| Aspergillus niger | 12 μL/mL (MIC); 25 μL/mL (MMC) | 81 | |||
| Bacillus cereus | 12.5 μg/mL (MIC) | 60 | |||
| Bacillus subtilis | 12 μL/mL (MIC); 20 μL/mL (MMC) | 81 | |||
| Escherichia coli | 12 μL/mL (MIC); 40 μL/mL (MMC) | 81 | |||
| Escherichia coli | 25 μg/mL (MIC) | 60 | |||
| Klebsiella pneumoniae | 16 μL/mL (MIC); 40 μL/mL (MMC) | 81 | |||
| Micrococcus luteus | 12.5 μg/mL (MIC) | 60 | |||
| Proteus mirabilis | 50 μg/mL (MIC) | 60 | |||
| Pseudomonas aeruginosa | 16 μL/mL (MIC) ; 25 μL/mL (MMC) | 81 | |||
| Pseudomonas aeruginosa | 25 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 25 μg/mL (MIC) | 60 | |||
| Salmonella enteritidis | 16 μL/mL (MIC); 40 μL/mL (MMC) | 81 | |||
| Salmonella enteritidis | 25 μg/mL (MIC) | 60 | |||
| Sarcina lutea | 12 μL/mL (MIC); 25 μL/mL (MMC) | 81 | |||
| Sarcina lutea | 12.5 μg/mL (MIC) | 60 | |||
| Staphylococcus aureus | 16 μL/mL (MIC); 30 μL/mL (MMC) | 81 | |||
| Staphylococcus aureus | 12.5 μg/mL (MIC) | 60 | |||
| Antifungal | Candida albicans | 50 μg/mL (MIC) | 60 | ||
| Candida albicans | 12 μL/mL (MIC); 16 μL/mL (MMC) | 81 | |||
| H. perforatum | Antibacterial | Agrobacterium tumefaciens | 25 μg/mL (MIC) | 60 | |
| Bacillus cereus | 12.5 μg/mL (MIC) | 60 | |||
| Escherichia coli | 25 μg/mL (MIC) | 60 | |||
| Escherichia coli | 7 mm GIZ (1 μL) | 137 | |||
| Micrococcus luteus | 12.5 μg/mL (MIC) | 60 | |||
| Micrococcus luteus | 6 mm GIZ (1 μL) | 137 | |||
| Proteus mirabilis | 50 μg/mL (MIC) | 60 | |||
| Pseudomonas aeruginosa | 50 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 25 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 3 mm GIZ (1 μL) | 137 | |||
| Salmonella enteritidis | 25 μg/mL (MIC) | 60 | |||
| Salmonella enteritidis | 4 mm GIZ (1 μL) | 137 | |||
| Salmonella typhimurium | 4 mm GIZ (1 μL) | 137 | |||
| Sarcina lutea | 12.5 μg/mL (MIC) | 60 | |||
| Staphylococcus aureus | 12.5 μg/mL (MIC) | 60 | |||
| Staphylococcus aureus | 5 mm GIZ (1 μL) | 137 | |||
| Staphylococcus epidermidis | 4 mm GIZ (1 μL) | 137 | |||
| Antifungal | Aspergillus niger | 15 μg/mL (MIC) | 137 | ||
| Candida albicans | 5 mm GIZ (2.5 μL oiL); 50 μg/mL (MIC) | 60, 137 | |||
| Cladosporium cladosporioides | 15 μg/mL (MIC) | 137 | |||
| Penicillium funiculosum | 15 μg/mL (MIC) | 137 | |||
| Trichoderma viride | 15 μg/mL (MIC) | 137 | |||
| H. tetrapterum | Antibacterial | Bacillus subtilis | 78 μg/mL (MIC) | 43 | |
| H. triquetrifolium | Antibacterial | Bacillus brevis | 10 mm GIZ (40 μg/disc) | 136 | |
| Bacillus cereus | 12 mm GIZ (40 μg/disc) | 136 | |||
| Escherichia coli PBR 322 | 10 mm GIZ (40 μg/disc) | 136 | |||
| Escherichia coli PUC 9 | 10 mm GIZ (40 μg/disc) | 136 | |||
| Pseudomonas aeruginosa | 12 mm GIZ (40 μg/disc) | 136 | |||
| Staphylococcus aureus | 16 mm GIZ (40 μg/disc) | 136 | |||
| Antifungal | Candida albicans | 12 mm GIZ (40 μg/disc) | 136 | ||
| H. hirsutum | Antibacterial | Agrobacterium tumefaciens | 50 μg/mL (MIC) | 60 | |
| Bacillus aureus | 78 μg/mL (MIC) | 43 | |||
| Bacillus cereus | 12.5 μg/mL (MIC) | 60 | |||
| Escherichia coli | 50 μg/mL (MIC) | 60 | |||
| Micrococcus luteus | 25 μg/mL (MIC) | 60 | |||
| Pseudomonas tolaasii | 50 μg/mL (MIC) | 60 | |||
| Salmonella enteritidis | 50 μg/mL (MIC) | 60 | |||
| Sarcina lutea | 12.5 μg/mL (MIC) | 60 | |||
| Staphylococcus aureus | 39 μg/mL (MIC) | 43 | |||
| Staphylococcus aureus | 25 μg/mL (MIC) | 60 | |||
| Taeniocarpium | H. linarioides | Antifungal | Verticillium albo-atrum | 36% GI (5 mg/mL) | 91 |
| Rhizoctonia solani (AG-9) | 56.7% GI (2.5 mg/mL) | 91 | |||
| Trigynobrathys | H. cordatum | Antibacterial | Staphylococcus aureus | moderate GI | 94 |
| Antifungal | Cladosporium cladosporioides | moderate GI | 94 | ||
| Cladosporium sphaerospermum | moderate GI | 94 |
GI = growth inhibition; GIZ = growth inhibitory zone; MIC = minimum inhibitory concentrations; MMC = minimum microbicidal (lethal) concentrations;
Taxonomic ranking below species not specified.
A recent study additionally revealed that healthy H. perforatum plants produced higher amounts of essential oil (0.75%) than plants infected with a ribosomal group 16SrVII phytoplasma known as Ash Yellows (0.11%), and that a higher sesquiterpene to monoterpene and aliphatics ratio was observed in infected plants [113]. Increased accumulation of hyperforin has been observed after elicitation of H. perforatum seedlings with the fungal pathogen Colletotrichum gloeosporioides [138]. How bacterial (e.g. Agrobacterium tumefaciens) or fungal infections alter the essential oil (or other lipophilic constituent) profile of H. perforatum, or other Hypericum species, is a promising area of future investigation.
The identification of particular chemotypes of H. perforatum, displaying a dominant production of β-caryophyllene, caryophyllene oxide, germacrene D, α- and β-pinene [61,116,126] has led to the development of a hypothesis that particular populations (or chemotypes) of this species are rich in either β-caryophyllene/caryophyllene oxide (e.g sesquiterpene hydrocarbons) or α-/β-pinene (e.g. monoterpene hydrocarbons), but not produce both groups of compounds in higher amounts simultaneously [see 52,55,58,68,71,91,106,108,114,115]. This hypothesis, however, has not been supported by the results of essential oil analyses from other Hypericum species (see Tables 1 and 2), or by data from particular samples of H. perforatum from France and Italy (see Table 3). Another hypothesis states that there are Hypericum populations (e.g. chemotypes) that produce higher amounts of non-terpenoid constituents to the exclusion of terpenoids [68,120]. An interesting study that compared volatiles from the essential oils, air and/or heat-dried, and lyophilized tissue of 13 Hypericum species from Portugal using instrumental olfactroscopy indicated that a decrease in n-methyloctane and α-pinene frequently correlated with an increase in β-caryophyllene and germacrene D, and vice-versa [139], but again such observations are not consistently supported (see Table 1).
Hypotheses of this nature, therefore, while potentially applicable when examining a limited number of populations, collected or originating from a defined geographic region, and examined within a specific time window (e.g. state of phenology), are not supported when examining evidence upon a broader scale (e.g. geographic range, taxonomic rank, seasonality). This point is emphasized because several recent publications have proposed using volatile constituents as chemotaxonomic markers, examining data with multivariate statistical techniques such as principal coordinate analysis (PCO), principal component analysis (PCA) and canonical discriminate analysis (CDA) [58,61]. Phylogenetic reconstruction using CDA resulted in the division of the analyzed Hypericum species into taxonomic sections in accordance with those delineated by morphological characters [1,58], while analyses using PCO and/or PCA either did [64] or did not result in such separations [58,61]. It is important to note that, despite the excellent scientific quality of these studies, the plants were collected across a relatively restricted geographic range (respectively, Portugal [64], southeastern Serbia [61] and the Hellenic arc [58]). The authors noted that, although samples were taken during different phenological stages, from different populations within the restricted geographic range and various extraction procedures were used, that the results of their analyses allowed the grouping of samples into accepted taxonomic clusters [58,64]. At the same time, the volatile oil compositions reported for particular species in these studies differed significantly from those reported for the same species from other geographic regions (e.g. H. perfoliatum, compare [63] and [64]). Petrakis et al. [58] discussed the implications of such findings and described in detail specifically why such methods can be potentially used to separate species samples (at least collected within a restricted geographic range), but should not be used to infer phylogeny.
Most studies with Hypericum have been conducted almost exclusively on wild-collected material and without repetition, limiting their usefulness. They do, however, provide an initial platform from which more detailed studies may be launched. In addition to the wealth of information available for H. perforatum, a considerable amount of data are available for essential oil content and composition in H. androsaemum, H. hyssopifolium, H. maculatum, H. perfoliatum, H. scabrum and H. triquetrifolium (see Table 1), providing a basis for comparison. H. androsaemum and H. triquetrifolium are cultivated to a limited extent, the former for the production of cut-flower stems and the latter, for the isolation of hypericin [140-141]. These species along with H. perforatum would, therefore, be ideal targets for further controlled studies on the influence of genotype on essential oil yield and constitution. The implications of the multi-dimensional (i.e. at the subspecies, population, plant, tissue, and even cellular level) phytochemical variability, such as that which has been reported in the Hypericum literature, have long since been appreciated by researchers in the fields of metabolomics and systems biology. Correspondingly, much energy has been invested in the development of appropriate experimental designs to ask specific questions about tracking variation in plant biochemistry in these fields [142,143]. Genetic engineering studies, aimed toward modifying aspects of the biosynthesis of essential oil components [144,145], are highly relevant to the field of essential oil research. The immediate future of research on the essential oil and volatiles chemistry of Hypericum, as for many other plant genera, lies in the development of targeted breeding programs for species with the potential to biosynthesize medicinally and economically valuable constituents. A slightly more distant future prospective includes the design of studies to genetically modify such biosynthetic pathways, to tailor both content and constituent output.
Acknowledgments
Brian Lawrence (Winston-Salem, NC, USA), Filippo Maggi (Univ. of Camerino, Italy) and Chlodwig Franz (Univ. of Veterinary Medicine, Vienna, Austria) are gratefully acknowledged for providing selected reference literature. Xin Liu (Karl-Franzens-Univ. Graz, Austria) is thanked for providing selected Chinese references and aid in translation. K. H. C. Başer (Anadolu Univ., Eskisehir, Turkey) is sincerely thanked for several years of fruitful collaboration on essential oil research. Funding for the author was provided through a Hertha-Firnberg Stipend (T345) from the Fonds zur Förderung der Wissenschaftlichen Forschung (FWF) in Austria.
References
- [1].Robson NKB. Hypericum botany. In: Ernst E, editor. Hypericum: The genus Hypericum. Taylor and Francis; New York, USA: 2003. pp. 1–22. [Google Scholar]
- [2].Crockett SL, Robson NKB. Taxonomy and chemotaxonomy of the genus Hypericum. Medicinal and Aromatic Plant Science and Biotechnology. (in press) [PMC free article] [PubMed] [Google Scholar]
- [3].Müller WE, editor. St. John’s Wort and its active principles in depression and anxiety. Birkhäuser Verlag; Basel, Switzerland: 2005. [Google Scholar]
- [4].Council of Europe . European Pharmacopoeia. 4th edition Strasbourg, France: 2001. [Google Scholar]
- [5].American Botanical Council . St. John’s wort. In: Blumenthal M, Goldberg A, editors. The complete German Commission E monographs: Therapeutic guide to herbal medicines. Austin, Texas: 1998. pp. 214–215. [Google Scholar]
- [6].Commission Suisse de Pharmacopée . Pharmacopoea Helvetica. 8th ed Département fédéral de l’intérieur; Berne, Switzerland: 1997. [Google Scholar]
- [7].Berghöfer R. Analytik und Isolierung phenolischer Inhaltsstoffe von Hypericum perforatum L. aus Anbau und Wildvorkommen und Vergleich mit anderen heimischen Hypericum-Arten. Cramer; Berlin/Stuttgart, Germany: 1987. Dissertationes Botanicae. [Google Scholar]
- [8].Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St. John’s wort for depression: an overview and meta-analysis of randomised clinical trials. British Medical Journal. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miraldi E, Biagi M, Giachetti D. Chemical constituents and effects of topical application of Oleum Hyperici on skin sensitivity to simulated sun exposure. Natural Product Communications. 2006;1:209–213. [Google Scholar]
- [10].Zdunić G, Gođevac D, Milenković M, Vučlćevicć D, Šavikin K, Menković N, Petrović S. Evaluation of Hypericum perforatum oil extracts for an anti-inflammatory and gastroprotective activity in rats. Phytotherapy Research. 2009;23:1559–1564. doi: 10.1002/ptr.2809. [DOI] [PubMed] [Google Scholar]
- [11].Maisenbacher P, Kovar KA. Analysis and stability of Hyperici oleum. Planta Medica. 1992;58:351–354. doi: 10.1055/s-2006-961483. [DOI] [PubMed] [Google Scholar]
- [12].Isacchi B, Bergonzi MC, Carnevalli F, van der Esch SA, Vincieri FF, Bilia AR. Analysis and stability of the constituents of St John’s wort oils prepared with different methods. Journal of Pharmaceutical and Biomedical Analysis. 2007;45:756–761. doi: 10.1016/j.jpba.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [13].European Herb Growers Association (EUROPAM) Production of medicinal and aromatic plants in Europe. [Accessed 19 May 2010]. Available online: http://www.europam.net/index.php.
- [14].Lis-Balchin M. Aromatherapy with essential oils. In: Başer KHC, Buchbauer G, editors. Handbook of essential oils: Science, technology and applications. Taylor and Francis; New York: 2010. pp. 549–584. [Google Scholar]
- [15].Lavagna SM, Secci D, Chimenti P, Bonsignore L, Ottaviani A, Bizzarri B. Efficacy of Hypericum and Calendula oils in the epithelial reconstruction of surgical wounds in childbirth with cesarean section. Farmaco. 2001;56:451–453. doi: 10.1016/s0014-827x(01)01060-6. [DOI] [PubMed] [Google Scholar]
- [16].Personal communications: Aura Cacia (Urbana, Iowa, USA) and Mountain Rose Herbs (Eugene, Oregon, USA).
- [17].Stjepanović L, Corović M, Nikolić R, Palović S, Zivanović P. Essential oil content and number of oil vesicles in Hypericum species from various habitats of Tara mountain. Arhiv za Farmaciju. 1965;15:177–188. [Google Scholar]
- [18].Roth L. Hypericum, hypericin: Botanik, Inhaltstoffe, Wirkung. Ecomed Verlagsgesellschaft GmbH; Landsberg, Germany: 1990. [Google Scholar]
- [19].Omidbaigi R, Azizi M. Effect of time of harvest on hypericin and essential oil content of Hypericum perforatum L. from Iran. Iran Agricultural Research. 2000;19:155–164. [Google Scholar]
- [20].Lutz R. The essential oils of different St. John’s worts. Ätherische Öle, Riechstoffe, Parfümerien, Essenzen und Aromen. 1952;2:137–139. [Google Scholar]
- [21].Šmelcerović A, Lepojević Z, Djordjević S. Sub-and supercritical CO2-extraction of Hypericum perforatum L. Chemical Engineering & Technology. 2004;27:1327–1329. [Google Scholar]
- [22].Glišić SB, Popadić SV, Skala DU. St. John’s wort (Hypericum perforatum L.) - Supercritical fluid extraction and antimicrobial and antidepressant activity of the extract and some components. Hemijska Industrija. 2006;60:61–71. [Google Scholar]
- [23].Wurdack KJ, Davis CC. Malpighiales phylogenetics: Gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. American Journal of Botany. 2009;96:1551–1570. doi: 10.3732/ajb.0800207. [DOI] [PubMed] [Google Scholar]
- [24].Stevens PF. Clusiaceae-Guttiferae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. IX. Springer; Berlin, Germany: 2007. pp. 48–66. [Google Scholar]
- [25].Ciccarelli D, Andreucci AC, Pagni AM. Translucent glands and secretory canals in Hypericum perforatum L. (Hyperiacaceae): morphological, anatomical and histochemical studies during the course of ontogenesis. Annals of Botany. 2001;88:637–644. [Google Scholar]
- [26].Lu H, Hu Z. Study on development of secretory structure and their secretory product accumulation of Hypericum perforatum. Xibei Zhiwu Xuebao. 2001;21:287–292. [Google Scholar]
- [27].Ciccarelli D, Andreucci AC, Pagni AM. The “black nodules” of Hypericum perforatum L. subsp. perforatum: morphological, anatomical, and histochemical studies during the course of ontogenesis. Israel Journal of Plant Sciences. 2001;49:33–40. [Google Scholar]
- [28].Soelberg J, Jørgensen LB, Jäger AK. Hyperforin accumulates in the translucent glands of Hypericum perforatum. Annals of Botany. 2007;99:1097–1100. doi: 10.1093/aob/mcm057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zobayed SMA, Afreen F, Goto E, Kozai T. Plant–environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum. Annals of Botany. 2006;98:793–804. doi: 10.1093/aob/mcl169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Adam P, Arigoni D, Bacher A, Eisenreich W. Biosynthesis of hyperforin in Hypericum perforatum. Journal of Medicinal Chemistry. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- [31].Croteau R. Biosynthesis and catabolism of monoterpenoids. Chemical Review. 1987;87:929–954. [Google Scholar]
- [32].Piovan A, Filippini R, Caniato R, Borsarini A, Maleci LB, Cappelletti EM. Detection of hypericins in the “red glands” of Hypericum elodes by ESI-MS/MS. Phytochemistry. 2004;65:411–414. doi: 10.1016/j.phytochem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- [33].Giuliani C, Pellegrino RM, Tirillini B, Bini LM. The role of secreting structures position on the leaf volatile organic compounds of Hypericum androsaemum. Natural Product Communications. 2010;5:107–110. [PubMed] [Google Scholar]
- [34].Lu H, Hu Z. Distribution of hypericin and volatile oils in Hypericum erectum. Zhongcaoyao. 2000;31:773–775. [Google Scholar]
- [35].Lu H, Liu W, Hu Z. Secretory structures in seniawin St. Johns wort (Hypericum seniawinii) Zhongcaoyao. 1999;30:290–293. [Google Scholar]
- [36].Fornasiero RB, Maffi L, Benvenuit S, Bianchi A. Morphological and phytochemical features of secretory structures in Hypericum richer (Clusiaceae) Nordic Journal of Botany. 2000;20:427–434. [Google Scholar]
- [37].Sun D-l, Zhou C-h, Shoa M-m, Lu H-f, Hu Z-h. Morphological identification of 20 medicinal species in Hypericum. Zhongguo zhongyao zazhi. 2007;32:893–898. [PubMed] [Google Scholar]
- [38].Hölscher D, Shroff R, Knop K, Gottschaldt M, Crecelius A, Schneider B, Heckel DG, Schubert US, Svatoš A. Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant Journal. 2009;60:907–918. doi: 10.1111/j.1365-313X.2009.04012.x. [DOI] [PubMed] [Google Scholar]
- [39].Mathis C, Ourisson G. Chemotaxonomic study of the genus Hypericum. III. Distribution of saturated hydrocarbons and monoterpenes in the essential oils of Hypericum. Phytochemistry. 1964;3:133–141. [Google Scholar]
- [40].Gudžić B, Dorđević S, Nedeljković J, Šmelcerović A. Essential oil composition of Hypericum atomarium Boiss. Hemijska Industrija. 2004;58:413–415. [Google Scholar]
- [41].Crockett SL, Demirci B, Başer KHC, Khan IA. Analysis of the volatile constituents of five African and Mediterranean Hypericum L. (Clusiaceae, Hypericoideae) species. Journal of Essential Oil Research. 2007;19:302–306. [Google Scholar]
- [42].Kartnig T, Gruber A, Sauer H. Comparative phytochemical investigations of Hypericum species. Planta Medica. 1989;55:215. [Google Scholar]
- [43].Maggi F, Cecchini C, Cresci A, Coman MM, Tirillini B, Sagratini G, Papa F, Vittori S. Chemical composition and antimicrobial activity of the essential oils from several Hypericum taxa (Guttiferae) growing in central Italy (Appennino Umbro-Marchigiano) Chemistry & Biodiversity. 2010;7:447–466. doi: 10.1002/cbdv.200900091. [DOI] [PubMed] [Google Scholar]
- [44].Santos PAG, Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC. Composition of the essential oil of Hypericum foliosum Aiton from five Azorean islands. Flavour and Fragrance Journal. 1999;14:283–286. [Google Scholar]
- [45].Hosni K, Msaada K, Ben Taarit M, Ouchikh O, Kallel M, Marzouk B. Essential oil composition of Hypericum perfoliatum L. and Hypericum tomentosum L. growing wild in Tunisia. Industrial Crops and Products. 2008;27:308–314. [Google Scholar]
- [46].Morteza-Semnani K, Saeedi M. The essential oil composition of Hypericum androsaemum L. leaves and flowers from Iran. Flavour and Fragrance Journal. 2005;20:332–334. [Google Scholar]
- [47].Bertoli A, Pistelli L, Morelli I, Spinelli G, Menichini F. Constituents of Hypericum hircinum oils. Journal of Essential Oil Research. 2000;12:617–620. [Google Scholar]
- [48].Demirci B, Başer KHC, Crockett SL, Khan IA. Analysis of the volatile constituents of Asian Hypericum L. (Clusiaceae, Hypericoideae) species. Journal of Essential Oil Research. 2005;17:659–663. [Google Scholar]
- [49].Erken S, Malyer H, Demirci F, Demirci B, Başer KHC. Chemical investigations on some Hypericum species growing in Turkey-I. Chemistry of Natural Compounds. 2002;37:434–438. [Google Scholar]
- [50].Yu J, Liu X, Gu L, Zhou X. Analysis of chemical constituents and bacteriostasis of essential oils from Hypericum kouytcheouense (sic) Zhongguo Yaoxue Zazhi (Beijing, China) 2002;37:900–902. [Google Scholar]
- [51].Zhang L, Dong G, Liu G. Chemical constituents from essential oil of Hypericum patulum. Zhongyaocai. 2009;32:224–226. [PubMed] [Google Scholar]
- [52].Maggi F, Ferretti G. Essential oil comparison of Hypericum perforatum L. subsp. perforatum and subsp. veronense (Schrank) Ces. from Central Italy. Journal of Essential Oil Research. 2008;20:492–494. [Google Scholar]
- [53].Demirci F, Başer KHC. Volatiles of Hypericum bupleuroides Griseb. Journal of Essential Oil Research. 2006;18:650–651. [Google Scholar]
- [54].Sajjadi SE, Rahiminezhad MR, Mehregan I, Poorassar A. Constituents of essential oil of Hypericum dogonbadanicum Assadi. Journal of Essential Oil Research. 2001;13:43–44. [Google Scholar]
- [55].Javidnia K, Miri R, Soltani M, Gholami M, Khosravi AR. Essential oil composition of four Hypericum species from Iran. Chemistry of Natural Compounds. 2008;44:374–377. [Google Scholar]
- [56].Vera RR, Chane-Ming J, Fraisse DJ. Essential oil of Hypericum lanceolatum Lam. from Reunion. Rivista Italiana EPPOS. 1996;7:639–644. [Google Scholar]
- [57].Schwob I, Bessiere JM, Dherbomez M, Viano J. Composition and antimicrobial activity of the essential oil of Hypericum coris. Fitoterapia. 2002;73:511–513. doi: 10.1016/s0367-326x(02)00171-5. [DOI] [PubMed] [Google Scholar]
- [58].Petrakis PV, Couladis M, Roussis V. A method for detecting the biosystematic significance of the essential oil composition: The case of five Hellenic Hypericum L. species. Biochemical Systematics and Ecology. 2005;33:873–898. [Google Scholar]
- [59].Cardona ML, Marco JA, Sendra JM, Seoane E, Ibanez JT. Waxes and volatile oils in Hypericum ericoides (Guttiferae) Lipids. 1983;18:439–442. [Google Scholar]
- [60].Saroglou V, Marin PD, Rancic A, Veljic M, Skaltsa H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochemical Systematics and Ecology. 2007;35:146–152. [Google Scholar]
- [61].Šmelcerović A, Spiteller M, Ligon AP, Šmelcerović Z, Raabe N. Essential oil composition of Hypericum L. species from southeastern Serbia and their chemotaxonomy. Biochemical Systematics and Ecology. 2007;35:99–113. [Google Scholar]
- [62].Couladis M, Baziou P, Petrakis PV, Harvala C. Essential oil composition of Hypericum perfoliatum L. growing in different locations in Greece. Flavour and Fragrance Journal. 2001;16:204–206. [Google Scholar]
- [63].Touafek O, Nacer A, Kabouche A, Kabouche Z. Analysis of the essential oil of Algerian Hypericum perfoliatum (L) Flavour and Fragrance Journal. 2005;20:669–670. [Google Scholar]
- [64].Nogueira T, Marcelo-Curto MJ, Figueiredo AC, Barroso JG, Pedro LG, Rubiolo P, Bicchi C. Chemotaxonomy of Hypericum genus from Portugal: Geographical distribution and essential oils composition of Hypericum perfoliatum, Hypericum humifusum, Hypericum linarifolium and Hypericum pulchrum. Biochemical Systematics and Ecology. 2008;36:40–50. [Google Scholar]
- [65].Ferretti G, Maggi F, Tirillini B. Essential oil composition of Hypericum richeri VIII. from Italy. Flavour and Fragrance Journal. 2005;20:295–298. [Google Scholar]
- [66].Couladis M, Chinou IB, Tzakou O, Petrakis PV. Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. apollinis (Boiss. & Heldr.) Phytotherapy Research. 2003;17:152–154. doi: 10.1002/ptr.1093. [DOI] [PubMed] [Google Scholar]
- [67].Mathela DK, Mathela CS, Dev V. Volatile constituents of Hypericum elodeoides Chois. Journal of the Indian Chemical Society. 1984;61:792–793. [Google Scholar]
- [68].Ghasemi Y, Khalaj A, Mohagheghzadeh A, Khosravi AR, Morowvat MH. Composition and antimicrobial activity of the essential oil and extract of Hypericum elongatum. Journal of Applied Sciences. 2007;7:2671–2675. [Google Scholar]
- [69].Çakir A, Kordali S, Zengin H, Izumi S, Hirata T. Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flavour and Fragrance Journal. 2004;19:62–68. [Google Scholar]
- [70].Schwob I, Viano J, Jann-Para G, Bessiere J-M, Dherbomez M. Composition and antimicrobial activity of the essential oil of Hypericum hyssopifolium ssp. hyssopifolium from southeast France. Journal of Essential Oil Research. 2006;18:469–471. [Google Scholar]
- [71].Toker Z, Kizil G, Özen HC, Kizil M, Ertekin S. Compositions and antimicrobial activities of the essential oils of two Hypericum species from Turkey. Fitoterapia. 2006;77:57–60. doi: 10.1016/j.fitote.2005.08.019. [DOI] [PubMed] [Google Scholar]
- [72].Başer KHC, Özek T, Nuriddinov HR, Demirci AB. Essential oils of two Hypericum species from Uzbekistan. Chemistry of Natural Compounds. 2002;38:54–57. [Google Scholar]
- [73].Çakir A, Duru ME, Harmandar M, Ciriminna R, Passannanti S, Piozzi F. Comparison of the volatile oils of Hypericum scabrum L. and Hypericum perforatum L. from Turkey. Flavour and Fragrance Journal. 1997;12:285–287. [Google Scholar]
- [74].Kudryashev S. Essential oils from wild plants of the central region of the Gissar Mountains. Parfums de France. 1932;12:98–101. [Google Scholar]
- [75].Kurbanov MK, Mutayev MM. Contents of biologically active substances in Hypericum scabrum L. growing in different regions of Tajikistan. Rastitel’nye Resursy. 1993;29:40–43. [Google Scholar]
- [76].Xiong Y-j, Ilyas K, Xie C-x, Li Y. Constituents in essential oil of Hypericum scabrum. Zhongchengyao. 2006;28:865–867. [Google Scholar]
- [77].Morteza-Semnani K, Saeedi M, Changizi S. The essential oil composition of Hypericum scabrum L. from Iran. Flavour and Fragrance Journal. 2006;21:513–515. [Google Scholar]
- [78].Ozkan AMG, Demirci B, Başer KHC. Essential oil composition of Hypericum thymopsis Boiss. Journal of Essential Oil Research. 2009;21:149–153. [Google Scholar]
- [79].Dong J. Chemical constituents of volatile oils of Hypericum attenuatum Choisy. Zhongcaoyao. 2004;35:734–736. [Google Scholar]
- [80].Radulović NS, Ethorethević AS, Palić RM. The intrasectional chemotaxonomic placement of Hypericum elegans Stephan ex Willd. inferred from the essential-oil chemical composition. Chemistry & Biodiversity. 2010;7:943–952. doi: 10.1002/cbdv.200900252. [DOI] [PubMed] [Google Scholar]
- [81].Gudžić B, Djoković D, Vajs V, Palić R, Stojanović G. Composition and antimicrobial activity of the essential oil of Hypericum maculatum Crantz. Flavour and Fragrance Journal. 2002;17:392–394. [Google Scholar]
- [82].Vasileva R, Georgieva V, Vojnova J, Milkova T. GC-MS study on the essential oils of Bulgarian Hypericum maculatum Grantz and Hypericum perforatum L. Dokladi na Bulgarskata Akademiya na Naukite. 2003;56:71–74. [Google Scholar]
- [83].Zeng H-y, Zhou P-h. Analysis of the chemical constituents of the essential oils from the leaves of two Hypericum plants. Xiangtan Daxue Ziran Kexue Xuebao. 2001;23:52–54. [Google Scholar]
- [84].Pavlovic M, Tzakou O, Petrakis PV, Couladis M. The essential oil of Hypericum perforatum L., Hypericum tetrapterum Fries and Hypericum olympicum L. growing in Greece. Flavour and Fragrance Journal. 2006;21:84–87. [Google Scholar]
- [85].Bertoli A, Menichini F, Mazzetti M, Spinelli G, Morelli I. Volatile constituents of the leaves and flowers of Hypericum triquetrifolium Turra. Flavour and Fragrance Journal. 2003;18:91–94. [Google Scholar]
- [86].Karim H, Kamel M, Mouna BT, Thouraya C, Brahim M. Essential oil composition of Hypericum triquetrifolium Turra. aerial parts. Italian Journal of Biochemistry. 2007;56:40–46. [PubMed] [Google Scholar]
- [87].Crockett SL, Demirci B, Başer KHC, Khan IA. Volatile constituents of Hypericum L. section Myriandra (Clusiaceae): species of the H. fasciculatum Lam. alliance. Journal of Essential Oil Research. 2008;20:244–249. [Google Scholar]
- [88].Gudžić B, Dorđević S, Palić R, Stojanović G. Essential oils of Hypericum olympicum L. and Hypericum perforatum L. Flavour and Fragrance Journal. 2001;16:201–203. [Google Scholar]
- [89].Brunarska Z. Some hypericin species of the genus Hypericum. II. Hypericum polyphyllum. 1962;14:89–96. Dissertationes Pharmaceuticae. [Google Scholar]
- [90].Gudžić BT, Šmelcerović A, Dorđević S, Mimica-Dukić N, Ristic M. Essential oil composition of Hypericum hirsutum L. Flavour and Fragrance Journal. 2007;22:42–43. [Google Scholar]
- [91].Çakir A, Kordali S, Kilic H, Kaya E. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochemical Systematics and Ecology. 2005;33:245–256. [Google Scholar]
- [92].Abreu IN, Reis MG, Marsaioli AJ, Mazzafera P. Essential oil composition of Hypericum brasiliense Choise. Flavour and Fragrance Journal. 2004;19:80–82. [Google Scholar]
- [93].Ferraz ABF, Limberger RP, Bordignon SAL, von Poser GL, Henriques AT. Essential oil composition of six Hypericum species from southern Brazil. Flavour and Fragrance Journal. 2005;20:335–339. [Google Scholar]
- [94].Ladeira AM, da Silva GB, Raggi L, Young MCM, Agripino DG, Lima MEL, Moreno PRH. Chemical composition and antimicrobial activities of the essential oil of Hypericum cordatum (Vell. Conc.) N. Robson (Hypericaceae) Journal of Essential Oil Research. 2009;21:558–560. [Google Scholar]
- [95].Yu J, Gu L, Zhou X. Study on chemical constituents in essential oil of stems, flowers and leaves of Hypericum japonicum. Zhongguo Yaoxue Zazhi (Beijing, China) 2001;36:199–200. [Google Scholar]
- [96].Sköld M, Karlberg AT, Matura M, Börje A. The fragrance chemical β-caryophyllene: air oxidation and skin sensitization. Food Chemistry and Toxicology. 2006;44:538–545. doi: 10.1016/j.fct.2005.08.028. [DOI] [PubMed] [Google Scholar]
- [97].Mathis C, Ourisson G. Chemotaxonomic study of the genus Hypericum. II. Identification of the essential oils in Hypericum. Phytochemistry. 1964;3:115–131. [Google Scholar]
- [98].Mathis C, Ourisson G. Chemotaxonomic study of the genus Hypericum. IV. Distribution of sesquiterpenes, monoterpene alcohols, and saturated aldehydes in the essential oils of Hypericum. Phytochemistry. 1964;3:377–378. [Google Scholar]
- [99].Weyerstahl P, Splittgerber U, Marschall H, Kaul VK. Constituents of the leaf essential oil of Hypericum perforatum L. from India. Flavour and Fragrance Journal. 1995;10:365–370. [Google Scholar]
- [100].Wang X, Dong X, Yan S. Chemical constituents of volatile essential oil in Hypericum perforatum. Xibei Zhiwu Xuebao. 2006;26:1259–1262. [Google Scholar]
- [101].Li H, Zhang B. Analysis of volatile components in Hypericum perforatum L. from northwestern China. Baoji Wenli Xueyuan Xuebao, Ziran Kexueban. 2006;26:200–203. [Google Scholar]
- [102].Yiliyasi K, Xie C-x, Xiong Y-j, Li Y. Analysis of chemical components of volatile oil extracted from Xinjiang Hypericum perforatum. Zhongchengyao. 2007;29:441–442. [Google Scholar]
- [103].Meng X, Guo L, Yang M, Li Y. Analysis of the essential oils from Hypericum perforatum L. Fenxi Huaxue. 2003;31:689–693. [Google Scholar]
- [104].Zeng H-y, Zhou P-h. Studies on chemical components of the volatile oil from the leaves of Hypericum perforatum. Zhongyaocai. 2000;23:752–754. [PubMed] [Google Scholar]
- [105].Rabier J, Charchoglyan A, Mevy J-p, Viano J, Masotti V. Production of hypericin and pseudohypericin in shoot cultures of Hypericum perforatum L., Actes des 18th journées “Huiles essentielles et extraits” (special issue) Rivista Italiana EPPOS. 1999;10:625–630. [Google Scholar]
- [106].Schwob I, Bessiere J-M, Masotti V, Viano J. Changes in essential oil composition in Saint John’s wort (Hypericum perforatum L.) aerial parts during its phenological cycle. Biochemical Systematics and Ecology. 2004;32:735–745. [Google Scholar]
- [107].Bruneton J. Pharmacognosie, phytochimie, plantes médicinales. Collection Tec et Doc; Lavoisier, Paris: 1993. pp. 367–369. [Google Scholar]
- [108].Schwob I, Bessiere J-M, Viano J. Composition of the essential oils of Hypericum perforatum L. from southeastern France. Comptes Rendus Biologies. 2002;325:781–785. doi: 10.1016/s1631-0691(02)01489-0. [DOI] [PubMed] [Google Scholar]
- [109].Chatzopoulou PS, Koutsos TV, Katsiotis ST. Chemical composition of the essential oils from cultivated and wild grown St. John’s Wort (Hypericum perforatum) Journal of Essential Oil Research. 2006;18:643–646. [Google Scholar]
- [110].Kakhky AM, Rustaiyan A, Masoudi S, Tabatabaei-Anaraki M, Aboly J. Chemical composition of the essential oils from flowers, leaves, stems and roots of Hypericum perforatum L. from Iran. Journal of Essential Oil-Bearing Plants. 2008;11:548–552. [Google Scholar]
- [111].Morteza-Semnani K, Saeedi M, Vahedi M. Volatile constituents of Iranian Hypericum perforatum L. Oriental Journal of Chemistry. 2002;18:443–444. [Google Scholar]
- [112].Azizi M. Change in content and chemical composition of Hypericum perforatum L. oil at three harvest times. Journal of Herbs, Spices & Medicinal Plants. 2007;13:79–85. [Google Scholar]
- [113].Bruni R, Pellati F, Maria G Bellardi, Benvenuti S, Paltrinieri S, Bertaccini A, Bianchi A. Herbal drug quality and phytochemical composition of Hypericum perforatum L. affected by ash yellows phytoplasma infection. Journal of Agricultural and Food Chemistry. 2005;53:964–968. doi: 10.1021/jf0487654. [DOI] [PubMed] [Google Scholar]
- [114].Pintore G, Chessa M, Boatto G, Cerri R, Usai M, Tirillini B. Essential oil composition of Hypericum perforatum L. var. angustifolium DC growing wild in Sardinia (Italy) Journal of Essential Oil Research. 2005;17:533–535. [Google Scholar]
- [115].Radušienė J, Judzentiene A, Bernotiene G. Essential oil composition and variability of Hypericum perforatum L. growing in Lithuania. Biochemical Systematics and Ecology. 2005;33:113–124. [Google Scholar]
- [116].Mockute D, Bernotiene G, Judzentiene A. Volatile compounds of the aerial parts of wild St. John’s wort (Hypericum perforatum L.) plants. Chemija. 2003;14:108–111. [Google Scholar]
- [117].Guedes APSP. Essential oils from plants and in vitro shoot cultures of Hypericum androsaemum L., H. perforatum L. and H. undulatum Schousboe ex. Willd. University of Minho; Portugal: [Accessed 20 May 2010]. 2009. Ph.D. Dissertation. Available online: http://repositorium.sdum.uminho.pt/bitstream/1822/9876/1/Tese.pdf. [Google Scholar]
- [118].Nogueira T, Duarte F, Venâncio R, Tavares M, Lousã C, Bicchi PR. Aspectos quimiotaxonómicos do género Hypericum L. em Portugal. Silva Lusitana. 1998;6:55–61. [Google Scholar]
- [119].Šmelcerović A, Mimica-Dukic N, Ðorđevic S. Essential oil composition of Hypericum perforatum L. ssp. angustifolium from south Serbia. Journal of Essential Oil-Bearing Plants. 2004;7:275–278. [Google Scholar]
- [120].Mimica-Dukić N, Ivancev-Tumbas I, Igić R, Popović M, Gašić O. The content and composition of essential oil of Hypericum perforatum from Serbia. Pharmaceutical and Pharmacological Letters. 1998;8:26–28. [Google Scholar]
- [121].Gudžić B, Nedeljković JM, Ðorđević S, Čomor JJ. Composition and anti-microbial activity of essential oil of Hyperici herb (Hypericum perforatum L.) from Vlasina Region. Facta Universitatis. 1997;1:47–51. [Google Scholar]
- [122].Akhbari M, Batooli H. Composition of Hypericum perforatum L. volatile oil from kashan oil composition of Hypericum perforatum L. American-Eurasian Journal of Sustainable Agriculture. 2009;3:107–110. [Google Scholar]
- [123].Nahrstedt A, Butterweck V. Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry. 1997;30(Suppl.):129–134. doi: 10.1055/s-2007-979533. [DOI] [PubMed] [Google Scholar]
- [124].Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 4 (2). Section 9. Hypericum sensu lato (part 2): subsection 1. Hypericum series 1. Hypericum. Bulletin of the British Museum of Natural History (Botany) 2002;32:61–123. [Google Scholar]
- [125].Martz F, Peltola R, Fontanay S, Duval RE, Julkunen-Tiitto R, Stark S. Effect of latitude and altitude on the terpenoid and soluble phenolic composition of juniper (Juniperus communis) needles and evaluation of their antibacterial activity in the boreal zone. Journal of Agricultural and Food Chemistry. 2009;57:9575–9584. doi: 10.1021/jf902423k. [DOI] [PubMed] [Google Scholar]
- [126].Mockute D, Bernotiene G, Judzentiene A. The essential oils with dominant germacrene D of Hypericum perforatum L. growing wild in Lithuania. Journal of Essential Oil Research. 2007;20:128–131. [Google Scholar]
- [127].Warnke PH, Becker ST, Podschun R, Sivananthan S, Springer IN, Russo PAJ, Wiltfang J, Fickenscher H, Sherry E. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. Journal of Cranio-Maxillofacial Surgery. 2009;37:392–397. doi: 10.1016/j.jcms.2009.03.017. [DOI] [PubMed] [Google Scholar]
- [128].Buchbauer G. Biological activities of essential oils. In: Başer KHC, Buchbauer G, editors. Handbook of essential oils: science, technology and applications. Taylor and Francis; New York: 2010. pp. 235–280. [Google Scholar]
- [129].Buchbauer G. Über biologische Wirkungen von Duftstoffen und Ätherischen Ölen. Wiener Medizinische Wochenschrift. 2004;154:539–547. doi: 10.1007/s10354-004-0121-9. [DOI] [PubMed] [Google Scholar]
- [130].Pauli A, Schilcher H. In vitro antimicrobial activities of essential oils monographed in the European Pharmacopoeia. In: Başer KHC, Buchbauer G, editors. Handbook of essential oils: science, technology and applications. 6th Edition CRC Press; Boca Raton, Florida: 2010. pp. 353–548. [Google Scholar]
- [131].Uribe S, Ramirez J, Pena A. Effects of β-pinene on yeast membrane functions. Journal of Bacteriology. 1985;161:1195–1200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hayouni EA, Bouix M, Abedrabba M, Leveau J-y, Hamdi M. Mechanism of action of Melaleuca armillaris (Sol. ex Gaertu) Sm. essential oil on six LAB strains as assessed by multiparametric flow cytometry and automated microtiter-based assay. Food Chemistry. 2008;111:707–718. [Google Scholar]
- [133].Al-Banna L, Darwish RM, Aburjai T. Effect of plant extracts and essential oils on root-knot nematode. Phytopathologia Mediterranea. 2003;42:123–128. [Google Scholar]
- [134].Yildirim E, Kesdek M, Kordali S. Effects of essential oils of three plant species on Tribolium confusum du Val and Sitophilus granarius (L.) (Coleoptera: Tenebrionidae and curculionidae) Fresenius Environmental Bulletin. 2005;14:574–578. [Google Scholar]
- [135].Yildirim E, Kesdek M, Aslan I, Calmasur O, Sahin F. The effects of essential oils from eight plant species on two pests of stored product insects. Fresenius Environmental Bulletin. 2005;14:23–27. [Google Scholar]
- [136].Kizil G, Toker Z, Özen HÇ, Aytekin C. The antimicrobial activity of essential oils of Hypericum scabrum, Hypericum scabroides and Hypericum triquetrifolium. Phytotherapy Research. 2004;18:339–341. doi: 10.1002/ptr.1460. [DOI] [PubMed] [Google Scholar]
- [137].Rancic A, Sokovic M, Vukojevic J, Simic A, Marin P, Duletic-Lausevic S, Djokovic D. Chemical composition and antimicrobial activities of essential oils of Myrrhis odorata (L.) Scop, Hypericum perforatum L and Helichrysum arenarium (L.) Moench. Journal of Essential Oil Research. 2005;17:341–345. [Google Scholar]
- [138].Gibson D, Sirvent T. Induction of hypericins and hyperforin in Hypericum perforatum L. in response to biotic and chemical elicitors. Physiological and Molecular Plant Pathology. 2002;60:311–320. [Google Scholar]
- [139].Nogueira T, Duarte F, Tavares R, Curto MJM, Capelo J, Freitas A. Comparative study of the aromas of Hypericum L. species from Portugal using olfactroscopy. Flavour and Fragrance Journal. 1999;14:195–199. [Google Scholar]
- [140].European and Mediterranean Plant Protection Organization (OEPP) Service d’Information 2005, No. 6 Bulletin. [Accessed 20 May 2010]. 2005. Available online: http://archives.eppo.org/EPPOReporting/2005/Rsf-0506.pdf.
- [141].Alali F, Tawaha K, Al-Eleimat T. Determination of hypericin content in Hypericum triquetrifolium Turra (Hypericaceae) growing wild in Jordan. Natural Product Research. 2004;18:147–151. doi: 10.1080/14786410310001608046. [DOI] [PubMed] [Google Scholar]
- [142].Sumner LW, Amberg A, Barrett D, Beger R, Beale MH, Daykin C, Fan TW-m, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Higashi R, Kopka J, Lindon JC, Lane AN, Marriott P, Nicholls AW, Reily MD, Viant MR. [Accessed 20 May 2010];Proposed minimum reporting standards for chemical analysis. 2007 doi: 10.1007/s11306-007-0082-2. Available online http://msi-workgroups.sourceforge.net/chemical-analysis/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kopka J, Fernie A, Weckwerth W, Gibon Y, Stitt M. Metabolite profiling in plant biology: platforms and destinations. Genome Biology. 2004;5:109–117. doi: 10.1186/gb-2004-5-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Franz C, Novak J. Sources of essential oils. In: Başer KHC, Buchbauer G, editors. Handbook of essential oils: science, technology and applications. Taylor and Francis; New York: 2010. pp. 39–82. [Google Scholar]
- [145].Wildung MR, Croteau RB. Genetic engineering of peppermint for improved essential oil composition and yield. Transgenic Research. 2005;14:365–372. doi: 10.1007/s11248-005-5475-2. [DOI] [PubMed] [Google Scholar]


