Abstract
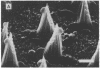
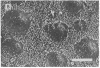
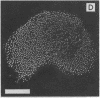
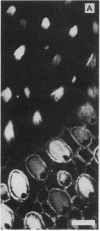
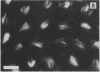
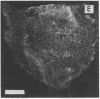
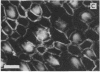
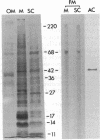
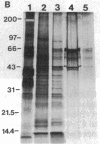
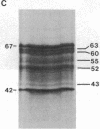
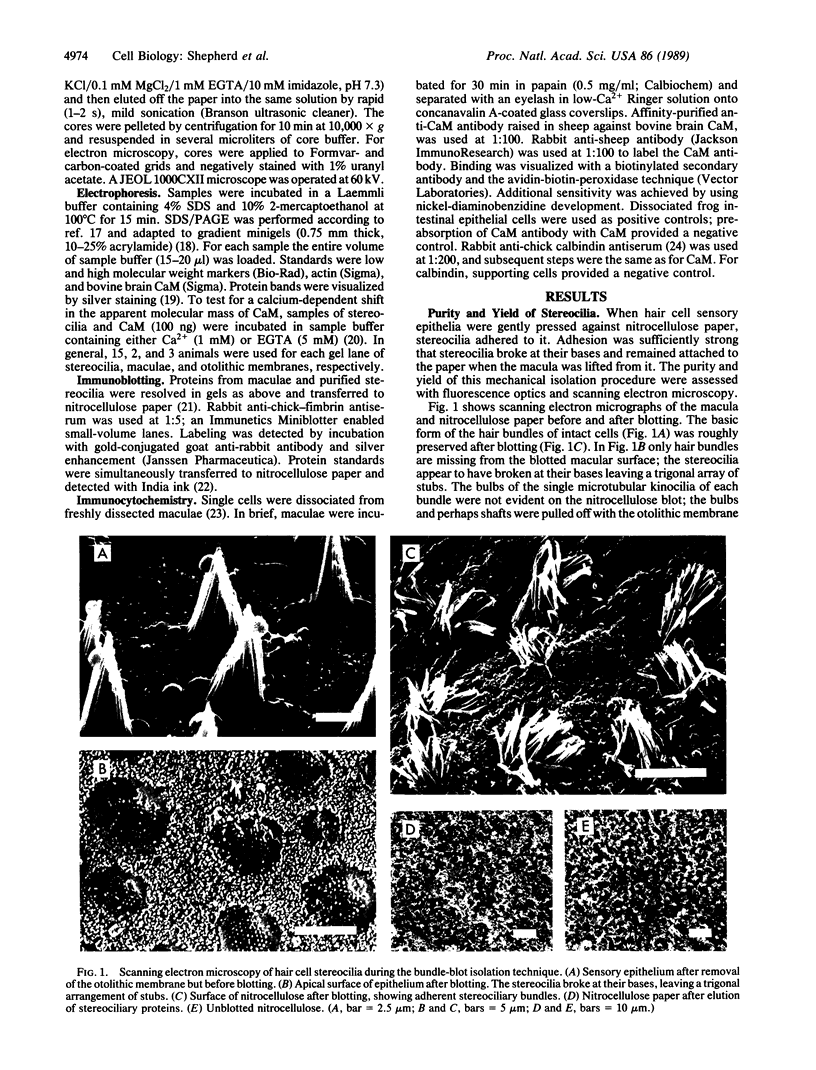
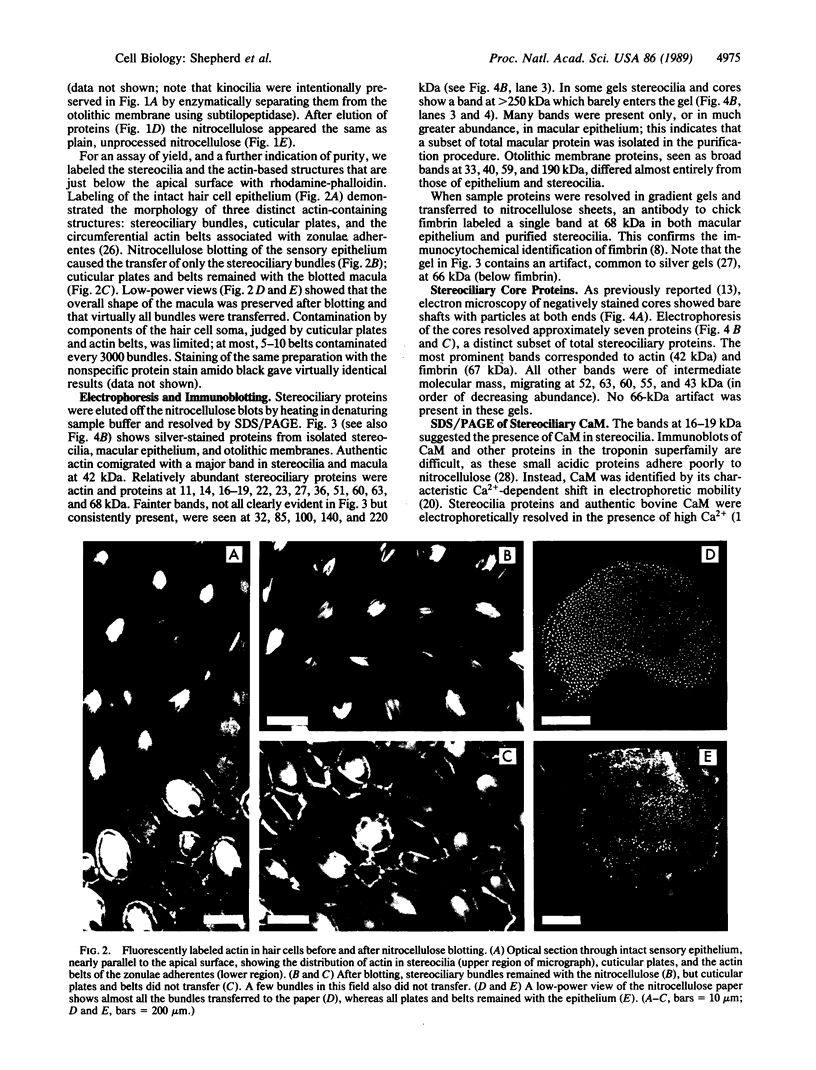
Stereocilia were isolated from bullfrog (Rana catesbeiana) saccular hair cells by nitrocellulose adhesion. The high purity and high yield of the preparation were demonstrated by microscopy. SDS/PAGE of stereociliary proteins resolved 12-15 major bands. Actin, previously identified as a component of the stereociliary core, was identified in purified stereocilia as a band comigrating with authentic actin and by phalloidin labeling of intact isolated stereocilia. Fimbrin was identified in immunoblots of purified stereocilia. The most abundant other proteins migrated at 11, 14, 16-19, 27, and 36 kDa. Demembranated stereociliary cores consisted primarily of protein bands corresponding to actin and fimbrin and several proteins ranging from 43 to 63 kDa. Because the adaptation mechanism in hair cells is calcium-sensitive and seems localized to stereocilia, we sought evidence for calcium-binding proteins in stereocilia. Calmodulin and calbindin antibodies labeled stereocilia in intact cells. A protein band in purified stereocilia exhibited a Ca2+-dependent shift in electrophoretic mobility identical to that of authentic calmodulin, and the 27-kDa band may represent calbindin. These biochemical data demonstrate that stereocilia consist of a relatively small set of proteins. Most of these, including those involved in transduction and adaptation, are as yet uncharacterized. The availability of purified stereocilia should prove useful in further studies of structure-function relationships in these mechanically sensitive organelles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assad J. A., Hacohen N., Corey D. P. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2918–2922. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Purification of microvilli and an analysis of the protein components of the microfilament core bundle. Exp Cell Res. 1978 Oct 15;116(2):397–407. doi: 10.1016/0014-4827(78)90463-9. [DOI] [PubMed] [Google Scholar]
- Chen Z., Lancet D. Membrane proteins unique to vertebrate olfactory cilia: candidates for sensory receptor molecules. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1859–1863. doi: 10.1073/pnas.81.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Kellner J., Mannherz H. G., Gröschel-Stewart U., Kendrick-Jones J., Scholey J. Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature. 1982 Dec 9;300(5892):531–532. doi: 10.1038/300531a0. [DOI] [PubMed] [Google Scholar]
- Eatock R. A., Corey D. P., Hudspeth A. J. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci. 1987 Sep;7(9):2821–2836. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A., Bretscher A., Weber K. Immunohistochemical localization of several cytoskeletal proteins in inner ear sensory and supporting cells. Hear Res. 1982 May;7(1):75–89. doi: 10.1016/0378-5955(82)90082-x. [DOI] [PubMed] [Google Scholar]
- Flock A., Cheung H. C. Actin filaments in sensory hairs of inner ear receptor cells. J Cell Biol. 1977 Nov;75(2 Pt 1):339–343. doi: 10.1083/jcb.75.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Comparison of Ca++-regulated events in the intestinal brush border. J Cell Biol. 1985 Mar;100(3):754–763. doi: 10.1083/jcb.100.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Cytoskeletal architecture of the chicken hair cells revealed with the quick-freeze, deep-etch technique. Hear Res. 1986;22:41–54. doi: 10.1016/0378-5955(86)90076-6. [DOI] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci U S A. 1987 May;84(9):3064–3068. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K. A. Proteins transferred to nitrocellulose for use as immunogens. Anal Biochem. 1985 Jun;147(2):285–288. doi: 10.1016/0003-2697(85)90273-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979 Dec;83(3):667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Neugebauer D. C., Thurm U. Chemical dissection of stereovilli from fish inner ear reveals differences from intestinal microvilli. J Neurocytol. 1984 Oct;13(5):797–808. doi: 10.1007/BF01148494. [DOI] [PubMed] [Google Scholar]
- Oberholtzer J. C., Buettger C., Summers M. C., Matschinsky F. M. The 28-kDa calbindin-D is a major calcium-binding protein in the basilar papilla of the chick. Proc Natl Acad Sci U S A. 1988 May;85(10):3387–3390. doi: 10.1073/pnas.85.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1983 Dec;135(2):470–474. doi: 10.1016/0003-2697(83)90714-5. [DOI] [PubMed] [Google Scholar]
- Pansini A. R., Christakos S. Vitamin D-dependent calcium-binding protein in rat kidney. Purification and physiocochemical and immunological characterization. J Biol Chem. 1984 Aug 10;259(15):9735–9741. [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984 Aug;15(2):103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Rabié A., Thomasset M., Legrand C. Immunocytochemical detection of calcium-binding protein in the cochlear and vestibular hair cells of the rat. Cell Tissue Res. 1983;232(3):691–696. doi: 10.1007/BF00216440. [DOI] [PubMed] [Google Scholar]
- Sans A., Brehier A., Moniot B., Thomasset M. Immuno-electronmicroscopic localization of 'vitamin D-dependent' calcium-binding protein (CaBP-28k) in the vestibular hair cells of the cat. Brain Res. 1987 Dec 1;435(1-2):293–304. doi: 10.1016/0006-8993(87)91612-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Wolchok S. R. Conditions for reproducible detection of calmodulin and S100 beta in immunoblots. Biochem Biophys Res Commun. 1984 Nov 14;124(3):752–759. doi: 10.1016/0006-291x(84)91022-2. [DOI] [PubMed] [Google Scholar]