1. Introduction
The dorsolateral prefrontal cortex (DLPFC) has figured prominently as abnormal in schizophrenia. Weinberger et al. (Weinberger et al., 1986) performed a now classic study implicating DLPFC in the working memory impairments observed in patients with schizophrenia. However, while the fMRI signal was attributed in this study to the site of working memory in DLPFC, the anatomical location of this activation pattern in the prefrontal cortex was not entirely clear, nor is it clear today.
An alternative and successful approach to localizing the site of working memory in DLPFC comes from work on nonhuman primates, where neurons in Brodmann area (BA) 46 and 9 exhibit persistent activity during working memory tasks (Goldman-Rakic, 1987). BA46 and BA9 also play an important role in working memory in humans (Petrides et al., 1993), and patients with schizophrenia show deficits in physiological activation especially in the BA 46 (Cannon et al., 2005; Perlstein et al., 2001). BA 46 is, however, difficult to localize with neuroimaging methods because neuroimaging methods tend to use gyrification pattern as a reference system, and BA46, defined based on cytoarchitectonic findings on post-mortem brains, does not follow the gyrification pattern. Brodmann originally mapped one human brain into various cytoarchitectonic areas (Brodmann, 1909). Later, the mapping of area 46 was done on five human brains (Rajkowska Goldman-Rakic, 1995) (Figure 1B) and was found to have major regions of overlap located between coronal Talairach coordinates +50 and +29 in the AP plane (Figure 1C). In comparison to Brodmann’s definition, area 46 is more rostral and is of smaller extension. In morphometric nomenclature, the region of BA46 is a part of the middle frontal gyrus (MFG) (Rajkowska Goldman-Rakic, 1995).
Fig. 1. Definitions of prefrontal cortex and BA46.
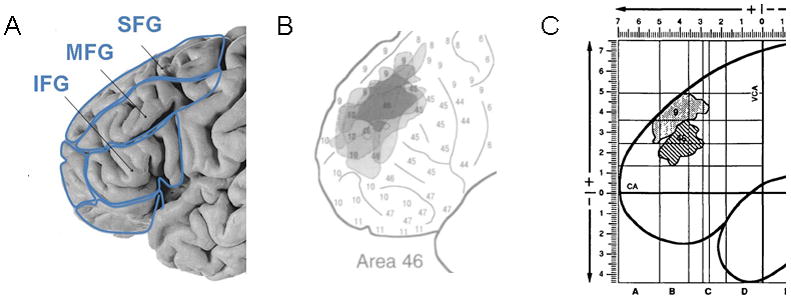
Panel A: The frontal lobe is divided into superior frontal gyrus (SFG), middle frontal gyrus (MFG) and inferior frontal gyrus (IFG) based on the gyrification pattern of the brain. Panel B: Superimposition of five reconstructions of BA46 displays the variation (in gray) and the common zone (in black). Reproduced from (Rajkowska Goldman-Rakic, 1995) with permission of Oxford University Press. Panel C: Definition of BA46 (striped area), as the overlap region of individual extension of BA46 mapped on five postmortem human brains. The overlay BA46 is located between the +5cm and +2.9cm coordinates that translate into coronal Talairach coordinates +50 and +29. Reproduced from (Rajkowska Goldman-Rakic, 1995) with permission of Oxford University Press.
Two morphometric studies have explored BA46 in human subjects. The localization of BA46 is difficult to confirm because of the high variability of the gyral and sulcal patterns in individual subjects (Bartley et al., 1997; Noga et al., 1996) and because Brodmann areas do not always follow gyral patterns. Thus presently it is impossible to exactly determine the borders of BA46 in living subjects. Al-Hakim and co-workers nonetheless developed semi-automated rules to define an “average” BA46 in the MFG to be used in structural and functional imaging studies in living human subjects (Al-Hakim, 2006).
The second study, performed by Zuffante and coworkers (Zuffante et al., 2001), attempted to delineate the region of BA46 in schizophrenia subjects. These investigators were able to draw on coronal slices between the Talairach coordinates y=32 and y=45, but experienced technical difficulties between Talairach coordinate y=45 and y=50, and for this reason did not include this part. The problems encountered by Zuffante and colleagues are not surprising. The complexity and variation of the gyrification pattern of the cortex in the rostral part of MFG makes manual drawings in a 2-dimensional (2-D) view very challenging.
A promising approach to delineate the prefrontal cortex is based on 3D-reconstructions of the brain surface. Previously, 3D brain surface reconstruction were used as a visual guide to manually draw the ROIs of the prefrontal areas (Buchanan et al., 1998; Wible et al., 1997). In the current study, we used FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) to create a 3D reconstruction of the white matter surface. This software also automatically parcellates the brain into subregions, with the MFG delineated into rostral and caudal parts with high accuracy (Desikan et al., 2006). The caudal part of the MFG corresponds to the premotor area, while the rostral portion encapsulates a number of Brodmann areas, including BA46 (personal communication with Rahul Desikan). In addition, FreeSurfer has a tool that cuts brain regions along the coronal plane, which makes it possible to focus still further on the rostral part of the MFG by using the anterior and posterior boundaries as suggested by the cytoachitectonic studies. For the purposes of this study, we more narrowly delineated FreeSurfer based parcellations of the rostral MFG in order to depict BA46 and we refer to this region as “mid-rostral middle frontal gyrus” (MR-MFG).
2. Methods
2.1 Subjects
Twenty male patients with chronic schizophrenia were recruited from the VA Boston Healthcare System, Brockton Division and were diagnosed with schizophrenia by trained raters using DSM-IV criteria, based on information from the Structured Clinical Interview for DSM-III-R (Spitzer, 1990b) and from the medical records. Twenty male normal control subjects were recruited through newspaper advertisement and screened using the Structured Clinical Interview (SCID Non-Patient Edition) (Spitzer, 1990a) by the same trained interviewers who evaluated the patients. No control subjects had an Axis-I psychiatric disorder or a first-degree relative with an Axis-I psychiatric disorder. Control subjects were group-matched to patients on age, sex, handedness, and parental social economic-status (PSES).
The inclusion criteria for all subjects were age between 18 and 55 years, no history of neurologic illness or major head trauma, no electroconvulsive therapy, no alcohol or drug dependence in the last 5 years, and no significant alcohol or drug abuse within the last year.
Premorbid IQ was assessed using the WRAT3 (Wide Range Achievement Test 3, scaled score)(Wilkinson, 1993) and current IQ was assessed using the WAIS-III (Wechsler Adult Intelligence Scale—3rd Edition) (Wechsler, 1997). The WRAT3 is a brief achievement test measuring reading recognition, spelling, and arithmetic computation. The test decodes skills acquired before the onset of the disease and these skills remain preserved during the disease (Dalby 1986). In contrast, the WAIS-III scale measures current adult and adolescent intelligence. The WAIS-III verbal IQ test combines verbal comprehension and working memory, and the WAIS-III performance IQ test includes perceptual organization and processing speed. The patients and the control subjects were administered both neuropsychological tests, the WRAT3 and the WAIS-III, to characterize intellectual abilities in the patient group (Weickert et al., 2000).
This study was approved by the VA Boston Healthcare System, Harvard Medical School, and Brigham and Women’s Hospital Institutional Review Boards. Written informed consent was obtained from all subjects prior to study participation.
2.2 MRI acquisition, image processing and automated parcellation
2.21 MRI acquisition
MR images were acquired on a 3-Tesla whole body MRI Echospeed system General Electric scanner (GE Medical Systems, Milwaukee) at Brigham and Women’s Hospital. An eight channel coil was used in order to perform parallel imaging using ASSET (Array Spatial Sensitivity Encoding techniques, GE) with a SENSE-factor (acceleration) of 2. The structural MRI acquisition protocol included the following pulse sequence and parameters: contiguous inversion-prepared spoiled gradient-recalled acquisition (fastSPGR), TR=7.4ms, TE=3ms, TI=600, 10 degree flip angle, 25.6cm2 field of view, matrix=256×256. The voxel dimensions were 1×1×1 mm. Images were realigned using the line between the anterior and posterior commissures and the sagittal sulcus to correct for head tilt.
2.22 Image processing and automated parcellation
The MRI scans were analyzed using the FreeSurfer software package (version 3.0.5) (Dale et al., 1999; Fischl, Sereno Dale, 1999; Fischl et al., 2004), which is freely available on-line at http://surfer.nmr.mgh.harvard.edu/. FreeSurfer has a specialized tool for automated parcellation of the neocortical gray matter. The hallmarks of the process are the computation of the curvature of the gray and white matter interface in order to characterize the sulci and gyri, and inflation of the whole brain into a sphere for the purpose of registering subjects to the standard atlas (Desikan et al., 2006; Fischl Dale, 2000; Fischl et al., 2001; Fischl, Sereno, Tootell et al., 1999; Fischl et al., 2004). Mapping between subjects and the atlas is performed using a non-rigid registration on the inflated surface. The end result is the parcellation of the human cortex into 34 regions of interest in each hemisphere (Desikan et al., 2006). The parcellation for rostral and caudal middle frontal gyri (Figure 2A) was visually inspected, compared to the parcellation method as suggested by Crespo-Facorro and coworkers (Crespo-Facorro et al., 1999), and no correction was needed. The FreeSurfer automated parcellation method has been cross-validated in healthy and diseased subjects (Desikan et al., 2006) and applied successfully in schizophrenia subjects (Venkatasubramanian et al., 2008).
Fig. 2. Pial surface reconstruction representing the parcellated subregions of MFG.
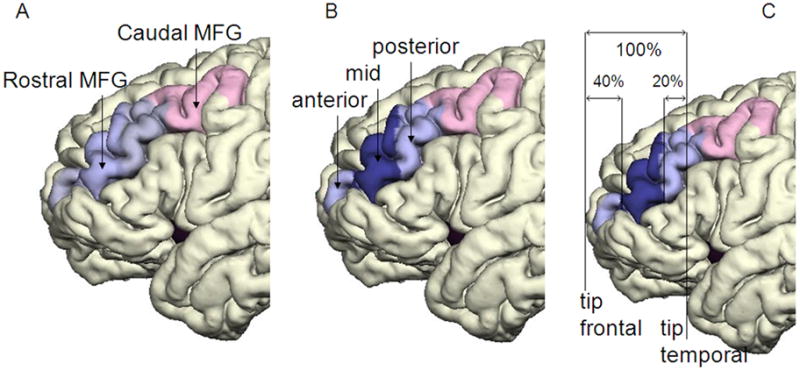
Panel A shows caudal MFG (colored pink) and rostral MFG (light blue). Panel B shows the three subregions of the rostral MFG: the anterior rostral MFG, mid-rostral MFG (MR-MFG)(dark blue) and posterior rostral MFG. Panel C demonstrates the anterior and posterior boundaries of MR-MFG, adapted from Al-Hakim and colleagues (Al-Hakim, 2006).
2.23 Delineation of MR-MFG
Rostral MFG was further subdivided into anterior, mid and posterior portions (see Figure 2B); the MR-MFG was the region of main interest. Localization rules for MR-MFG were based on cytoarchitectonic data of the BA46 between the anterior and posterior Talaraich coordinates +50 and +29 (Rajkowska Goldman-Rakic, 1995), and implemented as proportional divisions (40%, 40%, and 20%) of the distance between the tip of the temporal and frontal lobe to account for the brain size variability in human brain (Figure 2C). The middle part of those divisions corresponds to the BA46, respectively to MR-MFG. These delineation rules were applied previously in a semi-automatic approach to isolate this area (Al-Hakim, 2006). The tip of the lobe was identified as the most anterior coronal plane that just showed gray matter and was expressed in the coordinates of the appropriate coronal planes in each subject. The coordinates were used to determine the percentages of the divisions. The new coordinates were used in the script to cut the label map of rostral MFG and generate the MR-MFG label map. The cortical volumes were calculated using the statistical module of the FreeSurfer software, which integrates the area of each surface triangle and multiplies it by the mean thickness over the entire ROI.
2.24 Volume normalization
Gray matter volumes were normalized by the combined volumes of the cerebral cortex on the left and right hemisphere, a measure of the neocortical gray matter, which was derived from FreeSurfer.
2.25 Statistical Analysis
Statistical analyzes were performed using the Statistical Package for Social Sciences (SPSS) (version 16.0; SPSS Inc., Chicago, IL, USA). We conducted MANOVA analyses for each region separately with one within factor of side and one between factor of group, and followed with MANOVAs on the sub-regions of rostral MFG in order to characterize precisely the source of the group differences for this region (one within factor of side and one between factor of group). The main effect of side and group for each region are reported, but the side by group interaction was not statistically significant in any model so these results are not reported. We corrected all group comparisons for multiple comparisons using a Bonferroni correction. In particular, for the first stage of analysis, we multiplied the p-values by two based on the regions of interest (rostral and caudal), and for the second stage of analysis, we multiplied the p-values by three based on the regions of the rostral MFG (anterior, posterior, and middle sub-regions).
Socio-demographic data were analyzed for group differences using ANOVA. Scores on WRAT3, WAIS-III Verbal IQ, and WAIS-III Performance IQ tests were analyzed to compare group differences using ANOVA.
3. Results
3.1 Subjects
Patients and controls were group matched on the following variables: age at MRI scan (p=0.67), Parental Socio-economical Status (PSES, p=0.54), and handedness score (p=0.14). All subjects were male. Additionally, there were no significant group differences in academic abilities as revealed by the WRAT3 score (p=0.09), an estimate of premorbid IQ (Table 1). Patients with schizophrenia did, however, show significantly lower current socio-economical status than normal control subjects (SES, p=0.001), lower scores on WAIS-III Verbal IQ (p=0.010) and on WAIS-III Performance IQ (p=0.005), as well on years of education (p=0.007), which is consistent with impairment resulting from the illness (Table 1)(Weickert et al., 2000).
Table 1.
Demographic and clinical characteristics of study groups.
| Variable | Schizophrenic patients |
Healthy control subjects |
Group difference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | range | Mean | SD | n | range | p | |
| Age (years) | 38.2 | 9.6 | 20 | 22–55 | 36 | 11 | 20 | 21–55 | 0.668 |
| Gender | males | males | |||||||
| Handednessa | 0.70 | 0.25 | 20 | 0.25 –1.00 | 0.80 | 0.17 | 20 | 0.47–1.00 | 0.140 |
| Socioeconomic statusb | |||||||||
| Subject’s own | 3.6 | 1.1 | 20 | 2 | 0.7 | 20 | 0.001** | ||
| Parental | 2.6 | 1.2 | 20 | 2.3 | 1.2 | 19 | 0.538 | ||
| Education (school years) | 13.2 | 2 | 20 | 15 | 1.9 | 20 | 0.007** | ||
| WRAT3 - scaled reading score | 96.7 | 12.4 | 17 | 103.8 | 11.3 | 18 | 0.087 | ||
| WAIS-III Verbal IQ | 98.7 | 16.7 | 20 | 114.8 | 16.7 | 12 | 0.010 | ||
| WAIS-III Performance IQ | 91.5 | 13.1 | 20 | 109.6 | 20.1 | 11 | 0.005** | ||
| Symptom onset (years) | 23.5 | 5.6 | 18 | NA | |||||
| Duration of illness (years) | 16.1 | 10.0 | 17 | NA | |||||
| Antipsychotic medication dosagec | 330 | 281 | 20 | NA | |||||
| PANSS (total score) | 85.6 | 28 | 20 | NA | |||||
WRAT3 = Wide Range Achievement Test 3, scaled score (Wilkinson, 1993); WAIS-III = Wechsler Adult Intelligence Scale—3rd Edition (Wechsler, 1997); IQ = intelligence quotient; PANSS = Positive and Negative Syndrome Scale (Kay et al., 1987); SD = standard deviation, n = number of participants, p = significance 2-tailed,
significance of p<0.01, NA = data not applicable.
Handedness was evaluated using the Edinburgh inventory and right-handedness is above 0.
Higher scores indicate lower socioeconomic status (Hollingshead, 1965).
Chlorpromazine equivalent (mg).
3.2 Volume Comparison
Table 2 provides relative volumes of gray matter for MFG and for each of the subregions of MFG in subjects with schizophrenia and healthy controls. The regions and subregions are represented in Figure 2A and B. The MFG is the combined area of rostral and caudal MFG, colored light blue and pink in Figure 2A. The rostral MFG is further divided into the anterior-rostral MFG, light blue in Figure 2B, MR-MFG, dark blue, and posterior-rostral MFG, light blue. The delineation of the mid rostral MFG is represented in Figure 2C. The three regions of highest interest, the MFG, the rostral MFG and MR-MFG, are highlighted in bold in Table 2 as they show statistically significant differences in gray matter volumes between schizophrenia and healthy subjects. Figure 3 shows scatterplots of those three regions, the relative volumes of MFG, rostral MFG and MR-MFG for both hemispheres in schizophrenia and healthy subjects.
Table 2.
Gray matter volumes of MFG and its subregions in patients and controls.
| Brain region | Descriptive data of relative volume (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia group (n=20) |
Healthy control group (n=20) |
group differences | |||||||
| left hemisphere |
right hemishere |
left hemisphere |
right hemishere |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| MFG | 3.315 | 0.277 | 3.599 | 0.246 | 3.570 | 0.352 | 3.787 | 0.303 | p < 0.008 |
| Caudal MFG | 1.403 | 0.176 | 1.311 | 0.136 | 1.461 | 0.180 | 1.360 | 0.176 | ns |
| Rostral MFG | 1.911 | 0.169 | 2.288 | 0.183 | 2.108 | 0.296 | 2.426 | 0.224 | p < 0.014 |
| Anterior-Rostral MFG | 0.237 | 0.127 | 0.360 | 0.141 | 0.232 | 0.088 | 0.301 | 0.079 | ns |
| Mid-Rostral MFG (MR-MFG) | 0.785 | 0.142 | 0.883 | 0.120 | 0.911 | 0.186 | 1.005 | 0.127 | p < 0.012 |
| Posterior-Rostral MFG | 0.652 | 0.255 | 0.772 | 0.200 | 0.715 | 0.281 | 0.842 | 0.224 | ns |
The gray matter volumes of the MFG, and its subregions, the rostral MFG and the MR-MFG, show significant group differences, whereas the remaining regions do not, indicating that the group differences detected in MFG is solely driven by the MR-MFG region.
MFG = Middle Frontal Gyrus, ns = non significant, p = significance 2-tailed, SD = standard deviation.
Fig. 3. Volume comparison of MFG and its subregions.
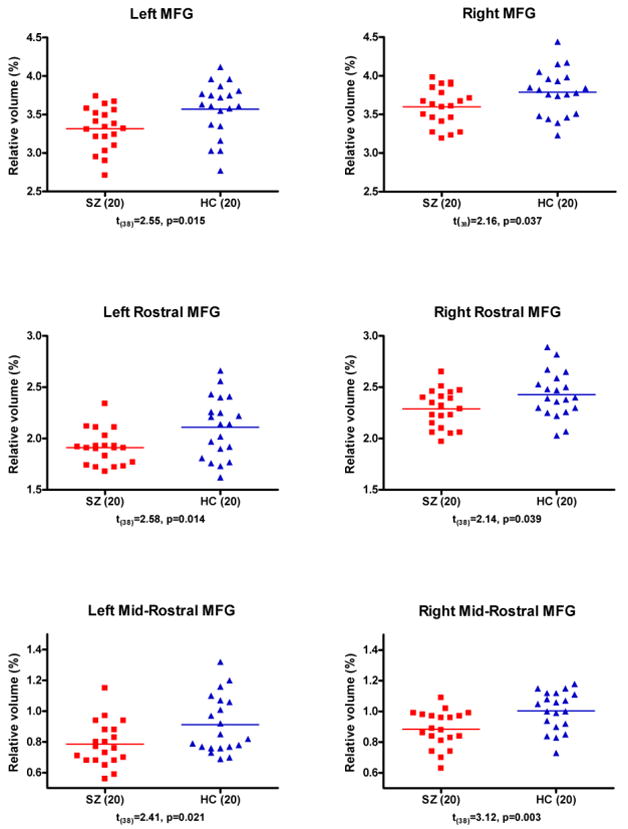
Relative Volume = [Absolute Volume (cm3)/Cortex Volume (cm3)] × 100 (%). Bars indicate the means. Probabilities are from independent-sample t-tests. Abbreviations: MFG = middle frontal gyrus; HC = healthy controls; SZ = schizophrenia subjects.
The initial MANOVA analyses suggested a significant group difference in the rostral [F(1, 38)=8.27, p< 0.007× 2 = 0.014] but not caudal MFG [F(1, 38)=1.67, p< 0.205 × 2 = 0.41]. The follow-up MANOVAs on the rostral region pointed to the mid-rostral region as the brain region driving the group difference: anterior rostral MFG: [F(1, 38)=1.12, p< 0.297 × 2 = 0.594]; mid-rostral MFG: [F(1,38)=9.69, p< 0.004 × 2 = 0.008]; post-rostral MFG: [F(1, 38)=0.99, p< 0.326 × 2 = 0.652]. For all regions and in both groups the left side was smaller than the right side: rostral MFG [F(1,38) = 75.64, p<0.001]; caudal MFG [F(1,38)=8.87, p < 0.005].
Thus, to summarize, the gray matter volumetric differences in MFG were driven by the MR-MFG and none of the other subregions. In the MR-MFG region, schizophrenic subjects, relative to controls, evinced reduced cortical gray matter.
4. Discussion
It was our goal to use morphometric images to delineate a part of middle frontal gyrus where substantial overlap of BA46 maps was previously observed and to explore differences in gray matter volume of this region between the schizophrenia and the control group.
DLPFC has been explored widely as it is believed to be one of the sites of working memory. The definition of DLPFC varies in the extent of the region depending on the study. Some studies consider MFG and SFG to be part of the DLPFC and some vary in the anterior or posterior extension of the gyri (Gur et al., 2000; Tisserand et al., 2002). Due to the inconsistent definition of DLPFC we have concentrated on the MFG, where PET and fMRI have demonstrated working memory deficits in schizophrenia patients (e.g, (Cannon et al., 2005; Glahn et al., 2005; Perlstein et al., 2001; Weinberger et al., 1986) and in unaffected relatives (Meda et al., 2008), and where BA46, a region that has been shown to be involved in memory in experiments in primates, is located (Goldman-Rakic, 1987).
Several morphometric studies have investigated MFG. A meta-analysis of 31 peer-reviewed voxel-based-morphometry studies with a total of 1195 patients with schizophrenia and 1162 healthy controls showed gray matter density reduction in the left MFG in patients with schizophrenia (Glahn et al., 2008). Cortical thinning of MFG has also been reported to be bilaterally in schizophrenia patients (Kuperberg et al., 2003; Nesvag et al., 2008). Moreover, studies using manual delineation as a method report reduced gray matter volume in MFG in schizophrenia subjects (Goldstein et al., 1999; Gur et al., 2000; Kasparek et al., 2007; Thompson et al., 2001), as well reduction in right MFG (Gaser et al., 1999). Goldstein et al. reported 10% reduction in patients with schizophrenia compared with healthy controls, which reconfirms the magnitude of changes we have measured, namely a reduction of gray matter volume in the MFG of about 8% in the left and 5% in the right hemisphere in schizophrenia group. In summary, morphometric studies suggest gray matter volume reductions in the MFG in patients with schizophrenia, but have not explored whether the whole or just a part of this region is contributing. Our study narrows this finding specifically to mid-rostral MFG.
The only study, to our knowledge, to explore the rostral part of MFG in schizophrenia subjects is the work by Zuffante and colleagues (Zuffante et al., 2001), who also evaluated BA46, as defined by Rajkowska and Goldman-Rakic (Rajkowska Goldman-Rakic, 1995). These investigators delineated this area by manually drawing on coronal slices. They did not find any changes in the gray matter volumes between patients and controls. The discrepancy between findings from the Zuffante study and findings from the current study are likely due to the fact that they were not able to include the anterior portion of BA46.
It might be surprising that we have devoted our study to a single and small area in the prefrontal lobe, when the current trend in exploration of the etiology of schizophrenia is to investigate the connectivity between brain regions. We note, however, that DLPFC is currently too loosely defined and too large to investigate connections to other brain regions involved in working memory. Therefore we focused on delineating a small region within the DLPFC that we believe is likely important in schizophrenia and will be most meaningful for future studies investigating connectivity abnormalities in the brain in schizophrenia.
One limitation of the study lies in the resolution of the MRI and the partial delineation of the whole BA46. Cytoarchitectonic studies show that the extensions of BA46 vary across subjects and across gyri (Rajkowska Goldman-Rakic, 1995). Nevertheless, Rajkowska and Goldman-Rakic were able to identify a major region of overlap between subjects for BA46. We focused on this region of overlap because structural MRI currently does not provide the resolution to visualize cytoarchitectonically related regions. In order to delineate further the whole area of BA46, it might be necessary to combine data from fMRI and structural MRI and to delineate the region in each subject individually.
In this study we successfully applied FreeSurfer, a software program based on 3-dimensional views of reconstructed brain surfaces. Using this method we showed volumetric differences between the chronic schizophrenia and the control group that were accounted for by differences in gray matter volume located exclusively in the MR-MFG, and none of the other subregions of MFG. This finding is important as the MR-MFG corresponds to BA46, a region reported to be involved in working memory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hakim R, Fallon JH, Nain D, Melonakos J, Tannebaum A. A dorsolateral prefrontal cortex semi-automatic segmenter. Proceedings of the SPIE. 2006;6144:170–177. [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120 (Pt 2):257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalizationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth; Leipzig: 1909. [Google Scholar]
- Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry. 1998;155:1049–1055. doi: 10.1176/ajp.155.8.1049. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim JJ, Andreasen NC, O’Leary DS, Wiser AK, Bailey JM, Harris G, Magnotta VA. Human frontal cortex: an MRI-based parcellation method. Neuroimage. 1999;10:500–519. doi: 10.1006/nimg.1999.0489. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research. Neuroimage. 1999;10:107–113. doi: 10.1006/nimg.1999.0458. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of of primate prefrontal cortex and regulation of behaviour by representional memory. In: Brooks B, editor. Handbook of Physiology, the Nervous System V. 1987. [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Jr, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Two factor index of socialposition. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Kasparek T, Prikryl R, Mikl M, Schwarz D, Ceskova E, Krupa P. Prefrontal but not temporal grey matter changes in males with first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:151–157. doi: 10.1016/j.pnpbp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Meda SA, Bhattarai M, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD. An fMRI study of working memory in first-degree unaffected relatives of schizophrenia patients. Schizophr Res. 2008;104:85–95. doi: 10.1016/j.schres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, Agartz I. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Noga JT, Bartley AJ, Jones DW, Torrey EF, Weinberger DR. Cortical gyral anatomy and gross brain dimensions in monozygotic twins discordant for schizophrenia. Schizophr Res. 1996;22:27–40. doi: 10.1016/0920-9964(96)00046-1. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci U S A. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams JBW, Gibbson M, First M, editors. The structured clinical interview for DSM-III-R-non-patient edition (SCID-NP) American Psychiatric Association; Washington, DC: 1990a. [Google Scholar]
- Spitzer R, Williams JBW, Gibbson M, First M, editors. The structured clinical interview for DSM-III-R (SCID) American Psychiatric Association; Washington, DC: 1990b. [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2008;117:420–431. doi: 10.1111/j.1600-0447.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. The Psychological Corporation. Harcourt Brace & Company; San Antonio Texas: 1997. [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Fischer IA, Allard JE, Kikinis R, Jolesz FA, Iosifescu DV, McCarley RW. Parcellation of the human prefrontal cortex using MRI. Psychiatry Res. 1997;76:29–40. doi: 10.1016/s0925-4927(97)00060-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test Administration Manual. Wide Range, Inc; 15 Ashley Place, Suite 1A., Wilmington, DE: 1993. [Google Scholar]
- Zuffante P, Leonard CM, Kuldau JM, Bauer RM, Doty EG, Bilder RM. Working memory deficits in schizophrenia are not necessarily specific or associated with MRI-based estimates of area 46 volumes. Psychiatry Res. 2001;108:187–209. doi: 10.1016/s0925-4927(01)00124-x. [DOI] [PubMed] [Google Scholar]


