EFFECTS of the drugs FK 506 and CyA on lymphocyte function have been mostly studied in vitro and in vivo in animal models.1–5 Little information is available about changes in the immune system of patients who, having undergone orthotopic liver transplantation (OLT), are treated with immunosuppressive drugs. To evaluate cellular immunity of patients treated for prolonged periods with FK 506 or CyA, serial immunologic monitoring was performed in liver transplant recipients. The purpose of this monitoring was to compare long-term effects of CyA and FK 506 on immunologic function and absolute numbers of circulating lymphocyte subsets in liver transplant recipients, to determine if these drugs had similar or different immunosuppressive effects in vivo.
METHODS AND RESULTS
The patients monitored for immunologic parameters represent a subset of liver transplant recipients participating in the randomized FK 506/CyA trial conducted at the University of Pittsburgh Medical Center in 1990–91. A total of 96 immunologically monitored patients were randomized as follows: 55 to the CyA arm, 51 to FK 506 arm. Forty-one patients, initially randomized to CyA, were crossed over to FK 506.
Immunological parameters monitored included 20 endpoints as follows: total T, B. and NK cells; subsets ofT, B, and NK cells; activated T, B, and NK cells; CD4/CD8 ratio; natural killer (NK) activity; Iymphokine-activated killer (LAK) cell generation; proliferative responses to OKT3 MAb; in vitro production of IFN-γ; and in vitro production of IL-2.
Peripheral blood mononuclear cells (PBMNC) of the patients were collected at the multiple time points, starting with a baseline (day 1) specimen obtained immediately before transplantation, and then continuing after transplantation at regular intervals (see Fig 1), and, additionally, at the time of major events such as viral infection, rejection, or a second transplant. To date, a partial data analysis has been completed for 96 liver transplant recipients monitored for up to 180 days.
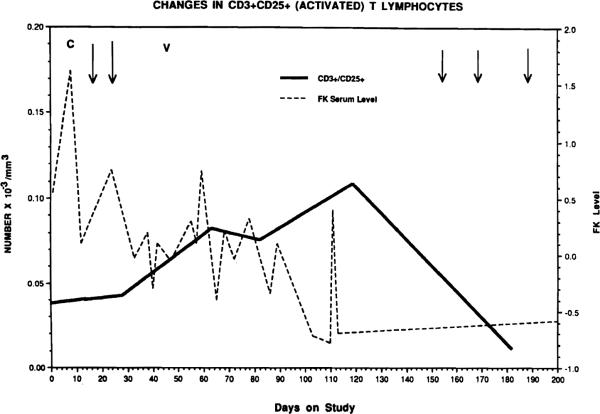
Fig 1.
Serial immunologic monitoring of a liver transplant recipient. Absolute numbers of circulating IL-2R+ T lymphocytes were measured by two-color flow cytometry. Arrows denote episodes of histologically documented rejection. V = viral infection. C = crossover from CyA to FK 506. FK 506 serum level was measured by ELISA.
The study was performed at the Immunologic Monitoring and Diagnostic Laboratory (lMDL) of the Pittsburgh Cancer Institute. Freshly harvested PBMNC were used for two-color flow cytometry, 4-hour 51Cr-release NK cell assays, and to generate LAK cells in response to 1000 Cetus U/mL of IL-2. Cells were cryo-preserved for in vitro proliferation in response to OKT3 MAb and phorbol ester as well as OKT3-induced IL-2 or IFN-γ production. Methods used for serial monitoring of the above parameters have been described by us earlier.6,7 All flow cytometry data are presented as absolute numbers of circulating cells. The results were analyzed using a statistical model, which did not adjust for infections or rejections experienced by the CyA-treated vs FK 506-treated patients, but which adjusted for crossovers from CyA to FK 506. All major events occurring during monitoring, such as a crossover from CyA to FK 506; rejection episodes which were confirmed histologically; viral, bacterial, or fungal infections; development of PTLD; immunosuppression as reflected by the blood level of FK 506 or CyA (determined by ELISA); and the administration of other medications, eg, solumedrol, were documented and recorded for each patient studied.
An example of immunologic monitoring as performed in this study is shown in Fig 1. Changes in the absolute number of circulating CD3+CD25+ (IL-2R+) T lymphocytes during a posttransplant immunosuppressive therapy in a single patient treated with FK 506 are shown. Note that the serum levels of FK 506 as well as episodes of rejection or viral infection are shown also to indicate that in this, and all other patients monitored, the immunologic data were interpreted with respect to these parameters.
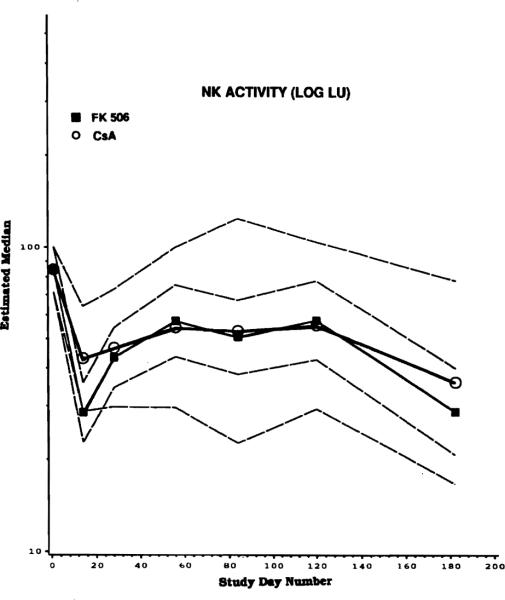
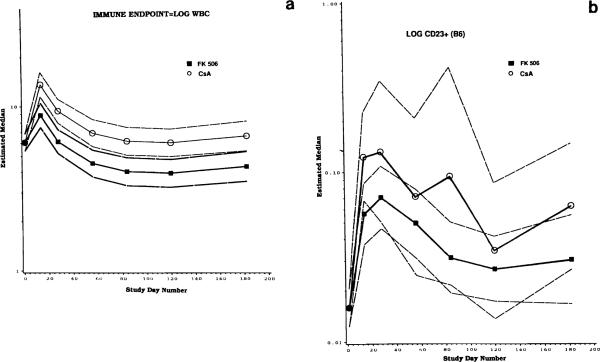
Initially, analyses were performed to compare long-term effects of the drug on various immunologic parameters in the cohort of patients treated with CyA vs those treated with FK 506. The results of this comparison showed that no significant differences could be detected between functions of lymphocytes obtained from patients treated with FK 506 or CyA. For example, NK activity (Fig 2) was comparable in patients treated with FK 506 vs CyA. Patients treated with CyA had significantly higher (P < .02) WBC counts than those treated with FK 506, with an estimated difference of >50% between CyA vs FK 506 groups (Fig 3a). Although no statistically significant differences were observed between the two patient groups in absolute numbers of lymphocytes, lymphocyte subsets, or activated lymphocytes, patients on CyA generally had higher numbers of these cells in the blood than patients treated with FK 506. For example, a >50% difference in B cells (CD23+), with a higher number of circulating B cells in CyA-treated individuals, was observed (Fig 3b). As Fig 3b indicates, a large spread in the data presumably accounts for a lack of statistical significance in the observed differences.
Fig 2.
A comparison of NK activity in patients randomized to FK 506 vs CyA. The solid lines are medians, and interrupted lines denote 5% and 95% confidence limits. No statistically significant difference was observed between patients treated with FK 506 and CyA.
Fig 3.
A comparison of absolute numbers of WBC (a), and circulating B lymphocytes (b) in patients randomized to FK 506 vs CyA. In (a), P < .02 for CyA vs FK 506. In (b), P < .06 for CyA vs FK 506. See legend to Fig 2 for symbol description.
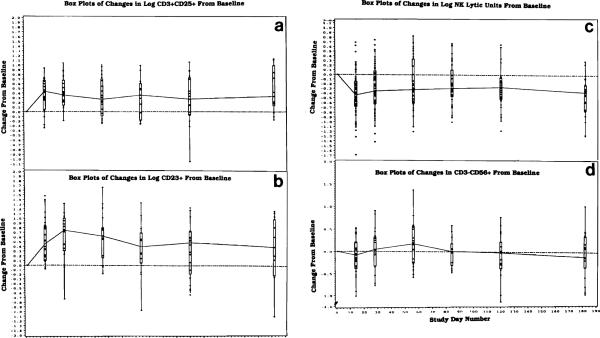
In addition to comparing immune cell numbers and function in patients treated with FK 506 vs CyA, it was also possible to inquire if significant changes from baseline were observed in both immune function as well as absolute numbers of various lymphocyte subsets during long-term therapy. A series of statistical analyses were performed in which immunologic measurements over time obtained for each patient after transplantation were compared to his or her own baseline (ie, measurement immediately before transplantation). The data obtained for patients on FK 506 or CyA were combined for this analysis, so that all 96 patients were treated as one group. The results are presented as “boxplots” (shown in Fig 4).
Fig 4.
Long-term effects of immunosuppressive therapy with FK 506 or CyA in all 96 liver transplant recipients. Shown are changes from baseline (pretransplant) during 180 days of therapy for absolute numbers of circulating CD3+CD25+ T lymphocytes (a) or CD23+ B lymphocytes (b), and for NK activity (c) or absolute numbers of CD3−CD56+ NK cells (d). The solid line is the median of differences from the baseline (interrupted line), with “boxes” indicating the 25th and 75th quartlles, and “whiskers” showing extended ranges for each parameter. All changes are significant at P < .001 or lower.
The following changes from baseline were observed during long-term posttransplant immunosuppression. The absolute number of total lymphocytes was significantly increased (P < .001) throughout therapy. This increase involved total T lymphocytes (results not shown) and B lymphocytes (Fig 4b) but not NK (CD3−CD56+) cells (Fig 4d). In terms of lymphocyte subsets, numbers of activated T cells (CD3+HLA-DR+; CD3+CD25+ in Fig 4a), non–MHC–restricted T cells (CD3+CD56+) and B cells (CD23+ as well as CD21+) were significantly and persistently elevated in the circulation during therapy. The CD4/CD8 ratio was decreased, due mainly to a more substantial rise in numbers of CD8+ cells, which was consistently greater than that in CD4+ cells (data not shown). Although the absolute numbers ofNK cells did not change, their proportion in relation to T and B lymphocytes was decreased and, thus, not surprisingly, NK activity was significantly (P < .001) depressed in these patients (Fig 4c). In contrast, the ability of patients' PBMNC to respond to IL-2 in vitro by activation and generation of killing function remained unchanged relative to baseline (results not shown). The changes in numbers or function of PBMNC listed above were all highly significant (see Fig 4) for all time points following transplantation.
Next, NK and LAK activities prior to (at baseline) and after transplantation (during immunosuppressive therapy) were compared to those determined in our laboratory for a large group (n = 120) of normal, healthy volunteers. As Fig 5a shows, NK activity of patients on immunosuppressive drugs, as a group, was significantly lower than that of a normal control group. Nevertheless, there were many patients whose NK activity was within a normal range (Fig 5a). On the other hand, LAK activity in the patient group was comparable to that in normal volunteers, both at baseline and during therapy with FK 506 or CyA. In addition, PBMNC obtained from a small subgroup of patients (n = 12) on immunosuppressive therapy with FK 506 or CyA were induced in vitro with OKT3 MAb (100 ng/mL)/phorbolester (PMA, 20 ng/mL), and proliferation as well as IL-2 or IFN-γ production were measured in the supernatants of these cells. In parallel experiments, PBMNC obtained from normal volunteers (n = 12) were similarly induced in vitro. When compared to normal controls, PBMNC of patients treated with immunosuppressive drugs had significantly decreased functions in vitro (Fig 5b). Nevertheless, these functions were within a lower normal range (Fig 5b). These results led us to conclude that lymphocytes obtained from patients on long-term therapy with immunosuppressive drugs, when washed off from the drug, retain the capability to respond in vitro to various specific (eg, OKT3) or nonspecific (eg, IL-2) signals.
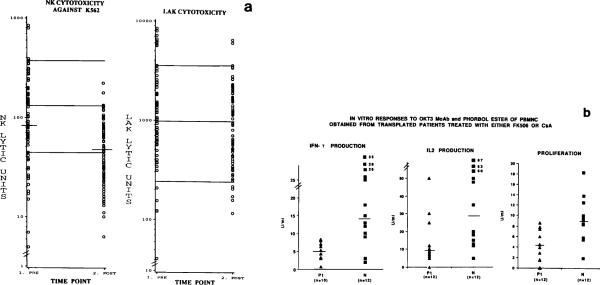
Fig 5.
Functional characteristics of PBMNC obtained from patients treated with FK 506 or CyA. (a) NK and LAK activities of fresh PBMNC, before and during therapy are shown. The solid horizontal lines are medians, at 5% and 95% confidence limits, for 120 normal PBMNC. Median NK activity (in LU) was significantly (P < .01) lower for PBMNC obtained during therapy than those obtained before therapy. LAK activity was unchanged pre- and post-therapy. (b) In vitro responses of patient and normal PBMNC to OKT3/PMA are compared in terms of prOliferation and IL-2 or IFN-γ production. Medians are indicated by horizontal lines. Differences between patient and normal cells were significant at P < .01.
DISCUSSION
Longitudinal immunologic monitoring of 96 patients who underwent OLT, and were randomized to receive FK 506 or CyA therapy, was performed, and the data available for various time points up to 180 days posttransplantation were used for preliminary statistical analysis. The hypothesis tested in this analysis concerned a greater in vivo immunosuppressive potential of FK 506, which was shown to be 10 to 100 times more efficient than CyA in suppressing immune responses of human lymphocytes in vitro1,8 and of the immune system in rodents in vivo.1 The comparison of FK 506 vs CyA effects in liver transplant recipients indicated that lower numbers of WBC, lymphocytes, and activated lymphocyte subsets were present in the peripheral blood of patients treated with FK 506. At the same time, no differences were observed in functional properties (ie, NK activity or the ability to respond to IL-2) of PBMNC obtained from the blood of those two groups of patients at anytime in the course of monitoring. Also, no statistically significant differences were detected in the frequency of rejection or infection episodes in this subset of patients randomized to FK 506 or CyA. Thus, it appears that FK 506 may be more efficient than CyA in controlling the entry of activated T lymphocytes and CD23+ B cells into the peripheral circulation from lymphoid organs. FK 506 seemed not to be more effective than CyA in down-regulating functions of PBMNC in the circulation.
To further define long-term effects of FK 506 and CyA on immune cell numbers and function, changes relative to pretransplant baseline levels in these parameters were examined. Highly significant increases in the number of circulating T and B lymphocytes, but not NK cells, and especially, of activated lymphocyte subsets observed in these patients, persisted through the entire posttransplant period. It could be argued that such rises in activated T-lymphocyte numbers, including non-MHC-restricted cytolytic T cells (CD3+ CD56 +), are not consistent with the state of drug-induced immunosuppression in patients treated with FK 506 or CyA. However, it is possible that activated T and B lymphocytes are being continuously released into the circulation from lymphoid organs, where an intense immune response to the graft is ongoing, despite therapy with immunosuppressive drugs. An alternative explanation might be that extravasation and/or migration of lymphocytes from the blood to lymphoid and other tissues is inhibited or altered by the drugs, resulting in accumulation of these cells in the peripheral circulation. On the other hand, the presence of activated lymphocytes in the peripheral blood of these transplanted and immunosuppressed patients may be related to frequent viral and other infections, much like elevated numbers of circulating activated T cells in patients with AIDS and opportunistic infections.9 In addition, persistently elevated numbers of activated B cells in the circulation of our patients might be a factor contributing to the development of posttransplant lymphoproliferative disease (PTLD), known to occur in a small proportion of transplanted individuals treated with immunosuppressive therapy.10
Functional evaluations of lymphocytes Qbtained from patients treated with FK 506 or CyA showed that their ability to respond to IL-2 was normal, but NK activity and in vitro responses to the TCR-mediated signal (OKT3) were depressed relative to those of normal lymphocytes. Thus, despite the observed increased numbers of circulating activated lymphocytes, immunologic functions ofthese cells appeared to be tempered by the presence of FK 506 or CyA in the serum. In individual patients, these functions did not seem to be lower during episodes of rejection or elevated at the time of viral infections. However, only a small group of 12 patients was so far analyzed for changes in immune functions relative to rejection or infection episodes or serum drug levels. Further analysis of the data is in progress to more precisely examine the relationship between these parameters and immune function.
Overall, this first longitudinal study of immune cells in liver transplant recipients treated with either FK 506 or CyA for a prolonged period of time indicated that these drugs appear to have profound effects on lymphocyte kinetics, and somewhat less pronounced but detectable effects on certain other, but clearly not all, lymphocyte functions.
REFERENCES
- 1.Thomson A. Immunol Today. 1989;10:6. doi: 10.1016/0167-5699(89)90057-1. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber SL. Science. 1991;251:283. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Duncan JI. Cyclosporin: Mode of Action and Clinical Application. Kluwer; London: 1989. p. 50. [Google Scholar]
- 4.Kroczed RA, Black CDV, Barbet J, et al. J Immunol. 1987;139:3597. [PubMed] [Google Scholar]
- 5.Kasaian MT, Biron CA. J Immunol. 1990;144:299. [PubMed] [Google Scholar]
- 6.Whiteside TL, Bryant J, Day R, et al. J Clin Lab Analysis. 1990;4:102. doi: 10.1002/jcla.1860040207. [DOI] [PubMed] [Google Scholar]
- 7.Emstoff MS, Gooding W, Nair S, et al. Am J Oncol. in press. [Google Scholar]
- 8.Zeevi A, Eiras G, Todo S, et al. Transplant Proc. 1990;22:Hl6. [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgi JV, Detels R. Clin Immunol Immunopathol. 1989;52:10. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 10.Ho M, Jaffe R, Miller G, et al. Transplantation. 1988;45:719. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]