Results from early experimentation with hepatic homotransplantation were discouraging. The technical procedures used for insertion of the homograft carried a high risk (30, 60, 61, 82, 83, 102). The first efforts to potentiate homograft survival with immunosuppressive drugs or whole body irradiation were relatively unsuccessful (62, 84, 90, 91). More recently, laboratory experimentation has conclusively shown that homotransplantation of the liver can be carried out in dogs with success comparable to that attainable with the kidney (93). The purpose of this account is to describe such advances in liver homotransplantation as they have evolved from laboratory studies, with proper emphasis on that unique body of information which could be obtained only by clinical trial.
In the past, important steps were made toward characterizing the microscopic features of the liver homograft, principally early after transplantation. Unfortunately, the pathologists who studied various features of the problem had access to only one phase of the over-all picture. Some reviewed the histologic changes following orthotopic transplantation to the untreated (10, 51, 61, 83) or treated (62, 90, 91) dog; others described the changes after auxiliary canine homotransplantation in which the homograft is placed in a heterotopic site and the animal’s own liver retained (30, 33, 54, 66, 77, 91, 99, 102), and still others described human liver homotransplants relatively early after operation (62, 88–91). For the present report, an attempt was made to review all tissues available from our previous transplantation research. In this way, the pathologist among us (K.A.P.) was able to obtain a complete picture of the early and late changes in both the host and the liver homograft (58, 72, 93).
1. Requirements of Tissue Preservation
Most of the research on whole organ liver transplantation has been done with the dog. The use of this animal has some distinct disadvantages. Unlike man, there is a high incidence of endogenous bacterial colonization of the dog liver (28, 104), providing a fertile ground for sepsis in the event of cell necrosis following rejection or a moderately severe ischemic injury. In addition, the canine liver is peculiarly susceptible to ischemic injury, which may result in a syndrome termed “outflow block.” When this complication develops, the revascularized homograft entraps large quantities of blood (82). The inability of blood to escape is probably due to constriction of the small intraparenchymal hepatic veins studied by Deysach (19) and by Thomas and Essex (98). These and larger vessels in the dog were demonstrated by Arey (3) to have extraordinarily well-developed muscular coats.
The congestion seen in the severely ischemic hepatic homograft is nonspecific and can be produced in the liver of the intact dog in a variety of physiologic circumstances including anaphylaxis and peptone shock (50, 80, 101), temporary devascularization of the liver (38, 94), histamine injection (5, 50), isolated perfusion of the liver (42, 48, 49), endotoxin shock (47) and hemorrhagic shock (103). The canine liver is thus the target organ for a variety of injurious influences, one of the most important being anoxia.
The first consequence of outflow block is self-perpetuating injury to the liver. A large number of hepatocytes undergo dissolution (Fig. 1), the principal loss being around the central veins (82). Grave secondary events follow. Inasmuch as portal flow through the tense and swollen liver is retarded, portal hypertension develops within a few minutes or hours. Hemorrhagic gastroenteritis is the almost invariable consequence, with fatal gastrointestinal hemorrhage following shortly thereafter (82).
Fig. 1.

Outflow block in a canine hepatic homograft 4 days after transplantation. The centrilobular sinusoids (arrows) are distended with blood, and adjacent hepatocytes are necrotic. There is accumulation of fat in the liver cells of the middle lobular zone. Only hepatocytes adjacent to the portal tracts (P) are normal. Hematoxylin-eosin; ×40.
If attempts are made to transplant the uncooled dog liver, outflow block inevitably results if the anoxic interval exceeds 20 or 30 minutes (30, 82). When the donor animal is rendered hypothermic (29–31°C.) and additional rapid cooling carried out by means of intraportal perfusion with chilled lactated Ringer’s solution (Fig. 2), an anoxic interval of 75–120 minutes is reasonably well tolerated (82). This can be most effectively done if the flow of perfusate through the portal vein is started while the animal is being exsanguinated. The liver temperature is lowered in this way while the hepatic arterial circulation is still intact, and the final stages of cooling are then carried out after the animal is dead. With this technic, the central hepatic temperature usually reaches approximately 15°C. Subsequent rewarming is gradual during insertion of the homograft (Fig. 3).
Fig. 2.
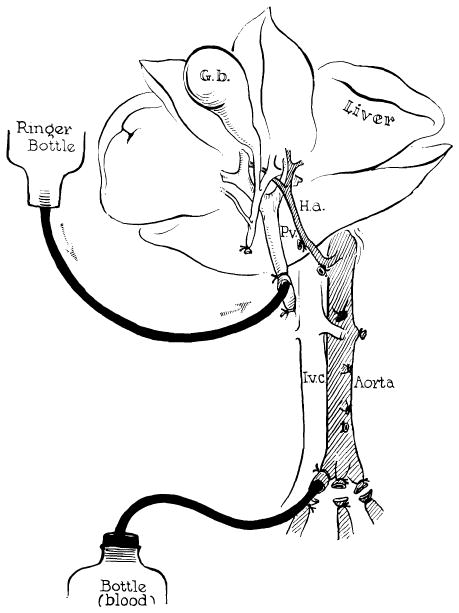
Method of further cooling a liver homograft just before its removal. Donor animals are operated on with total body hypothermia of 29–31°C. Cold lactated Ringer’s solution is infused through the portal vein at the same time the donor animal is exsanguinated. (Figs. 2, 3 and 17 from Starzl et al. [82].)
Fig. 3.
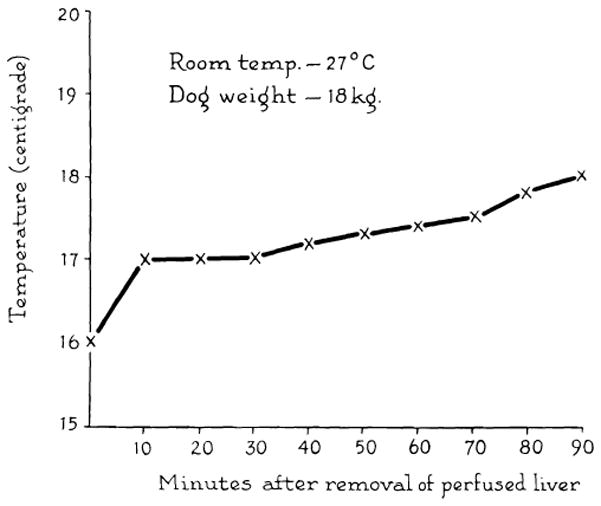
Rewarming curve of interior of donor liver exposed to room temperature for 1½ hr. Cooling was achieved by the method in Figure 2.
For clinical application, this simple and efficient method of organ preservation has serious limitations. First, most potential cadaveric donors would not be under the influence of total body hypothermia at the time of death. Second, removal of a hepatic homograft is sufficiently complicated in man so that time is needed for deliberate and careful excision. Finally, it is necessary to prepare and operate on the recipient patient, a process which would usually require two or more hours.
To meet the requirements for clinical homotransplantation, a method of postmortem extracorporeal support was devised (57) which may prove to have utility in the transplantation of liver or other organs for which there are complex technical requirements. Immediately after death, cannulas are inserted into the abdominal aorta and inferior vena cava and perfusion is begun (Fig. 4). A bubble oxygenator and heat exchanger are incorporated into the circuit, which has been primed with heparinized glucose or electrolyte solution. The temperature of the cadaver can usually be reduced to 20 or 25°C. within 30 minutes.
Fig. 4.
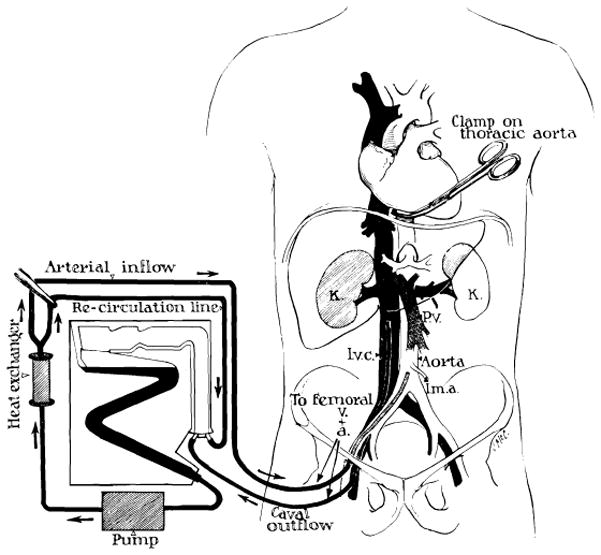
Technic of extracorporeal cadaver perfusion. Catheters are inserted via the femoral vessels into the aorta and vena cava as soon as possible after death. The extracorporeal circuit is primed with heparinized glucose or electrolyte solution to which procaine is added. The cadaver is anticoagulated with first surge of the pump. Temperature control is provided by the heat exchanger. (From Starzl [86].)
Although postmortem preservation of the liver is possible for several hours with this approach, varying degrees of injury to the dog liver were always observed (Fig. 5), and prolonged survival (more than five days) could not be obtained in laboratory animals (57). With clinical use of the technic, better results were obtained, although here also there was tissue damage (Fig. 6). In clinical application, the degree of injury to the homograft probably reflects not only deficiencies of the perfusion method but also the tissue anoxia which occurs during the terminal hours of life.
Fig. 5.
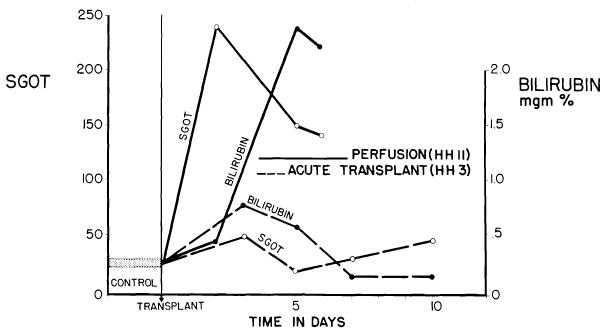
Acute liver injury after use of cadaveric canine homograft (solid lines). Note sharp increases of SGOT and bilirubin following orthotopic transplantation of cadaveric homograft (HH 11), which had a total ischemic interval of 2 hr., 17 min. Contrasting minor abnormalities after use of a living donor are shown (broken lines). (From Marchioro et al. [57].)
Fig. 6.
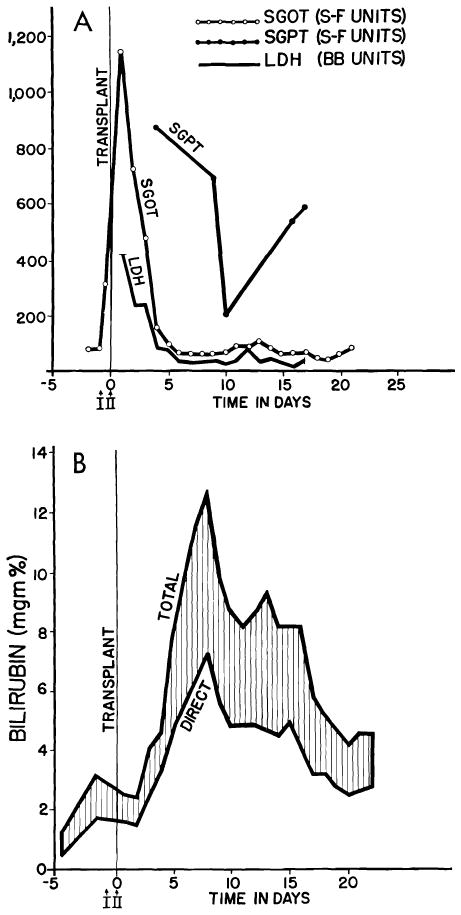
Serious injury to human liver homograft (Case 2, Colorado series). Time from donor death to revascularization in the recipient was 152 min. during 98 of which, extracorporeal perfusion was carried out. A, acute increases of serum enzyme values, which reversed in a few days. B, deepening jaundice in first 8 days. Both enzyme alterations and acute hyperbilirubinemia were probably due to ischemia rather than rejection. (Figs. 6, 41 and 42 from Starzl et al. [88].)
For the Denver case of auxiliary transplantation, a modification of this technic was employed which seemed to have distinct advantages. Immediately upon death of the donor patient, external cardiac massage was instituted with maintenance of a good peripheral blood pressure. This was continued for 108 minutes before perfusion was begun. During the ensuing 105 minutes of extracorporeal perfusion, open cardiac massage was continued, providing two mechanisms of artificially sustained circulation. The use of manual massage greatly increased the venous return to the extracorporeal circuit. In this case, the time from donor death to revascularization in the recipient was 262 minutes, but despite this long interval the postoperative serum glutamic oxalacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) in the recipient patient did not exceed 150 units.
Whether or not the aforementioned elaborate systems of organ procurement will be necessary or even practical remains to be seen (15). It is noteworthy that both Moore (63) and Demirleau (18) performed their human liver transplants by almost the same method as has been described in dogs (82). The degree of ischemic injury which can be tolerated by the liver probably varies in different species. In the pig, for example, van Wyck and his associates (105) have shown that moderately good function could still be expected after removal of the liver for three hours or more at room temperature. Similarly, it has seemed from experience with the clinical liver homotransplants that anoxic injury to the human liver is far more reversible than in the dog, probably because outflow block does not readily occur in man. Nevertheless, the consequence of employing an irreversibly damaged liver homograft in man is immediate fatality, as manifested by the operating-table death of Patient 1 in the Colorado series. The homograft had undergone extensive autolysis before it was implanted.
Investigation of means to provide long-term preservation of hepatic tissue will undoubtedly be a fertile area of future research. In an intriguing recent study, Brown and his associates (11) showed that canine livers, which were dehydrated and preserved for as long as five days in glycerol or dimethylsulfoxide, resumed measurable function after subsequent transplantation. During the past 10 years, important progress has also been made in normothermic perfusion technics, with an oxygenator in the circuit. With perfusion of both portal venous and arterial systems and with proper attention to control of pH and other physiologic variables, it has been possible to obtain function of canine (42), porcine (25) and bovine (12) livers for many hours or even days. Recently, Liem, Waltack and Eiseman (46) have used a modification of this technic to resuscitate patients with liver failure, employing extracorporeal pig livers.
2. Pharmacologic Factors in Liver Transplantation
Host injury by the agents used to prevent rejection is one of the chief deterrents to successful transplantation of any organ, accidental bone marrow depression and leukopenia being common lethal complications. Even if overdosage is avoided, the animals lose weight and become anemic within a few days or weeks (Fig. 7). Moreover, immunosuppression is uniquely hazardous after canine hepatic homotransplantation. The drug azathioprine, which forms the cornerstone of therapy, is definitely toxic for the canine liver (14, 71, 93). Recent toxicity studies in 18 nonoperated dogs revealed acute rises of SGOT, SGPT and alkaline phosphatase in each dog (Fig. 7) on a dose schedule comparable to that employed after transplantation. The biochemical abnormalities were manifest within a few days after the beginning of therapy and tended to improve spontaneously during the next 30 or 40 days until discontinuance of the study (Fig. 7). Although none of the animals became jaundiced, the severe enzyme alterations were reflected in anatomic changes.
Fig. 7.
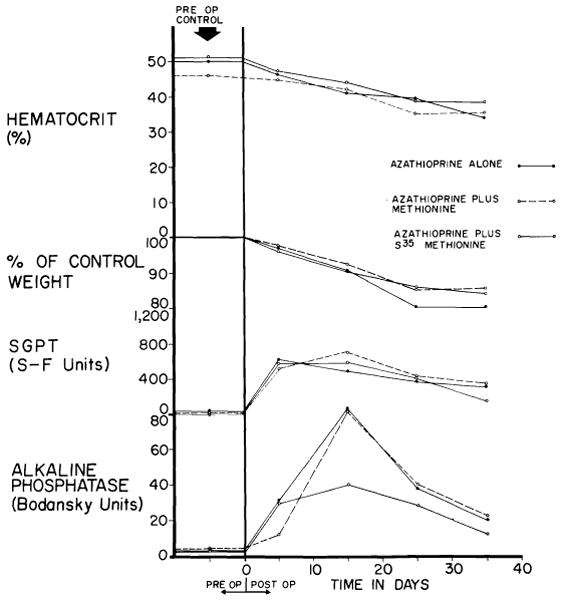
Toxicity of azathioprine when used alone, with S35 methionine and with methionine. Six dogs were in each of the 3 test groups. Despite abnormalities of liver function, jaundice did not develop. (Figs. 7, 8, 18, 23–28, 30, A, 33 and 36 from Starzl et al. [93].)
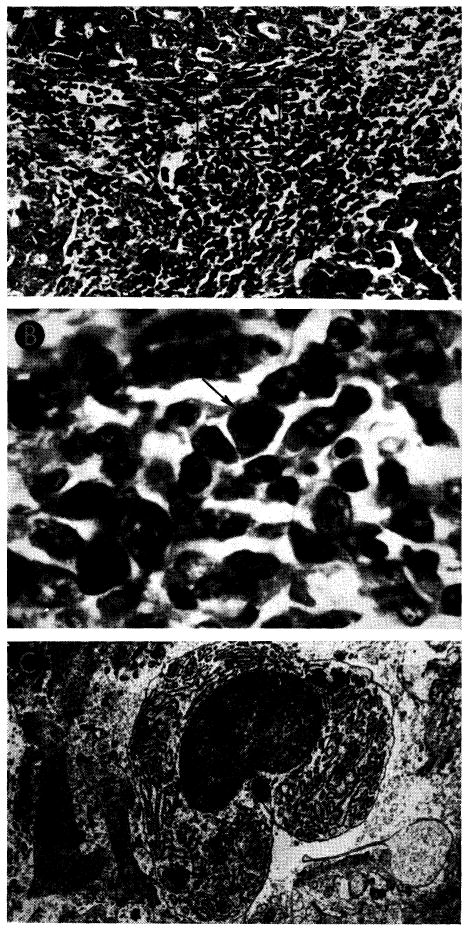
Grossly, many of the livers had a fine granular surface, frequently with pitted and shrunken areas, and when the tissue was cut it had increased resistance. Microscopic studies showed that in 7 of the azathioprine livers there was necrosis of the hepatocytes in the centers of all of the lobules (Fig. 8). This lesion was accompanied in five of the livers by bile “thrombi” in the central and midzonal bile canaliculi; in two by accumulation of fat in the cytoplasm of the midzonal hepatocytes, and in two more by scattered focal necrosis. One in the group was examined in the electron microscope after the animal had been receiving azathioprine for 40 days. This confirmed that the central zonal hepatocytes were necrotic and demonstrated that in the cytoplasm of the liver cells in the middle zone of the lobule there was loss of glycogen and rough endoplasmic reticulum, with an increase of lipofuscin granules and fat-containing vacuoles. The bile canaliculi were dilated and many were blocked by bile casts. The microvilli were thin and short (Fig. 9, B).
Fig. 8.
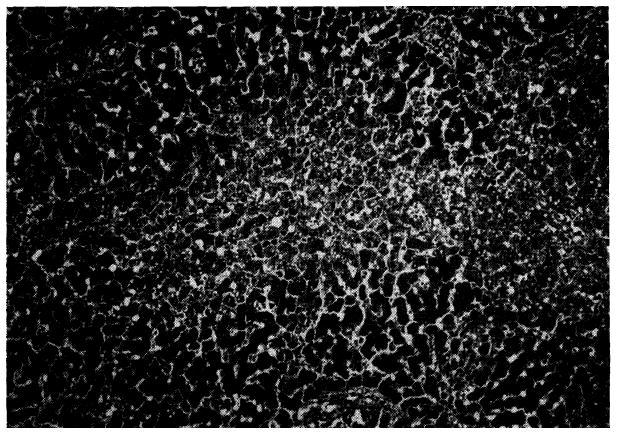
Liver of a dog given azathioprine for 26 days. There is necrosis of liver cells in center of the lobule. Hematoxylin-eosin; × 40.
Fig. 9.
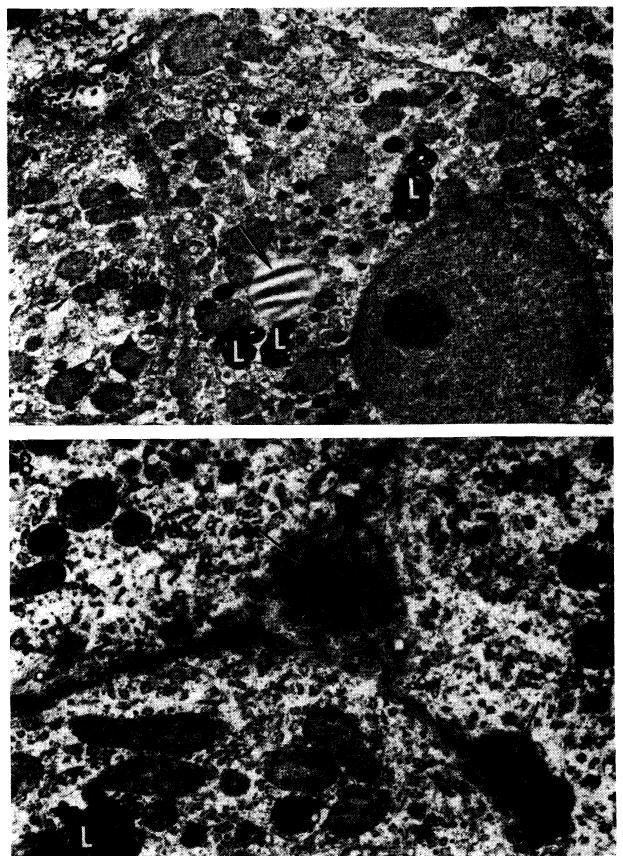
Ultrastructural changes caused by azathioprine in canine liver (from nontransplanted animals). A, after 40 days of azathioprine treatment. With light microscopy, there was pallor of centrilobular hepatocytes but no necrosis. This electron micrograph shows that cytoplasm lacks glycogen and the normal amount of rough endoplasmic reticulum, but contains an excess of lipofuscin (L) and lipid (arrow). Bile canaliculi are unaltered; ×2000. B, more severely damaged liver after azathioprine treatment for 40 days. Centrilobular hepatocyte necrosis was evident on light microscopy, similar to that in Figure 8. Two bile canaliculi (arrows) are blocked by inspissated bile. Microvilli in the larger of the canaliculi are scanty and short. Adjoining hepatocytes lack glycogen and normal amount of rough endoplasmic reticulum and contain excess lipofuscin (L). Electron micrograph; ×4000.
In five other livers, in which centrilobular necrosis had not occurred, there was pallor of the hepatocytes in the central zone of each liver lobule. Two of these livers were examined ultrastructurally, and the hepatocytes in this part of the lobule were found to be lacking in both glycogen and rough ergastoplasm. Smooth endoplasmic reticulum was present together with many lipofuscin granules (Fig. 9, A). Dilatation of the bile canaliculi with shortening of their microvilli was present in one of the two livers.
Six of the livers appeared to be perfectly normal under light microscopy. Only one of them was examined ultrastructurally; it showed in the centrilobular hepatocytes less rough endoplasmic reticulum and glycogen than normal and an increase of lipofuscin granules.
In man, azathioprine hepatotoxicity has not been observed except in isolated cases, although a high incidence of liver injury has been well documented with the use of 6-mercaptopurine (23, 53). In a recent autopsy study, the problem of azathioprine toxicity was investigated by examination of livers from patients who had received kidney transplants from a few days to 13 months previously (71). All of the patients had been treated with continuous azathioprine and high-dose steroid therapy and almost all had received intermittent doses of actinomycin C intravenously. Liver function studies in most instances had been made during life, and serious biochemical aberrations were present in only three of the 37 patients, all three of whom had jaundice terminally.
Despite the paucity of abnormalities of liver function during life, three of the livers studied showed reticulin and fibrous bands linking portal tracts (Fig. 10, A); in seven there was centrizonal cholestasis (Fig. 10, B); in 9 there was necrosis of the centrilobular liver cells, and many contained excess lipofuscin granules and lipid droplets in the centrizonal hepatocytes (Fig. 11).
Fig. 10.
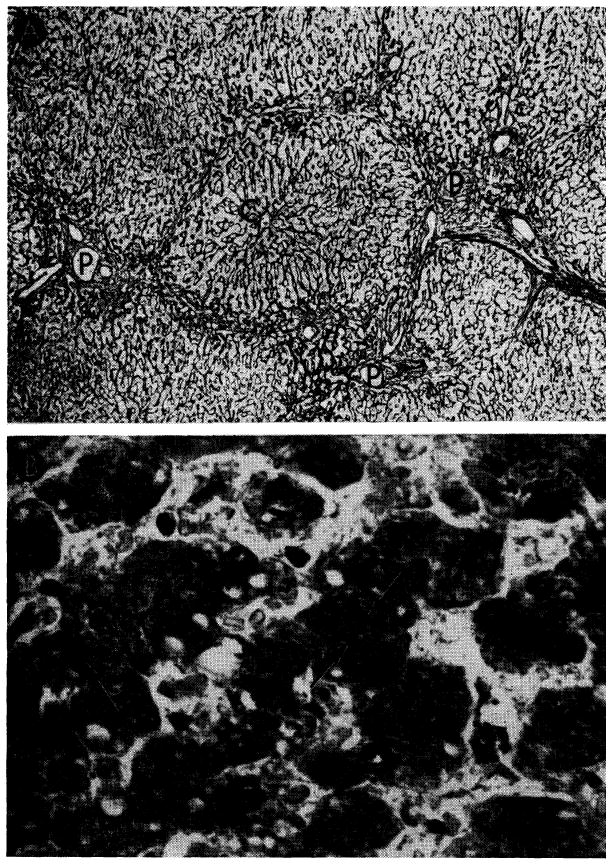
Hepatotoxicity of immunosuppressive agents in human recipients of renal homografts. A, liver from a patient who died 155 days after renal homograft. During the whole time she received azathioprine and prednisone. Reticulin is increased around the portal tracts (P), which are linked by bands of connective tissue. Central vein (C) is normal. Silver stain for reticulin; ×30. B, centrilobular cholestasis in liver from a patient who died 207 days after receiving a renal homograft; he had azathioprine throughout that period. Inspissated bile plugs the canaliculi (arrow). Hematoxylin-eosin; ×600.
Fig. 11.
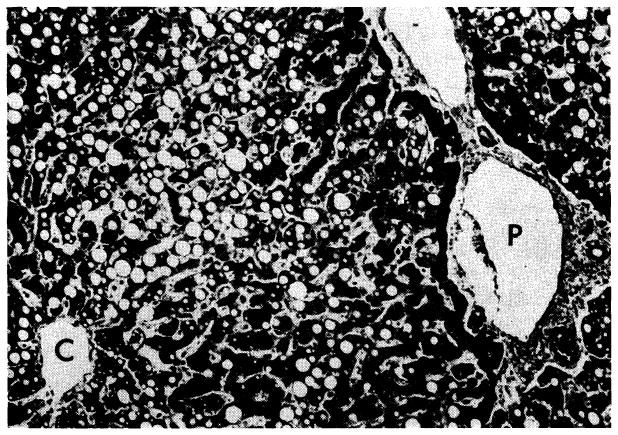
Widespread fat accumulation in liver of a patient who died 50 days after renal homotransplantation. He was treated throughout with azathioprine and prednisone. C, central vein; P, portal tract. Hematoxylin-eosin; × 100.
In the human cases, it is difficult to separate the hepatotoxic stigmas of azathioprine from those which may have been caused by prednisone since steroids have been shown in the rat (4, 40, 67), rabbit (2, 74), dog (2) and man (95, 97) to cause fatty infiltration and/or focal necrosis of the liver, and in dogs and rabbits these changes progressed to an increase of hepatic connective tissue (2).
Thus it would seem that the use of azathioprine, or even prednisone, is to some extent self-defeating in that the drugs used to prevent immunologic repudiation of the hepatic tissue are also responsible for severe liver injury. Fortunately, the human liver seems somewhat less susceptible to azathioprine, at least as judged by the relative absence of changes of liver function even after prolonged administration. In dogs, the problem is seemingly greater. The hepatotoxic effect of azathioprine is most severe during the first few days after the beginning of administration. The greatest risk from rejection is present during this time, and so apparently is the most severe damage from drug toxicity.
The direct drug toxicity of azathioprine is not unlike that observed with carbon tetrachloride, pyridine, butter-yellow and certain other liver poisons. Protection against injury or potentiation of recovery after exposure to some of these hepatotoxins has been reported with some of the lipotropic substances, including methionine. The use of lipotropic substances in an attempt to mitigate azathioprine toxicity is discussed later.
3. Nutritional and Metabolic Influences on Hepatic Morphology and Function
During the first half of the 20th Century, conflicting opinions and differing evidence were presented concerning the possible specific effect of splanchnic venous blood flow on hepatic morphology and function. After Eck’s initial observation that portal venous diversion in dogs did not result in death (22), the role of splanchnic venous flow was minimized. Within a few years, however, Hahn (35), working with Pavlov, demonstrated that animals with this operation had weight loss and serious neurologic aberrations which could be triggered by protein ingestion (9, 96). Since animals with Eck fistula have a reduced total hepatic blood flow (34, 36), the unresolved question was whether these adverse consequences were simply due to the quantitative flow change or whether they resulted from depriving the liver of some specific substance in the intestinal venous blood.
In 1953, the issue seemed to have been settled by the observation by Child’s group (13), later reinforced by more detailed studies by Silen et al. (78), working with portacaval transposition. With this experimental preparation, the portal venous flow is diverted from the liver as with an Eck fistula, but the venous component of total hepatic blood flow is replaced by systemic blood from the inferior vena cava and the total hepatic blood flow is unchanged or increased (36, 85). Dogs with portacaval transposition were thought by Child to have normal capacity for liver regeneration, and numerous subsequent studies of hepatic function showed normal values except for a reduced capacity to eliminate an intravenous ammonia load (78). These observations suggested that the critical factor in the maintenance of hepatic integrity was not the source of the total hepatic flow but rather the quantity. This simplified concept requires revision.
Although the aforementioned studies with transposition seemed to indicate that the livers retained essentially normal function, it was recently observed that this operation caused severe deglycogenation of hepatic parenchymal tissue (92), more than 50% of the glycogen stores being lost within the first six or eight weeks after operation (Fig. 12). This deglycogenation suggests that important and subtle changes of hepatic metabolism are produced when splanchnic flow is diverted from primary passage through the liver despite its replacement from systemic venous sources. Such effects can be greatly accentuated by use of a recently devised preparation (59) in which one half of the liver is perfused with systemic venous blood while the other half retains its normal splanchnic flow (Fig. 13). In these circumstances the liver fraction receiving alimentary return from the gastrointestinal tract remains large, whereas the other portion which is revascularized with vena caval blood undergoes a striking atrophy (Fig. 14). In the atrophic portion, there is centrilobular reticulin collapse associated with loss of hepatocytes around the central veins. There are also general atrophy of the surviving liver cells in this half of the liver, shrinkage of the individual liver lobules and severe depletion of glycogen in the centrizonal cells (Fig. 15). The part of the liver receiving normal splanchnic venous blood contains enlarged lobules composed of hypertrophied hepatocytes with abundant cytoplasmic glycogen (Fig. 15, B).
Fig. 12.
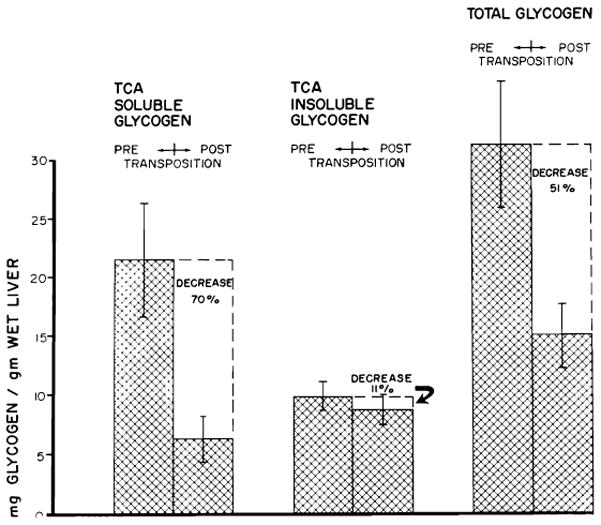
Mean changes in liver glycogen fractions of 17 dogs following transposition. Vertical lines represent ±1 SE. (Figs. 12–14 from Starzl et al. [92].)
Fig. 13.
Split liver preparation employed to test influence of splanchnic venous flow on liver morphology. Half of the liver receives splanchnic flow but the other half receives inflow from the inferior vena cava, making it analogous to a portacaval transposition. A, right half of the liver perfused with systemic venous blood. B, left half supplied by systemic venous blood. (From Marchioro et al [59].)
Fig. 14.
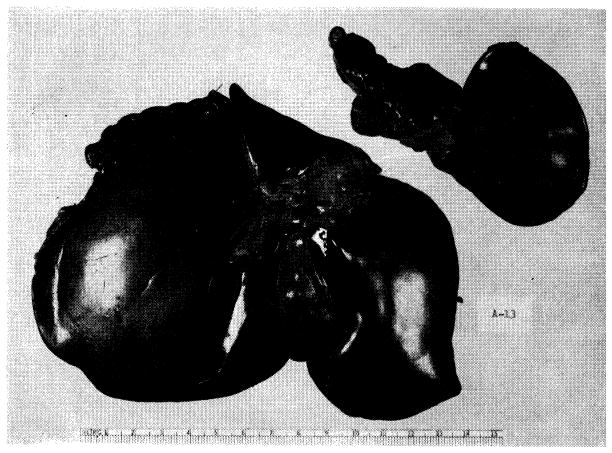
Result of experiment in Figure 13, B. The atrophic left portion the liver (52 Gm.) has been detached from the normal right fraction (234 Gm.). Specimen was obtained 73 days after operation.
Fig. 15.
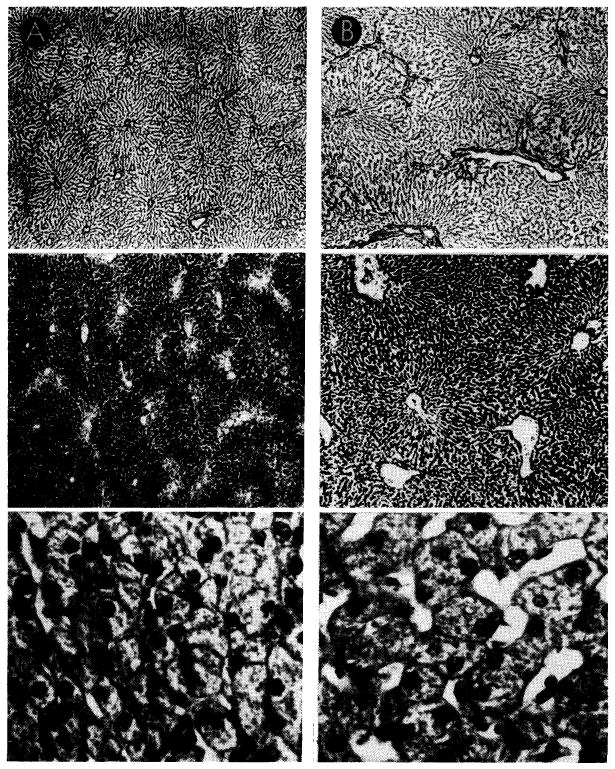
Canine liver, one-half perfused with blood from the inferior vena cava (A), the other half retaining normal portal venous supply (B). Top sections were impregnated with silver to show lobular reticulin framework; center sections, glycogen stained with periodic acid-Schiff reagent; bottom sections, hematoxylin-eosin. Magnifications are the same for the two sides. Liver which received vena caval blood (A) shows shrinkage of liver lobules, depletion of centrilobular glycogen and atrophy of hepatocytes when compared to the part which received portal blood (B).
Such studies are of vital importance in defining the physiologic requirements which must be met for successful liver transplantation. Failure to appreciate the role of splanchnic venous flow in maintenance of hepatic integrity may severely limit the possibility of successful transplantation, especially when an auxiliary liver is used without removal of the host organ.
4. Orthotopic Liver Transplantation in Untreated Recipients
The immediate risk of orthotopic hepatic homotransplantation is small in untreated dogs. Among 23 animals subjected to orthotopic transplantation, there was only one operative death (93). The other 22 animals lived for at least two days, and 19 of them lived for six days or longer (Fig. 16).
Fig. 16.
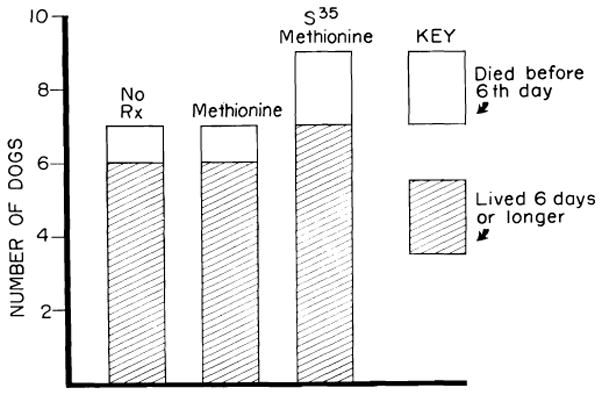
Results of orthotopic transplantation in dogs not receiving immunosuppressive therapy. Seven of the animals received no treatment; 7 had methionine, intravenously each day; 9 received radioactive methionine. Neither methionine nor its isotope potentiated homograft survival. Mean survival of the 22 dogs which survived operation was 7.1 days.
Such uniform results were obtained only after extensive experience in the laboratory. With practice, the time required for the operation tends to decrease, and the incidence of anastomotic failure becomes negligible. During actual placement of the homograft, it is necessary to provide drainage for the blocked splanchnic and inferior vena caval venous systems. This can be done with an external by-pass from both the inferior vena cava and the portal vein to the jugular veins as practiced by Moore’s group (61), or, alternatively, a preliminary portacaval shunt can be carried out in the recipient animal (Fig. 17) so that the two venous pools can be decompressed with a single by-pass (82). After insertion of the homograft, the temporary portacaval shunt is removed, leaving the animal with a physiologically normal vascular reconstruction. It is convenient to remove the entire donor aorta with the specimen (82) in order to allow an arterial anastomosis of larger vessels (Fig. 18). Internal biliary drainage is provided with a cholecystojejunostomy or with a cholecystoduodenostomy (Fig. 18).
Fig. 17.
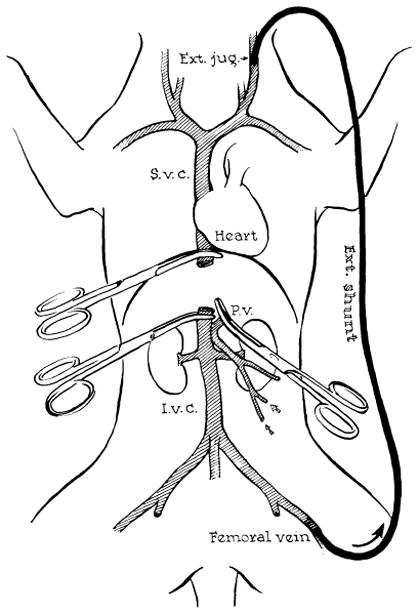
Method for decompression of inferior vena caval and splanchnic systems during removal of recipient liver and replacement with a homograft. Note that a preliminary portacaval shunt has been placed. By means of this temporary anastomosis, the two venous systems are connected, allowing their decompression with a single external by-pass.
Fig. 18.
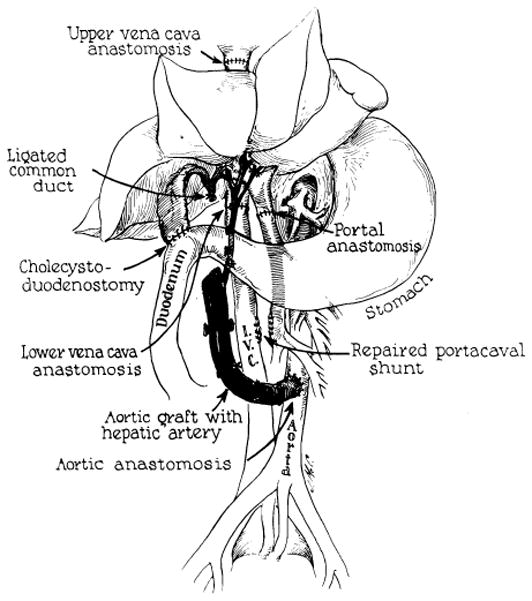
Reconstruction after orthotopic liver homotransplantation in the dog. Internal biliary drainage is with a cholecystoduodenostomy. Aorta is transplanted in continuity with the hepatic artery of the homograft.
After a technically satisfactory procedure, the subsequent events are subject to little variation. Almost all animals die before the 10th postoperative day. The animals are relatively healthy for the first few days. By the 3rd day there are almost always increases of the alkaline phosphatase level, and by the 4th day virtually every animal has hyperbilirubinemia; these changes are progressive (Fig. 19) in all but the rare case until the time of death (61, 83). Late in the course, hypoglycemia is a relatively common finding (83). An occasional dog may live for an extended period, the longest reported course in an untreated recipient being 21 days (83). Unquestionably, such extended survival is the fortuitous consequence of a highly favorable antigenic match between donor and recipient.
Fig. 19.
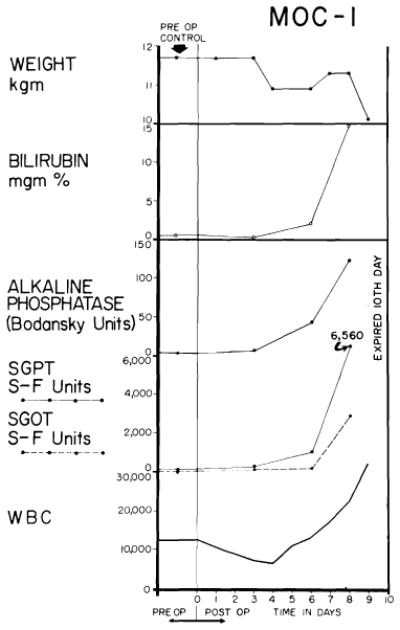
Biochemical changes after orthotopic liver transplantation to the untreated recipient. A brief period of good function follows operation, but deterioration of the biochemical pattern is unrelenting once it has begun.
At autopsy, the rejecting or rejected liver homografts usually have an increased size and weight. The liver is firm with rounded edges of a darker than normal color and with a firm, gritty feel to section. The cut surface of the liver has a nutmeg appearance, not unlike that seen in cardiac failure.
In the first 48 hours, congestion of the central zone of the hepatic lobule, necrosis of a few hepatocytes adjacent to the central vein, interstitial edema and prominence of Kupffer cells occur. These changes are nonspecific and occur in hepatic autografts (51). They are probably related to the ischemia inevitably associated with transplantation and perhaps to some minor degree of outflow block.
From the 3rd day onward, changes characteristic of rejection occur (10, 51, 61, 83). Almost always there is a mononuclear cell infiltration around and within the walls of the small portal and central veins (Figs. 20 and 21). Approximately 40% of these cells possess pyroninophilic cytoplasm, but a few are typical mature plasma cells. The majority are difficult to classify and are the cells variously called large lymphoid (75), hemocytoblasts (26) and immunoblasts (17). Occasionally, one of these large cells is seen in mitosis. After the 6th day, the infiltration is generally dense in all the small portal tracts and contains a higher proportion of plasma cells. There is necrosis of hepatocytes in the central and middle zones of the liver lobules (Fig. 20); ultimately only a thin rim of hepatic cells remains around the portal tract. The central zones of the lobules are occupied by distorted sinusoids, which contain red cells and infiltrating mononuclear cells adhering to the endothelium. Kupffer cells are hypertrophied and filled with hemosiderin and particles of bile pigment. Intrahepatic bile stasis and destruction of bile duct epithelium are common. Dilatation of the lymphatics in the walls of the central hepatic and portal veins is sometimes present. Foci of fibrinoid necrosis in the walls of small hepatic arteries are seen in about 25% of animals (Fig. 21, C). Later, collapse of the central part of the lobular reticulin framework leads to condensation of fibers and some early fibrosis around the central veins in the longest survivors.
Fig. 20.
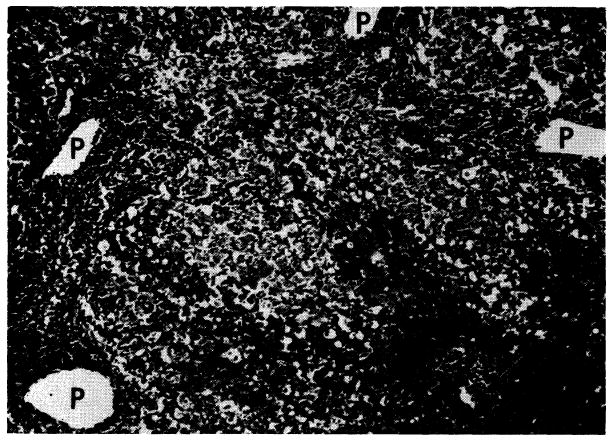
Untreated canine hepatic homograft at 6 days. Portal veins (P) and central veins (arrow) are surrounded by dense cellular infiltration. There is centrilobular necrosis with hemorrhage. Cytoplasm of surviving hepatocytes in the middle and peripheral zones of the lobules contains abundant lipid. Hematoxylin-eosin; ×30.
Fig. 21.
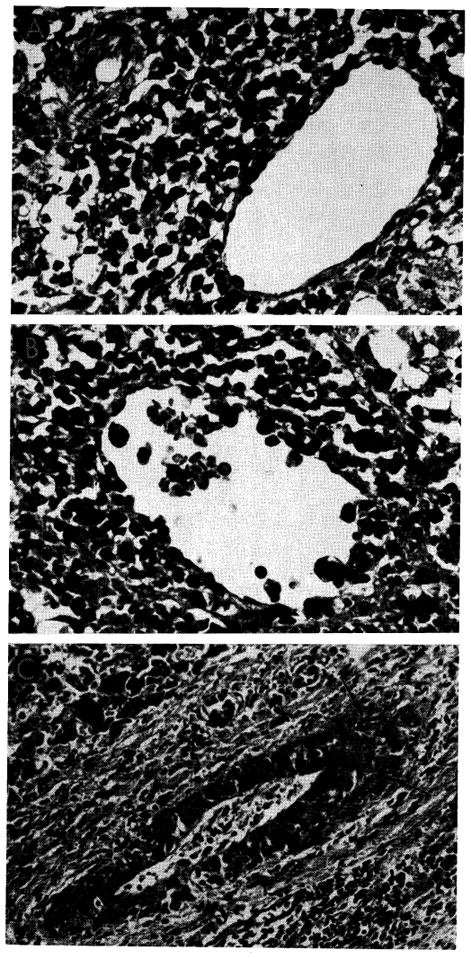
Vascular changes in homografts of untreated recipients. A, portal tract of untreated canine hepatic homograft shown in Figure 20. Portal vein is surrounded by mononuclear cells, some of which can be seen in the vessel wall. Hepatic artery is unaffected. Hematoxylin-eosin; ×500. B, central vein, surrounded by mononuclear cells, from untreated canine homograft shown in Figures 9 and 10. Some cells are adhering to the endothelium and others are infiltrating the vein wall. Hematoxylin-eosin; ×500. C, focus of fibrinoid necrosis (arrows) in wall of a small branch of the hepatic artery in a canine liver 7 days after homotransplantation. Hematoxylin-eosin; ×500.
5. Orthotopic Homografts in Animals Treated with Immunosuppressive Agents
The earliest attempts to potentiate homograft survival with total body irradiation were total failures (84). Early reports of the use of azathioprine showed definite potentiation of survival, but with the longest period of maintenance of life being 31 days (90, 91). Subsequently, it has been unequivocally demonstrated that protracted homograft survival can be obtained in the dog with the use of azathioprine and that between one fourth and one fifth of all animals receiving a satisfactory technical procedure may be expected to live two months or longer (93).
In the preceding section, it was pointed out that the mortality was only 17% operatively and during the first six days following transplantation to unmodified recipients. The risk of early death is higher in animals treated with azathioprine (Fig. 22), a fact not difficult to explain in view of the systemic and specific hepatic toxicity of this agent. More than 27.5% of the animals failed to live as long as a week (93), the principal cause of death being postanesthetic pneumonitis.
Fig. 22.
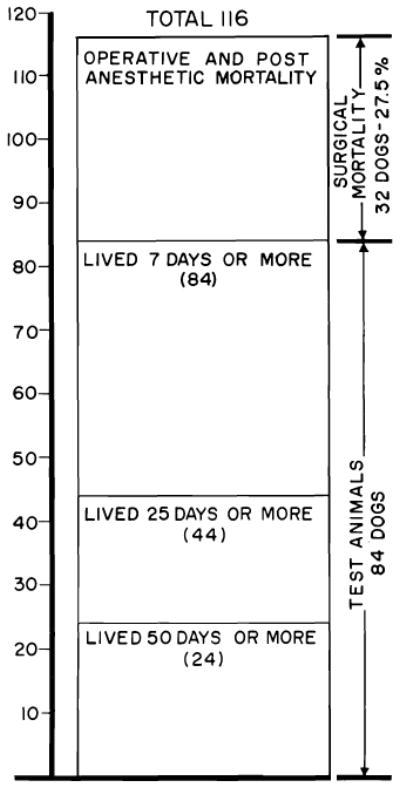
Survival after 116 orthotopic homotransplantation experiments in dogs. Mortality in the 1st week was 27.5%, almost always the result of acute pulmonary sepsis. Animals living longer than 1 week had a better than 50% chance of living 25 days or more and about a 30% chance of living 50 days or more. Maximal survival has been 11½ months.
Despite the increased acute risk of orthotopic transplantation to drug-modified recipients, a substantial number of those dogs which survived for as long as one week had prolonged homograft function (Fig. 22). Of 84 such animals, 44 lived at least 25 days, and 24 of these lived for 50 days or longer (93). Ten of the residual group died between the 50th and the 120th day of causes listed in Table 1. Liver function was abnormal in all but one of these 10, although other lethal complications were present in each. Two other dogs which died after discontinuance of azathioprine therapy are discussed below. Twelve dogs were still alive from 82 to 344 days after operation (Table 1).
TABLE 1.
Orthotopic Canine Homotransplantation with Immunosuppression (Fate of 24 animals which lived 50 days or longer; survival figures for dogs still living is brought up to Mar., 1965.)
| Group | Survival Days | Gross Cause of Death | Last Bilirubin, Mg. | Last Alkaline Phosphatase, B.U.* | Last SGPT† |
|---|---|---|---|---|---|
| 1 | (1) 344 | Alive‡ | 0.1 | 1.8 | 28 |
| (2) 98 | Pneumonitis; liver failure | 5.7 | 503 | 760 | |
| (3) 129 | Pneumonitis | 0.4 | 58 | 22 | |
| (4) 110 | Alive | 0.1 | 43 | 56 | |
| 2 | (1) 120 | GI hemorrhage; liver failure | 3.7 | 296 | 870 |
| (2) 58 | Pneumonitis | 1.3 | 300 | 220 | |
| (3) 248 | Alive‡ | 0.7 | 63 | 330 | |
| (4) 70 | GI hemorrhage; liver failure | 5.9 | 516 | 1,660 | |
| (5) 201 | Alive‡ | 5.7 | 207 | 1,260 | |
| 3 | (1) 238 | Alive‡ | 0.3 | 12 | 136 |
| (2) 101 | Pneumonitis; liver failure | 5.6 | 436 | 580 | |
| (3) 221 | Alive‡ | 0.2 | 8 | 100 | |
| 4 | (1) 163 | Bleeding duodenal ulcer; liver failure‡ | 4.7 | 166 | 110 |
| (2) 103 | Postbiopsy hemorrhage; liver failure | 5.7 | 746 | 380 | |
| 5 | (1) 132 | Alive | 5.1 | 993 | 840 |
| 6 | (1) 69 | Pneumonitis; liver failure; GI hemorrhage | 3.9 | 657 | 1,200 |
| (2) 50 | Pneumonitis; liver failure | 4.8 | 354 | 250 | |
| (3) 93 | Liver failure; GI hemorrhage | 3.9 | 268 | 1,068 | |
| 7 | (1) 102 | Alive | 4.0 | 742 | 2,380 |
| (2) 101 | Alive | 0.3 | 145 | 640 | |
| (3) 97 | Alive | 2.9 | 239 | 420 | |
| 8 | (1) 92 | Alive | 3.5 | 245 | 740 |
| (2) 77 | Pneumonia; ascites | 2.7 | 299 | 198 | |
| (3) 82 | Alive | 4.5 | 311 | 700 |
B.U. = Bodanskv units.
SGPT in Sigma-Frankel units.
Azathioprine stopped after 116–123 days. Subsequent course without immunosuppression.
Hematologic changes
After orthotopic homotransplantation to the treated host, anemia is a common early problem in virtually all animals. This seems to be due to at least two different causes. First, azathioprine administration to the nontransplanted dog causes a progressive fall of hematocrit (Fig. 7). Since this occurs without a reduction of red cell survival time, it is probably due to inhibition of erythropoiesis.
An important contributing factor after liver homotransplantation is a decreased red cell half-life (93). This finding, also noted in homotransplantation of the spleen (27, 56), was observed in each of seven dogs studied and followed the course shown in Figure 23. The maximal effect occurred early after operation, with values gradually returning toward normal after the 1st month in animals which survived beyond this time. The red cell destruction may have been due to erythroclastic activity of the liver (41), a possibility supported by the high iron content observed microscopically in the homografts. Whether or not this represents a graft-versus-host reaction directed against recipient red cells is not known, but the spontaneous regression of the phenomenon as well as its failure to recur after discontinuance of immunosuppressive therapy are encouraging observations (Fig. 23).
Fig. 23.
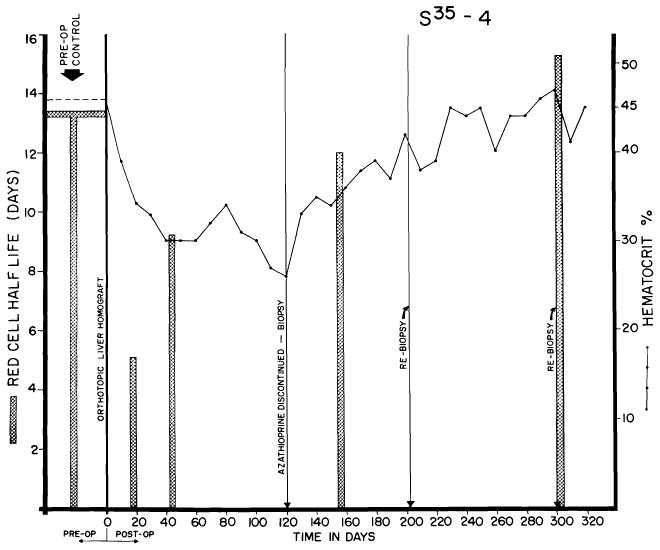
Red cell survival and hematocrits in dog alive 344 days after orthotopic transplantation. Note sharp reduction of red cell half-life in 1st postoperative month, with gradual return toward normal. Red cell survival was not altered by withdrawal of azathioprine at end of 4 months, but the depressed hematocrit rose sharply in succeeding months.
Course after transplantation
After homotransplantation to the treated animal, there was a great variability in the vigor of the rejection. About one fifth of the 84 dogs alluded to previously never had any clinically detectable rejection (93). An example is shown in Figure 24. The dog received a homograft in March, 1964, had only minor early abnormalities of liver function, had all therapy discontinued in four months and has been in good health since then. Most of the cases in which really long survival was obtained came from this favored group of 19 dogs, in which a fortuitous good match between donors and recipients seemed to have existed which simplified the problem of immunologic control.
Fig. 24.
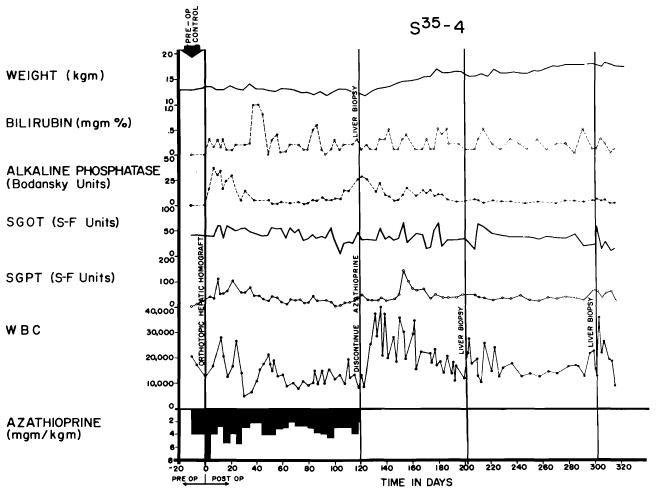
Course of an animal which never had any clinically evident homograft rejection. Note rapid weight gain following cessation of therapy at 4 months. The pronounced leukocytosis after withdrawal of immunosuppression was commonly seen. This animal, given adjuvant therapy with S35 methionine, was still alive after 344 days.
At the other end of the spectrum, observed in about one third of the animals, was an inexorable rejection characterized by relentless deterioration of liver function, progressive jaundice and death in all instances in 41 days or less (Fig. 25). Rises of SGOT, SGPT and alkaline phosphatase values (Fig. 25) were similar to but of generally greater magnitude than those produced by azathioprine administration to the nontransplanted dog. Terminal convulsions or coma, intractable vomiting and hypoglycemia were common. All 24 animals died in less than 41 days, with mean survival of only 15 days.
Fig. 25.
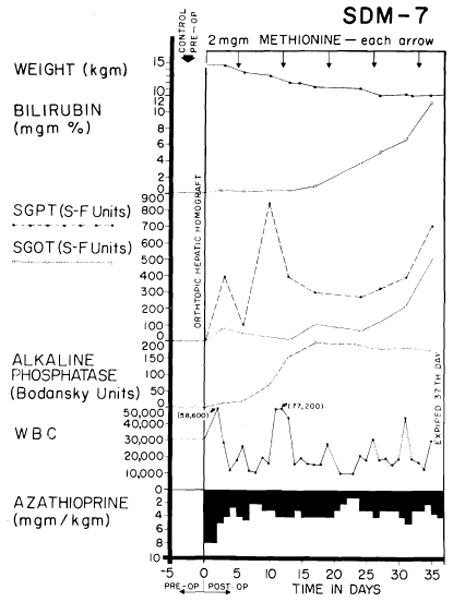
Example of inexorable rejection despite immunosuppressive therapy. Serum bilirubin was the most useful measurement for following the course after homotransplantation, since the other abnormalities of liver function depicted can also be caused by azathioprine.
Finally, a rejection of intermediate intensity was observed in 41 of the 84 experiments (48.8%) despite the fact that hyperbilirubinemia and rises of SGOT, SGPT and alkaline phosphatase levels were also present (Figs. 26 and 27), sometimes to the same degree as in the animals with inexorable rejection. However, the biochemical indices of hepatic injury were partially (Fig. 27), or even relatively completely, reversible (Fig. 26). At the peak of rejection, which lasted as long as six weeks in some cases, these dogs often appeared to be in a terminal state, with clay-colored stools, dark urine, profound anorexia and rapid weight loss. As liver function subsequently improved, most of the animals had a return of appetite. A number of the dogs ultimately died of complications of chronic liver insufficiency such as gastrointestinal hemorrhage or of pneumonitis, but others continued to live for many weeks or months with gradually improving liver function. The longest survival of any animal with this type of reversible rejection was 161 days (Fig. 27). Sixteen others lived for 50 days or more, indicating that protracted survival in these unfavorable circumstances was not rare.
Fig. 26.
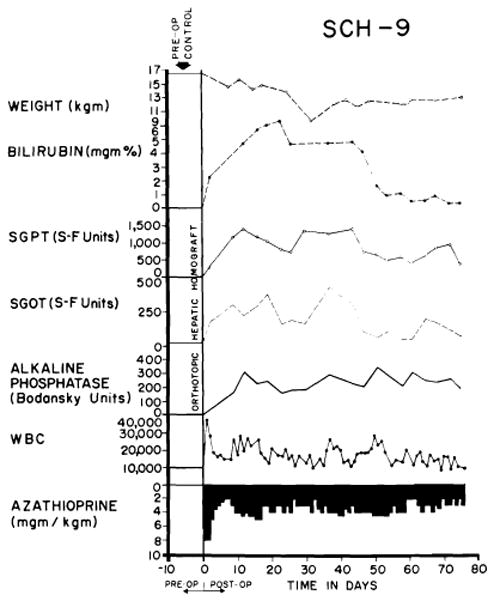
Virtually complete reversal of severe hepatic rejection. Note severe jaundice, which ultimately disappeared. The animal was still alive at time of writing. Reversal was accomplished without alteration of immunosuppressive therapy. The animal received adjuvant S35 methionine, methionine and choline.
Fig. 27.
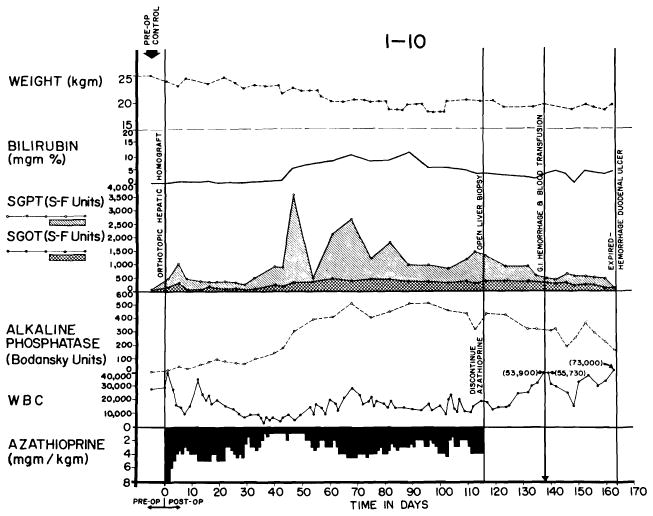
Reversal of rejection in an animal treated with azathioprine. Serum bilirubin rose to more than 10 mg., then declined. Improvement of liver function continued after discontinuance of azathioprine therapy at 116 days. Death 45 days later was due to massive hemorrhage from a large duodenal ulcer.
Perhaps the most interesting feature of the more than 40 examples of rejection reversal was that the ultimate improvement was not dependent upon intensification of immunosuppressive therapy, since neither increased doses of azathioprine nor additional drugs were used, and in one instance the phenomenon was noted in the absence of all therapy (Fig. 28). It seems probable that rejection of any tissue is characterized by a tendency to remission. Although prednisone (31, 55, 87), and less certainly actinomycin C or local irradiation, may be of life-saving value in promoting the process after renal homotransplantation, the use of such additional measures is probably not requisite in some instances irrespective of the type of homograft used. The spontaneity with which reversal may occur makes caution necessary in attributing this effect to any preceding variation in therapy.
Fig. 28.
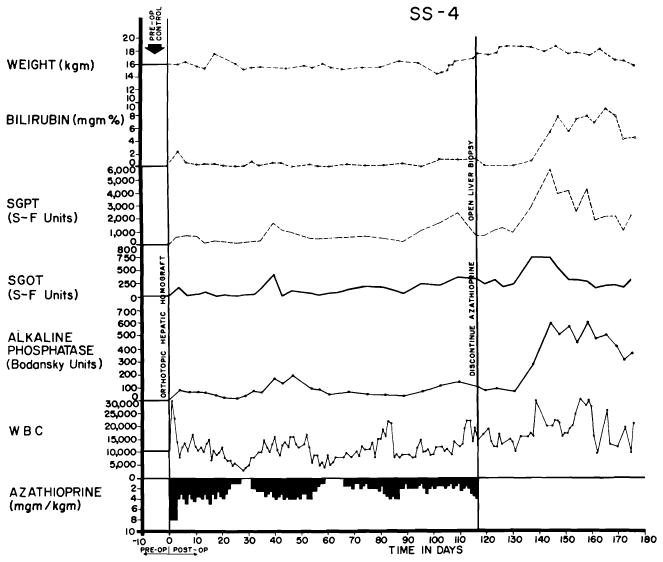
Course of an animal which had no clinically evident rejection during the first 4 months. After azathioprine was discontinued, liver function deteriorated, though the animal appeared healthy and had little weight loss. The late rejection partially reversed without reinstitution of therapy.
Chronic liver function
The last routine observations of liver chemistry of all dogs living beyond 50 days are shown in Table 1. In six of these animals, other determinations were performed from 98 to 300 days postoperatively. Retention of BSP at 45 minutes was 5% or less in four, 8.2% in one, and 34% in one. Prothrombin times were 52 to 90%. Total protein content was 4.8–5.8 Gm./100 ml.
Host-graft nonreactivity
Although the early recovery after liver homotransplantation has many hazards, as described above, chronic convalescence is no more difficult than after the use of less complicated organs. Indeed, the frequency and rapidity with which dogs could be withdrawn from immunosuppression without an ensuing fatal rejection is remarkable. In our laboratory, six nonjaundiced dogs had all therapy discontinued 100–123 days after homotransplantation. Five were still alive 83, 101, 120, 129 and 224 days later; the only death occurred of pneumonitis in the presence of excellent liver function. One of the six animals had a nonfatal delayed rejection, beginning two weeks after azathioprine was withdrawn, but the hyperbilirubinemia and enzyme increases ultimately stabilized and reversed without treatment (Fig. 28). The others had no subsequent deterioration of homograft function (Fig. 24). In all cases, irregular increases of the peripheral white blood count were observed during the first two months after cessation of therapy.
A seventh animal had serious liver malfunction while on azathioprine therapy (Fig. 27), a maximal bilirubin value of more than 10 mg./100 ml. being present on the 90th postoperative day. By the 120th day, this was decreasing and continued to decline after azathioprine therapy was stopped. The animal ultimately died after 161 post-transplant days of a bleeding duodenal ulcer.
The consistency of this apparent state of host-graft nonreactivity and the rapidity with which it seemed to develop exceeds that reported after canine renal homotransplantation (52, 64, 69, 86). The explanation for this is not apparent, but conceivably the large antigenic mass could play a role, or perhaps the liver with its enormous regenerative capacity is simply capable of sustained function in the face of continuing but minimal chronic rejection. As will be described later, the findings in the serial biopsy specimens obtained after discontinuance of therapy do not support the latter hypothesis, but the amount of histologic material available is still too scanty to warrant conclusions.
Effect of methionine or its radioisotope
The therapeutic dilemma posed by the necessity of using a hepatotoxic immunosuppressive agent has already been described. Since certain lipotropic factors have been demonstrated to protect against or facilitate recovery from various liver poisons (7, 20, 70), it seemed reasonable to evaluate methionine, which has been reported to prevent the toxicity of bromobenzene (43), pyridine (6, 16), chloroform (21), Mapharsen (29) and butter-yellow (32) and to accelerate recovery from chronic carbon tetrachloride poisoning (76).
In the nontransplanted dogs described earlier, the pattern of hepatic injury with azathioprine therapy was compared with that observed when methionine or its radioisotope was also administered intravenously. The degree and duration of the abnormal changes of liver chemical values were not influenced (Fig. 7). Moreover, administration of methionine or its radioisotope did not potentiate homograft survival after homotransplantation to the otherwise untreated host. In spite of these negative findings, slightly better results were obtained after transplantation to the modified recipient when methionine or its S35 isotope was given in addition to azathioprine. The improvement of survival was, however, of negligible statistical significance (P = 0.17). The difficulty of evaluating such adjuvant agents is greatly increased by the extreme variability of host response to the foreign tissue, which in the present study ranged from complete absence of rejection to uncontrollable repudiation of the liver homograft. This spectrum undoubtedly conformed to the degree of chance antigen matching between donors and recipients. The presence in outbred populations of this powerful and unpredictable influence makes conclusions about minor therapeutic alterations meaningless unless hundreds or even thousands of experiments are performed. The inability to demonstrate a statistically significant advantage of methionine or its isotope may have been due to the system of testing in which the variables of uncontrolled donor-recipient selection were of far greater importance in the individual experiment than the therapeutic variable being examined.
Pathologic Changes in Orthotopic Homografts of Treated Dogs
The histologic alterations in the liver after homotransplantation to the treated recipient have been studied in more than 100 dogs from a few to longer than 300 days after operation. Detailed analyses of the early and late changes in the homografts have been described elsewhere (72, 93). Here, an attempt will be made to relate the pathologic findings to the foregoing clinical observations and to evolve a more general view of both rejection and recovery from the damage caused by this process.
Although it can be delayed and attenuated, rejection probably cannot be completely avoided in any instance despite the use of immunosuppressive agents at present available, although the magnitude of the immunologic insult obviously differs in individual experiments. If the rejection is mild, the host cells invading the homograft may be few and the loss of function negligible. If it is severe, the cellular invasion tends to be extensive, with loss of many hepatocytes and varying degrees of hepatic failure. In either case, there is strong evidence that the principal damage occurs relatively early, that the invading cells subsequently tend to leave the homograft, that this emigration corresponds to the clinical reversal of rejection and that most of the ultimate histologic features of repair are dictated by the degree and localization of the early injury. The facts that this sequence occurs clinically after transplantation of both kidneys (86) and livers (93) and that the same events have been observed histologically (58) are among the most encouraging features that have emerged from all previous experience with whole organ homografting procedures. They indicate that the central problem of immunologic control occurs at a relatively early time. Furthermore, management of rejection is not entirely dependent on immunosuppressive agents; a key factor involves a dynamic biologic process in which the immunologic relationship between the host and the graft changes rapidly.
The delay of onset of graft repudiation in the modified host can be appreciated by comparison with the earlier described untreated series in which progressive histologic evidence of rejection was present from the 2nd day onward. In contrast, only about 25% of animals receiving azathioprine had cellular infiltration during the 1st week, and mononuclear cells with pyroninophilic cytoplasm were rare (72, 93). Other changes were similar to those before the 3rd day in the untreated animal. In spite of the paucity of abnormal findings by light microscopy, electron micrographs obtained in one dog at six days showed a few lymphoid cells in both portal and central areas; similar cells were found adhering to, and possibly injuring, the sinusoidal endothelium.
Although delayed in onset, the peak of rejection in animals receiving azathioprine seemed to occur seven to 15 days after operation (72, 93). The outstanding microscopic features were similar to those of the untreated homografts after four to seven days: cellular infiltration and marked centrizonal and midzonal hepatocyte necrosis. In these livers, the portal tracts and central veins were infiltrated by mononuclear cells (Fig. 29, A), 30–40% of which possessed pyroninophilic cytoplasm. Many were fairly large primitive cells, among which mitoses were sometimes seen (Fig. 29, B); others resembled plasma cells. The infiltration around the central vein was usually much lighter. Centrilobular necrosis of hepatocytes usually accompanied the cellular infiltration, and in about 65%, the midzonal hepatocytes were also necrotic (Fig. 30, A). Where hepatocytes were destroyed, there were centrilobular collapse and condensation of the reticulin. The animals that survived longest in this group frequently had bands of condensed reticulin linking adjacent central veins. Mild to moderate centrilobular congestion was usual. Kupffer cells, filled with hemosiderin and bile pigment, and dilated sinusoids occupied the necrotic centers of the lobules. Intra-hepatic cholestasis and destruction of bile canaliculi in the affected part of the liver lobule were common. Interstitial edema was almost always present.
Fig. 29.
Liver homograft rejection in animals treated with azathioprine. In dogs that died 1–2 weeks after operation, pathologic changes are similar to those with homotransplantation to the unmodified recipient, differing only in that the sequence of destruction is delayed. A, treated canine hepatic homograft, undergoing rejection at 7 days. Portal tract is heavily infiltrated by mononuclear cells. Outlined rectangular area is shown in greater detail in B. Hematoxylin-eosin; ×250. B, details of cellular infiltration in portal tract of hepatic homograft in A. Many of the cells are large blast cells with several nucleoli and pyronin-positive cytoplasm; one is in mitosis (arrow). Hematoxylin-eosin; ×600. C, treated hepatic homograft at 8 days. A plasma cell with abundant rough endoplasmic reticulum in its cytoplasm is seen in a portal tract. Electron micrograph; × 2,400.
Fig. 30.
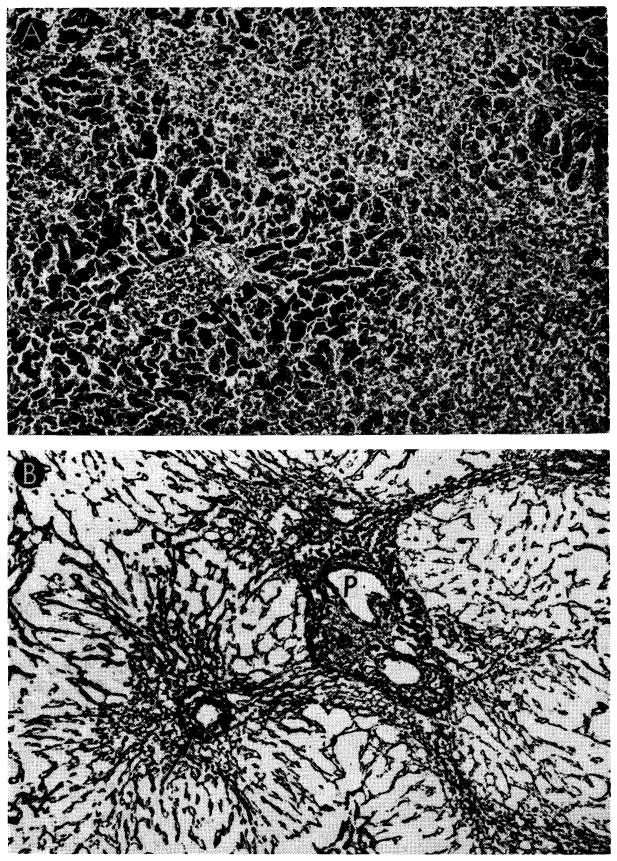
Unsuccessful control of rejection after orthotopic liver transplantation. A, hepatic homograft rejected in 15 days despite azathioprine treatment. There is widespread destruction of hepatocytes in central and middle zones of the lobules. Only a rim of liver cells remains around the small portal tract (arrow), which is heavily infiltrated by mononuclear cells. Hematoxylin-eosin; ×40. B, early distortion of architecture resulting from canine liver rejection in the modified host. These changes determine the pattern of healing in animals which survive this critical period. Centrilobular loss of hepatocytes has been followed by collapse and condensation of reticulin around the central vein (arrow). P, portal tract. Reticulin stain; ×40.
Preliminary ultrastructural examination of three such homografts at eight, nine and 10 days showed that in the portal tracts and sinusoids, plasma cells and their precursors, with abundant rough endoplasmic reticulum in their cytoplasm (Fig. 29, C), had appeared among the lymphoid cells seen at six days. In many places the sinusoidal epithelium had disintegrated. Most of the hepatocytes in the central parts of the lobules were necrotic, and those in the middle zone were severely damaged and exhibited nuclear changes as well as the changes in cytoplasmic organelles noted at six days. Dilatation and destruction of bile canaliculi with microvillar loss and obstruction by masses of bile pigment appeared to be common.
From 15 days onward, most of the livers appeared to have survived the first assault of rejection and were settling down to repair; delayed death was commonly due to pneumonitis or other nonhepatic causes. Although some cellular infiltration was usually present around the portal and central veins, it tended to be light and few of the cells were pyroninophilic. The primitive “large lymphoid” cells were uncommon in specimens from 15 to 120 days (Fig. 31). Numerous hemosiderin-laden macrophages and Kupffer cells were present. There was centrilobular atrophy, the hepatocytes often containing fat droplets or excess lipofuscin, or both, and lacking rough ergastoplasm. In many homografts, the centrilobular bile canaliculi had loss of microvilli or were plugged with bile.
Fig. 31.
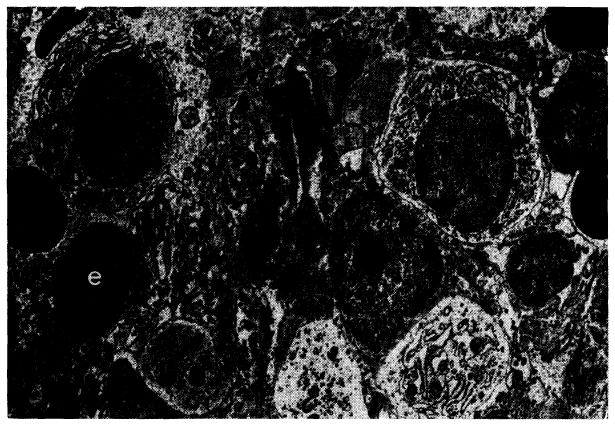
Treated canine hepatic homograft at 25 days. Plasma cells (p), small lymphocytes (ly) and erythrocytes (e) are present in portal tract. Primitive “large lymphoid” cells are not present. Electron micrograph; × 1,600.
The dominant features after 15 days were, however, those of healing and regeneration. The centrilobular hepatocyte death or atrophy was usually accompanied by collapse and condensation of the central part of the lobular reticulin framework (Fig. 30, B). Collagen fibers were deposited in these perivenous areas, and linking of the centrilobular areas with bands of connective tissue was noted in almost half the specimens studied after 25 days. Increased connective tissue in the portal area was a less consistent feature, but when present, the portal and central scars were sometimes connected.
Evidence of regeneration was occasionally observed between 25 and 50 days and commonly thereafter. Hepatocytes at the periphery of the liver lobules showed increased basophilia, occasional mitoses and polyploidy indicative of proliferation. In some, proliferation of small bile ducts and ductules had occurred. Regeneration nodules were present in about a third of the homografts after 50 days, and where fibrosis was heavy these contributed to a pattern of true cirrhosis.
The serial biopsies of three long-term survivors are of special interest since their tissues were examined at 120–123 days and again after 77–182 subsequent days without therapy. One animal was unique in that all tissues appeared to be within normal limits on both light and electron microscopy (Fig. 32).
Fig. 32.
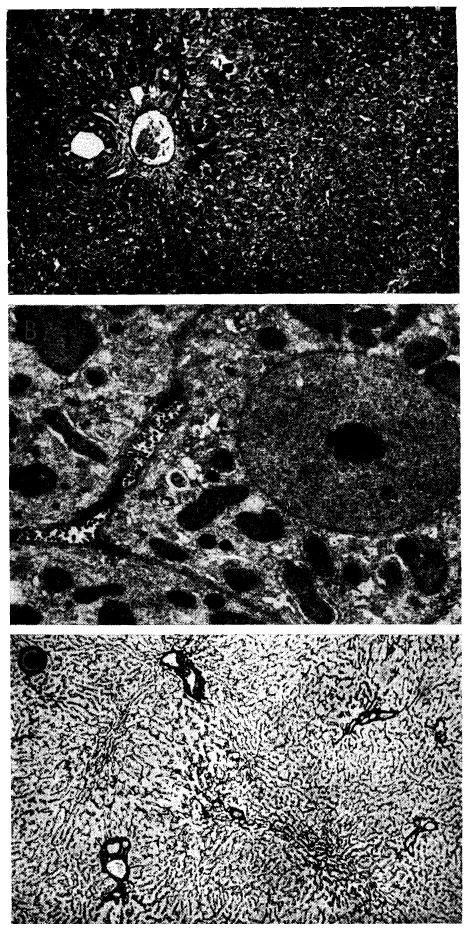
Tissues from the longest surviving orthotopic canine liver homograft. This animal had normal liver function almost 1 year after operation. A, biopsy at 120 days. There is evidence of regeneration of hepatocytes at the periphery of the lobule, but no other abnormality. Hematoxylin-eosin; ×40. B, biopsy 82 days after all immunosuppressive drugs had been stopped. The homograft appears normal. Electron micrograph; ×1,600. C, biopsy 182 days after all immunosuppressive drugs had been withdrawn and 302 days after transplantation. The lobular reticulin pattern appears normal. Reticulin stain; × 30.
A second dog had a moderately heavy cellular infiltration of the hepatic homograft at the time treatment was discontinued (Fig. 33, A). The portal fibrous tissue was increased, and connective tissue bands, containing many new biliary ductules, linked portal tracts to each other and occasionally to central veins (Fig. 34, upper). Pseudolobules of actively regenerating liver cells were fairly common. The centrilobular hepatocytes had undergone atrophy; those remaining lacked glycogen and rough ergastoplasm and contained many lipid droplets. Seventy-seven days later there were fewer infiltrating cells, the fibrosis was less conspicuous, and the hepatocytes had normal-looking cytoplasm (Figs. 33, B, and 34, B).
Fig. 33.
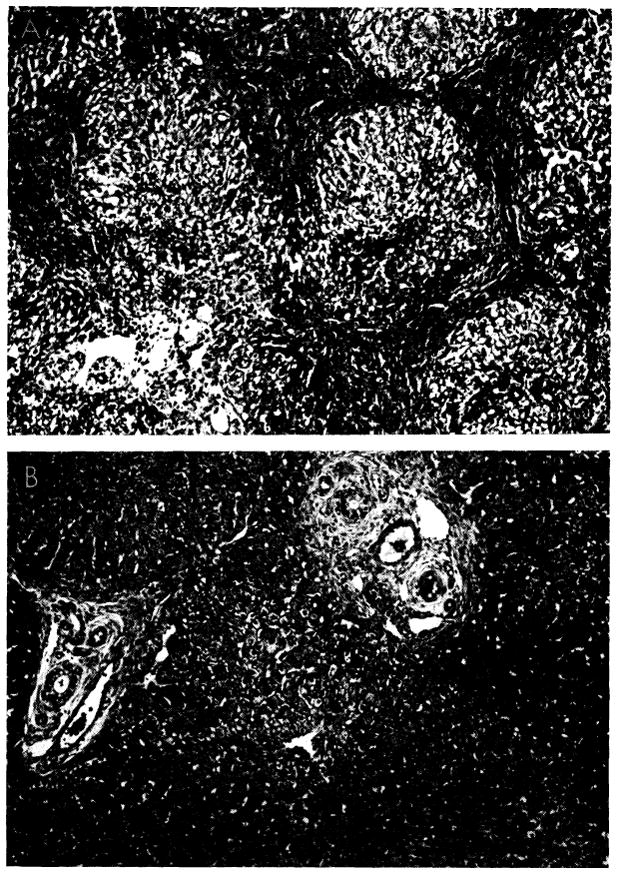
Improvement of histologic appearance of an orthotopic liver homograft after discontinuance of azathioprine therapy. The animal was in excellent health 238 days after operation. A, biopsy at 121 days. The lobular architecture is distorted by thick bands of connective tissue. Hepatocytes in pseudolobules of regenerating liver contain much lipid. B, all therapy was stopped after 121 days, and this specimen was obtained 77 days later. Fibrosis is less conspicuous and hepatocytes now appear normal. Hematoxylin-eosin; × 40.
Fig. 34.
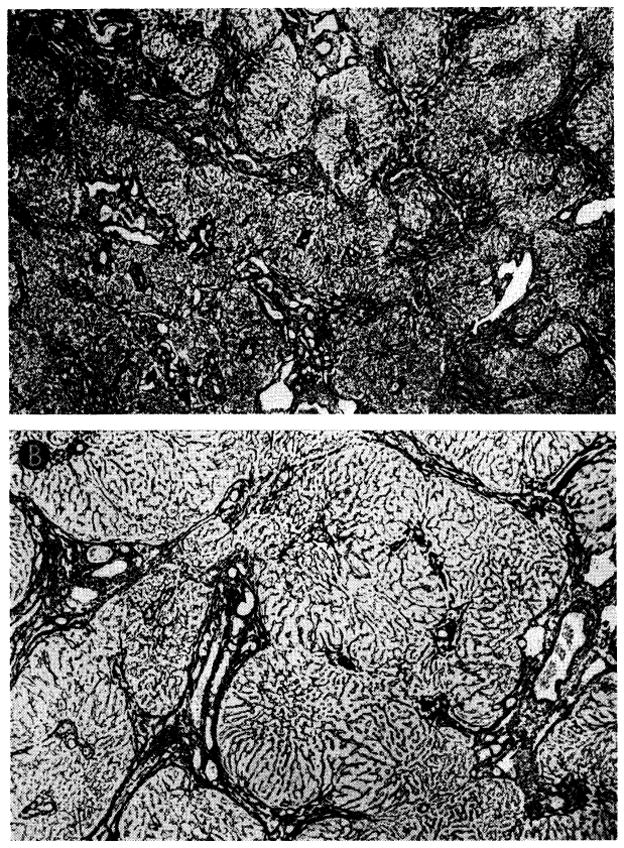
Changes in reticulin network in biopsies shown in Figure 33. A, at 121 days, thick bands of reticulin link portal tracts to each other and to central veins, destroying normal lobular pattern. Reticulin stain; ×20. B, 77 days later, reticulin bands seem less coarse and not so frequent. Reticulin stain; × 30.
The third dog had irregular increases of serum enzyme values during the first four months which were essentially unchanged after cessation of therapy (Fig. 35). The first biopsy showed abnormalities (Fig. 36, A). The portal tracts showed some increase in connective tissue, widespread proliferation of small biliary ductules and a slight to moderately heavy patchy infiltration by mononuclear cells. Eighty-four days later (Fig. 36, B) the cellular infiltration was much heavier than before. The percentage of pyroninophilic cells remained about the same, but in some portal tracts there were now many neutrophils. Most of the hepatocytes, especially those in the centers of the lobules, contained multiple small droplets of fat. This deterioration appeared to be due in part to a homograft rejection and in part to the effects of cholangitis. Despite these alarming findings, the dog remains in good health 248 days after homotransplantation.
Fig. 35.
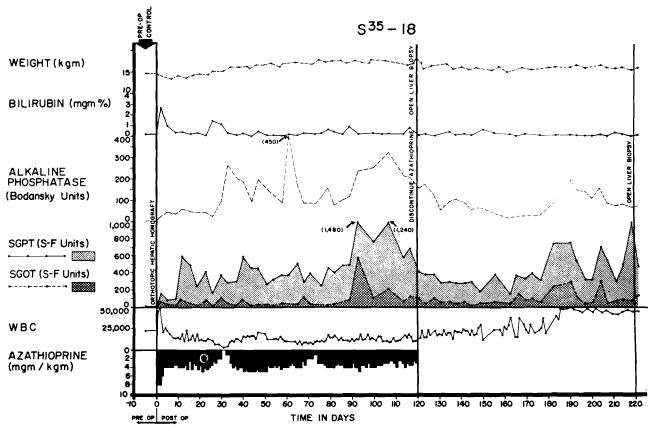
Course of dog in good health 248 days after orthotopic liver transplantation. Jaundice was never present, although there were chronic elevations of SGOT, SGPT and alkaline phosphatase values. Azathioprine was stopped at 123 days. Biopsies from this animal are shown in Figure 36.
Fig. 36.
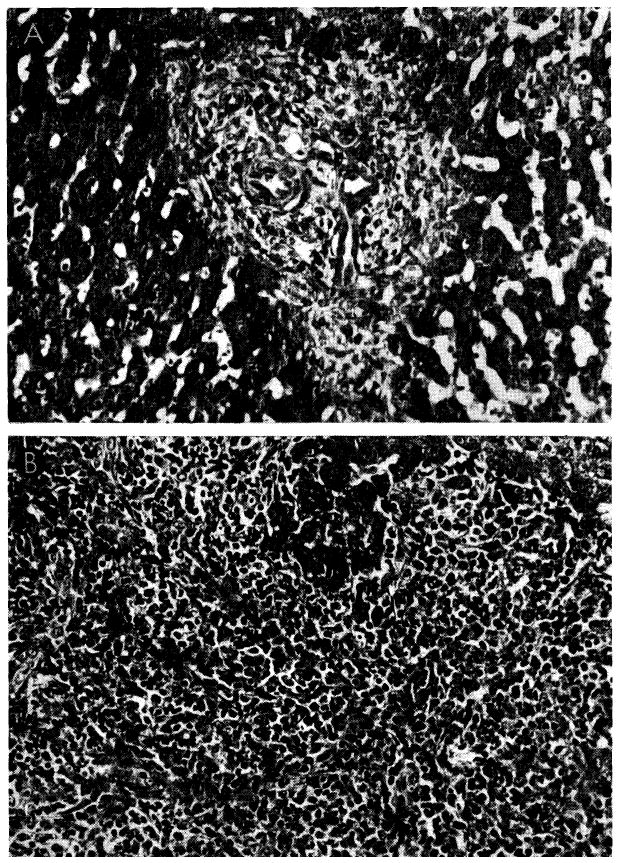
Biopsies from animal whose clinical course is shown in Figure 35. A, at 123 days there are some increase of portal connective tissue, proliferation of small biliary ductules and slight cellular infiltration. B, 84 days after cessation of immunosuppressive treatment, there has been marked deterioration. Portal tract is heavily infiltrated by a mixture of mononuclears and neutrophils. Hematoxylin-eosin; ×300.
In the examinations of these canine homografts, histologic evidence indicting a vascular component of hepatic rejection was particularly sought in view of previous hypotheses that a reduction of blood flow was immunologically induced at either the sinusoidal level (51, 83) or more proximally in the larger arteries of the portal tracts (62). The findings in the above livers were compatible only with the former possibility. There was evidence in some of the electron micrographs of damage to the sinusoidal endothelium following contact with host lymphoid cells, a finding analogous to that reported in renal homografts (44) and one which could plausibly explain the centrilobular localization of the hepatocyte necrosis. The possibility of occlusion of larger vessels was unsupported, since the degree of tissue injury was relatively homogeneous without areas of selective destruction. Furthermore, injury to the hepatic artery or portal vein was not a prominent feature in the untreated series and was virtually never seen in the treated animals.
6. Changes in Host Organs after Canine Orthotopic Transplantation
After orthotopic transplantation to either the unmodified or the treated recipient, there is a high incidence of abnormalities of other organ systems (10, 51, 61, 83). Many of the dogs have duodenal ulcers with bleeding or perforation. Intussusception and pancreatitis are common. Atelectasis and pneumonitis are the commonest causes of early death. Such changes can be viewed as complications of surgical technic and anesthesia, of immunosuppression and of altered hepatic metabolism.
The etiology of certain other host changes has been less easy to classify. In early reports from our laboratories (10, 83), findings were described after liver homotransplantation to unmodified recipients which included lymph node enlargement with cortical thinning, decrease of follicles and increase of lymphocytes and plasma cells. These alterations as well as histologic abnormalities in the bone marrow, kidneys and lungs were thought to be part of a “general host reticulo-endothelial response to the antigenic stimulus of the homograft.” Mc-Bride and his associates (51) contended that the host changes, with the possible exception of those in the lymph nodes and spleen, were nonspecific since most were also present in their experience after hepatic autotransplantation.
The question of host alteration after liver transplantation has since been subjected to reappraisal (72, 93). In dogs not treated with immunosuppression, changes in the character of the recipient lymphoid tissue were regularly observed, the shifts of differential cell population being similar to those of the cells invading the homograft. The lymph nodes of these animals were enlarged. Microscopically there was a proliferation of large cells with pyroninophilic cytoplasm around the post-capillary venules. Macrophages containing hemosiderin and debris were present in larger numbers than normal. In animals surviving beyond the 7th day, the medullary cords became filled with plasma cells. A similar proliferation of large pyroninophilic cells and later of plasma cells was seen in the spleens of animals dying from the 3rd day onward. In dogs receiving azathioprine despite which the transplant was rejected, there were also lymphoid changes which paralleled those in the homograft.
Widespread fibrinoid necrosis in host vessels such as that observed by Murray et al. (64) in dogs after renal homotransplantation was not seen, and changes in the recipient lungs, kidneys, adrenals and pancreas either were not present or were nonspecific.
These findings support McBride’s conclusion that changes in organs other than the host’s lymphoid tissue are unlikely to be directly related to the response of the host to the homograft. They also, however, provide evidence that a general lymphoid reaction to the hepatic homograft always occurs in the untreated recipient and can be seen in the treated animal when immunosuppression is inadequate. In these circumstances, the spleen and lymph nodes are sites of intense proliferation of large primitive lymphoid cells and later of plasma cells which are cotemporaneously found in the homograft. These changes resemble those that occur in the nodes draining a skin homograft (75) and in the spleen and lymph nodes after a renal homograft (73).
Although it thus appears that a generalized response to the homograft occurs in the host lymphoid tissues, it is encouraging to note that little clinical or pathologic evidence of a significant graft-versus-host reaction could be obtained. The danger of this possibility was first appreciated by Billingham and Brent (8), who were able to produce “runt disease” by inoculating fetal hosts with lymphoid homografts from mature donors. Later, Trentin (100) described a “secondary homologous disease” which was thought to result from the attack of mature lymphoreticular homografts upon irradiated and immunologically defenseless adult hosts. In the animals in both types of experiments, weight loss, cachexia, diarrhea and loss of hair were observed (8, 100). After liver transplantation, some dogs exhibited one or more of these phenomena, but only in the presence of poor homograft function. In animals with good liver function, the shortened red cell half-life described earlier (Fig. 23) was the only evident adverse effect on the host, a finding which may be comparable to the graft-versus-host auto-hemolytic disease produced by Simonsen (79) in chick embryos.
7. Auxiliary Canine Liver Homotransplantation
The attractive features of auxiliary liver homotransplantation have been recognized by many authors, beginning with Welch and his colleagues (30, 102), who first described the “secondary liver homograft” in 1954. For clinical use it would be desirable to retain whatever function is possessed by the diseased host liver so that immediate dependence on the transplant would not be necessary. In addition, the difficult technical problems presented by resection of the diseased liver could be avoided, a matter of practical consideration in treatment of patients with benign disease who almost always have portal hypertension and extensive venous collaterals.
Many studies of the pathology and physiology of the auxiliary liver transplant have been performed (33, 54, 58, 66, 77, 91, 99). Unfortunately, the findings from most of these investigations were interpreted with the assumption that the observed changes were caused solely by immunologic forces. However, in Section 3, it was shown that the splanchnic blood flow makes a unique contribution to hepatic structure and function.
As would be expected, similar physiologic considerations are of vital concern in the auxiliary liver homotransplant. When the second liver is placed in the pelvis and revascularized so that systemic blood is directed into the homograft portal vein (Fig. 37, A), there is a very rapid shrinkage of the transplant even in the recipients treated with immunosuppression, beginning within two or three weeks after operation (91,99). The animal’s own liver, which is supplied with splanchnic venous blood, retains its normal size (Fig. 38). The marked reduction of size is accompanied by striking histologic changes. There are hepatocyte loss around the central veins and accompanying collapse of the supporting reticulin. Many of these auxiliary livers exhibit no more evidence of rejection than orthotopic livers at a comparable postoperative interval, but the degree of liver cell loss is far greater.
Fig. 37.
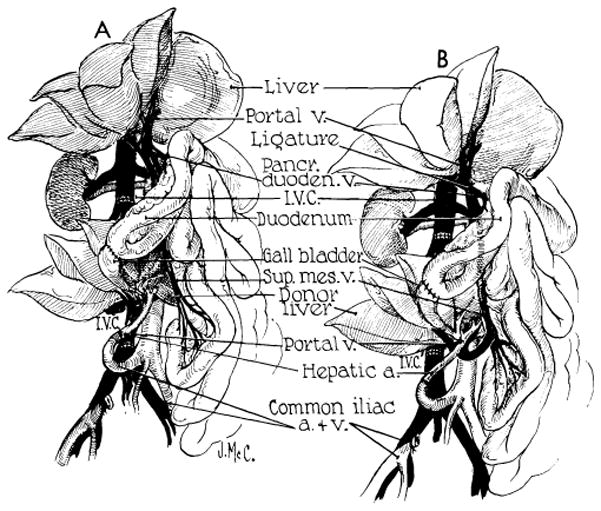
Auxiliary liver transplantation. A, method of Welch. Note that portal venous inflow is from the inferior vena cava. The homograft undergoes rapid atrophy. B, modification of Welch method in which nonhepatic splanchnic flow is diverted through the homograft. With this preparation, the homograft retains its size and the animal’s own liver shrinks. It is usually more convenient to bring the hepatic artery behind rather than in front of the portal vein as depicted. (Figs. 37, 39 and 40 from Marchioro et al. [58].)
Fig. 38.
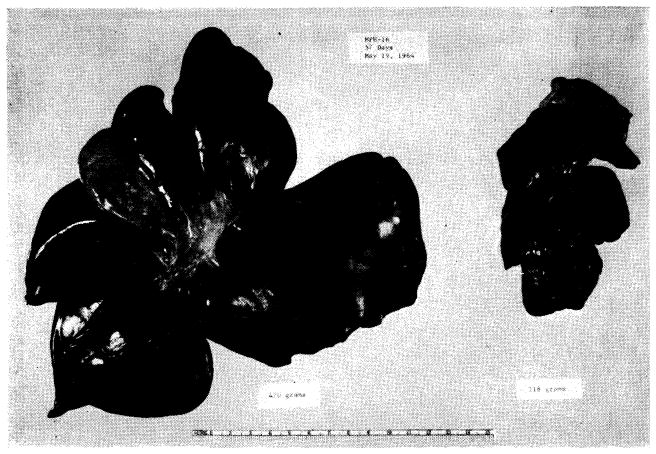
Results with auxiliary liver transplantation when revascularized by Welch method (Fig. 37, A). Note marked atrophy of the homograft (right) and no change in the animal’s own liver (left). General morphology of the homotransplant is quite recognizable. The two specimens were obtained 45 days after auxiliary transplantation.
The explanation for this acute atrophy of the homotransplant has been described in Section 3. If the vascular reconstruction to the homograft is altered so that the transplanted tissue receives splanchnic venous flow and the animal’s own liver does not, a similar atrophic process occurs in the autologous hepatic tissue (Table 2). The histologic features of centrilobular hepatocyte dissolution and reticulin collapse are now present in the animal’s own liver (Fig. 39). In addition to good retention of its general morphologic features, the optimally vascularized auxiliary homograft has also been demonstrated to be able to sustain life for protracted intervals in dogs (58). In several animals receiving this operation, the autologous liver was removed at a second-stage operation 60–77 days after the initial transplantation. All animals on whom this was attempted woke promptly from anesthesia, and one dog lived for an additional 56 days, or 126 days total survival after homotransplantation (Fig. 40).
TABLE 2.
Results after Auxiliary Homotransplantation by Modified Welch Method (Fig. 36, B)
| Wt. of Donor, Kg. | Wt. of Recipient, Kg. | Total Survival, Days | Removal Host Liver | Survival after Hepatectomy, Days | Wt. of Homograft at autopsy, Gm. | Wt. of Host Liver at Autopsy or Hepatectomy Gm. |
|---|---|---|---|---|---|---|
| 14.1 | 15.2 | 28 | No | Not done | 325 | 322 |
| 14.5 | 19.5 | 25 | No | Not done | 380 | 306 |
| 15.4 | 18.2 | 126 | Yes | 49 | 656 | 225 |
| 18.6 | 20.5 | 69 | Yes | 8 | 478 | 211 |
| 18.2 | 18.6 | 52 | No | Not done | 440 | 300 |
| 20.0 | 22.9 | 101 | Yes | 28 | 492 | 310 |
Note that the auxiliary organ outweighed recipient animal’s own liver in every instance, despite the fact that the donor dogs were of smaller size.
Fig. 39.
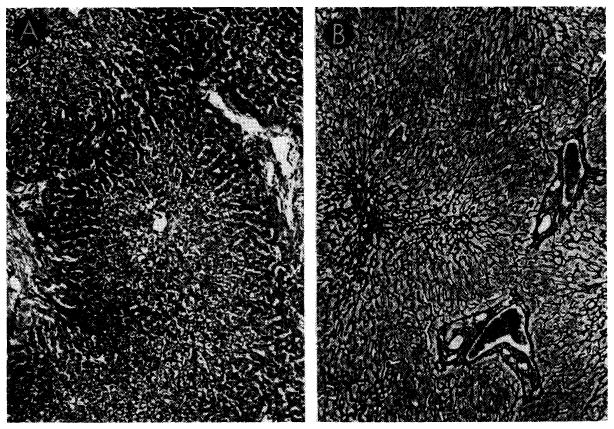
Biopsies of their own livers from two dogs revascularized as shown in Figure 37, B. A, 35 days after operation, there is centrizonal necrosis of the liver cells. Hematoxylin-eosin; orig mag. ×30. B, 51 days after operation, centrilobular reticulin has collapsed. Reticulin stain; orig. mag. ×45.
Fig. 40.
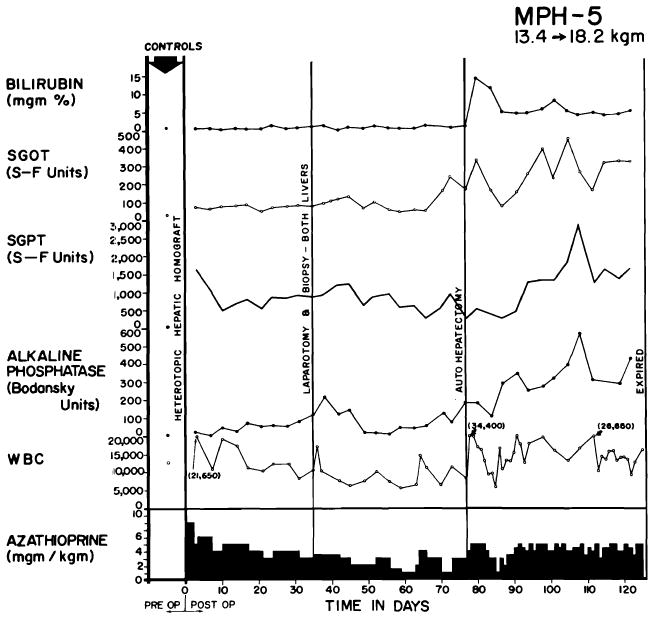
Clinical course of a dog whose homograft was vascularized by method shown in Figure 37, B. Note abrupt bilirubinemia which followed removal of the dog’s own liver (autohepatectomy). The dog lived for 49 days with sole dependence on the homograft, ultimately dying of wound dehiscence and evisceration which followed repeat biopsy.
These experiments have done much to clarify the physiologic requirements for homotransplantation of an auxiliary liver. Apparently there is a competition between the coexisting livers for some metabolite or other substance in the portal venous blood. That organ which has first access to the portal flow retains its functional and morphologic integrity. The other organ, whether it be the homograft or the autologous liver, undergoes atrophy predominantly affecting the centrizonular area.
Whether this substrate competition will prove to be of important clinical significance is not known. In the benign diseases for which liver transplantation might be contemplated, there would be pre-existing failure of the recipient patient’s liver, so that it might be incapable of efficient metabolite extraction. Should this prove to be the case, the exact method of auxiliary homograft revascularization will be less critical.
8. Orthotopic Liver Transplantation in Man
Seven attempts have been made at orthotopic homotransplantation of the liver in man. The world experience is summarized in Table 3. The indication for operation was primary carcinoma of the liver in four instances, metastatic carcinoma in two and biliary atresia in the other. All seven patients died in 23 days or less.
TABLE 3.
World Experience of Human Orthotopic Liver Transplantation
| Case & Date* | Donor-Recipient Blood Types | Donor-Recipient Age & Sex | Recipient Disease | Cause of Donor Death | Donor Death to Revascularization | Survival | Cause of Death | ||
|---|---|---|---|---|---|---|---|---|---|
| 1. Denver (88–91) 3/1/63 | A+ | A+ | 3 M | 3 M | Biliary artresia | Cardiac arrest during craniotomy | 420 min. | 4 hours | Operative hemorrhage |
| 2. Denver (88–91) 5/5/63 | A+ | A+ | 55 M | 48 M | Cirrhosis & hepatoma | Terminal brain tumor | 152 min. | 22 days | Pulmonary emboli |
| 3. Denver (88–91) 6/24/63 | O− | O+ | 69 M | 67 M | Cholangio carcinoma | Storke | 192 min. | 7½ days | Pulmonary emboli; GI bleeding |
| 4. Denver (88–91) 7/16/63 | O+ | A− | 73 M | 52 M | Cirrhosis & hepatoma | Coronary | 174 min. | 6½ days | Pulmonary emboli; congestive heart failure |
| 5. Denver (88–91) 10/4/63 | O+ | O+ | 64 M | 29 F | Cirrhosis & hepatoma | GSW head (suicide) | 164 min. | 23 days | Common duct necrosis; bile peritonitis; ? rejection with hepatic failure; hemorrhagic dilathesis |
| 6. Moore (62, 63) 9/16/63 | B+ | A+ | 35 M | 58 M | Metastatic colon carcinoma | GSW head (homicide) | 170 min. | 11 days | Pneumonitis; liver abscesses; hepatic failure |
| 7. Demirleau (18) 1/64 | O | A | 71 M | 75 M | Metastatic colon carcinoma | Bilateral gangrene of legs | 180 min. | 3 hours | Operative hemorrhage |
Reference numbers in parentheses.
Technical considerations
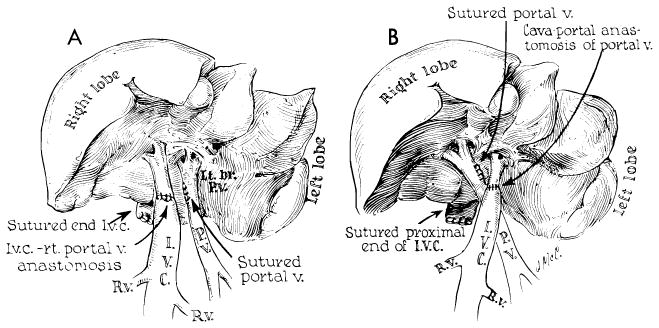
Orthotopic transplantation was performed as shown in Figures 41 and 42 (88). The operation differs from that performed by us in the dog in that a direct end-to-end hepatic arterial anastomosis is performed (Fig. 42, B). The surgical details of implantation involve for the most part utilization of well-standardized surgical methods. The vascular anastomoses frequently must be done with short cuffs and with limited exposure, necessitating intraluminal suturing technics (Fig. 42, C). The greatest hazard is in performance of the inferior vena caval anastomosis at the diaphragm. Both donor and recipient segments of the vena cava often have orifices of small posterior phrenic tributaries which are unknowingly severed when the respective livers are removed. Such open orifices are located a short distance from the cut edge of, and can only be seen from the inside of, the vessel. Failure to suture the openings during the caval-caval anastomosis results in later hemorrhage at a time when the presence of the homograft makes secondary exposure of this area almost impossible. The double-suture method developed in dogs for this anastomosis has virtually eliminated this problem (Fig. 42, C).
Fig. 41.
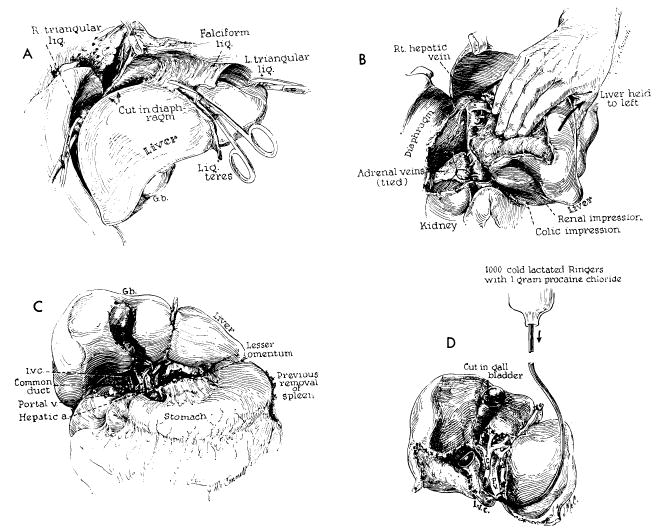
Technical steps in removal of cadaveric homograft. All maneuvers except D are also carried out in excising the recipient patient’s own diseased liver. A, incision of restraining ligaments of liver. B, dissection of raw area and ligation of adrenal veins. C, completed dissection of gastrohepatic ligament. D, cold perfusion of excised homograft. Note that gallbladder is opened. Failure to remove bile may result in autolysis of the extrahepatic biliary system during period of devascularization.
Fig. 42.
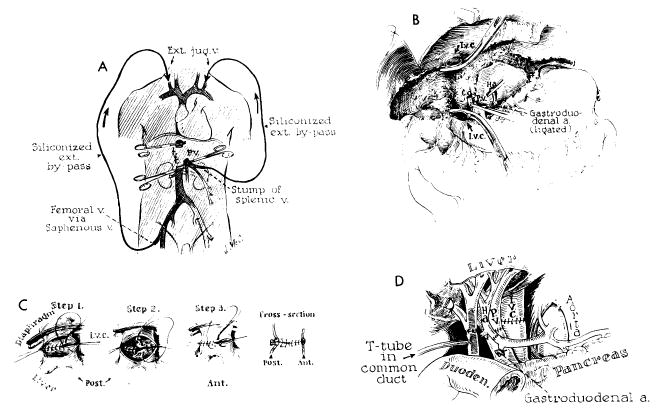
Technical steps in human homotransplantation. A, external bypass systems used for decompression of blocked venous pools. External shunt from the portal system was shown to be unnecessary and only vena caval bypass is required. B, operative field after removal of recipient’s diseased liver. C, anastomosis of suprahepatic vena cava. Great care must be exercised to prevent suture-line leakage, because exposure of this suture line is difficult after homograft is in place. D, completed anastomoses of portal structures. Note that T-tube is placed through a stab wound in recipient portion of reconstructed common duct. An alternative method of internal biliary drainage is with cholecystoenterostomy.
Reconstitution of internal biliary drainage in infants will probably be most effectively done with a Roux-en-Y cholecystjejunostomy. In all of the six adults treated with orthotopic homotransplantation, choledochocholedochostomy has been performed, stenting the anastomosis with a T-tube inserted through the recipient portion of the common duct (Fig. 42, D). This metbod of biliary reconstruction may prove to carry an increased hazard since the principal arterial supply to the common duct comes from retroduodenal sources. Although Parke, Michels and Ghosh (65) have demonstrated that vascular contributions to the common duct also come retrograde from the hepatic arteries in the hilus of the liver, whether these will consistently insure viability of the homograft portion of the duct system can be learned only by further experience. The potential danger of ischemic necrosis can be appreciated from the fact that one of the fatalities in the Denver series resulted from slough of the homograft common duct (89, 91). Absolon’s (1) recipient of an auxiliary liver homograft died of the same cause.
A special note should be made about decompression of the venous systems which must be temporarily occluded during orthotopic homotransplantation. As mentioned earlier, failure to obtain satisfactory drainage from these venous pools in the dog results in certain failure, particularly when any degree of venous hypertension is allowed to develop in the splanchnic bed. In man, the necessity for providing drainage from at least the portal system is probably not so important. Child has demonstrated that acute occlusion of the portal vein in monkeys and human beings is usually well tolerated, presumably because collateral channels are more abundant in primates than in lower forms. It has, in fact, been demonstrated in the clinical cases of orthotopic homotransplantation that drainage of the portal system is not mandatory. In six of the seven patients treated thus far, external portal by-passes were not used, and in the seventh, flow ceased after a few minutes. The conclusion seems justified that a single effective by-pass from the inferior vena cava to the superior vena cava is all that is required for adequate venous return to the heart from both the inferior vena caval and the portal systems. In the Denver cases, the external shunt has been connected from the leg veins to the jugular system (Fig. 41, A). An alternative use of the external by-pass is that described by Moore (63) and Demirleau (18), who inserted the lower end of the external by-pass directly into the abdominal vena cava.
Changes in coagulation
It would be superfluous to recount all the details of this limited clinical experience. Nevertheless each well-studied case has contributed to that body of knowledge previously accumulated in animal work. One of the features of hepatic transplantation which had been incompletely appreciated from laboratory studies concerned the changes in coagulation which occur after the operative procedure. It was known from canine experiments that acute fibrinolysis was common during removal of the diseased liver and its replacement. Similar bleeding problems have been observed in the clinical cases, and in two instances these resulted in fatal operative hemorrhage (18, 88). The hemorrhagic tendency presumably did not result from an acute deficiency of those clotting factors which are synthesized in the liver. Analysis of plasma fibrinogen during the liverless phase in two cases of the Denver series (88) did not reveal a clinically significant drop, a finding in accord with many experimental studies which show that a substantial decrease of clotting factors after total hepatectomy requires several hours to develop. Instead, the important finding appeared to be an explosive increase of the plasma fibrinolytic activity which developed within minutes, both during manipulation of the liver and after its removal. The exact cause of this change is open to some speculation. As a working hypothesis, it might be assumed that the liver normally elaborates a substance which inhibits conversion of plasminogen to plasmin (fibrinolysin), presumably by a deterrent action on a plasminogen activator. Absence of the liver or severe hepatic injury during operative manipulation could conceivably permit uncontrolled conversion of plasminogen to plasmin by removal of such a restraining influence on the activator system.
To promote clotting during the critical anhepatic phase, purified human fibrinogen and epsilon amino caproic acid (EACA) were administered to Patients 2–4 in the Denver series. Unquestionably, these therapeutic maneuvers decreased the hemorrhagic tendency at the time of operation, but the delayed state of hypercoagulability which followed during the next few days had not been anticipated. In all three cases, thrombosis of the terminal inferior vena cava, iliac or femoral veins occurred, leading to pulmonary emboli which in each case were directly responsible for or an important contributing cause of death.
The problem of coagulation control is thus a complex one in which the danger of bleeding is succeeded by that of hypercoagulation. Many therapeutic approaches have been evaluated in the laboratory, including total body heparinization during or after the transplantation with or without neutralization, promotion of clotting during homotransplantation with purified fibrinogen and differing doses of EACA and therapeutic transfusion with fresh blood. In Case 5 of the Denver series the patient was heparinized with 2 mg./kg. during the anhepatic phase and no neutralizing antiheparin agents were used thereafter. The patient survived the operation but only after a massive blood loss of 9,000 cc. We believe that the operation should be done without any iatrogenic interference with the coagulation mechanism either to prevent or to promote clotting. Instead, all efforts are directed to mechanical control of hemorrhage. Insertion of the external by-pass is carried out with care to prevent intimal venous injury.
Immunosuppression
The most effective agent for prevention or treatment of rejection is azathioprine, as outlined earlier. In all of the human cases, this drug was used. In addition, prednisone was employed (Fig. 43). This steroid, which has been of unequivocal value in controlling or reversing rejection of renal homografts (31, 55, 86), cannot be effectively tested in dogs since it regularly produces gastrointestinal ulcerations and hemorrhage in this species. Actinomycin C and azaserine have also been intermittently administered to the human patients, but with unknown effect.
Fig. 43.
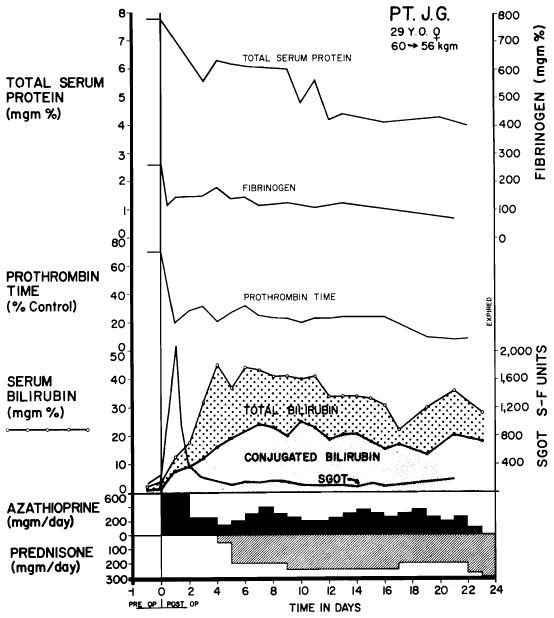
Serial chemical studies in Patient 5 of Colorado series. Note serious abnormalities of various measurements. SGOT increase immediately after operation was the highest observed in any case. Immunosuppressive therapy is depicted at the bottom. (Figs. 43, 44 and 49 from Starzl et al. [91].)
Homograft function
The function obtained after human orthotopic liver homotransplantation has to some extent reflected the unfavorable circumstances which surround procurement of the donor organ. In the first patient of the Denver series, the use of a dying liver contributed to the operating room death. In each of the other cases, more or less severe anoxic injury was documented (Figs. 43 and 44), and one of the critical problems in care has been to differentiate the effects of ischemic damage from those caused by rejection. In Moore’s patient (62, 63), relentlessly developing jaundice could not be halted even with maximal immunosuppressive therapy, and it was difficult from the autopsy findings to differentiate the changes due to protracted anoxia from those due to immunologic attack. Patient 5 in the Denver series posed a similar problem. After operation, the plasma bilirubin level rose to 42 mg./100 ml. before reversal of the trend was seen. Other liver function was poor through life (Fig. 43), beginning with severe transitory rises of SGOT and SGPT and continuing with depressed plasma protein levels, prolonged prothrombin time and elevations of serum pyruvate and lactate values. The degree of injury in three of the Colorado cases was less severe and more reversible (Fig. 44), suggesting that suitable cadaveric livers can be obtained for human use in appropriate circumstances. Since the liver is a major source of protein synthesis, an observation of its function in Patient 5 of the Denver series is of unique interest. The haptoglobin type of the donor patients was determined to be Smithies’ 2-2 (81). The recipient plasma contained only type 2-1. After operation, the only haptoglobin found in the recipient was type 2-2, providing strong evidence that the liver is the only site of haptoglobin synthesis (91).
Fig. 44.
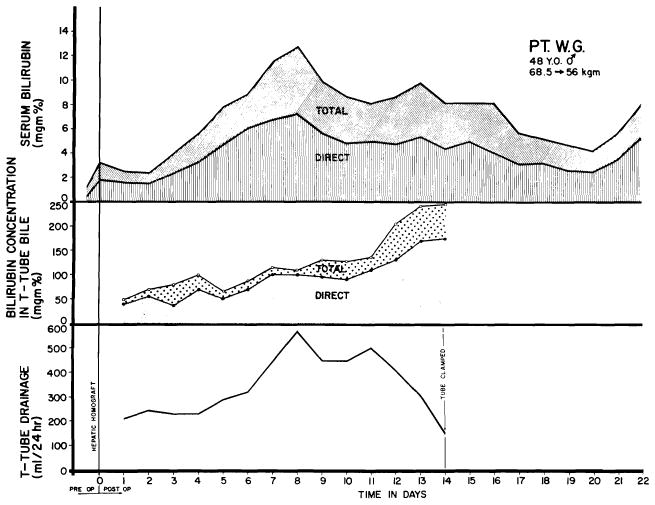
Course of Patient 2 of Colorado series, showing relationships of serum bilirubin, T-tube drainage volume and bilirubin content of T-tube bile. Note temporary worsening of jaundice after transplantation, probably due to ischemic injury rather than rejection.
Donor selection
From the foregoing remarks and from those in section 1, it is evident that more careful selection of cadaveric donors will be necessary in the future. A postmortem liver is suitable for use only in very special circumstances in which good circulation is maintained until shortly before death, and in a setting in which extracorporeal perfusion may be instituted almost immediately thereafter. The ideal donor is one in the terminal phase of a nonmalignant disease process. Patients with cerebral gliomas, massive strokes or extensive traumatic injuries of the central nervous system are examples. In some of these disorders, part of the accepted treatment of the malady from which the patient is dying is total body hypothermia, and the prior utilization of this form of therapy is probably of great coincidental benefit in postmortem tissue preservation. Such was the situation with the donor for Moore’s orthotopic homograft (62, 63).
The utilization of cadaveric donors who have had a sudden death is usually not feasible. To obtain prompt cadaveric perfusion, it is necessary to have an entire team of surgical and supporting personnel at the bedside at the moment of death. Permission from the family must be obtained in advance. Observations on hepatic function of the dying patient are essential. Moment-to-moment recording of urinary output during the final hours of life is of value in assessing the general circulatory adequacy of vital organs. Finally, preparation of the recipient patient must be begun several hours in advance. Thus the most suitable candidates for cadaveric donation are those in whom death is known, with a reasonable degree of certainty, to be impending for several hours.
Because of the special circumstances surrounding both the prospective donor and his family at this time, it is imperative that the transplantation team not be involved with terminal medical care. The physicians caring for the intended donor must continue their therapy up to and including the ultimate certification of death without consideration of the events to follow. Only after death can the transplant team insinuate itself. Its role is simply that of performing an immediate, rather than delayed, autopsy.
From postmortem studies of Peters and his associates (68), it has been discovered that the vascular supply of the liver is almost never seriously involved by atherosclerosis. Consequently, the influence of age on the quality of the liver homograft is not so great as with other organs such as the kidney. The mode of death is the factor of greatest importance in obtaining suitable tissue. An aged patient dying in the circumstances described above would be preferable to a young, healthy one in whom the conditions of death did not fulfill the general requirements cited. In all age groups, it is necessary to inquire about drinking habits, previous jaundice, neoplasia and chronic infectious disease. It is possible that prospective donors who have had a chronic debilitating disease with inadequate nutrition should be excluded.
A special note should be made concerning the practice of seeking donors who have the same blood type as the recipient. With renal homografts, it has been found that the use of donors with different blood types is feasible, provided the general scheme in use in blood banks is followed in which O is the universal donor and AB the universal recipient (86). With livers, this type of mismatch may be dangerous, inasmuch as the liver is capable of erythroclastic activity (41) and conceivably could cause an autohemolytic disease similar to, but more severe than, that described earlier in dogs. In one case in the Denver series of liver transplants, an O+ liver was transplanted to an A+ recipient without demonstrably harmful effects. Mismatches in the other direction which violate the universal donor scheme are known from experience with renal homotransplantation to be hazardous (86) and should definitely be avoided in future work with livers.
Survival after orthotopic homotransplantation
The Causes of failure after orthotopic homotransplantation are listed in Table 3. Two patients died immediately of operative hemorrhage. The five others lived for 6½ to 23 days. Two died of hepatic failure (Table 3), which may have been due more to extensive ischemic injury of the homograft than to failure to prevent its rejection. In one of these two patients there was necrosis of the donor portion of the reconstructed common duct. The primary factor in the deaths of the other three patients was pulmonary embolization (88–91). A contributing cause of death in three instances was late gastrointestinal hemorrhage due to acute ulceration of the esophagus, stomach or duodenum (88–91).
Pathology of the human homograft
Detailed accounts of the changes in Moore’s and Demirleau’s cases have not been published. The pathologic features in the five human homografts carried out at the University of Colorado were previously reported (88–91) but were recently reviewed and reinterpreted by one of us (K.A.P.) in light of the vast amount of animal pathologic material which had subsequently been analyzed. One homograft was unusual (Case 1) in that the whole liver had undergone autolysis, probably due to the seven hours of partial or complete ischemia.
Patient 4 died after 6½ days. The cytoplasm of all of the hepatocytes was filled with very fine fat droplets. There was a small amount of centrilobular atrophy, but only the hepatocytes immediately adjacent to the central veins were affected (Fig. 45, A). In this central zone the sinusoids were dilated and congested. The reticulin framework of the lobules was intact. A moderate number of infiltrating cells was present in the connective tissue of the portal tracts (Fig. 45, B). Most of these cells were small lymphocytes, but a few were larger lymphoid cells with pyronin-positive cytoplasm. Plasma cells and neutrophils were also present in small numbers. This cellular infiltrate was patchy, being dense around some portal veins. There were no infiltrating cells around the central veins or sinusoids. Several small hepatic artery branches showed focal areas of fibrinoid necrosis affecting the intima and media; a few others showed a small amount of intimal thickening (Fig. 45, C). In some of the smaller portal tracts there was proliferation of bile ductules (Fig. 45, D).
Fig. 45.
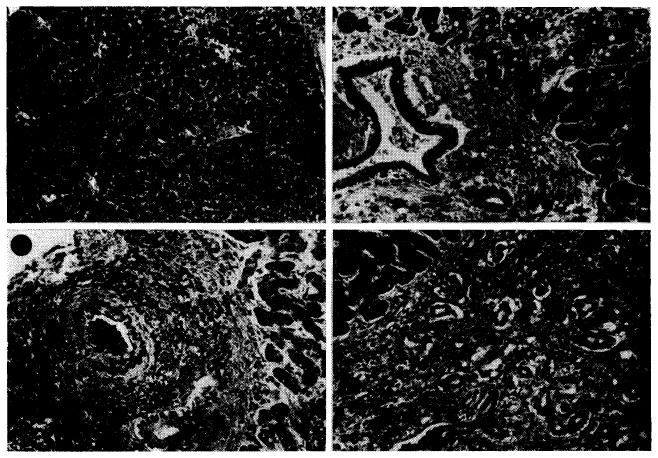
Case 4 of Colorado series; treated human orthotopic hepatic homograft at 6½ days. A, lobular pattern is normal but hepatocytes contain fine fat droplets. Hematoxylin-eosin; ×15. B, portal tract is filtrated by a few cells, most of them lymphocytes. Many of the hepatocytes contain lipid droplets in their cytoplasm. Hematoxylin-eosin; ×150. C, small branch of the hepatic artery shows thickening of its intima. Weigert stain for elastic tissue counterstained with van Gieson’s; ×100. D, a small portal tract is heavily infiltrated by mononuclear cells and contains many proliferating bile ducts. Hematoxylin-eosin; ×150.
In Case 3, the homograft was examined after 7½ days. The cytoplasm of the centrizonal and midzonal hepatocytes contained large droplets of fat (Fig. 46, A). The hepatocytes immediately adjacent to the central vein had disappeared and those just peripheral to this area were atrophic and contained large amounts of lipofuscin and fat in their cytoplasm (Fig. 46, B). In this central zone the sinusoids were dilated and congested. There was collapse of the centrilobular part of the reticulin framework with condensation of reticulin around the central veins. A small amount of centrilobular cholestasis was present. A moderate number of infiltrating cells was found around the portal and central veins. Although many of these cells were mononuclears, some were neutrophils and a few, eosinophils. Of the mononuclears, the majority were lymphocytes, but about 5% were large pyronin-positive cells and plasma cells. In many small hepatic artery branches, foci of fibrinoid necrosis involved the whole thickness of the wall (Fig. 46, C). A few of the larger hepatic artery branches showed marked fibroelastic thickening of the intima. There was slight proliferation of bile ductules in the smaller portal tracts.
Fig. 46.
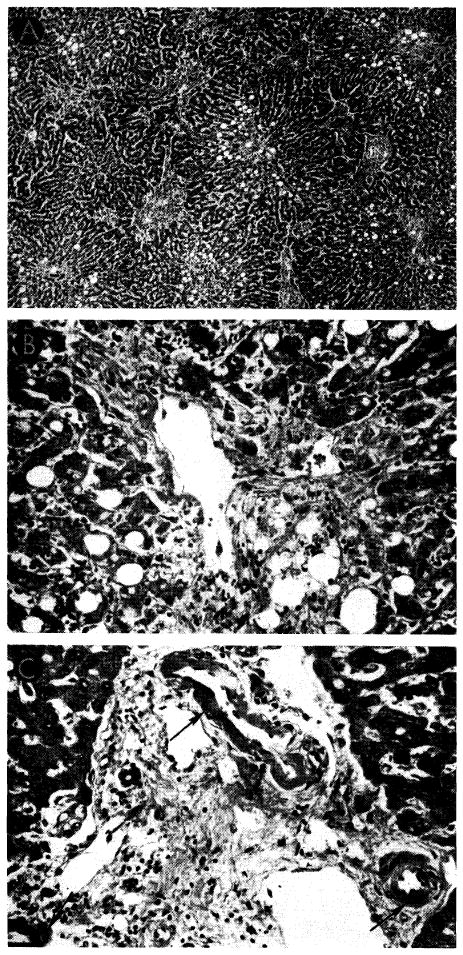
Case 3 of Colorado series: treated human orthotopic hepatic homograft at 7½ days. A, cytoplasm of centrizonal and midzonal hepatocytes contains large droplets of fat. Portal tracts are conspicuous because of some bile duct proliferation and cellular infiltration. Hematoxylin-eosin; ×20. B, hepatocytes immediately adjacent to the vein have disappeared and those just peripheral to this area are atrophic and contain fat droplets in their cytoplasm. Cellular infiltration around the hepatic vein is slight. Hematoxylin-eosin; ×250. C, three small branches of hepatic artery (arrows) show fibrinoid necrosis of their walls. Connective tissue contains a few infiltrating mononuclear cells. Hematoxylin-eosin; ×250.
Patient 2 lived for 22 days. The centrilobular hepatocytes contained much lipofuscin, and those cells immediately adjacent to the central vein were atrophic (Fig. 47, A–C). A few areas of extensive necrosis were present, each involving several hepatic lobules; in these foci the lobular reticulin framework had collapsed (Fig. 47, D–F). Elsewhere the lobular architecture was intact. The portal connective tissue was increased and in a few places reticulin bands linked adjacent portal tracts. There was a moderate amount of cellular infiltration around the veins in the smaller portal tracts. Although most of these cells were small lymphocytes, a few were plasma cells. No larger pyroninpositive cells were seen. Macrophages, full of hemosiderin, were common in the portal connective tissue. Several of the bile ducts in the portal tracts contained casts of bile pigment. There were proliferation of bile ductules in the smaller portal tracts and centrilobular and midzonal cholestasis. The Kupffer cells throughout the lobules were laden with hemosiderin, lipofuscin and some bile particles.
Fig. 47.
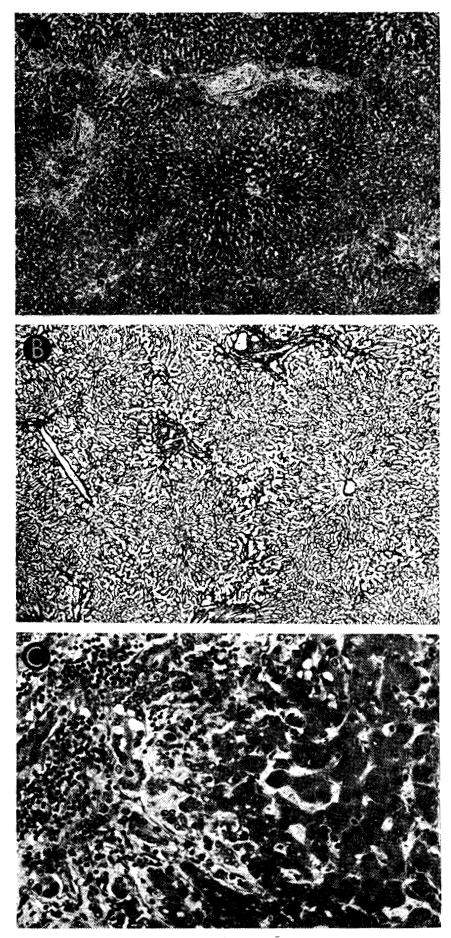
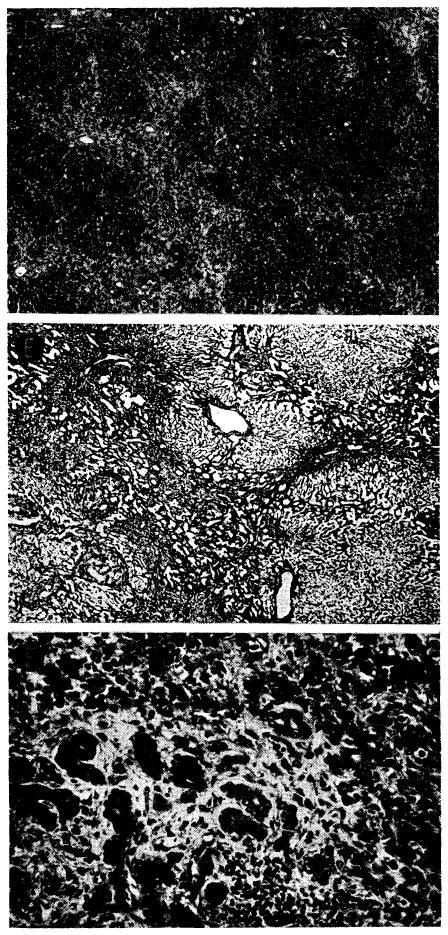
Case 2 of Colorado series: treated human orthotopic hepatic homograft at 22 days. A, B and C show typical appearance of the tissue; D–F show most severely damaged porting, these necrotic areas being focal and uncommon. A, centers of lobules appear dark because the hepatocytes contain excess lipofuscin. Increase of portal connective tissue was probably present before homotransplantation. There is patchy cellular infiltration, particularly in smaller portal tracts. Hematoxylin-eosin; ×20. B, lobular architecture is essentially normal. Reticulin stain; ×20. C, portal tract is infiltrated by mononuclear cells. There is proliferation of small bile ducts. Hematoxylin-eosin; ×250. D, area in which whole lobules have undergone necrosis, being replaced by collagen and reticulin fibers, many small bile ducts and foci of infiltrating mononuclear cells. Hematoxylin-eosin; ×20. E, area of massive necrosis. Reticulin has collapsed, destroying normal lobular pattern. Reticulin stain; ×20. F, massive necrosis has occurred and hepatocytes are now replaced by collagen and reticulin fibers, proliferating bile ducts and foci of infiltrating mononuclear cells. Hematoxylin-eosin; ×250.
Survival for Patient 5 was 23 days. The hepatocytes in the central and middle zones of the lobules were necrotic: only those in the peripheral zone, adjacent to the portal tracts, remained viable (Fig. 48, A). In the necrotic areas the sinusoids were dilated and congested. Some hemorrhages were present in the central zone. The central part of the lobular reticulin framework had collapsed (Fig. 48, B), and there was linking of the collapsed zones one to another. A few collagen fibers were present in these bands and zones of condensed reticulin fibers. Cholestasis was present in the middle and peripheral parts of the lobules. Kupffer cells, laden with hemosiderin, remained in the centrilobular necrotic areas. Cellular infiltration was generally slight in extent, although a few foci of heavier infiltration were present. The cells were only present in the portal tracts and consisted entirely of mononuclears, of which only about 10% had pyroninophilic cytoplasm; the rest were small lymphocytes. The bile ducts and branches of the hepatic artery were normal.
Fig. 48.
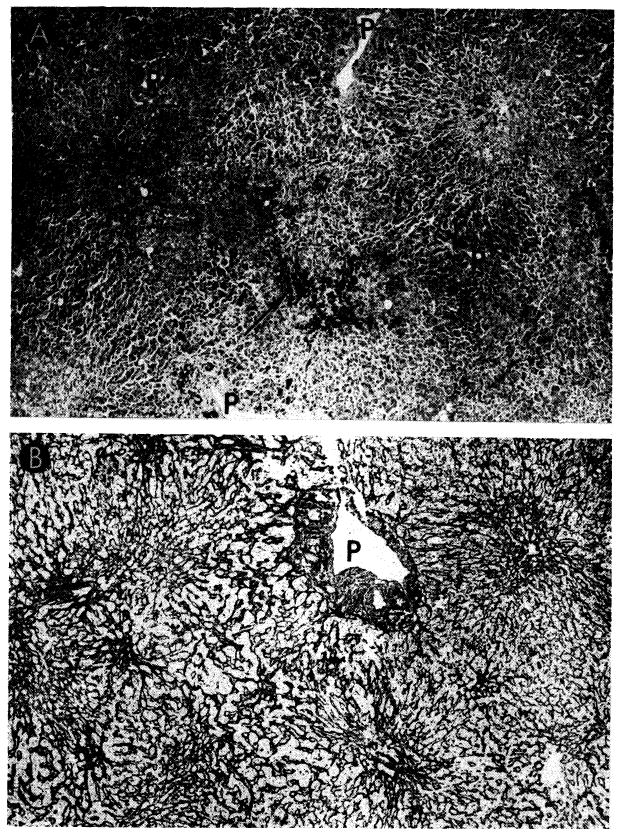
Case 5 of Colorado series; orthotopic homograft at 23 days. A, there is centrizonal and midzonal necrosis of liver cells. Only those near portal tracts (P) survive. Some hemorrhages are present in central zones (arrow). Hematoxylin-eosin; ×30. B, There is collapse of the supporting centrilobular reticulin where liver cells have undergone necrosis. P, portal tract. Reticulin stain; ×30.
In summary, the homografts in the four patients surviving operation all had some cellular infiltration around the portal veins. Although varying in density from one portal tract to another, it was never severe and was often mild. The larger portal tracts were rarely more than lightly infiltrated. Eighty-five to 90% of the cells were small lymphocytes, between 5 and 10% were pyroninophilic cells, and the rest were neutrophils and the occasional eosinophil. Of the pyroninophilic cells, some were larger lymphoid types, while a few were plasma cells. Only in one case were these cells present around the central veins. There were foci of fibrinoid necrosis in the walls of several of the small branches of the hepatic artery in two of the homografts. Centrilobular and midzonal necrosis of hepatocytes was the outstanding feature in the longest-lived patient, but the others only showed atrophy and disappearance of the liver cells immediately adjacent to the central veins. Various degrees of collapse of the central part of the lobular reticulin framework were present in three of the four cases and were accompanied by centrilobular cholestasis.
It is interesting that there was relatively good preservation of the general lobular architecture and cell structure in three of these four cases, that only one had the extensive centrilobular liver cell necrosis that is so common in treated canine hepatic homografts and that cellular infiltration was not severe in any. These histologic findings support the contention that the homograft reaction played no decisive role in the death of four of the patients. The centrizonal and midzonal necrosis in the homograft from the patient who lived 23 days may have been due to a homograft reaction, but other factors could have accounted partly or entirely for these changes.
9. Auxiliary Liver Transplantation in Man
Single attempts at auxiliary transplantation have been made in Minneapolis (1), Richmond (39), Denver and Cleveland (37) in an effort to treat biliary atresia, nutritional cirrhosis or hepatic carcinoma (Table 4). All 4 patients died within the first 22 postoperative days.
TABLE 4.
World Experience of Human Auxiliary Liver Transplantation
| Case & Date* | Donor-Recipient Blood Types | Donor-Recipient Age & Sex | Recipient Disease | Cause of Donor Death | Donor Death to Revascularization | Survival | Cause of Death | ||
|---|---|---|---|---|---|---|---|---|---|
| 1. Absolon (1) 11/3/64 | A+ | O− | 2F | 1M | Biliary atresia | Cardiac surgery | About 4 hr. | 13 da. | Bile peritonitis & sepsis |
| 2. Hume (39) 12/5/64 | O+ | O+ | 55M | 17F | Malignant hepatoma | Head injury | About 3½ hr. | 2 da. | Pneumothorax & respiratory insufficiency |
| 3. Hermann (37) 2/12/65 | A+ | O− | 5M | 1F | Biliary atresia | Carniopharyngioma | 345 min. | 1 da. | Severe acidosis |
| 4. Denver 2/20/65 | A+ | A+ | 67M | 50M | Laennec’s cirrhosis | Cardiac arrest | 262 min. | 22 da. | Pneumonitis |
Reference numbers in parentheses.
The first trial was by Absolon (1) on Nov. 3, 1964. The recipient patient was a 13-month-old boy of O— blood type who had intra- and extra- hepatic biliary atresia. Before operation the total serum bilirubin content was 22 mg./ml. and alkaline phosphatase, 150 Somogyi units. The cadaveric liver was obtained from a 2-year-old girl of A+ blood type who died during open heart correction of a cardiac malformation. Death was pronounced while she was on pump-oxygenator support, and hypothermic perfusion was continued until the liver was removed. The period of complete absence of circulation during insertion of the liver was 37 minutes, despite which significant ischemic injury occurred inasmuch as the recipient SGOT rose acutely to a high of 1,200 units during the first three postoperative days. Excessive bleeding was not encountered during transplantation, the estimated blood loss being 50 ml.
Certain technical problems were encountered by Absolon which may be troublesome in future cases. Apparently, the original plan was to perform splenectomy and place the homograft in the splenic fossa. Severe space limitations were encountered. Ultimately, left nephrectomy was also performed to obtain more room, and the transplant was positioned in the left paravertebral gutter. The hepatic artery was attached end-to-end to the divided left iliac artery and the subhepatic vena cava to the common iliac vein. The vena cava above the liver was ligated and the homograft portal vein then anastomosed to the lower vena cava of the graft to provide an extra route of vascular decompression. Thus the auxiliary liver had only an arterial supply. The common duct was ligated and internal biliary drainage provided with a Roux-en-Y cholecystenterostomy.
In spite of the physiologically disadvantageous method of revascularization, there was unequivocal evidence of homograft function. The serum bilirubin level dropped to 5 mg./1 ml. and alkaline phosphatase to 25 Somogyi units. The patient’s anti-A isoagglutinin titer fell sharply after operation, suggesting that this pre-existent humoral antibody was being bound by the hepatic tissue. After nine days, bile peritonitis resulted from slough of the ligated homograft common duct. The child died after 13 days of klebsiella and pseudomonas sepsis with pneumonia, wound infection and septicemia. During life, immunosuppression was provided with azathioprine and steroids. At autopsy, the homograft was said to have had no evidence of rejection.
A physiologically more normal method of revascularization was used by Hume (39) and Hermann (37). In their cases, splenectomy was also performed and the homograft placed in the left upper abdomen. The hepatic artery was anastomosed to the splenic artery in one instance and to the aorta in the other. Both patients had portal hypertension. The venous inflow to the homograft portal vein was from the distal end of the splenic vein, thus providing retrograde nonhepatic splanchnic flow for the homograft. The suprahepatic vena cava was ligated above the entrance of the hepatic veins, and the vena cava below the liver was anastomosed to the left renal vein. Unfortunately, the patients died after one and two days, respectively.
In both cases, there may have been unacceptably protracted ischemia. Both donors had nephrectomy for renal homotransplantation before extracorporeal perfusion was started. Abnormalities of carbohydrate metabolism were evident postoperatively. Hume’s patient, whose own liver was removed after completion of transplantation, required continuous glucose infusion to prevent hypoglycemia. In the child treated by Hermann, profound metabolic acidosis developed. This has also been observed in three of the Denver cases after orthotopic transplantation (88, 91), with sharp increases of serum pyruvate and lactate values (Fig. 49).
Fig. 49.
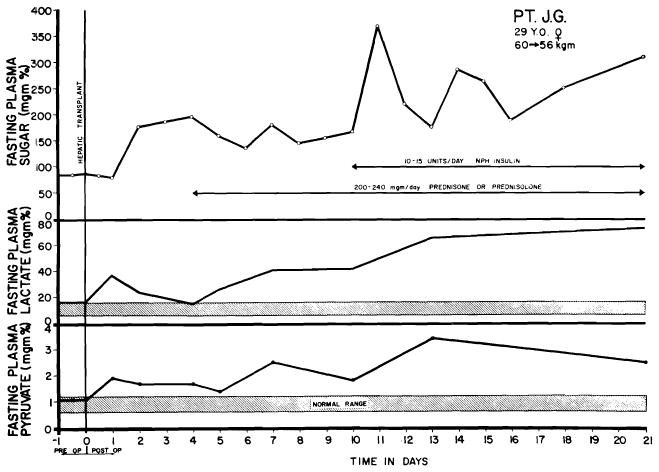
Changes in serum lactate and pyruvate in Case 5 of Denver orthotopic series. Transitory elevation immediately followed operation, with subsequent decline toward normal. The secondary rise beginning the 4th day was progressive. Note normal to increased levels of plasma sugar. Shaded bars indicate normal ranges for serum lactate and pyruvate. Severe acidosis, presumably of similar etiology, caused the early death of Hermann’s patient with auxiliary liver transplantation.
Hume’s case illustrates a technical problem which may be of the utmost importance in patients who do not have massive pre-existing ascites. Before operation, pneumoperitoneum was induced in his patient in order to provide increased space. Despite this precaution, it was impossible to close the wound, necessitating removal of the recipient’s own diseased liver.
The longest survival after auxiliary liver transplantation was achieved in a 50-year-old patient of A+ blood type who was treated with this procedure at the Denver Veterans Administration Hospital on Feb. 20, 1965, and who lived for more than three weeks. He had been known to have cirrhosis since 1957. A short time after hospitalization because of severe liver failure, serum bilirubin content was 45 mg./1 ml. Prothrombin time was 20–30% and serum albumin 2.68 gm./100 ml. On Feb. 17, 1965, he had a massive esophageal hemorrhage which was controlled by an emergency end-to-side portacaval shunt.
Three days later, a homograft was obtained from a 67-year-old cadaver, also of A+ blood type. The donor died in the operating room at the conclusion of a popliteal thrombectomy. External and open cardiac massage were provided without interruption for the next two hours, after which hypothermic extracorporeal perfusion (see Fig. 4) was carried out for the ensuing 105 minutes. During the cadaveric perfusion, it was noticed that the venous return was three or four times greater if massage were continued than when it was temporarily stopped. The cadaveric liver was removed in a cooled state 213 minutes after death and was revascularized 49 minutes later.
The technic of transplantation was very similar to the canine operation shown in Figure 50, differing in principle only in that an Eck fistula had been previously constructed. The right colon was reflected and the celiac axis anastornosed to the side of the recipient’s abdominal aorta. The liver was interposed into an excised segment of inferior vena cava so that the portal vein was supplied by systemic venous blood (Fig. 50). Internal biliary drainage was with a Roux-en-Y cholecystojejunostomy. The recipient patient was in terminal condition at the beginning of the operation and at its conclusion required a tracheostomy. Since no clot formed, the mechanical control of hemorrhage was extremely difficult and time-consuming. Due to the previous presence of massive ascites, there was no difficulty in closing the abdomen around the extra organ.
Fig. 50.
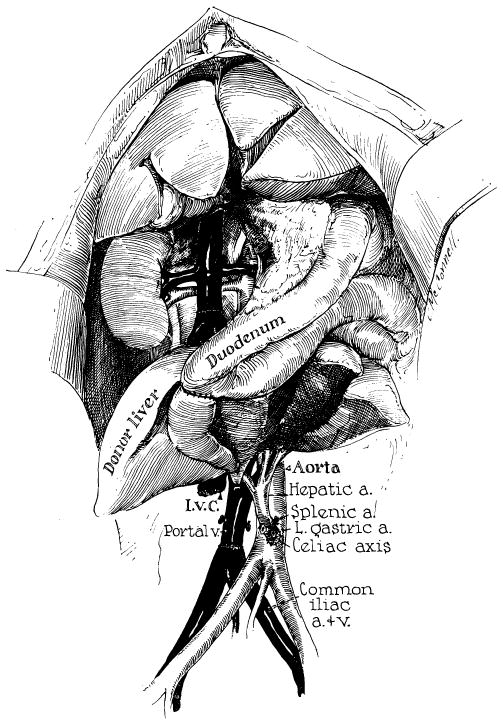
Technic of canine auxiliary liver transplantation similar to that used for a patient at the University of Colorado, except that an end-to-side portacaval shunt had already been placed in the patient and a Roux-en-Y cholecystojejunostomy was employed rather than the method of internal biliary drainage shown above. (From Starzl et al. [90].)
As mentioned in section 7, the method of portal revascularization can have a profound influence on both structure and function of hepatic tissue when two organs are simultaneously present since they appear to be in competition for metabolites present in the venous blood returning from the intestine. Unfortunately, the exact method of transplantation used for this patient has never been evaluated in the laboratory, but it seemed likely that the problem of substrate competition between the patient’s own liver and the homograft would not be a serious one. First, the autologous liver was so diseased that its function was already inadequate to sustain life. Second, any splanchnic flow which it had been receiving was eliminated at the time of the portacaval anastomosis.
The early course after operation appeared to confirm this expectation. Within two days, the bilirubin level had dropped to 7 mg./1 ml. Prothrombin time rose to 50–80%. There was improvement of the patient’s semicomatose state. The degree of acute ischemic injury was surprisingly slight, presumably because the combination of extracorporeal cadaveric perfusion and cardiac massage was highly effective; the highest postoperative SGOT or SGPT value was 150 units. Protracted ileus and acute renal failure were life-threatening extrahepatic complications, but by the 12th postoperative day the patient resumed an oral diet and began to walk. Red cell survival studies during the first two weeks revealed a half-life of 7 days, a finding similar to that described earlier in the animal experiments.
Immunosuppression for this patient consisted of administration of azathioprine, prednisone and actinomycin C. A rejection crisis was diagnosed on the 10th postoperative day on the basis of a secondary rise of bilirubin and a decline of prothrombin time to 13%. This was reversed by increased steroid doses plus local homograft irradiation. The patient died of pulmonary sepsis after 22 days.
Footnotes
Aided by USPHS grants no. AM 06283, AM 06344, HE 07735, AM 07772, AI 04152 and FR 00051, and by a grant from the Medical Research Council of Great Britain.
References
- 1.Absolon KB. Personal communication. 1964.
- 2.Anderson MC, Bergan JJ, Salan JR. Experimental production of hepatocellular damage with cortisone. S Forum. 1960;11:13. [PubMed] [Google Scholar]
- 3.Arey LB. Throttling veins in the livers of certain mammals. Anat Rec. 1941;81:21. [Google Scholar]
- 4.Baker BL, et al. The effect on liver structure of treatment with adreno-corticotropin under varied dietary conditions. Am J Anat. 1948;82:79. doi: 10.1002/aja.1000820104. [DOI] [PubMed] [Google Scholar]
- 5.Bauer W, et al. The control of circulation through the liver. J Physiol (London) 1932;74:343. doi: 10.1113/jphysiol.1932.sp002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter JH. The effects of pyridine administration and their prevention by methionine. Proc Am Fed Clin Res. 1945;2:77. [Google Scholar]
- 7.Best CH, Taylor NB. The Physiological Basis of Medical Practice. Baltimore: Williams & Wilkins Company; 1955. [Google Scholar]
- 8.Billingham RE, Brent L. The reaction of injected homologous lymphoid tissue cells against the host. Transplant Bull. 1957;4:177. [Google Scholar]
- 9.Bollman JL. The animal with an Eck fistula. Physiol Rev. 1961;14:607. [Google Scholar]
- 10.Brock DR, Starzl TE. Histopathologic alterations associated with the transplanted homologous dog liver. Exper & Molec Path. 1962;1:187. doi: 10.1016/0014-4800(62)90020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown H, et al. Cold preservation of liver for homotransplantations. S Forum. 1964;15:215. [PubMed] [Google Scholar]
- 12.Chapman ND, et al. Function of the ex-vivo pig liver perfused with human blood. S Forum. 1964;15:90. [PubMed] [Google Scholar]
- 13.Child CG, III, et al. Liver regeneration following portacaval transposition in dogs. Ann Surg. 1953;138:600. doi: 10.1097/00000658-195310000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke DA, et al. 6-Mercaptopurine: Effects in mouse sarcoma 180 and in normal animals. Cancer Res. 1953;13:593. [PubMed] [Google Scholar]
- 15.Couch NP, Curran WJ, Moore FD. The use of cadaver tissues in transplantation. New England J Med. 1964;271:691. doi: 10.1056/NEJM196410012711401. [DOI] [PubMed] [Google Scholar]
- 16.Coulson RA, Brazda FG. Influence of choline, cystine and methionine on toxic effects of pyridine and certain related compounds. Proc Soc Exper Biol & Med. 1948;69:480. doi: 10.3181/00379727-69-16762. [DOI] [PubMed] [Google Scholar]
- 17.Dameshek S. “Immunoblasts” and “immunocytes”—An attempt at a functional nomenclature. Blood. 1953;21:243. [PubMed] [Google Scholar]
- 18.Demirleau, et al. Tentative d’homogreffe hépatique. Mem Acad chir. 1964 Feb 3;M-179 [PubMed] [Google Scholar]
- 19.Deysach LJ. The nature and location of the “sphincter mechanism” in the liver as determined by drug actions and vascular injections. Am J Physiol. 1941;132:713. [Google Scholar]
- 20.Drill VA. Hepatotoxic agents. Mechanism of action and dietary inter-relationship. Pharmacol Rev. 1952;4:1. [PubMed] [Google Scholar]
- 21.Drill VA. Lipotropic effects of vitamin B12 and other factors. Ann New York Acad Sc. 1954;57:654. doi: 10.1111/j.1749-6632.1954.tb36442.x. [DOI] [PubMed] [Google Scholar]
- 22.Eck NVK. Voprosu o perevyazkie vorotmois veni: Predvaritelnoye soobshtshjenye. Voenno-med zhur. 1877;130:1. [Google Scholar]
- 23.Einhorn M, Davidsohn I. Hepatotoxicity of mercaptopurine. JAMA. 1964;188:802. doi: 10.1001/jama.1964.03060350028007. [DOI] [PubMed] [Google Scholar]
- 25.Eiseman B, et al. Isolated liver perfusion for reducing blood ammonia. Arch Surg. 1961;83:356. doi: 10.1001/archsurg.1961.01300150030004. [DOI] [PubMed] [Google Scholar]
- 26.Fagraeus A. Nomenclature of Immunologically Competent Cells. In: Wolstenholme GEW, O’Connor M, editors. Cellular Aspects of Immunity. Boston: Little, Brown & Company; 1960. p. 3. [Google Scholar]
- 27.Fiscus WG, et al. Effect of spleen homotransplants in animals subjected to sublethal doses of total body irradiation. S Forum. 1963;14:182. [PubMed] [Google Scholar]
- 28.Ford WW. Bacteriology of healthy organs. Tr A Am Physicians. 1900;15:389. [Google Scholar]
- 29.Goodell JPB, Hanson PC, Hawkins WB. Methionine protects against mapharsen liver injury in protein-depleted dogs. J Exper Med. 1944;79:625. doi: 10.1084/jem.79.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodrich EO, et al. Homotransplantation of the canine liver. Surgery. 1956;39:244. [PubMed] [Google Scholar]
- 31.Goodwin WE, et al. Human renal transplantation: Clinical experiences with 6 cases of renal homotransplantations. J Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 32.György P, Goldblatt H. Treatment of experimental dietary cirrhosis of liver in rats. J Exper Med. 1949;90:73. doi: 10.1084/jem.90.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagihara P, Absolon KB. Experimental studies on homologous heterotopic liver transplantation. Surg, Gynec & Obst. 1964;119:1297. [PubMed] [Google Scholar]
- 34.Hallett EB, et al. Liver blood flow, hepatic glucose production and splanchnic oxygen consumption in normal dogs and following Eck fistula: Liver blood flow before and after splenectomy. Surg, Gynec & Obst. 1952;95:401. [PubMed] [Google Scholar]
- 35.Hahn M, et al. Die Eck’sche Fistel zwischen der unteren Hohlvene und der Pfortader und ihre Folgen für den Organismus. Arch exper Path u Pharmakol. 1893;32:161. [Google Scholar]
- 36.Heer FW, Silvius DE, Harper HA. The relation between hepatic blood flow and function in normal dogs and dogs with cirrhosis after portacaval shunt or transposition. S Forum. 1960;9:282. [PubMed] [Google Scholar]
- 37.Hermann R. Personal communication. 1965.
- 38.Hines JR, Roncoroni M. Acute hepatic ischemia in dogs. Surg, Gynec & Obst. 1956;102:689. [PubMed] [Google Scholar]
- 39.Hume D. Personal communication. 1965.
- 40.Ingle DJ, Meeks RC. Comparison of some metabolic and morphologic effects of cortisone and hydrocortisone given by continuous injection to rats. Am J Physiol. 1952;170:77. doi: 10.1152/ajplegacy.1952.170.1.77. [DOI] [PubMed] [Google Scholar]
- 41.Jacob HS, MacDonald RA, Jandl JH. The regulation of spleen growth and sequestering function. J Clin Invest. 1962;41:1367. doi: 10.1172/JCI104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kestens PJ, Austen WG, McDermott WV., Jr Biochemical and physiologic studies on the viable extracorporeal liver. S Forum. 1959;10:225. [PubMed] [Google Scholar]
- 43.Koch-Weser D, de la Huerga J, Popper H. Effect of choline supplements on fatty metamorphosis and liver cell damage in choline and protein deficiency. J Nutrition. 1953;49:443. doi: 10.1093/jn/49.3.443. [DOI] [PubMed] [Google Scholar]
- 44.Kountz SL, et al. Mechanism of rejection of homotransplanted kidneys. Nature (London) 1963;199:257. doi: 10.1038/199257a0. [DOI] [PubMed] [Google Scholar]
- 45.Kukral JC, et al. Hepatic function after canine liver transplantation. Arch Surg. 1962;85:157. doi: 10.1001/archsurg.1962.01310010161022. [DOI] [PubMed] [Google Scholar]
- 46.Liem DS, Waltuch TL, Eiseman B. Function of the ex-vivo pig liver perfused with human blood. S Forum. 1964;15:90. [PubMed] [Google Scholar]
- 47.MacLean LD, Weil MH. Hypotension (shock) in dogs produced by Escherichia coli endotoxin. Circulation Res. 1956;4:546. doi: 10.1161/01.res.4.5.546. [DOI] [PubMed] [Google Scholar]
- 48.Maegaith BG. Microanatomy of the hepatic vascular system. Tr. 10th Conf; May 21–22, 1951; New York: Josiah Macy, Jr., Foundation; p. 181. [Google Scholar]
- 49.Makravarti M, Tripod J. The action in the perfused liver of acetylcholine, sympatheticomimetic substances and local anesthetics. J Physiol. 1940;97:316. doi: 10.1113/jphysiol.1940.sp003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mautner H, Pick EP. Ueber die durch Schockgifte erzeugten Zirkulationsveranderungen; der Einfluss der Leber auf Blutdruck und Schlagvolumen. Arch exper Path. 1929;142:271. [Google Scholar]
- 51.McBride RA, et al. Homotransplantation of the canine liver as an orthotopic vascularized graft. Am J Path. 1962;41:501. [PMC free article] [PubMed] [Google Scholar]
- 52.McGavic JD, et al. Analysis of mechanisms of drug induced tolerance in canine renal homotransplants. S Forum. 1963;14:210. [PubMed] [Google Scholar]
- 53.McIlvanie SK, McCarthy JD. Hepatitis in association with prolonged 6-mercaptopurine therapy. Blood. 1959;14:80. [PubMed] [Google Scholar]
- 54.Mehrez IO, et al. Homotransplantation of the canine liver: A new technic. Ann Surg. 1964;159:416. doi: 10.1097/00000658-196403000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marchioro TL, et al. The role of adrenocortical steroids in reversing established homograft rejection. Surgery. 1964;55:412. [PMC free article] [PubMed] [Google Scholar]
- 56.Marchioro TL, et al. Splenic homotransplantations. Ann New York Acad Sc. 1964;120:626. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marchioro TL, et al. The use of extracorporeal perfusion for obtaining postmortem grafts. Surgery. 1963;54:900. [PMC free article] [PubMed] [Google Scholar]
- 58.Marchioro TL, et al. Physiologic requirements for auxiliary liver homotransplantation. Surg, Gynec & Obst. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 59.Marchioro TL, et al. The role of splanchnic venous blood in hepatic physiology. S Forum. 1965;16:280. [PMC free article] [PubMed] [Google Scholar]
- 60.Moore FD, et al. One-stage homotransplantation of the liver following total hepatectomy in dogs. Transplant Bull. 1959;6:103. doi: 10.1097/00006534-195901000-00041. [DOI] [PubMed] [Google Scholar]
- 61.Moore FD, et al. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 62.Moore FD, et al. Immunosuppression and vascular insufficiency in liver transplantation. Ann New York Acad Sc. 1964;120:729. doi: 10.1111/j.1749-6632.1964.tb34765.x. [DOI] [PubMed] [Google Scholar]
- 63.Moore FD. Personal communication. 1965.
- 64.Murray JE, et al. Analysis of mechanism of immunosuppressive drugs in renal homotransplantations. Ann Surg. 1964;160:449. doi: 10.1097/00000658-196409000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parke WW, Michels NA, Ghosh GM. Blood supply of the common bile duct. Surg, Gynec & Obst. 1963;117:47. [PubMed] [Google Scholar]
- 66.Paronetto F, et al. Immunologic observations on homografts: I. The canine liver. Transplantation. 1965;3:303. doi: 10.1097/00007890-196505000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Patrick RS. The influence of cortisone and ACTH on experimental zonal necrosis of the liver. J Path & Bact. 1955;70:377. doi: 10.1002/path.1700700214. [DOI] [PubMed] [Google Scholar]
- 68.Peters GN, et al. In preparation. [Google Scholar]
- 69.Pierce JC, Varco RL. Effects of long term 6-mercaptopurine treatment upon kidney homotransplants in dogs. Surgery. 1963;54:124. [PubMed] [Google Scholar]
- 70.Popper H, Schaffner F. Liver: Structure and Function. New York: McGraw-Hill Book Company, Inc; 1957. [Google Scholar]
- 71.Porter KA, et al. Pathologic studies on immunosuppressive drug toxicity in dogs and man. Unpublished observations. [Google Scholar]
- 72.Porter KA, et al. Pathologic studies on rejection of the canine liver in unmodified and treated recipients. (in preparation) [Google Scholar]
- 73.Porter KA, et al. The role of lymphocytes in the rejection of canine renal homotransplants. Lab Invest. 1964;13:1080. [PubMed] [Google Scholar]
- 74.Rich AR, Cochran TH, McGoon DC. Marked lipemia resulting from administration of cortisone. Bull Johns Hopkins Hosp. 1951;88:101. [PubMed] [Google Scholar]
- 75.Scothorne RJ, MacGregor IA. Cellular changes in lymph nodes following skin homografting in the rabbit. J Anat. 1955;89:283. [PMC free article] [PubMed] [Google Scholar]
- 76.Sellers EA, Lucas CC, Best CH. Lipotropic factors in experimental cirrhosis. Brit M J. 1948;1:1061. doi: 10.1136/bmj.1.4561.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sicular A, et al. Studies of rejection of the homotransplanted canine liver. S Forum. 1963;14:202. [PubMed] [Google Scholar]
- 78.Silen W, et al. Studies of hepatic function in dogs with Eck fistula or portacaval transposition. Arch Surg. 1957;74:964. doi: 10.1001/archsurg.1957.01280120142017. [DOI] [PubMed] [Google Scholar]
- 79.Simonsen M. Biological incompatibility in kidney transplantation in dogs: Serological investigations. Acta path et microbiol scandinav. 1953;32:36. [PubMed] [Google Scholar]
- 80.Simonds JP. Fundamental physiologic reactions in anaphylactic and peptone shock. JAMA. 1919;73:1437. [Google Scholar]
- 81.Smithies O. Zone electrophoresis in starch gels and its application to studies of serum proteins. In: Anfensen CB Jr, et al., editors. Advances in Protein Chemistry. Vol. 14. New York: Academic Press, Inc; 1959. [DOI] [PubMed] [Google Scholar]
- 82.Starzl TE, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg, Gynec & Obst. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 83.Starzl TE, et al. Studies on the rejection of the transplanted homologous dog liver. Surg, Gynec & Obst. 1961;112:135. [PMC free article] [PubMed] [Google Scholar]
- 84.Starzl TE, et al. Canine liver homotransplants: The effect of host and graft irradiation. Arch Surg. 1962;85:460. doi: 10.1001/archsurg.1962.01310030108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Starzl TE, et al. Studies on hepatic blood flow and the rate of brom-sulphalein clearance in dogs with portacaval transposition. Surgery. 1962;52:660. [PMC free article] [PubMed] [Google Scholar]
- 86.Starzl TE. Experience in Renal Transplantation. Philadelphia: W. B. Saunders Company; 1964. [Google Scholar]
- 87.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg, Gynec & Obst. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 88.Starzl TE, et al. Homotransplantation of the liver in humans. Surg, Gynec & Obst. 1963;117:659. [PMC free article] [PubMed] [Google Scholar]
- 89.Starzl TE, et al. Clinical experience with organ transplantation. South M J. 1965;58:131. doi: 10.1097/00007611-196502000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Starzl TE, et al. Experimental and clinical homotransplantation of the liver. Ann New York Acad Sc. 1964;120:739. doi: 10.1111/j.1749-6632.1964.tb34766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Starzl TE, et al. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Starzl TE, et al. The effect of portacaval transposition upon carbohydrate metabolism: Experimental and clinical observations. Surgery. 1965;57:687. [PMC free article] [PubMed] [Google Scholar]
- 93.Starzl TE, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131. [PMC free article] [PubMed] [Google Scholar]
- 94.State D, Lichtenstein I. A study of the genesis of shock associated with experimentally induced hepatic necrosis in dogs. Surgery. 1956;39:12. [PubMed] [Google Scholar]
- 95.Steinberg H, Webb WM, Rafsky HA. Hepatomegaly with fatty infiltration secondary to cortisone therapy. Gastroenterology. 1952;21:304. [PubMed] [Google Scholar]
- 96.Svec MH, Freeman S. Effect of impaired hepatic circulation on plasma free amino acids of dogs. Am J Physiol. 1949;159:357. doi: 10.1152/ajplegacy.1949.159.2.357. [DOI] [PubMed] [Google Scholar]
- 97.Thaler H. Fatty liver and fat embolism. Deutsche med Wschnschr. 1962;87:1207. doi: 10.1055/s-0028-1111888. [DOI] [PubMed] [Google Scholar]
- 98.Thomas WD, Essex HE. Observations on the hepatic venous circulation with special reference to the sphincteric mechanism. Am J Physiol. 1949;158:302. doi: 10.1152/ajplegacy.1949.158.2.303. [DOI] [PubMed] [Google Scholar]
- 99.Thomford NR, Shorter RG, Hallenbeck GA. Homotransplantation of the canine liver: Survival and histology with and without Imuran. Arch Surg. 1965;90:527. doi: 10.1001/archsurg.1965.01320100071012. [DOI] [PubMed] [Google Scholar]
- 100.Trentin JJ. Tolerance and homologous disease in irradiated mice protected with homologous bone marrow. Ann New York Acad Sc. 1958;73:799. doi: 10.1111/j.1749-6632.1959.tb40859.x. [DOI] [PubMed] [Google Scholar]
- 101.Weil WR. Studies in anaphylaxis: XXI. Anaphylaxis in dogs; a study of the liver in shock and peptone poisoning. J Immunol. 1917;2:525. [Google Scholar]
- 102.Welch CS. A note on the transplantation of the whole livers in dogs. Transplant Bull. 1955;2:54. [Google Scholar]
- 103.Wiggers CJ, Opdyke DF, Johnson JR. Portal pressure gradients under experimental conditions including hemorrhagic shock. Am J Physiol. 1947;146:192. doi: 10.1152/ajplegacy.1946.146.2.192. [DOI] [PubMed] [Google Scholar]
- 104.Wolbach SB, Saiki T. A new anaerobic spore bearing bacterium commonly present in livers of healthy dogs and believed to be responsible for many changes attributed to aseptic autolysis of liver tissue. J M Res. 1909;21:267. [PMC free article] [PubMed] [Google Scholar]
- 105.Wyck Jvan, et al. Function of cadaver liver. Surgery. 1965;58:120. [PubMed] [Google Scholar]
- 106.Zukoski CF, Callaway JM. Adult tolerance induced by 6-methyl mercaptopurine to canine renal homograft. Nature (London) 1963;198:706. doi: 10.1038/198706a0. [DOI] [PubMed] [Google Scholar]


